Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review
Abstract
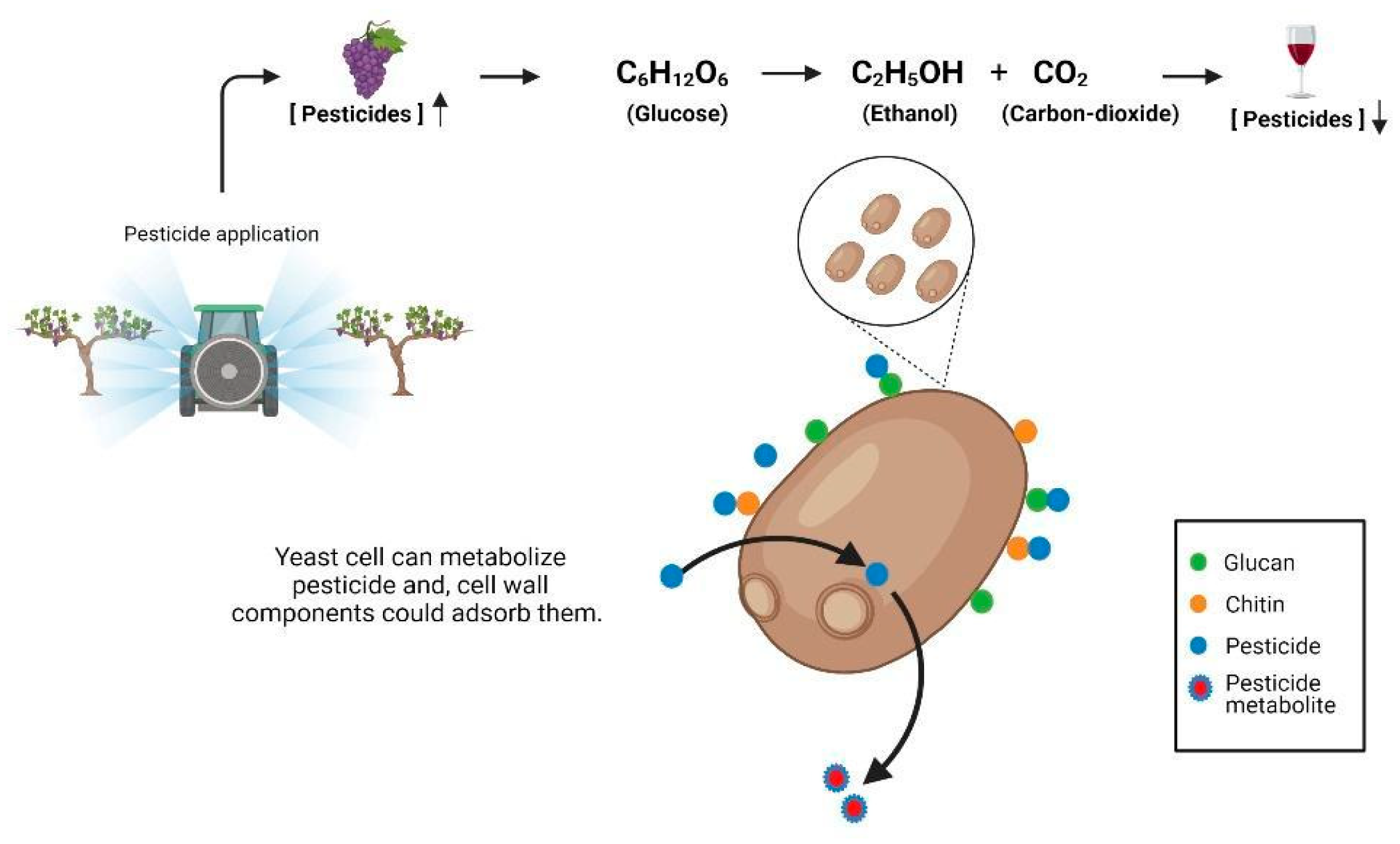
1. Introduction
2. Influence of Pesticide on Yeasts
Molecular Studies
3. Effect of Yeast over Pesticide Dissipation
4. Conclusions and Future Approach
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- González-Rodríguez, R.M.; Rial-Otero, R.; Cancho-Grande, B.; Gonzalez-Barreiro, C.; Simal-Gándara, J. A Review on the Fate of Pesticides during the Processes within the Food-Production Chain. Crit. Rev. Food Sci. Nutr. 2011, 51, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G.; Satya, S.; Naik, S.N. Food Processing a Tool to Pesticide Residue Dissipation—A Review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Bajwa, U.; Sandhu, K.S. Effect of Handling and Processing on Pesticide Residues in Food—A Review. J. Food Sci. Technol. 2014, 51, 201–220. [Google Scholar] [CrossRef] [PubMed]
- FoodChain ID Regulatory Limits Website. Available online: https://bcglobal.bryantchristie.com/db#pesticides/query (accessed on 27 February 2023).
