Abstract
The aim of this study was to distinguish effects due to diet composition from those triggered by ruminal pH on fermentation patterns and microbial profiles in a continuous culture system (RUSITEC). The study followed a 2 × 2 factorial design, with two diets varying in the proportions of forage and concentrate and two pH levels in the culture medium. RUSITEC fermenters were used to simulate rumen fermentation and feed digestibility, fermentation end-products, microbial protein synthesis, microbial community, and long-chain fatty acid profiles in the digesta were determined. Multivariate analyses were applied to summarize the overall results. High concentrate (34% cereal grain, 32% hay) diets were more digestible (p < 0.05) than high forage (10% cereal grain, 78% hay) diets, resulting in a greater (p < 0.05) formation of most fermentation end-products and microbial protein in the rumen. However, there were no significant (p > 0.05) differences between diets in methane production. Ciliate protozoa, anaerobic fungi, some fibrolytic bacteria, hydrogenation of oleic acid, and relative proportion of conjugated linoleic acid were increased (p < 0.05) with high forage diets. A decline in rumen pH from 6.8 to 6.4 decreased (p < 0.05) feed digestibility, protein degradability, and the daily outputs of some fermentation end-products (gas, VFA, acetate, ammonia) but had no effect (p > 0.05) on the synthesis of microbial protein, and on the output of methane, propionate, butyrate or lactate. Minor changes in microbial community profile or the fatty acid relative proportions were observed within this pH range. The overall multivariate analysis revealed a clear discrimination between high-concentrate and high-forage diets, with subtler and less-defined pH effects on ruminal fermentation and microbial communities.
1. Introduction
Intensive meat (beef, lamb) and dairy production systems are reliant on the use of high-concentrate diets to meet the nutritional requirements of high-yielding ruminants [1]. The inclusion of starch and protein-rich grains in these diets is increased at the expense of more fibrous feedstuffs aiming to increase the energy and protein concentrations in the ration. Feed efficiency is, in most cases, improved by increasing the consumption of grains by ruminants. As higher levels of production are intended, the inclusion of supplementary fats or oils becomes necessary to reach the high energy density required in these diets [2]. High inclusion levels of these concentrate feedstuffs will increase productivity but may alter the rumen ecosystem, raising the occurrence of undesirable nuisance in the way of ruminal acidosis or milk fat depression in cows [1,3]. The rumen is well-known as an anaerobic microbial fermenter, highly efficient in the degradation of fiber [4,5]. However, this valuable attribute can be endangered under suboptimal conditions, such as a low pH or restricted availability of fiber [6].
The rapid fermentation of starch and sugars in the rumen gives rise to the accumulation of short-chain organic acids (including lactate) and a decline in pH in the rumen. These changes cause a substantial shift in ruminal fermentation [7,8]. In the animal, feeding diets with higher availability of readily fermentable carbohydrates and less fiber is associated with a decline in ruminal pH, and it is challenging to elucidate which factor (diet composition or ruminal pH) is more determinant of the fermentation pattern [6,9]. Additionally, different types of substrate (fibrous or starchy) differentially promote the proliferation of one or other microbial communities, more or less adapted to the enzymatic digestion of the available substrate [9]. In turn, the predominant microbes (those groups or species prevailing under the conditions generated by the type of diet) will constrain the fermentation process to a defined pattern [6]. The situation becomes more intricate when diets are supplemented with unsaturated oils because fat can depress some ruminal microorganisms, the fatty acid biohydrogenation processes will be affected, and the oil-induced effects on fermentation can be different for high-forage or high-grain diets [10,11].
Over the last decades, one of the main challenges in the studies on rumen fermentation has been to distinguish between the effect of the nature of the diet (per se) and that triggered by the ruminal pH on the pattern of fermentation, the microbial composition and the fatty acid hydrogenation in the rumen [12,13]. Calsamiglia et al. [12] concluded that changes in the ruminal fermentation pattern in response to a high-grain diet were caused by the joined effects of the substrate (low fiber) and acidic pH. It is important to elucidate this question for designing the most appropriate feeding strategies in high-yielding ruminants. This goal is difficult to achieve with animals in vivo, as both factors are highly interdependent (pH will decline with a high-grain diet), and any results will be affected by confounding effects [12]. Thus, the use of in vitro continuous cultures simulating rumen fermentation under controlled conditions, such as the RUSITEC system, are valuable models to approach these objectives [14]. In these bioreactors, pH is maintained fairly stable regardless of the composition of the diet by using a strong buffer solution, and all outputs (fermentation gas, undigested residue, outflow of effluent) can be quantitatively collected and precisely sampled for analytical determinations [14]. Our hypothesis is that the forage-to-concentrate ratio in the diet and the ruminal pH can show distinctive effects on the microbiota and the fermentation pattern in the rumen. Thus, the objective of this work was to study the effects of the composition of the diet (high proportion of either concentrate or forage) used as a substrate of fermentation and of the pH at which rumen fermentation occurs on the fermentation pattern, the microbiota, and the relative proportion of long-chain fatty acids (and their hydrogenation) in the rumen. The RUSITEC continuous culture system is used as the model to simulate ruminal fermentation and to study simultaneously both factors discriminating their specific effects (effects of the forage-to-concentrate ratio of the diet regardless of rumen pH, or how a high-forage or a high-concentrate diet is fermented when rumen pH is decreased).
2. Materials and Methods
The in vitro fermenters (single flow continuous culture) used were specifically designed to mimic ruminal fermentation, referred to as RUSITEC (RUmen SImulation TEChnique, [14]).
2.1. Experimental Design
The experiment was planned according to a 2 × 2 factorial design, with two diets varying in the proportions of forage and concentrate (high forage diet F and high concentrate diet C) used as the fermentation substrate and two pH levels (higher or lower) in the culture medium. The ingredients and chemical composition of the experimental diets are shown in Table 1. The experimental diets differed in their forage-to-concentrate ratio, and both were supplemented with 3% sunflower oil to assess the effects of the sources of variation (diet and pH) on long-chain fatty acid hydrogenation. Each diet was prepared every three days and stored at 4 °C until use.

Table 1.
Ingredients and chemical composition of experimental diets 1.
To obtain the two different pH levels in the culture medium, two types of buffer solutions were infused into the fermenters. Thus, half of the fermenters received the mineral solution prepared according to McDougall [15]. This so-called artificial saliva is conventionally used in in vitro rumen fermenters intending to maintain a pH = 6.8 (close to neutral) in the culture medium. Modified saliva was infused into the other units. The composition of this buffer was formulated to reduce the pH, promoting a more acidified medium in the ruminal cultures (pH = 6.4). The composition of the two buffer solutions infused in the fermentation units to reach either high or low pH is shown in Table 2.

Table 2.
Composition of the two buffer solutions infused into the continuous cultures to obtain higher or lower pH in the culture medium.
2.2. RUSITEC in Vitro Continuous Cultures
Sixteen RUSITEC units arranged in two separate sets were used. Each unit was inoculated with rumen digesta to establish a continuous culture of mixed ruminal microorganisms. Samples of rumen contents from sheep were collected from a commercial abattoir. Four adult sheep from a farm where animals were fed mixed rations composed of forage and concentrates were selected for the collection of samples. When animals were slaughtered and eviscerated, rumina were opened and emptied, collecting well-mixed samples of rumen contents in thermos flasks. These samples were immediately carried to the laboratory so that the time from digesta collection to the culture inoculation was less than one hour. Samples of rumen contents were strained through several layers of gauze to separate the liquid (rumen fluid) and particulate rumen digesta. The inoculum for each fermentation unit consisted of 400 mL of filtered rumen fluid diluted in 250 mL of artificial saliva and 80 g of the particulate digesta remaining in the gauze. After inoculation, each fermenter was operated daily following the procedures described in detail by García-González et al. [16] and Vargas et al. [17]. Incubation was maintained at a stable temperature of 39 °C with a continuous infusion of the corresponding buffer solution at a rate of 556 mL/day in each vessel. Feed was provided in a nylon bag (100 µm pore size) containing 15 g of diet and put in a plastic container placed inside each fermentation vessel. The bag was withdrawn after 48 h of incubation. Therefore, when each fermenter was opened every day, two bags were present in the feed container, one bag that had been incubated for 48 h (which was removed and replaced with a new bag) and the bag that had been incubated for 24 h (which was left in the fermenter for the next 24 h).
