Abstract
In this study, the influence of solar inclination angles on biomass growth and the biomolecule profile of Chlorella pyrenoidosa (C. pyrenoidosa) were analyzed in the vertical flat–panel photobioreactor (FPPBR). The growth of C. pyrenoidosa was analyzed at three different solar inclination angles (32.9°, 47.9°, and 90°) in a natural open environment with BG-11 medium and poultry excreta leachate (PEL). The maximum yield of biomass was obtained at 2.40 g/L with PEL and 1.45 g/L with BG-11 medium at a solar inclination angle of 47.9°. The biomass yield with PEL and BG-11 was 5.09–10.26%, 3.94–5.72%, respectively, while biomass productivity with PEL and BG-11 was 5.27–10.63%, 4.06–5.90% higher at a solar inclination angle of 47.9° as compared to 32.9°. The average temperature and radiation of FPPBR were recorded ≈3.90 ± 0.40% and ≈17.28 ± 2.23% higher at a solar inclination angle of 47.9° as compared to a solar inclination angle of 32.9°. The inclined radiation was acquired the maximum area of FPPBR. Results indicated that solar inclination angles enhanced the productivity of algae in FPPBR.
1. Introduction
Algae are cultivated in open ponds and closed photobioreactors (PBR). Closed PBR are sophisticated cultivation systems that require high-cost and technical expertise, whereas open ponds are cost-effective and easier to scale-up. PBR has a culture of purity and has a high rate of productivity [1,2,3,4,5]. However, most of the algal biomass is produced in open ponds. Tubular PBR have higher biomass productivity than the flat-panel closed photobioreactors (FPPBR), but the problems with the tubular PBR are oxygen accumulation, turbulence streaming, a higher surface-to-volume ratio, and biofouling [1,6,7]. However, high photosynthetic efficiency, high volumetric productivity, and low operation and maintenance costs make the FPPBR suitable for algae cultivation [2,3,8]. Thus, FPPBR configurations have a low-cost and are energy efficient, and from an experimental point of view, FPPBR is more reliable than the other PBRs [8,9].
Solar light energy utilization for the cultivation of photosynthetic algae is the primary objective of transparent PBRs, and light energy is the most influential parameter for algal growth [7,9]. Algae are temperature-sensitive organisms that require an optimum temperature range of 15–34 °C for a high biomass yield [7,10]. Solar PBR is difficult to acquire at optimum light and thermal requirements [10] without the additional support of conventional energy because solar irradiance is not constant and temperature also alters. A building-integrated vertical PBR was also studied by Pruvost et al. [11] for algal biomass production modeling. Solar energy utilization depends mainly on the photobioreactor systems for algae growth, that is, how the direct solar radiation incidents on the surface of solar energy conversion technologies (such as photovoltaic panels and PBR), latitude (location), and the season of the year. The season and latitudinal location of a particular place are two important factors that cannot be changed. The optimum inclination (tilt) angle of PBR varies according to the position of the sun with respect to the earth, and it continuously changes year-round [12]. Thus, the solar energy conversion potential depends on the inclination angle of a particular place for the photobioreactor. The best way to optimize the solar inclination angle is by using solar tracking systems, which are costly and whose operation in PBRs is the most difficult task because photobioreactor systems are an assembly of multiple types of equipment with a liquid culture medium. Thus, the PBRs are fixed at an inclination angle.
The solar inclination angles are optimized for solar photovoltaic panels [12,13]. It is expected that the inclination of the PBR can alter the algae productivity because the inclined FPPBRs provide maximum exposure of the algae culture to the solar irradiance with significant control of temperature [14]. Thus, the main aim of this experimental study is to develop a self-sustaining FPPBR that does not need temperature (thermal) and light (radiation) because of the high cost and energy involved in the management of temperature and light for algae cultivation. In the present study, the optimum solar inclination angle for FPPBR in Katra (Jammu) was investigated, including the biomass growth and biomolecule composition of Chlorella pyrenoidosa (C. pyrenoidosa) at various solar inclination angles. Hence, to reduce the conventional energy load of the photobioreactor and to maintain the temperature and light conditions for the growth of algae, a self-dependent photobioreactor system was designed to utilize solar energy as one of the variables to complete the growth conditions (temperature and light).