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/mrls (accessed on 27 February 2023).
- Biblioteca del Congreso Nacional Biblioteca del Congreso Nacional. Available online: https://www.bcn.cl/leychile (accessed on 27 February 2023).
- Codex Alimentarius International Food Standards Website. Available online: https://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/commodities-detail/en/?c_id=113 (accessed on 27 February 2023).
- Dumitriu, G.-D.; Teodosiu, C.; Cotea, V.V. Management of Pesticides from Vineyard to Wines: Focus on Wine Safety and Pesticides Removal by Emerging Technologies. In Grapes and Wine; Morata, A., Loira, I., González, C., Eds.; IntechOpen: London, UK, 2021; pp. 1–27. [Google Scholar]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Removal of Pesticides from White and Red Wines by Microfiltration. J. Hazard. Mater. 2016, 317, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H. The Effects of Ozone-Enriched Storage Atmosphere on Pesticide Residues and Physicochemical Properties of Table Grapes. Ozone: Sci. Eng. 2019, 41, 404–414. [Google Scholar] [CrossRef]
- Nicolini, G.; Román Villegas, T.; Tonidandel, L.; Moser, S.; Larcher, R. Small Amounts of Charcoal during Fermentation Reduce Fungicide Residues without Penalising White Wine Aroma Compounds and Colour. Aust. J. Grape Wine Res. 2016, 22, 376–383. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Silva, D.; Filipe-Ribeiro, L.; Abrunhosa, L.; Nunes, F.M. Elimination of Ochratoxin A from White and Red Wines: Critical Characteristics of Activated Carbons and Impact on Wine Quality. LWT 2021, 140, 110838. [Google Scholar] [CrossRef]
- Carrau, F.M.; Medina, K.; Farina, L.; Boido, E.; Henschke, P.A.; Dellacassa, E. Production of âfermentation Aroma Compounds by Saccharomyces Cerevisiae wine Yeasts: Effects of Yeast Assimilable Nitrogen on Two Model Strains. FEMS Yeast Res. 2008, 8, 1196–1207. [Google Scholar] [CrossRef]
- Fleet, G. Yeast Interactions and Wine Flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Blanco, P.; Orriols, I.; Losada, A. Survival of Commercial Yeasts in the Winery Environment and Their Prevalence during Spontaneous Fermentations. J. Ind. Microbiol. Biotechnol. 2011, 38, 235–239. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of Yeast Species and Strains in Wine Flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Čuš, F.; Česnik, H.B.; Bolta, Š.V.; Gregorčič, A. Pesticide Residues in Grapes and during Vinification Process. Food Control. 2010, 21, 1512–1518. [Google Scholar] [CrossRef]
- Alister, C.; Araya, M.; Morandé, J.E.; Volosky, C.; Saavedra, J.; Cordova, A.; Kogan, M. Effects of Wine Grape Cultivar, Application Conditions and the Winemaking Process on the Dissipation of Six Pesticides. Cienc. E Investig. Agrar. 2014, 41, 19–20. [Google Scholar] [CrossRef]