2.3. Collection and Analysis of Samples
After a 7-day adaptation period, samples of liquid effluent (overflowing from each fermenter), digesta (incubation residue recovered in each bag), and fermentation gas (released to a tight bag) were collected for a period of 14 days. The volume of fermentation gas produced was measured daily, and a sample was collected to determine methane concentration. The volume of effluent liquid was also measured daily, and samples were taken for subsequent analysis of volatile fatty acids (VFAs), lactate, and ammonia. Dry matter (DM) and nutrient disappearance from the nylon bags after 48 h of incubation was assumed to represent the apparent digestibility (extent of degradation of the substrate by the processes of microbial digestion and fermentation). On day nine, (15NH4)SO4 (95% enriched, Sigma, Madrid, Spain) was infused into each fermenter for four days, and then samples of digesta and effluent were used to isolate a microbial pellet to estimate the synthesis of microbial protein using 15N as a marker [17]. On the last day of the experiment, composite samples (including fermenter supernatant, effluent, and incubation residues in bags) were collected, frozen at −80 °C, and freeze-dried [17]. These samples were prepared for analysis of long-chain fatty acids and DNA extraction [17].
2.4. Chemical Analyses
Feed diets and incubation residues were analyzed for DM content (AOAC method 934. 01), ash (AOAC method 942. 05), N (AOAC method 984. 13), and crude fat (bag filter AOCS procedure Am 5-04) as described by García-González et al. [16]. Determinations of neutral detergent fiber (NDF, AOAC method 2002.04), acid detergent fiber, and lignin (method AOAC 973.18) were made according to Van Soest et al. [18] using an ANKOM220 fiber analyzer (ANKOM Technology Corporation, Macedon, NY, USA). Methane (CH4) in fermentation gas and volatile fatty acid (VFA) in effluent were determined by gas chromatography, ammonia nitrogen and lactate in effluent samples by colorimetry, and non-ammonia N and 15N enrichment in digesta and microbial pellets by isotope ratio mass spectrometry according to the methods described in detail by García-González et al. [16]. Fatty acid profiles in rumen digesta were analyzed by gas chromatography after fat extraction and fatty acid methylation [19].
2.5. Microbial Community Profile by PCR Analyses
Copy numbers of major ruminal prokaryotic and eukaryotic domains (including methanogenic Archaea) and main ruminal bacterial species were determined by real-time qPCR using the Applied Biosystems StepOne PlusTM Real-Time PCR system (Applied Biosystems), with the primers detailed by Vargas et al. [17], and following the procedures described by Vargas et al. [20]. In the case of groups involved in fatty acid biohydrogenation, along with the quantitation of total Butyrivibrio spp. (group), some specific groups were also assessed, such as B. fibrisolvens, assumed to be responsible for the conversion of C18:2 c9c12 first to C18:2 c9t11 (rumenic acid) and then to C18:1 t11 (vaccenic acid) and the species B. proteoclasticus that would cause the complete hydrogenation of unsaturated C18 fatty acids to stearic acid. Standards used for copy number calculations were prepared as described by Andrés et al. [21].
2.6. Statistical Analyses
Data were analyzed using the GLM procedure of SAS [22] as a completely randomized experimental design with a 2 × 2 factorial arrangement: two diets (diet F or diet C) and two pH levels in the fermentation medium (higher or lower). The four experimental treatments were randomly assigned to 16 fermentation vessels. Each vessel was considered an independent experimental unit, resulting in four replicates per treatment. Variables measured at various times during the experiment were averaged across all sampling days to obtain a mean value for each fermenter. The following statistical model was used for the ANOVA:
where yijk represents the value for each individual observation, μ is the mean, αi is the effect of diet, βj the effect of rumen pH, αβ(ij) is the interaction between both factors, and εijk is the residual error (variability between replicates within each treatment). The Tukey test was used for multiple comparisons of means.
yijk = μ + αi + βj + αβ(ij) + εijk,
Hierarchical cluster analysis and principal components analysis (PCA) were used to assess the similarity and grouping of treatments based on all the data recorded in the study (feed digestibility, fermentation end-products, fatty acid profile and hydrogenation, microbial community profile). Multivariate analyses were run using the Paleontological Statistics (PAST) software package, version 4.03 [23].
3. Results
3.1. Ruminal Fermentation
The effects of diet and pH on rumen fermentation are shown in Table 3. The interaction between both factors was not significant (p > 0.05 for all variables).

Table 3.
Effect of diet and pH in the medium (high or low) on rumen fermentation in continuous cultures.
The rumen pH was lower with diet C (p = 0.031) because of the decrease in pH observed when this diet was incubated at a lower pH. Digestibility of OM, NDF, ADF, and cellulose was significantly higher for diet C than for diet F, with no significant differences between both diets in protein and fat digestibility (p > 0.05). The production of fermentation gas was increased (p < 0.01) when diet C was incubated, but diet had no effect (p > 0.05) on methane production. The production of VFA was also greater (p < 0.01) with diet C, and some differences in the fermentation pattern were observed. The daily production of butyrate, valerate, caproate, isoacids, and L-lactate, and the C2:C3 ratio were higher (p < 0.001) with diet C compared with diet F, whereas there were no significant differences (p > 0.05) between diets in acetate and propionate productions. Both the daily production of ammonia (p < 0.001) and the microbial protein synthesis (p < 0.05) were greater with diet C, but the efficiency of microbial protein synthesis (g/100 g FOM) was not affected (p > 0.05) by diet.
As expected, pH in the medium was affected by the strength of the buffer infused into the culture. The pH had a significant effect (p < 0.05) on the digestibility of OM, CP, and EE, which were decreased with a pH of 6.4 compared with a pH of 6.8. Digestible fiber (NDF, ADF, and cellulose) was not affected (p > 0.05) by pH. The fermentation gas production was higher (p < 0.05) at the higher pH, but pH did not affect (p > 0.05) methane production. Total VFA production tended to be higher (p = 0.05), and the production of acetate, iso-acids, and ammonia and the C2:C3 ratio were greater at pH 6.8 than at pH 6.4, but only when diet C was incubated. Rumen pH had no effect on the production of propionate, butyrate, and L-lactate. Microbial protein synthesis was not affected by pH in the culture medium.
3.2. Long-Chain Fatty Acid Profile in Rumen Digesta
The effects of diet (F or C) and medium pH (high or low) on the fatty acid profile in rumen digesta are shown in Table 4. The interaction between both factors was not significant (p > 0.05) for any variable. Fatty acid profile and hydrogenation rates in continuous cultures were not affected (p > 0.05) by rumen pH. In contrast, the proportions of saturated fatty acids C15:0 and C17:0 were lower (p < 0.01), and the sum of C18 fatty acids was higher (p < 0.05) with diet C than with diet F. As a proportion of total C18 fatty acids, monounsaturated C18:1 c12 and polyunsaturated C18:2 c9t11 and C18:3 c9c12c15 were greater (p < 0.05) with diet F than with diet C. However, C18:1 c9 in the digesta was higher (p < 0.05) with diet C. The biohydrogenation of oleic acid was higher (p < 0.05) with diet F, whereas that of linolenic acid tended to be higher (p < 0.10) with diet C. No significant (p > 0.10) differences were detected between diets in the overall hydrogenation of C18 fatty acids.

Table 4.
Effect of diet and pH in the medium (high or low) on fatty acid (FA) profile and hydrogenation in rumen digesta from continuous cultures.
3.3. Rumen Microbial Community Profile in Rumen Digesta
Effects of diet and pH in the medium (high or low) on rumen microbial community profile in the digesta of continuous cultures are illustrated in Figure 1a and 1b, respectively. Figure 1a shows the effects of diet as fold-change (in log2 units) of the high forage diet over the high concentrate diet. For total bacteria, methanogenic archaea, and most bacterial species, there were no significant (p > 0.05) differences between diets. There were significantly (p < 0.001) more copy numbers of protozoa, fungi, and Fibrobacter succinogenes and fewer of Streptococcus bovis with the high forage than with the high concentrate diet (Figure 1a). The effects of pH on the microbial community profile are shown as fold-change (in log2 units) of the higher pH (6.8) over the lower pH (6.4) (Figure 1b). There were no significant (p > 0.05) differences between both pH in the incubation medium except for S. bovis whose copy numbers were increased at a higher pH.
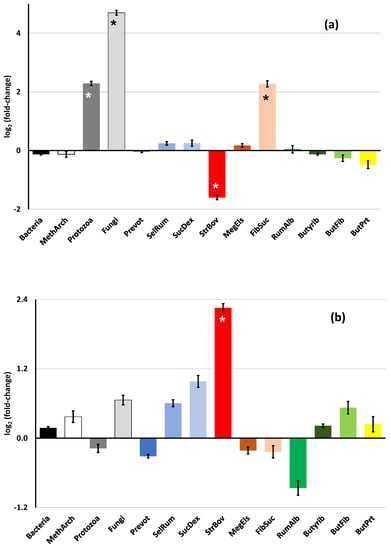
Figure 1.