2. Materials and Methods
2.1. Organism and Stock Culture
C. pyrenoidosa was acquired from the National Collection of Industrial Microorganisms (NCIM 2738), Pune, India. A phototrophic broth culture of C. pyrenoidosa was grown in a 250 mL Erlenmeyer flask filled with BG-11 medium and incubated at 30 ± 2 °C with an 8:16 h light/dark cycle and a 700 Lumens white light LED bulb of 7 W (Royal Energies RE7). Culture purity was analyzed by regular subculturing and microscopic observations.
2.2. Preparation of Wastewater
Poultry excreta were collected from the chicken shop near the university. Poultry excreta leachate was prepared according to our previous study, in which 250 g of poultry excreta were dissolved in 1 L of double-distilled water for 30 min, then vigorously stirred at 350 rpm at 30 °C, filtered with the help of a muslin cloth, and the collected filtrate was considered to be poultry excreta leachate. Previously optimized 25% concentrations of poultry excreta leachate with BG-11 medium (PEL) were selected for outdoor experimental studies [15].
2.3. Photobioreactor System
The vertical flat-panel closed photobioreactor (FPPBR) was utilized in this study, and the experiments were performed under different environmental conditions. The dimensions of the FPPBR are specified as 28 cm length, 25 cm width, and 12 cm height. The FPPBR was made using toughened glass (Saint-Gobain, Chennai, India) having a thickness of 8 mm, and the stand was made using iron with the feature of inclination at various angles (Figure 1). At the bottom of the FPPBR, a bubbler was placed, which produced bubbles for the agitation process. The aeration was provided by an air pump (SEBO SB-548A) having a capacity of 1.7.5 L/min with an air bubbler having a size of 4.5″ × 0.8″ × 0.5″/11.5 × 2 × 1.2 cm (Jardin Air Bubble) and the functioning volume of culture medium was 3.5 L. The measurements of solar irradiance were obtained by a solar power meter (Tenmars TM-207), and the determination of the temperature at the illuminated face of the FPPBR within the culture medium and ambient temperature was obtained by using thermometers. The thermometers had a temperature range of −10 °C to 110 °C.
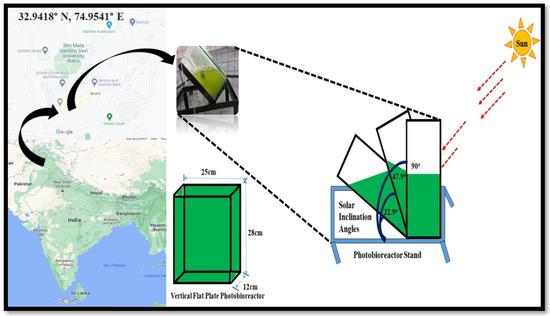
Figure 1.
Schematic experimental set-up of FPPBR at different solar inclination angles (32.9°, 47.9°, and 90°).
2.4. Outdoor Experiment
Outdoor experiments were conducted in the spring and autumn (February–March and October–November) seasons of two years. Due to COVID-19 pandemic restrictions, the experimental investigation was conducted in the spring and autumn seasons of 2019 and in the spring season of 2020; the rest of experimental investigations were continued in the autumn season of 2021 at 32.9417° N, 74.9541° E, Shri Mata Vaishno Devi University, Katra, India. Three solar inclination angles were selected for the study, which were 32.9°, 47.9°, and 90°, respectively. The latitude of 32.9° is the latitudinal location of the experimental site, which was considered the optimum inclination angle with respect to the Earth’s surface for obtaining maximum light [16], whereas the optimum inclination for solar devices is latitude +15 (±15°), i.e., 47.9°, as suggested by Duffie and Beckman [17], and 90° (vertical) is the horizontal position of the FPPBR, which was utilized in various studies at outdoor climatic conditions [11,18]. The maximum solar irradiance was observed at 900–1000 W/m2 (February–March and October–November). The temperature was dependent on the availability of solar irradiance, i.e., increasing solar irradiance increased the ambient temperature as well as the inside temperature of FPPBR, and decreasing solar irradiance reduced the ambient temperature and the inside temperature of FPPBR. Therefore, the ambient day temperature range was 20–30 °C, and the maximum temperature was between 1 pm and 2:30 pm. During the night, the temperature fell rapidly and was recorded at 3–5 °C. Therefore, a water heater (Sobo HS 100 Thermo Control Submersible Aquarium Immersion Heater) was utilized to maintain the temperature around 25 °C in each FPPBR. The evaporation rates of FPPBR at both solar inclination angles were analyzed by daily measurement of the height of the culture medium in the reactor, and the same amount of double-distilled water was added to the FPPBR so that algae could grow efficiently.