- Torija, M. Effects of Fermentation Temperature on the Strain Population of Saccharomyces Cerevisiae. Int. J. Food Microbiol. 2003, 80, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Angioni, A.; Garau, V.L.; Aguilera Del Real, A.; Melis, M.; Minelli, E.V.; Tuberoso, C.; Cabras, P. GC-ITMS Determination and Degradation of Captan during Winemaking. J. Agric. Food Chem. 2003, 51, 6761–6766. [Google Scholar] [CrossRef]
- Bizaj, E.; Čuš, F.; Raspor, P. Removal of Pyrimethanil and Fenhexamid from Saccharomyces Cerevisiae Liquid Cultures. Food Technol. Biotechnol. 2011, 49, 474–480. [Google Scholar]
- Čuš, F.; Raspor, P. The Effect of Pyrimethanil on the Growth of Wine Yeasts. Lett. Appl. Microbiol. 2008, 47, 54–59. [Google Scholar] [CrossRef]
- García, M.A.; Oliva, J.; Barba, A.; Cámara, M.Á.; Pardo, F.; Díaz-Plaza, E.M. Effect of Fungicide Residues on the Aromatic Composition of White Wine Inoculated with Three Saccharomyces Cerevisiae Strains. J. Agric. Food Chem. 2004, 52, 1241–1247. [Google Scholar] [CrossRef]
- Regueiro, J.; López-Fernández, O.; Rial-Otero, R.; Cancho-Grande, B.; Simal-Gándara, J. A Review on the Fermentation of Foods and the Residues of Pesticides-Biotransformation of Pesticides and Effects on Fermentation and Food Quality. Crit. Rev. Food Sci. Nutr. 2015, 55, 839–863. [Google Scholar] [CrossRef]
- Sala, C.; Fort, F.; Busto, O.; Zamora, F.; Arola, L.; Guasch, J. Fate of Some Common Pesticides during Vinification Process. J. Agric. Food Chem. 1996, 44, 3668–3671. [Google Scholar] [CrossRef]
- Caboni, P.; Cabras, P. Pesticides’ Influence on Wine Fermentation. Adv. Food Nutr. Res. 2010, 59, 43–62. [Google Scholar] [PubMed]
- Gava, A.; Emer, C.D.; Ficagna, E.; Fernandes de Andrade, S.; Fuentefria, A.M. Occurrence and Impact of Fungicides Residues on Fermentation during Wine Production—A Review. Food Addit. Contam. Part. A Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 943–961. [Google Scholar] [CrossRef] [PubMed]
- Abou-Arab, A.A.K. Degradation of Organochlorine Pesticides by Meat Starter in Liquid Media and Fermented Sausage. Food Chem. Toxicol. 2002, 40, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Angioni, A. Pesticide Residues in Grapes, Wine, and Their Processing Products. J. Agric. Food Chem. 2000, 48, 967–973. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Andrade, J.V.; Ferreira, I.C.; Estevinho, L.M. Toxicity Effects of Fungicide Residues on the Wine-Producing Process. Food Microbiol. 2006, 23, 393–398. [Google Scholar] [CrossRef]
- Sharma, J.; Satya, S.; Kumar, V.; Tewary, D.K. Dissipation of Pesticides during Bread-Making. Chem. Health Saf. 2005, 12, 17–22. [Google Scholar] [CrossRef]
- Russo, P.; Berbegal, C.; De Ceglie, C.; Grieco, F.; Spano, G.; Capozzi, V. Pesticide Residues and Stuck Fermentation in Wine: New Evidences Indicate the Urgent Need of Tailored Regulations. Fermentation 2019, 5, 23. [Google Scholar] [CrossRef]