Effects of diet (a) and pH in the medium (b) on rumen microbial community profile in continuous cultures. In Figure 1a, effects are shown as fold-change (in log2 units) of the high forage diet over the high concentrate diet. In Figure 1b, effects are shown as fold-change (in log2 units) of the higher pH (6.8) over the lower pH (6.4) in the incubation medium. The symbol “*” in the bar represents a significant (p < 0.001) difference; in bars with no “*” the difference between treatments did not reach statistical significance (p > 0.05). The abbreviations used for the microbial groups are MethArch = Methanogen Archaea; Prevot = Prevotella spp.; SelRum = Selenomonas ruminantium; SucDex = Succinivibrio dextrinosolvens; StrBov = Streptococcus bovis; MegEls = Megasphaera elsdenii; FibSuc = Fibrobacter succinogenes; RumAlb = Ruminococcus albus; Butyrib = Butyrivibrio group; ButFib = B. fibrisolvens; ButPrt = B. proteoclasticus.
3.4. Multivariate Analyses of Diet and pH Effects
Both cluster analysis (Figure 2) and PCA (Figure 3) revealed a clear differential grouping of vessels receiving diet F or diet C and a subtler separation attributed to the effect of medium pH (higher or lower). In the PCA plot, diets were clearly separated along component 1 (explaining more than 81% of the variance), indicating that this principal component would reflect the effects of the level of concentrate in the diet on the ruminal fermentation. Component 2 explained only 6.9% of the variance and did not reflect a clear discrimination of the vessels. From the loadings derived by the PCA, it was observed that the most influential variables on Component 1 were ammonia production and biohydrogenation of C18:3 c9c12c15 (higher on the positive side of the axis, where fermenters fed a high concentrate diet are located) and biohydrogenation of C18:2 c9c12 (higher on the negative side of the axis, where fermenters fed a high forage diet are located).
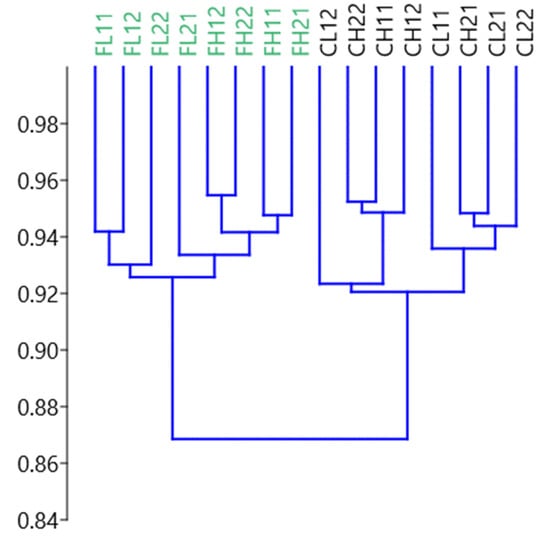
Figure 2.
Dendrogram derived from a multivariate hierarchical cluster analysis, showing the grouping of the 16 RUSITEC fermenters according to diet (F = high forage or C = high concentrate) and pH (H = higher or L = lower). The number after the treatment code indicates the RUSITEC system and replicate number (there were two RUSITEC systems and two replicates of each treatment in each system). The y-axis represents the Bray-Curtis similarity measure.
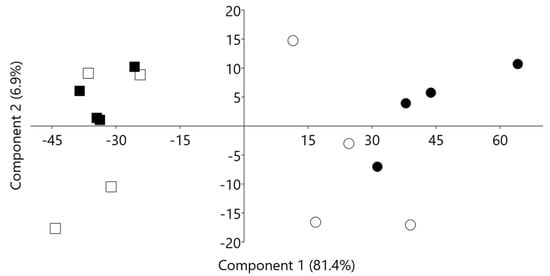
Figure 3.
Principal components plot derived from the analysis of rumen fermentation, fatty acid biohydrogenation, and microbial community profile data, showing the discrimination along principal components 1 and 2 of RUSITEC fermenters receiving either a high forage (squares) or a high concentrate (circles) under a higher pH (filled symbols) or a lower pH (hollow symbols) in the fermenter.
4. Discussion
4.1. Substrate Digestion, Fermentation Pattern, and Microbial Protein Synthesis in the Rumen
As expected, diet C (34% cereals, 32% forage) was more digestible than diet F (10% cereals, 78% forage) because starchy feeds are digested in the rumen to a greater extent than fibrous forages. Martinez et al. [13] did not observe differences in dry matter digestibility between high-forage and high-concentrate diets, although fiber digestibility was higher in diets with a higher proportion of forage, and concluded that the source of fiber is a factor that affects the extent of fermentation. Beet pulp was more than triple in diet C (7.6%) than in diet F (2.2%), and this feedstuff is rich in soluble fiber, so the degradation of NDF is almost complete after 48 h [24]. The lignin content was higher in diet F than in diet C, and this indigestible fraction hinders the degradation of cell wall polysaccharides [25]. The greater production of fermentation gas and VFA observed with diet C is consistent with its higher digestibility. Kargar et al. [11] found no differences between 40% and 60% concentrate diets supplemented with three different oils (sunflower, pomegranate, or garlic) in ruminal fermentation kinetics and concluded that a less concentrate diet could attenuate the adverse effects of the dietary supplementation with unsaturated fat on ruminal fermentation.
Anaerobic digestion of structural carbohydrates is associated with increased acetate and methane production. In our study, there were no diet effects on the production of methane or acetate. Martínez et al. [13] observed higher acetate production in RUSITEC fermenters fed a high-fiber diet in comparison with a high-concentrate diet, with no differences between both types of diet in the production of propionate. Unexpectedly, the acetate-to-propionate ratio was higher with diet C. Other studies have reported that the acetate-to-propionate ratio decreased with an increasing proportion of concentrate in the diet [13,26]. It seems that not only the proportion of forage in the diet but also the type of forage (alfalfa or grass hay) [13] or the source of inoculum for in vitro cultures [27] can affect the acetate-to-propionate ratio. A lower acetate-to-propionate ratio with a more concentrate diet is also associated with reduced fiber digestibility as the level of starchy feeds in the diet is increased [13,26]. The addition of lactic acid to RUSITEC fermenters fed a 50:50 forage: concentrate diet caused a decrease in the acetate-to-propionate ratio [28]. The unexpectedly high acetate-to-propionate ratio observed with diet C in our study can be associated with an atypical high molar proportion of butyrate relative to propionate [29]. Fuentes et al. [30] reported higher yields of butyrate in vitro in fermenters fed a high concentrate diet compared with a high forage diet, and the difference was larger as the pH was higher. It seems that butyrate-producing bacteria find favorable growth conditions in fermenters with high-concentrate diets, especially in a highly buffered medium that prevents the decrease in pH that normally occurs in the animal. Butyrate-producing bacteria are commonly classified into two groups according to their involvement in fatty acid biohydrogenation [31]. Increased butyrate production has also been associated with moderate levels (up to 10 g/kg DM) of unsaturated fat supplementation [32]. A low-fiber and high-starch diet, along with the addition of sunflower oil, could explain the increased formation of butyrate [33]. In addition to the high production of butyrate (C4), a high production of longer chain (C5 and C6) VFA has been observed in RUSITEC fermenters [2,13], substantially higher than values usually recorded in vivo. There is not an explanation for this observation, although it is possible that, in these fermenters with a highly buffered medium (no pH decline), the shorter-chain VFAs (acetic and propionic) are used as precursors of longer-chain VFAs (butyric, valeric, caproic). In this sense, it has been observed that some rumen bacteria (e.g., Eubacterium pyruvativorans, Clostridium kluyveri, or Megasphaera sp.) are able to synthesize medium-chain fatty acids (from 5 to 8 C) from acetic and propionic acids when cultivated in vitro [34,35]. The use of acetic and propionic acids in these processes would change their molar proportions and the C2:C3 ratio in the continuous cultures. L-Lactate concentration was increased with the high-concentrate diet, likely as the result of increased lactate production and/or reduced utilization of this metabolite [36]. The fermentation of rapidly degraded carbohydrates in the rumen leads to a high production of lactate that is linked to decreased rumen pH [7]. The main lactate-utilizing bacteria is Megasphaera elsdenii (estimated to metabolize 60 to 80% of lactate), a highly sensitive species to decreased pH [37,38]. However, in in vitro fermentation systems, the pH is maintained fairly stable, and any drop is attenuated due to the strong buffering capacity of the medium.