2.5. Calculation of Energy on FPPBR
The analysis of solar energy on the surface of the photobioreactor was calculated by the following Equation (1):
where SR is the solar radiation (W/m2), AFPPBR is the area of FPPBR, Tu is the transmissivity of the glass plate of FPPBR (0.85), TI is the solar energy absorption of uncoated clear glass (15%), and TT is time in seconds [19].
Energy (MJ) = SR × AFPPBR × TU × TI × TT/1,000,000
2.6. Biomolecule Profile
The protein content was investigated by the Lowry method [20] with bovine serum albumin (BSA) standards and an absorbance reading at 750 nm. 1 mL of fresh algal cell suspension was mixed with 3 mL of concentrated sulfuric acid and 1 mL of phenol (5% w/v). After that, the mixture was incubated in the water bath at 90 °C for 5 min. The absorbance of the sample was measured by a spectrophotometer at 490 nm and compared with the standard glucose curve [21]. The algal cell chlorophyll was determined by the methanol method with spectrophotometry [22]. The algal cell suspension was filtered through GF/C Whatman filter paper, then methanol was added, and the sample was stored in a water bath at 75 °C for 20 min. The sample was centrifugated at 4000 rpm for 10 min, and after that, the absorption values of the supernatant at 470 nm, 653 nm, and 660 nm were measured by a spectrophotometer.
The lipid content of algal biomass was examined by the sulfo-phospho-vanillin spectrophotometric method [23]. The standard stock of lipid was prepared by using olive oil (15 mg) in a 1 mL solvent mixture of chloroform and methanol (1:1) on the microplate. The solvent was evaporated at 90 °C and 180 µL sulfuric acid was added. After that, the microplate was heated in a dry heat bath at 100 °C for 10 min, cooled to room temperature, and for color development, 0.5 mL of phosphovanillin reagent was added. The mixture was heated at 37 °C for 15 min and incubated for 45 min in a dark box. Absorbance was taken at 530 nm. The phosphovanillin reagent was prepared by dissolving 6 mg vanillin in 100 mL hot water and diluting it with 85% phosphoric acid, then storing the solution in a dark place. Fresh phosphovanillin reagent was prepared before every test run of samples. For algal cell lipid estimation, carefully analyzed the algal cell weight in per mL by centrifugation (4000 rpm for 6 min) to ensure 0.41 mg/mL cell concentration. 20 µL of algal cell suspension was dissolved in 180 µL of sulfuric acid (98%) and heated at 100 °C for 10 min, then cooled at room temperature. A freshly prepared 0.5 mL of phosphor-vanillin reagent was added, and the sample was heated at 37 °C for 15 min. After this, the sample was stored in a dark box for 45 min, and absorbance was measured at 530 nm.
2.7. Algae Biomass Concentration and Biomass Productivity
Biomass growth was measured by taking the optical density (OD) at 680 nm with a UV-visible spectrophotometer (UV-1800, Shimadzu, Kyoto, Japan) [24]. The dry cell weight of algal biomass was analyzed by gravimetric analysis, in which algal biomass was collected by centrifugation for 10 min at 4 °C and 8000 rpm, twice washed with distilled water for removal of salts from the algal biomass, and then dried at 60 °C for 24 h [15]. Standard curves were made between the optical density and biomass weight of algae by MS Excel-2007 for BG-11 and PEL.