- Bizaj, E.; Curtin, C.; Čadež, N.; Raspor, P. Interactions Between Industrial Yeasts and Chemical Contaminants in Grape Juice Affect Wine Composition Profile. Food Technol. Biotechnol. 2014, 52, 222–231. [Google Scholar]
- Cabras, P.; Meloni, M.; Pirisi, F.M. Pesticide Fate from Vine to Wine. Rev. Environ. Contam. Toxicol. 1987, 99, 83–117. [Google Scholar]
- Scariot, F.J.; Jahn, L.M.; Delamare, A.P.L.; Echeverrigaray, S. The Effect of the Fungicide Captan on Saccharomyces Cerevisiae and Wine Fermentation. BIO Web Conf. 2016, 7, 02027. [Google Scholar] [CrossRef]
- Vadkertiová, R.; Sláviková, E. Influence of Pesticides on Yeasts Colonizing Leaves. Z. Naturforsch. C 2011, 66, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Cabras, P.; Garau, V.L.; Angioni, A.; Farris, G.A.; Budroni, M.; Spanedda, L. Interactions during Fermentation between Pesticides and Oenological Yeasts Producing H2S and SO2. Appl. Microbiol. Biotechnol. 1995, 43, 370–373. [Google Scholar] [CrossRef]
- Terpou, A.; Dimopoulou, M.; Belka, A.; Kallithraka, S.; Nychas, G.-J.E.; Papanikolaou, S. Effect of Myclobutanil Pesticide on the Physiological Behavior of Two Newly Isolated Saccharomyces Cerevisiae Strains during Very-High-Gravity Alcoholic Fermentation. Microorganisms 2019, 7, 666. [Google Scholar] [CrossRef]
- Causton, H.C.; Ren, B.; Koh, S.S.; Harbison, C.T.; Kanin, E.; Jennings, E.G.; Lee, T.I.; True, H.L.; Lander, E.S.; Young, R.A. Remodeling of Yeast Genome Expression in Response to Environmental Changes. Mol. Biol. Cell 2001, 12, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, E.; Takahashi, J.; Momose, Y.; Iwahashi, H. Effects of the Pesticide Thiuram: Genome-Wide Screening of Indicator Genes by Yeast DNA Microarray. Environ. Sci. Technol. 2002, 36, 3908–3915. [Google Scholar] [CrossRef]
- Simões, T.; Teixeira, M.C.; Fernandes, A.R.; Sá-Correia, I. Adaptation of Saccharomyces Cerevisiae to the Herbicide 2,4-Dichlorophenoxyacetic Acid, Mediated by Msn2p- and Msn4p-Regulated Genes: Important Role of SPI1. Appl. Environ. Microbiol. 2003, 69, 4019–4028. [Google Scholar] [CrossRef]
- Vallejo, B.; Picazo, C.; Orozco, H.; Matallana, E.; Aranda, A. Herbicide Glufosinate Inhibits Yeast Growth and Extends Longevity during Wine Fermentation. Sci. Rep. 2017, 7, 12414. [Google Scholar] [CrossRef]
- Bode, R.; Schüssler, K.; Schmidt, H.; Hammer, T.; Birnbaum, D. Occurrence of the General Control of Amino Acid Biosynthesis in Yeasts. J. Basic Microbiol. 1990, 30, 31–35. [Google Scholar] [CrossRef]
- Falco, S.C.; Dumas, K.S. Genetic Analysis of Mutants of Saccharomyces cerevisiae Resistant to the Herbicide Sulfometuron Methyl. Genetics 1985, 109, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.H.; Larossa, R.A.; Lee, J.M.; Rafalski, A.; Derose, E.; Gonye, G.; Xue, Z. Global Expression Profiling of Yeast Treated with an Inhibitor of Amino Acid Biosynthesis, Sulfometuron Methyl. Physiol. Genomics 2000, 3, 83–92. [Google Scholar] [CrossRef]