In relation to the nitrogen metabolism, the concentration of ammonia was higher in the high-concentrate diet, with a higher crude protein content, than in the high-forage diet, in agreement with other reports [11,13,26,39]. Additionally, daily microbial protein output (g microbial protein per day) was increased with the high-concentrate diet as a result of the greater availability of nitrogen (higher ammonia concentration) and energy (more fermenter organic matter) for microbial synthesis.
The type of saliva infused into the fermenters did not affect the digestibility of fiber, probably because, with both buffers, the pH was not below 6.2, which is considered the critical point to cause adverse effects on cellulolytic bacteria and feed digestibility [27,40]. Hu et al. [41] observed that cellulose digestibility was higher at pH values between 6.8 and 7.3. Guo et al. [42] reported decreased nutrient degradability in RUSITEC fermenters when pH was decreased from 7.0 to 6.0, using artificial saliva more diluted than that used herein. However, other authors did not observe an effect of pH on digestibility in continuous cultures [43,44]. The negative effect of low pH on microbial fermentation has been associated with the magnitude of the pH drop [45]. Farenzena et al. [46] observed lower ruminal degradability at a pH of 6.5 than at a pH of 7.0, related to a decrease in the adhesion of bacteria to the substrate, with no effect on the activity of fibrolytic enzymes. In agreement with our study, other reports have shown that protein degradability was decreased with a pH decline from 6.2–6.5 to 5.6–6.1 [30,47]. The higher concentrations of ammonia and iso-acids with a higher pH could be related to the greater protein degradability, especially with diet C. This result agrees with Calsamiglia et al. [48], who recorded that with a low pH, the degradation of the protein was reduced and, consequently, the concentration of ammonia in rumen fermenters. The decrease in fat digestibility with decreasing pH suggests a depressing effect of low pH on lipolysis [49], an effect that could be more drastic as the pH falls below 6.0. In addition, this effect can be amplified when oil is added to feed, probably due to a negative synergistic effect on the growth and metabolism of lipolytic bacteria (Anaerovibrio lipolytica), interfering with the activity of their lipases [30].
A decrease in ruminal pH below 5.5 may limit the uptake of H2 by methanogenic archaea [27]. With concentrate diets and at ruminal pH below 5.5, archaea may temporarily lose their ability to convert CO2 and H2 into methane [50]. Some studies have reported that methane is reduced by decreasing ruminal pH [43,44]. However, Hünerberg et al. [51] did not appreciate an effect of rumen pH (decreasing up to 5.2) on methane production in vitro, suggesting that methanogens can tolerate conditions of acidosis for relatively long periods.
Total VFA production, in particular of acetate, was decreased at a lower pH (6.4). A similar effect has been observed in other studies [27,43]. Broudiscou et al. [29] pointed out that the adaptation of the inoculum to the fermentation substrate (forage or starch) affects the VFA production and the molar proportions of the different acids when fermentation occurs in vitro at different pH (5.8, 6.3 or 6.8). Digestibility, gas production, VFA concentration, and acetate to propionate were found to be greater at pH 6.5 than at 5.8 in batch cultures [52]. In our study, the acetate-to-propionate ratio was decreased at the lower pH, significantly with diet C. A similar trend has been reported by other authors [30,43,53]. As in our study, Roman-Garcia et al. [43] observed that decreasing pH caused a reduction in branched-chain VFA (iso-acids), probably as a result of decreased protein degradation, with no significant effect on microbial protein synthesis. Li et al. [44] reported that the use of VFA carbon for microbial synthesis was not affected by ruminal pH.
4.2. Microbial Community Profile
Our study showed that a high-forage diet favored ciliate protozoa, anaerobic fungi, and fibrolytic F. succinogenes, whereas S. bovis was more abundant with diet C. A large study examining the microbial profile of hundreds of digesta samples from different ruminant species fed either forage or concentrate diets revealed that archaeal and protozoal groups were less affected by diet than bacteria, and within these, Bacteroidales and Ruminococcaceae (cellulose degraders) were more abundant in animals fed forages, whereas Prevotella and Succinivibrionaceae (propionate producers) were more abundant in animals fed concentrate-rich diets [54]. Fibrobacter was most abundant with forage diets, and Butyrivibrio with mixed forage and concentrate diets [54]. In RUSITEC fermenters, total bacteria and protozoa, and the relative abundance of Ruminococcus spp., methanogenic archaea, and anaerobic fungi were decreased with a fattening lamb diet (85% concentrate and 15% straw) compared with a dairy sheep diet (50% concentrate and 50% alfalfa hay) [55]. Kargar et al. [11] did not observe differences between 40% and 60% concentrate diets in the number of total protozoa in batch cultures of rumen microorganisms. In Holstein heifers, the forage-to-concentrate ratio of the diet had no effect on anaerobic fungi and archaea, but as the concentrate level was increased, there was a decrease in the relative abundance of cellulolytic bacteria and ciliates [56]. Pickett et al. [57] found that Bacteriodetes was the most abundant phylum in the rumen and increased with a concentrate diet, whereas Prevotella was not affected by the level of forage or concentrate in the diet, in agreement with our results. Increasing the level of concentrate in dairy cow diets led to increased P. ruminicola, B. fibrisolvens, and S. ruminantium in the rumen, whereas F. succinogenes and M. elsdenii were reduced [58]. Key enzymes and bacteria (Bacillus, Prevotella, Ruminobacter, Selenomonas, Vibrio) involved in propionate production were increased with a high-concentrate diet, whereas butyrate was produced by Prevotella in high-concentrate diets and by Bacteroides in high-fiber diets [58]. In Angus cows fed diets with varying forage-to-concentrate ratios, the relative abundances of Bacteroidetes, Fibrobacterota, and Prevotella decreased, and those of Ruminococcaceae, Saccharofermentans, and Spirochaetota increased in rumen digesta with increasing dietary concentrate level [26]. In the rumen of cashmere goats, phyla Actinobacteria and Proteobacteria and genera Prevotella and Selenomonas were increased, whereas the phylum Bacteroidetes and the anaerobic fungi Neocallimastigomycota were decreased when the proportion of dietary concentrate was increased [59]. In Holstein cows, Ramos et al. [39] found that a high-concentrate diet reduced rumen bacterial richness and diversity, increased Firmicutes and Ruminococcus, and decreased Bacteroidetes and Prevotella ruminicola. Wang et al. [60] also found higher microbial diversity in the rumen of dairy cows fed a high forage diet but did not detect differences with a high concentrate diet in the relative abundance of Firmicutes, Bacteroidetes, or Proteobacteria.
Roman-Garcia et al. [61] reported that a low pH was associated with a substantial shift in some bacterial communities, such as increased Prevotella and genera of the phylum Proteobacteria, reducing species within phylum Firmicutes. In contrast, Li et al. [44] observed that with a low pH, the relative abundance of some Firmicutes was increased (Ruminococcaceae), whereas others were decreased (Clostridium spp.). In RUSITEC fermenters, decreasing pH from 7.0 to 6.0 did not change the abundance of the most abundant phyla (Firmicutes, Bacteroidetes, and Proteobacteria) or of the main bacterial genera but decreased the numbers of the main fibrolytic species R. albus, R. flavefaciens, and F. succinogenes [42]. In batch cultures, fibrolytic F. succinogenes and R. flavefaciens were decreased at a lower pH [52]. Compared with a nearly neutral pH (6.8), a low pH (5.5) in RUSITEC fermenters decreased the abundance of fibrolytic R. albus and phylum Actinobacteria and increased the abundances of Prevotella spp., Pseudobutyrivibrio spp., and Selenomonas ruminantium, with no effect on F. succinogenes or Butyrivibrio spp. [62]. In this latter study, Lactobacillus spp. tended to increase at a lower pH, in contrast with our results showing that the abundance of S. bovis was higher at a higher pH.
Butyrivibrio sp. are the main microorganism involved in the processes of rumen biohydrogenation [33], so dietary effects on these bacteria could be behind the fatty acid profile in the digesta. Wanapat et al. [63] observed that with a high forage diet, there was an increase in the abundance of the fibrolytic Fibrobacter succinogenes, the lactate-utilizing Megasphaera elsdenii (producer of C18:2 t10c12) and Butyrivibrio fibrisolvens (considered the main producer of C18:2 c9t11 and C18:1 t11). Similarly, Mrazek et al. [64] observed that diets with a higher fiber content increased the population of Butyrivibrio sp. in the rumen, while with high-energy concentrate feeds, this bacterial group was drastically reduced. Zened et al. [65] concluded that Butyrivibrio fibrisolvens were highly sensitive to the fermentation of starchy diets. Jenkins et al. [66] concluded that all the bacteria that hydrogenate C18:2 c9c12 to C18:2 c9t11 and C18:1 t11 also produce butyrate, but not all butyrate-producing bacteria can form C18:2 c9t11 or C18:1 t11. Goel et al. [67] also concluded that not all butyrate-producing bacteria are active in biohydrogenation.