2.8. Instrumentation Analysis
The functional group analysis of algal biomass was observed by Fourier transform infrared spectroscopy (FTIR) between the 4000 cm−1 and 500 cm−1 regions (Perkin Elmer, Waltham, MA, USA), with the sample dispersed in approximately 1 mg transparent pellets of KBr.
2.9. Growth Kinetics
The specific growth rate and biomass productivity of algae were observed using the following Equation (2):
where, µ is the specific growth rate of algae that is measured per D, Bf, Bi, are biomass concentration in g/L at final, the initial day of the experiment and Ti and Tf are initial and final cultivation times in the day.
where, dt is the doubling time.
where BP is the biomass productivity of algae and Bmax, Bi is weight of biomass at cultivation time Tf, Ti respectively.
µ = ln (Bf/Bi)/(Tf − Ti)
dt = ln(2)/µ
BP = Bmax − Bi/(Tf − Ti)
Productivities of biomolecules of algae were examined by the following Equation (5) [25]:
where, PBM denotes biomolecules productivities and BM denotes biomolecules.
PBM = BP × BM (%)/100
2.10. Experimental Considerations
The reflectivity of solar radiation on the surface of FPPBR was considered almost negligible because FPPBR was made of transparent glass, as mentioned in Section 2.3. Solar irradiance (radiation) has increased the temperature of FPPBR, which consequently causes evaporative losses of water. The average evaporative loss rate of water for FPPBR was estimated to be 32.9°, 47.9°, and 90°, corresponding to 3 ± 0.8, 4 ± 0.3 mL/D and 2.1 ± 0.5 mL/D, respectively. Experimental studies were conducted in the autumn and spring seasons, when irradiance was not found to be intense and the clear sky in hilly regions did not hinder much of the solar irradiance. In a batch method, freshwater algae (C. pyrenoidosa) were grown in 8.4 L FPPBR with a 3.5 L working volume in North-South oriented 32.9°, 47.9°, and 90° inclined FPPBR for the spring and autumn seasons. The latitudinal position of the experimental site was 32.9417° N, 74.9541° E, on the rooftop of the School of Energy Management. In the spring and autumn seasons of 2019, spring of 2020, and autumn of 2021, two consistent experiments were conducted with BG-11 medium and a 25% concentration of poultry excreta leachate with BG-11 medium (PEL), respectively (2.4. Outdoor experiments). Ambient temperatures and FPPBR temperature with solar irradiance on the surface of inclined FPPBR were recorded at regular intervals of 30 min from 9 am to 5 pm. The FPPBR of BG-11 and PEL medium at individual solar inclination angles (32.9°, 47.9°, and 90°) received approximately the same amount of solar irradiance, and consequently, the temperature of the FPPBR was also recorded as being approximately the same. Similarly, the FPPBRs of BG-11 and PEL medium had also shown the same temperature; there could be a similar salt concentration between BG-11 and PEL medium. Therefore, the temperature of both BG-11 and PEL was considered the same in FPPBR at individual inclination angles. The biomass growth and biomolecule profile of C. pyrenoidosa were analyzed at regular intervals up to 20 days with BG-11 and PEL.