- Hinnebusch, A.G. Translational Regulation of Gcn4 and the General Amino Acid Control of Yeast. Annu. Rev. Microbiol. 2005, 59, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Crespo, J.L.; Powers, T.; Fowler, B.; Hall, M.N. The TOR-Controlled Transcription Activators GLN3, RTG1, and RTG3 Are Regulated in Response to Intracellular Levels of Glutamine. Proc. Natl. Acad. Sci. USA 2002, 99, 6784–6789. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.G. Transmitting the Signal of Excess Nitrogen in Saccharomyces Cerevisiae from the Tor Proteins to the GATA Factors: Connecting the Dots. FEMS Microbiol. Rev. 2002, 26, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.D.; Shadel, G.S.; Kaeberlein, M.; Kennedy, B. Replicative and Chronological Aging in Saccharomyces Cerevisiae. Cell Metab. 2012, 16, 18–31. [Google Scholar] [CrossRef]
- Sieiro-Sampedro, T.; Alonso-del-Real, J.; Briz-Cid, N.; Rial-Otero, R.; Querol, A.; Simal-Gandara, J. The Effect of Two Antifungal Commercial Formulations on the Metabolism of a Commercial Saccharomyces Cerevisiae Strain and Their Repercussion on Fermentation Evolution and Phenylalanine Catabolism. Food Microbiol. 2020, 92, 103554. [Google Scholar] [CrossRef]
- Sieiro-Sampedro, T.; Briz-Cid, N.; Pose-Juan, E.; Figueiredo-González, M.; González-Barreiro, C.; Simal-Gándara, J.; Cancho-Grande, B.; Rial-Otero, R. Tetraconazole Alters the Methionine and Ergosterol Biosynthesis Pathways in Saccharomyces Yeasts Promoting Changes on Volatile Derived Compounds. Food Res. Int. 2020, 130, 108930. [Google Scholar] [CrossRef]
- Gil, F.N.; Becker, J.D.; Viegas, C.A. Potential Mechanisms Underlying Response to Effects of the Fungicide Pyrimethanil from Gene Expression Profiling in Saccharomyces Cerevisiae. J. Agric. Food Chem. 2014, 62, 5237–5247. [Google Scholar] [CrossRef]
- Angioni, A.; Dedola, F.; Garau, V.L.; Schirra, M.; Caboni, P. Fate of Iprovalicarb, Indoxacarb, and Boscalid Residues in Grapes and Wine by GC-ITMS Analysis. J. Agric. Food Chem. 2011, 59, 6806–6812. [Google Scholar] [CrossRef]
- Cabras, P.; Farris, G.A.; Fiori, M.G.; Pusino, A. Interaction between Fenhexamid and Yeasts during the Alcoholic Fermentation of Saccharomyces Cerevisiae. J. Agric. Food Chem. 2003, 51, 5012–5015. [Google Scholar] [CrossRef]
- Navarro, S.; García, B.; Navarro, G.; Oliva, J.; Barba, A. Effect of Wine-Making Practices on the Concentrations of Fenarimol and Penconazole in Rosé Wines. J. Food Prot. 1997, 60, 1120–1124. [Google Scholar] [CrossRef]