There seems to be some inconsistency in the multiplicity of results reported, probably due to differences in the diets tested (not only in the forage to concentrate, but also in the types of forage or grains or other ingredients used in each diet) or the levels of pH tested. Methodological aspects (rumen digesta samples used, type of study, molecular biology techniques used) can also be crucial in the comparisons, with a large proportion of phyla and species still to be identified or classified or their enzymatic activities characterized.
4.3. Long-Chain Fatty Acid Profile and Hydrogenation
In our study, the proportion of C18:2 c9t11, but not that of C18:1 t11, was increased with the high-forage diet, suggesting that biohydrogenation may occur through metabolic pathways alternative to the production of C18:1 t11. Gudla et al. [68] observed that a high-concentrate diet increased C18:1 t10, C18:1 c9, and C18:2 c9c12 and decreased C18:2 c9t11 and C18:1 t11. The hydrogenation processes of C18:1 t10, C18:1 t11, and C18:2 c9t11 are highly influenced by the interaction between the type of the forage, and the source of fat supplemented [69]. Wang and Song [70] observed increased hydrogenation of C18:2 c9c12 when starch was the fermentation substrate (in comparison with cellulose), although this effect was linked to increased bacterial synthesis. The addition of sunflower oil rich in C18:2 c9c12 affected fatty acid biohydrogenation [65] so that with less concentrate diets, C18:2 c9t11 and C18:2 t10c12 in the rumen of cows accounted for 80 and 3% of the total conjugated C18:2, respectively. However, feeding a diet with more cereals decreased C18:2 c9t11 and increased C18:2 t10c12 [65]. In dairy cows, fatty acid hydrogenation in the rumen was greater with a low-concentrate diet supplemented with linseed oil, with no effects on the duodenal flow of C18:2 conjugated fatty acids [71]. Conjugated linoleic acid in beef [72] and milk [69,73] can be augmented by increasing the forage-to-concentrate ratio or by feeding sunflower oil or seeds. In our study, C18:2 c9t11 was also increased with diet F.
In addition to the proportions of forage and concentrate, the composition and characteristics of the dietary fiber and the soluble carbohydrates may also have an influence on the long-chain fatty acids profile in the rumen. Ribeiro et al. [74] observed that C18:1 t10 was lower with rations with fresh alfalfa than with grass hay, with no differences between both forages in C18:1 t11. The flow of C18:1 t12 to the duodenum in dairy cows increased linearly, the flow of C18:1 c12 and total C18:1 cis had a quadratic response, and fatty acid hydrogenation decreased linearly in response to sucrose supplementation [74]. The effects of sucrose on biohydrogenation seem to be caused by a change in bacterial communities through mechanisms that are independent of pH. Our results showed that C18:1 c9 hydrogenation was increased with the high-forage diet, regardless of the level of pH. Maia et al. [75] showed that C18:2 c9c12 had some in vitro bacteriostatic effects on B. fibrisolvens. The addition of large amounts of starch together with sunflower oil increased C18:1 t10 at the expense of C18:1 t11, whereas there was less C18:1 t10 than C18:1 t11 when the starchy diet was not supplemented with oil, and these changes were independent of pH [65]. The basal diet is an important determinant of the fatty acid hydrogenation in the rumen upon a dietary supplementation with unsaturated fats. When supplemented with a mixture of sunflower and fish oils, C18:2 c9t11 in milk was reduced when cows were fed a high-concentrate diet, whereas C18:2 t9c11 and C18:2 t10c12 were increased [76]. Fatty acids of microbial origin (iso + odd-chain) were decreased with diet C, in line with the observation that decreasing pH shifted bacterial populations and their fatty acid composition [61]. Zhang et al. [77] found that even-chain, odd- and branched-chain fatty acids, and conjugated linoleic acid were increased, whereas polyunsaturated fatty acids were decreased in the rumen digesta when the relative abundance of cellulolytic bacteria was greater.
Our results did not show any significant effects of ruminal pH (ranging from 6.4 to 6.8) on the fatty acid profile or hydrogenation in the rumen, in line with Wang and Song [70], who did not find significant pH effects on fatty acid hydrogenation in vitro. Other studies have found that, when oilseeds were incubated in vitro, increasing the pH in the culture medium was associated with a decrease in polyunsaturated fatty acids, an increase in C18:1 t11 and C18:2 c9t11, and a faster biohydrogenation rate in the rumen [78]. It has been suggested that bacteria hydrogenating fatty acids to C18:0 and C18:1 in the rumen are more sensitive to acidic conditions than C18:2 c9t11-producers or that decreasing pH promotes isomerization of C18:2 c9t11 [78]. The formation of C18:1 t10 and C18:2 c9c12 increased, and the proportions of C18:0, C18:1 t11, and C18:2 c9t11 decreased with a low rumen pH (at 5.7 compared with 6.4), characteristic of low-forage and high-concentrate diets [30]. Ribeiro et al. [79] reported a decreased biohydrogenation rate of C18:2 and C18:3, but not that of C18:2 c9t11 when pH was reduced from 6.5-7.0 to 5.8-6.6 in in vitro cultures. It is worth mentioning that in these reports, the range of pH was wider, up to a lower and more limiting pH, than that reached in our study. Fuentes et al. [30] observed that with a pH of 5.6, the population of lipolytic bacteria (A. lipolytica) almost disappeared after 5 days. The strain of B. fibrisolvens involved in the formation of C18:1 t11 was also hampered with this acidic pH, to a more extent with a high-concentrate diet. However, pH did not affect the Butyrivibrio sp. responsible for the complete saturation of fatty acids to stearic acid. Fuentes et al. [80] reported that a lower pH depressed both lipolysis and biohydrogenation of C18:2 c9c12 as a result of an inhibitory effect on A. lipolytica and C18:1 t11-forming Butyrivibrio strains at the expense of favoring C18:0-forming Butyrivibrio strains. Troegeler-Meynadier et al. [81] concluded that the isomerization of C18:2 was strongly inhibited by an acidic rumen pH and proposed that C18:2 c9t11 production can be improved with a rumen pH close to neutrality and with the addition of C18:2 c9c12. At a lower pH, both the isomerization of C18:2 c9c12 and the conversion of C18:1 t11 to C18:0 were decreased in vitro [82]. Zened et al. [65] confirmed that the optimal pH for the activity of the enzyme C18:2 c9c12-isomerase of Butyrivibrio fibrisolvens is between 7.0 and 7.2. In our study, pH over a rather narrow range did not affect the formation of C18:2 c9t11.
5. Conclusions
High-concentrate diets are more fermentable in the rumen than high-fiber diets, resulting in a greater formation of most fermentation end-products, such as total gas, total VFA, and some short-chain organic acids (L-lactic, butyric, valeric, caproic). Protein degradation, daily output of ammonia and volatile isoacids, and microbial protein synthesis are also increased when high-concentrate diets are digested in the rumen. However, there were no significant differences between the high forage and the high concentrate diets tested in our study in methane production or in the efficiency of microbial protein synthesis efficiency. A high-forage diet promotes ciliate protozoa, anaerobic fungi, and some fibrolytic bacteria (Fibrobacter succinogenes), and only S. bovis seemed to be favored by a less fibrous diet. By increasing the concentrate feeds in the diet, the hydrogenation of linolenic acid is increased, and that of oleic acid is reduced, with no differences observed in that of linoleic acid. As a result, there are changes in the fatty acid profile in the rumen digesta, in particular in the relative proportion of conjugated linoleic acid (C18:2 c9t11), which is increased with high forage diets.
A decline in rumen pH from 6.8 to 6.4 decreases feed digestibility, and the daily outputs of fermentation gas and short-chain fatty acids, mainly acetate. Protein degradability is also decreased by a lower ruminal pH, decreasing the release of ammonia and the formation of short-chain isoacids. The synthesis of microbial protein and the output of some fermentation end-products (methane, propionate, butyrate, or lactate) were not affected by pH. Within this range of pH (between 6.4 and 6.8), only subtle changes are observed in the microbial community profile or the fatty acid relative proportions in the digesta or in their hydrogenation rates.
With precise control of pH and incubation conditions in the RUSITEC fermenters, it was possible to differentiate dietary and pH effects on fermentation. The overall multivariate analysis reveals a clear discrimination between high-concentrate and high-forage diets, mainly driven by the hydrogenation rates of fatty acids. However, this multivariate analysis shows subtler and less-defined pH effects on ruminal fermentation and microbial communities.
Author Contributions
Conceptualization, J.E.V., S.A. and S.L.; methodology, J.E.V., L.L.-F., I.M. and S.L.; validation, J.E.V. and S.L.; formal analysis, L.L.-F., E.H.H. and S.L.; investigation, J.E.V., L.L.-F., S.A., E.H.H., I.M. and S.L.; resources, S.A. and S.L.; data curation, J.E.V., L.L.-F. and S.L.; writing—original draft preparation, J.E.V. and S.L.; writing—review and editing, J.E.V., L.L.-F. and S.L.; supervision, S.A. and S.L.; project administration, S.L.; funding acquisition, S.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Spanish Ministerio de Ciencia e Innovación (PID2021-126489OB-I00, MCIN/AEI/10.13039/501100011033) and co-funded by the European Regional Development Fund (ERDF-FEDER) ″A way to make Europe-Una manera de hacer Europa”). Funding for the payment of APC was provided by Universidad de León (Ayudas a Grupos de Investigación BB202).
Institutional Review Board Statement
Not applicable. This was an in vitro study in compliance with the principles of reduction and replacement established in the EU Directive 2010/63/EU on the protection of animals used for research.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Elmhadi, M.E.; Ali, D.K.; Khogali, M.K.; Wang, H. Subacute Ruminal Acidosis in Dairy Herds: Microbiological and Nutritional Causes, Consequences, and Prevention Strategies. Anim. Nutr. 2022, 10, 148–155. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; López-Ferreras, L.; Snelling, T.J.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Dietary Supplemental Plant Oils Reduce Methanogenesis from Anaerobic Microbial Fermentation in the Rumen. Sci. Rep. 2020, 10, 1613. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, H.F.; Faciola, A.P. Ruminal Acidosis, Bacterial Changes, and Lipopolysaccharides. J. Anim. Sci. 2020, 98, skaa248. [Google Scholar] [CrossRef] [PubMed]
- Annison, E.F.; Bryden, W.L. Perspectives on Ruminant Nutrition and Metabolism I. Metabolism in the Rumen. Nutr. Res. Rev. 1998, 11, 173–198. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.E.; Huws, S.A.; Kim, E.J.; Lee, M.R.F.; Kingston-Smith, A.H.; Scollan, N.D. Advances in Microbial Ecosystem Concepts and their Consequences for Ruminant Agriculture. Animal 2008, 2, 653–660. [Google Scholar] [CrossRef]
- Pitta, D.W.; Indugu, N.; Baker, L.; Vecchiarelli, B.; Attwood, G. Understanding Diet–Microbe Interactions to Enhance Productivity of Dairy Cows. J. Dairy Sci. 2018, 101, 7661–7679. [Google Scholar] [CrossRef]
- Dijkstra, J.; Ellis, J.L.; Kebreab, E.; Strathe, A.B.; López, S.; France, J.; Bannink, A. Ruminal pH Regulation and Nutritional Consequences of Low pH. Anim. Feed Sci. Technol. 2012, 172, 22–33. [Google Scholar] [CrossRef]
- González, L.A.; Manteca, X.; Calsamiglia, S.; Schwartzkopf-Genswein, K.S.; Ferret, A. Ruminal Acidosis in Feedlot Cattle: Interplay between Feed Ingredients, Rumen Function and Feeding Behavior (a Review). Anim. Feed Sci. Technol. 2012, 172, 66–79. [Google Scholar] [CrossRef]
- Gruninger, R.J.; Ribeiro, G.O.; Cameron, A.; McAllister, T.A. Application of Meta-Omics to Understand the Dynamic Nature of the Rumen Microbiome and how it Responds to Diet in Ruminants. Animal 2019, 13, 1843–1854. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Regulation and Nutritional Manipulation of Milk Fat: Low-Fat Milk Syndrome. Livest. Prod. Sci. 2001, 70, 15–29. [Google Scholar] [CrossRef]
- Kargar, S.; Taasoli, G.; Akhlaghi, A.; Zamiri, M.J. In vitro Rumen Fermentation Pattern: Insights from Concentrate Level and Plant Oil Supplement. Arch. Anim. Breed 2023, 66, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Calsamiglia, S.; Cardozo, P.W.; Ferret, A.; Bach, A. Changes in Rumen Microbial Fermentation are Due to a Combined Effect of Type of Diet and pH. J. Anim. Sci. 2008, 86, 702–711. [Google Scholar] [CrossRef] [PubMed]
- Martínez, M.E.; Ranilla, M.J.; Tejido, M.L.; Ramos, S.; Carro, M.D. Comparison of Fermentation of Diets of Variable Composition and Microbial Populations in the Rumen of Sheep and Rusitec Fermenters. I. Digestibility, Fermentation Parameters, and Microbial Growth. J. Dairy Sci. 2010, 93, 3684–3698. [Google Scholar] [CrossRef]
- Czerkawski, J.W.; Breckenridge, G. Design and Development of a Long-Term Rumen Simulation Technique (Rusitec). Br. J. Nutr. 1977, 38, 371–384. [Google Scholar] [CrossRef] [PubMed]
- McDougall, E.I. Studies on Ruminant Saliva. 1. The Composition and Output of Sheep’s Saliva. Biochem. J. 1948, 43, 99–109. [Google Scholar] [CrossRef]
- García-González, R.; González, J.S.; López, S. Decrease of Ruminal Methane Production in Rusitec Fermenters through the Addition of Plant Material from Rhubarb (Rheum spp.) and Alder Buckthorn (Frangula alnus). J. Dairy. Sci. 2010, 93, 3755–3763. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; Snelling, T.J.; López-Ferreras, L.; Yáñez-Ruíz, D.R.; García-Estrada, C.; López, S. Effect of Sunflower and Marine Oils on Ruminal Microbiota, in vitro Fermentation and Digesta Fatty Acid Profile. Front. Microbiol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Morán, L.; Giráldez, F.J.; Panseri, S.; Aldai, N.; Jordán, M.J.; Chiesa, L.M.; Andrés, S. Effect of Dietary Carnosic Acid on the Fatty Acid Profile and Flavour Stability of Meat from Fattening Lambs. Food Chem. 2013, 138, 2407–2414. [Google Scholar] [CrossRef]
- Vargas, J.E.; Andrés, S.; López-Ferreras, L.; López, S. Effects of Supplemental Plant Oils on Rumen Bacterial Community Profile and Digesta Fatty Acid Composition in a Continuous Culture System (RUSITEC). Anaerobe 2020, 61, 102143. [Google Scholar] [CrossRef] [PubMed]
- Andrés, S.; Bodas, R.; Tejido, M.L.; Giráldez, F.J.; Valdés, C.; López, S. Effects of the Inclusion of Flaxseed and Quercetin in the Diet of Fattening Lambs on Ruminal Microbiota, in vitro Fermentation and Biohydrogenation of Fatty Acids. J. Agric. Sci. 2016, 154, 542–552. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.3 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2011. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 8 February 2023).