- Navarro, S.; Barba, A.; Oliva, J.; Navarro, G.; Pardo, F. Evolution of Residual Levels of Six Pesticides during Elaboration of Red Wines. Effect of Wine-Making Procedures in Their Dissappearance. J. Agric. Food Chem. 1999, 47, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.J.; Oliva, J.; Barba, A.; Cámara, M.A. Fungicide Dissipation Curves in Winemaking Processes with and without Maceration Step. J. Agric. Food Chem. 2005, 53, 804–811. [Google Scholar] [CrossRef]
- Pérez-Serradilla, J.A.; de Castro, M.D.L. Role of Lees in Wine Production: A Review. Food Chem. 2008, 111, 447–456. [Google Scholar] [CrossRef]
- Chassagne, D.; Guilloux-Benatier, M.; Alexandre, H.; Voilley, A. Sorption of Wine Volatile Phenols by Yeast Lees. Food Chemistry 2005, 91, 39–44. [Google Scholar] [CrossRef]
- Caridi, A. New Perspectives in Safety and Quality Enhancement of Wine through Selection of Yeasts Based on the Parietal Adsorption Activity. Int. J. Food Microbiol. 2007, 120, 167–172. [Google Scholar] [CrossRef]
- Nogales, R.; Cifuentes, C.; Benítez, E. Vermicomposting of Winery Wastes: A Laboratory Study. J. Environ. Sci. Health B 2005, 40, 659–673. [Google Scholar] [CrossRef]
- Vasserot, Y.; Steinmetz, V.; Jeandet, P. Study of Thiol Consumption by Yeast Lees. Antonie Van Leeuwenhoek 2003, 83, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Pinna, M.V.; Budroni, M.; Farris, G.A.; Pusino, A. Fenhexamid Adsorption Behavior on Soil Amended with Wine Lees. J. Agric. Food Chem. 2008, 56, 10824–10828. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, D.; Cebollero, E.; Gonzalez, R. A Recombinant Saccharomyces Cerevisiae Strain Overproducing Mannoproteins Stabilizes Wine against Protein Haze. Appl. Environ. Microbiol. 2008, 74, 5533–5540. [Google Scholar] [CrossRef]
- de Figueiredo Vilela, L.; de Araujo, V.P.G.; de Sousa Paredes, R.; da Silva Bon, E.P.; Torres, F.A.G.; Neves, B.C.; Eleutherio, E.C.A. Enhanced Xylose Fermentation and Ethanol Production by Engineered Saccharomyces Cerevisiae Strain. AMB Express 2015, 5. [Google Scholar]
- Kutyna, D.R.; Varela, C.; Stanley, G.A.; Borneman, A.R.; Henschke, P.A.; Chambers, P.J. Adaptive Evolution of Saccharomyces Cerevisiae to Generate Strains with Enhanced Glycerol Production. Appl. Microbiol. Biotechnol. 2012, 93, 1175–1184. [Google Scholar] [CrossRef] [PubMed]
- Reyes, L.H.; Gomez, J.M.; Kao, K.C. Improving Carotenoids Production in Yeast via Adaptive Laboratory Evolution. Metab. Eng. 2014, 21, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Çakar, Z.P.; Petek Çakar, Z.; Turanlı-Yıldız, B.; Alkım, C.; Yılmaz, Ü. Evolutionary Engineering of Saccharomyces Cerevisiae for Improved Industrially Important Properties. FEMS Yeast Res. 2012, 12, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Cadière, A.; Aguera, E.; Caillé, S.; Ortiz-Julien, A.; Dequin, S. Pilot-Scale Evaluation the Enological Traits of a Novel, Aromatic Wine Yeast Strain Obtained by Adaptive Evolution. Food Microbiol. 2012, 32, 332–337. [Google Scholar] [CrossRef] [PubMed]
| Pesticide | EU [4] | US [5] | Chile [6] | Codex [7] |
|---|---|---|---|---|