- DePeters, E.J.; Fadel, J.G.; Arosemena, A. Digestion Kinetics of Neutral Detergent Fiber and Chemical Composition within Some Selected By-Product Feedstuffs. Anim. Feed Sci. Technol. 1997, 67, 127–140. [Google Scholar] [CrossRef]
- Fukushima, R.S.; Kerley, M.S.; Ramos, M.H.; Porter, J.H.; Kallenbach, R.L. Comparison of Acetyl Bromide Lignin with Acid Detergent Lignin and Klason Lignin and Correlation with in vitro Forage Degradability. Anim. Feed Sci. Technol. 2015, 201, 25–37. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effects of Dietary Forage to Concentrate Ratio on Nutrient Digestibility, Ruminal Fermentation and Rumen Bacterial Composition in Angus Cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- Russell, J.B. The Importance of pH in the Regulation of Ruminal Acetate to Propionate Ratio and Methane Production in vitro. J. Dairy Sci. 1998, 81, 3222–3230. [Google Scholar] [CrossRef]
- Bekendorf, T.; Abel, H. In vivo and in vitro (Rusitec)-Investigations on Lactate Metabolism in the Rumen of Sheep. J. Anim. Physiol. Anim. 1996, 75, 133–141. [Google Scholar] [CrossRef]
- Broudiscou, L.P.; Offner, A.; Sauvant, D. Effects of Inoculum Source, pH, Redox Potential and Headspace Di-Hydrogen on Rumen in vitro Fermentation Yields. Animal 2014, 8, 931–937. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Calsamiglia, S.; Cardozo, P.W.; Vlaeminck, B. Effect of pH and Level of Concentrate in the Diet on the Production of Biohydrogenation Intermediates in a Dual-Flow Continuous Culture. J. Dairy Sci. 2009, 92, 4456–4466. [Google Scholar] [CrossRef]
- Belenguer, A.; Toral, P.G.; Frutos, P.; Hervás, G. Changes in the Rumen Bacterial Community in Response to Sunflower Oil and Fish Oil Supplements in the Diet of Dairy Sheep. J. Dairy Sci. 2010, 93, 3275–3286. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Griinari, J.M. Milk Fatty Acid Composition in Response to Reciprocal Combinations of Sunflower and Fish Oils in the Diet. Anim. Feed Sci. Technol. 2006, 131, 358–369. [Google Scholar] [CrossRef]
- Maia, M.R.G.; Chaudhary, L.C.; Figueres, L.; Wallace, R.J. Metabolism of Polyunsaturated Fatty Acids and Their Toxicity to the Microflora of the Rumen. Antonie Van Leeuwenhoek 2007, 91, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.J.; McKain, N.; McEwan, N.R.; Miyagawa, E.; Chaudhary, L.C.; King, T.P.; Walker, N.D.; Apajalahti, J.H.A.; Newbold, C.J. Eubacterium pyruvativorans sp. nov., a Novel Non-Saccharolytic Anaerobe from the Rumen that Ferments Pyruvate and Amino Acids, Forms Caproate and Utilizes Acetate and Propionate. Int. J. Syst. Evol. Microbiol. 2003, 53, 965–970. [Google Scholar] [CrossRef]
- Jeon, B.S.; Choi, O.; Um, Y.; Sang, B.-I. Production of Medium-Chain Carboxylic Acids by Megasphaera sp. MH with Supplemental Electron Acceptors. Biotechnol. Biofuels 2016, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Mackie, R.I.; Gilchrist, F.M.C.; Heath, S. An in vivo Study of Ruminal Micro-Organisms Influencing Lactate Turnover and its Contribution to Volatile Fatty Acid Production. J. Agric. Sci. 1984, 103, 37–51. [Google Scholar] [CrossRef]
- Nagaraja, T.G.; Titgemeyer, E.C. Ruminal Acidosis in Beef Cattle: The Current Microbiological and Nutritional Outlook. J. Dairy Sci. 2007, 90, E17–E38. [Google Scholar] [CrossRef] [PubMed]
- Long, M.; Feng, W.J.; Li, P.; Zhang, Y.; He, R.X.; Yu, L.H.; He, J.B.; Jing, W.Y.; Li, Y.M.; Wang, Z.; et al. Effects of the Acid-Tolerant Engineered Bacterial Strain Megasphaera elsdenii H6F32 on Ruminal pH and the Lactic Acid Concentration of Simulated Rumen Acidosis in vitro. Res. Vet. Sci. 2014, 96, 28–29. [Google Scholar] [CrossRef]
- Ramos, S.C.; Jeong, C.D.; Mamuad, L.L.; Kim, S.H.; Kang, S.H.; Kim, E.T.; Cho, Y.i.; Lee, S.S.; Lee, S.S. Diet Transition from High-Forage to High-Concentrate Alters Rumen Bacterial Community Composition, Epithelial Transcriptomes and Ruminal Fermentation Parameters in Dairy Cows. Animals 2021, 11, 838. [Google Scholar] [CrossRef]
- Kozloski, G.V.; Lima, L.D.; Cadorin, R.L.; Bonnecarrère Sanchez, L.M.; Senger, C.C.D.; Fiorentini, G.; Härter, C.J. Microbial Colonization and Degradation of Forage Samples Incubated in vitro at Different Initial pH. Anim. Feed Sci. Technol. 2008, 141, 356–367. [Google Scholar] [CrossRef]
- Hu, Z.-H.; Wang, G.; Yu, H.-Q. Anaerobic Degradation of Cellulose by Rumen Microorganisms at Various pH Values. Biochem. Eng. J. 2004, 21, 59–62. [Google Scholar] [CrossRef]
- Guo, T.; Guo, T.; Cao, Y.; Guo, L.; Li, F.; Li, F.; Yang, G. Changes in the Fermentation and Bacterial Community by Artificial Saliva pH in RUSITEC System. Front. Nutr. 2021, 8, 760316. [Google Scholar] [CrossRef] [PubMed]
- Roman-Garcia, Y.; Mitchell, K.E.; Denton, B.L.; Lee, C.; Socha, M.T.; Wenner, B.A.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. II: Relation with Solid Passage Rate and pH on Neutral Detergent Fiber Degradation and Microbial Function in Continuous Culture. J. Dairy Sci. 2021, 104, 9853–9867. [Google Scholar] [CrossRef] [PubMed]
- Li, M.M.; Ghimire, S.; Wenner, B.A.; Kohn, R.A.; Firkins, J.L.; Gill, B.; Hanigan, M.D. Effects of Acetate, Propionate, and pH on Volatile Fatty Acid Thermodynamics in Continuous Cultures of Ruminal Contents. J. Dairy Sci. 2022, 105, 8879–8897. [Google Scholar] [CrossRef] [PubMed]
- Cerrato-Sánchez, M.; Calsamiglia, S.; Ferret, A. Effect of the Magnitude of the Decrease of Rumen pH on Rumen Fermentation in a Dual-Flow Continuous Culture System. J. Anim. Sci. 2008, 86, 378–383. [Google Scholar] [CrossRef]
- Farenzena, R.; Kozloski, G.v.; Mezzomo, M.P.; Fluck, A.C. Forage Degradability, Rumen Bacterial Adherence and Fibrolytic Enzyme Activity in vitro: Effect of pH or Glucose Concentration. J. Agric. Sci. 2014, 152, 325–332. [Google Scholar] [CrossRef]
- Cantalapiedra-Hijar, G.; Yanez-Ruiz, D.R.; Newbold, C.J.; Molina-Alcaide, E. The Effect of the Feed-to-Buffer Ratio on Bacterial Diversity and Ruminal Fermentation in Single-Flow Continuous-Culture Fermenters. J. Dairy Sci. 2011, 94, 1374–1384. [Google Scholar] [CrossRef]
- Calsamiglia, S.; Ferret, A.; Devant, M. Effects of pH and pH Fluctuations on Microbial Fermentation and Nutrient Flow from a Dual-Flow Continuous Culture System. J. Dairy Sci. 2002, 85, 574–579. [Google Scholar] [CrossRef] [PubMed]
- van Nevel, C.; Demeyer, D. Influence of pH on Lipolysis and Biohydrogenation of Soybean Oil by Rumen Contents in vitro. Reprod. Nutr. Dev. 1996, 36, 53–63. [Google Scholar] [CrossRef] [PubMed]
- van Kessel, J.A.; Russell, J.B. The Effect of pH on Ruminal Methanogenesis. FEMS Microbiol. Ecol. 1996, 20, 205–210. [Google Scholar] [CrossRef]
- Hünerberg, M.; McGinn, S.M.; Beauchemin, K.A.; Entz, T.; Okine, E.K.; Harstad, O.M.; McAllister, T.A. Impact of Ruminal pH on Enteric Methane Emissions. J. Anim. Sci. 2015, 93, 1760–1766. [Google Scholar] [CrossRef]
- Jiao, P.; Wei, C.; Sun, Y.; Xie, X.; Zhang, Y.; Wang, S.; Hu, G.; AlZahal, O.; Yang, W. Screening of Live Yeast and Yeast Derivatives for Their Impact of Strain and Dose on in vitro Ruminal Fermentation and Microbial Profiles with Varying Media pH Levels in High-forage Beef Cattle Diet. J. Sci. Food Agric. 2019, 99, 6751–6760. [Google Scholar] [CrossRef]
- Bhatta, R.; Tajima, K.; Kurihara, M. Influence of Temperature and pH on Fermentation Pattern and Methane Production in the Rumen Simulating Fermenter (RUSITEC). Asian-Australas. J. Anim. Sci. 2006, 19, 376–380. [Google Scholar] [CrossRef]
- Henderson, G.; Cox, F.; Ganesh, S.; Jonker, A.; Young, W.; Janssen, P.H.; Abecia, L.; Angarita, E.; Aravena, P.; Arenas, G.N.; et al. Rumen Microbial Community Composition Varies with Diet and Host, but a Core Microbiome Is Found across a Wide Geographical Range. Sci. Rep. 2015, 5, 14567. [Google Scholar] [CrossRef] [PubMed]
- Mateos, I.; Ranilla, M.J.; Saro, C.; Carro, M.D. Shifts in Microbial Populations in Rusitec Fermenters as Affected by the Type of Diet and Impact of the Method for Estimating Microbial Growth (15N v. Microbial DNA). Animal 2017, 11, 1939–1948. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, H.; Wang, Y.; Li, S.; Cao, Z.; Ji, S.; He, Y.; Zhang, H. Effect of Dietary Forage to Concentrate Ratios on Dynamic Profile Changes and Interactions of Ruminal Microbiota and Metabolites in Holstein Heifers. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Pickett, A.T.; Cooke, R.F.; Mackey, S.J.; Brandão, A.P.; Colombo, E.A.; Oliveira Filho, R.v.; de Melo, G.D.; Pohler, K.G.; Poole, R.K. Shifts in Bacterial Communities in the Rumen, Vagina, and Uterus of Beef Heifers Receiving Different Levels of Concentrate. J. Anim. Sci. 2022, 100, skac338. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Li, Y.; Zhang, Y. Effects of High Forage/Concentrate Diet on Volatile Fatty Acid Production and the Microorganisms Involved in VFA Production in Cow Rumen. Animals 2020, 10, 223. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Li, B.; Wang, X.; Chen, Y.; Yang, Y. Effect of Dietary Concentrate to Forage Ratios on Ruminal Bacterial and Anaerobic Fungal Populations of Cashmere Goats. Anaerobe 2019, 59, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, Y.; Zhang, Y.; Wang, L. The Effects of Different Concentrate-to-Forage Ratio Diets on Rumen Bacterial Microbiota and the Structures of Holstein Cows during the Feeding Cycle. Animals 2020, 10, 957. [Google Scholar] [CrossRef] [PubMed]
- Roman-Garcia, Y.; Mitchell, K.E.; Lee, C.; Socha, M.T.; Park, T.; Wenner, B.A.; Firkins, J.L. Conditions Stimulating Neutral Detergent Fiber Degradation by Dosing Branched-Chain Volatile Fatty Acids. III: Relation with Solid Passage Rate and pH on Prokaryotic Fatty Acid Profile and Community in Continuous Culture. J. Dairy Sci. 2021, 104, 9868–9885. [Google Scholar] [CrossRef] [PubMed]
- Guo, T.; Guo, T.; Guo, L.; Li, F.; Li, F.; Ma, Z. Rumen Bacteria Abundance and Fermentation Profile during Subacute Ruminal Acidosis and its Modulation by Aspergillus oryzae Culture in RUSITEC System. Fermentation 2022, 8, 329. [Google Scholar] [CrossRef]
- Wanapat, M.; Gunun, P.; Anantsook, N.; Kang, S. Changes of Rumen pH, Fermentation and Microbial Population as Influenced by Different Ratios of Roughage (Rice Straw) to Concentrate in Dairy Steers. J. Agric. Sci. 2014, 152, 675–685. [Google Scholar] [CrossRef]
- Mrázek, J.; Tepšič, K.; Avguštin, G.; Kopečný, J. Diet-Dependent Shifts in Ruminal Butyrate-Producing Bacteria. Folia Microbiol. 2006, 51, 294–298. [Google Scholar] [CrossRef]
- Zened, A.; Enjalbert, F.; Nicot, M.C.; Troegeler-Meynadier, A. Starch plus Sunflower Oil Addition to the Diet of Dry Dairy Cows Results in a Trans-11 to Trans-10 Shift of Biohydrogenation. J. Dairy Sci. 2013, 96, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, T.C.; Wallace, R.J.; Moate, P.J.; Mosley, E.E. Recent Advances in Biohydrogenation of Unsaturated Fatty Acids within the Rumen Microbial Ecosystem. J. Anim. Sci. 2008, 86, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Goel, G.; Arvidsson, K.; Vlaeminck, B.; Bruggeman, G.; Deschepper, K.; Fievez, V. Effects of Capric Acid on Rumen Methanogenesis and Biohydrogenation of Linoleic and Alpha-Linolenic Acid. Animal 2009, 3, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Gudla, P.; Ishlak, A.; AbuGhazaleh, A.A. The Effect of Forage Level and Oil Supplement on Butyrivibrio fibrisolvens and Anaerovibrio lipolytica in Continuous Culture Fermenters. Asian-Australas. J. Anim. Sci. 2011, 25, 234–239. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A. Dietary Lipids and Forages Interactions on Cow and Goat Milk Fatty Acid Composition and Sensory Properties. Reprod. Nutr. Dev. 2004, 44, 467–492. [Google Scholar] [CrossRef]
- Wang, J.H.; Song, M.K. Effect of Sources and Levels of Carbohydrates on Fermentation Characteristics and Hydrogenation of Linoleic Acid by Rumen Bacteria in vitro. Asian-Australas. J. Anim. Sci. 2001, 14, 48–53. [Google Scholar] [CrossRef]
- Loor, J.J.; Ueda, K.; Ferlay, A.; Chilliard, Y.; Doreau, M. Biohydrogenation, Duodenal Flow, and Intestinal Digestibility of Trans Fatty Acids and Conjugated Linoleic Acids in Response to Dietary Forage: Concentrate Ratio and Linseed Oil in Dairy Cows. J. Dairy Sci. 2004, 87, 2472–2485. [Google Scholar] [CrossRef]
- Mir, P.S.; Ivan, M.; He, M.L.; Pink, B.; Okine, E.; Goonewardene, L.; McAllister, T.A.; Weselake, R.; Mir, Z. Dietary Manipulation to Increase Conjugated Linoleic Acids and Other Desirable Fatty Acids in Beef: A Review. Can. J. Anim. Sci. 2003, 83, 673–685. [Google Scholar] [CrossRef]
- Kliem, K.E.; Shingfield, K.J. Manipulation of Milk Fatty Acid Composition in Lactating Cows: Opportunities and Challenges. Eur. J. Lipid Sci. Technol. 2016, 118, 1661–1683. [Google Scholar] [CrossRef]
- Ribeiro, C.V.D.M.; Karnati, S.K.R.; Eastridge, M.L. Biohydrogenation of Fatty Acids and Digestibility of Fresh Alfalfa or Alfalfa Hay plus Sucrose in Continuous Culture. J. Dairy Sci. 2005, 88, 4007–4017. [Google Scholar] [CrossRef] [PubMed]
- Maia, M.R.; Chaudhary, L.C.; Bestwick, C.S.; Richardson, A.J.; McKain, N.; Larson, T.R.; Graham, I.A.; Wallace, R.J. Toxicity of Unsaturated Fatty Acids to the Biohydrogenating Ruminal Bacterium, Butyrivibrio fibrisolvens. BMC Microbiol. 2010, 10, 52. [Google Scholar] [CrossRef]
- Shingfield, K.J.; Reynolds, C.K.; Lupoli, B.; Toivonen, V.; Yurawecz, M.P.; Delmonte, P.; Griinari, J.M.; Grandison, A.S.; Beever, D.E. Effect of Forage Type and Proportion of Concentrate in the Diet on Milk Fatty Acid Composition in Cows given Sunflower Oil and Fish Oil. Anim. Sci. 2005, 80, 225–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, X.; Li, F.; Li, F.; Guo, L. Ruminal Cellulolytic Bacteria Abundance Leads to the Variation in Fatty Acids in the Rumen Digesta and Meat of Fattening Lambs. J. Anim. Sci. 2020, 98, skaa228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Song, M.K. pH Affects the in vitro Formation of Cis-9, Trans-11 CLA and Trans-11 Octadecenoic Acid by Ruminal Bacteria When Incubated with Oilseeds. Asian-Australas. J. Anim. Sci. 2003, 16, 1743–1748. [Google Scholar] [CrossRef]
- Ribeiro, C.V.D.M.; Eastridge, M.L.; Firkins, J.L.; St-Pierre, N.R.; Palmquist, D.L. Kinetics of Fatty Acid Biohydrogenation in vitro. J. Dairy Sci. 2007, 90, 1405–1416. [Google Scholar] [CrossRef]
- Fuentes, M.C.; Calsamiglia, S.; Fievez, V.; Blanch, M.; Mercadal, D. Effect of pH on Ruminal Fermentation and Biohydrogenation of Diets Rich in Omega-3 or Omega-6 Fatty Acids in Continuous Culture of Ruminal Fluid. Anim. Feed Sci. Technol. 2011, 169, 35–45. [Google Scholar] [CrossRef]
- Troegeler-Meynadier, A.; Nicot, M.C.; Bayourthe, C.; Moncoulon, R.; Enjalbert, F. Effects of pH and Concentrations of Linoleic and Linolenic Acids on Extent and Intermediates of Ruminal Biohydrogenation in vitro. J. Dairy Sci. 2003, 86, 4054–4063. [Google Scholar] [CrossRef]
- Troegeler-Meynadier, A.; Bret-Bennis, L.; Enjalbert, F. Rates and Efficiencies of Reactions of Ruminal Biohydrogenation of Linoleic Acid According to pH and Polyunsaturated Fatty Acids Concentrations. Reprod. Nutr. Dev. 2006, 46, 713–724. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).