| Acetamiprid | 0.5 | 0.35 | 0.5 | 0.5 |
| Azoxystrobin | 3 | 2 | 2 | 2 |
| Boscalid | 5 | 5 | 5 | 5 |
| Buprofezin | 0.01 | 1 | 1 | 1 |
| Chlorothalonil | 0.01 | * | 3 | 3 |
| Chlorpyrifos | 0.01 | * | 0.5 | 0.5 |
| Cyprodinil | 3 | 3 | 3 | 3 |
| Dimethomorph | 3 | 3 | * | 3 |
| Fenarimol | 0.3 | * | 0.3 | * |
| Fludioxonil | 4 | 2 | 2 | 2 |
| Folpet | 20 | 50 | 10 | 10 |
| Imidacloprid | 0.7 | 1 | 1 | 1 |
| Indoxacarb | 2 | 2 | 2 | 2 |
| Iprovalicarb | 2 | 2 | * | * |
| Lamda-cyhalotrin | 0.2 | 0.01 | 0.2 | * |
| Mepanipyrim | 2 | 1.5 | * | * |
| Metalaxyl | 1 | 2 | 1 | 1 |
| Penconazole | 0.5 | * | 0.4 | 0.4 |
| Procymidone | 0.01 | 5 | * | * |
| Pyrimethanil | 5 | 5 | 4 | * |
| Tebuconazole | 1 | 6 | 6 | 6 |
| Vinclozolin | 0.01 | 6 | * | * |
| Pesticide | Pre A.F a | Wine | Reduction b (%) | Refs. |
|---|---|---|---|---|
| Acetamiprid | 0.681 | 0.474 | 30.4 | Alister et al., 2014 |
| Buprofezin | 4.643 | 1.218 | 73.8 | |
| Imidacloprid | 3.565 | 2.826 | 20.7 | |
| Lamda-cyhalotrin | 0.02 | 0.003 | 85.0 | |
| Pyrimethanil | 5.084 | 1.313 | 74.2 | |
| Tebuconazole | 7.601 | 0.732 | 90.4 | |
| Boscalid | 4.26 ± 1.28 | 1.00 ± 0.32 | 76.5 | Angioni et al., 2011 |
| Indoxacarb | 0.54 ± 0.14 | <0.018 | 96.7 | |
| Iprovalicarb | 0.79 ± 0.27 | 0.36 ± 0.12 | 54.4 | |
| Azoxystrobin | 0.13 | 0.09 | 30.8 | Cabras and Angioni, 2000 |
| Cyprodinil | 0.36 | 0.21 | 41.7 | |
| Fludioxonil | 0.39 | <0.05 | 87.2 | |
| Mepanipyrim | 0.16 | <0.01 | 93.8 | |
| Boscalid | 0.02 ± 0.01 | 0.01 ± 0.00 | 50.0 | Cus et al., 2010 |
| Chlorotalonil | 0.04 ± 0.01 | <0.01 | 75.0 | |
| Dimethomorph | 0.05 ± 0.01 | 0.008 ± 0.00 | 84.0 | |
| Folpet | 0.04 ± 0.02 | <0.02 | 50.0 | |
| Procymidone | 0.14 ± 0.02 | 0.07 | 50.0 | |
| Pyrimethanil | 0.18 ± 0.03 | <0.01 | 94.4 | |
| Chlorpyrifos | 2.005 ± 0.262 | 0.027 ± 0.005 | 98.7 | Navarro et al., 1999 |
| Fenarimol | 0.828 ± 0.187 | 0.143 ± 0.035 | 82.7 | |
| Metalaxyl | 0.650 ± 0.181 | 0.450 ± 0.044 | 30.8 | |
| Penconazole | 0.575 ± 0.061 | 0.093 ± 0.010 | 83.8 | |
| Vinclozolin | 1.133 ± 0.042 | 0.224 ± 0.020 | 80.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra, K.; Ghosh, S.; Godoy, L. Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review. Fermentation 2023, 9, 266. https://doi.org/10.3390/fermentation9030266
Becerra K, Ghosh S, Godoy L. Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review. Fermentation. 2023; 9(3):266. https://doi.org/10.3390/fermentation9030266
Chicago/Turabian StyleBecerra, Kevin, Soumya Ghosh, and Liliana Godoy. 2023. "Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review" Fermentation 9, no. 3: 266. https://doi.org/10.3390/fermentation9030266
APA StyleBecerra, K., Ghosh, S., & Godoy, L. (2023). Pesticide and Yeast Interaction in Alcoholic Fermentation: A Mini-Review. Fermentation, 9(3), 266. https://doi.org/10.3390/fermentation9030266