3. Results and Discussions
3.1. Biomass and Biomolecule Growth Pattern at a Solar Inclination Angle of 32.9°
This experiment was performed outdoors at a solar inclination angle of 32.9°, which was the latitudinal location, and the photoperiod was managed by an 8:16 h light:dark cycle. The experiment was conducted for 20 days in open solar irradiance, and the highest peak average solar irradiance was recorded in the months of February–March, 2019 (SPNY1), October–November, 2019 (ATMY1), and February–March, 2020 (SPNY2), October–November, 2021 (ATMY2), which were 698.35 W/m2, 523.08 W/m2, and 791.73 W/m2, 682 W/m2, respectively. The highest peak average ambient temperature was recorded at 27.6 °C (SPNY1), 30.36 °C (ATMY1), and 28.80 °C (SPNY1), 30.1 °C (ATMY2), whereas the lowest temperature was observed at 17.16 °C (SPNY1), 28.79 °C (ATMY1), and 21.36 °C (SPNY1), 20.87 °C (ATMY2) (Figure 2). In the autumn season, FPPBR obtained the highest average energy of 405.99 MJ, compared to 358.42 MJ in the spring season. The suitable range of temperature for the optimum growth of algae, as suggested by Molinuevo-Salces et al. [26]; Singh and Singh [27], ranged between 15–30 °C and 25 °C was considered suitable for freshwater algae belonging to the genus Chlorella. About 50% of the solar irradiance around the mid-infrared (750 nm) region was directly created to the heat effect in the culture medium [28]. Thermal regulations in the PBRs are critical issues that require proper management because algae are photosynthetic organisms that need to grow within the optimum range of temperature. The continuous solar irradiance in summer creates excess heat in the PBR that has the ability to create a lethal effect on the algae, and cooling the PBR is another challenge for outdoor cultivation. In winter, low temperatures require heating PBR for optimum biomass growth. However, the spring and autumn seasons have moderate temperatures and solar irradiance that help prevent excessive heat and cool conditions. The dependability over natural solar irradiance helps reduce the energy load of the FPPBR by managing the internal temperature.
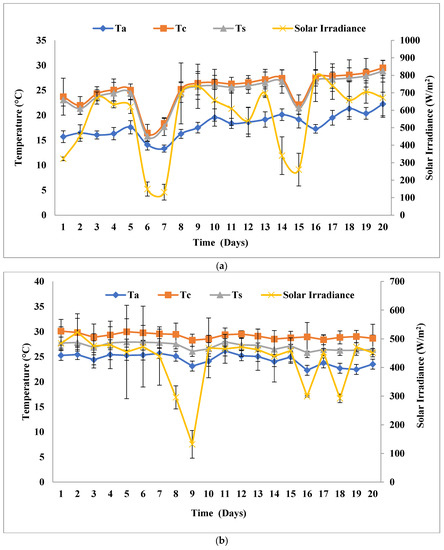
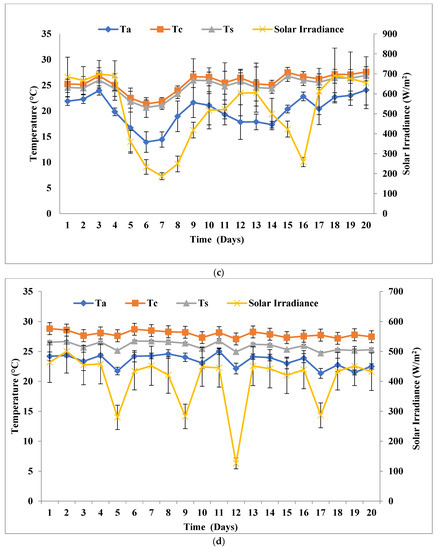
Figure 2.
Solar irradiance and temperature at a solar inclination angle of 32.9°: (a) SPNY1; (b) ATMY1; (c) SPNY2; (d) ATMY2.
Algal growth was analyzed at regular time intervals, and the initial biomass concentration was 0.076 g/L, used as inoculums for the BG-11 medium and PEL. The growth pattern of the C. pyrenoidosa revealed that the first three days were the acclimatization phase, which is why growth was very slow in outdoor conditions. After this, C. pyrenoidosa started a gradual increase in its biomass growth, as shown in Figure 3. Light and temperature are two important factors that affected the growth of algae in a nutrient-sufficient culture medium. Algae in outdoor climatic conditions were exposed for 2–3 days to diurnal and seasonal changes of light and temperature. Due to outdoor climatic conditions, natural solar irradiance and temperature were not constant and fluctuated throughout the day. Clouds and wind velocity may be influencing factors for solar irradiance and temperature, and they may change the incident solar irradiance on FPPBR and respectively fluctuate the ambient temperature as well as the internal temperature of FPPBR. In the laboratory, light and temperature are provided at optimal and constant rates, allowing algae to grow at a constant rate.
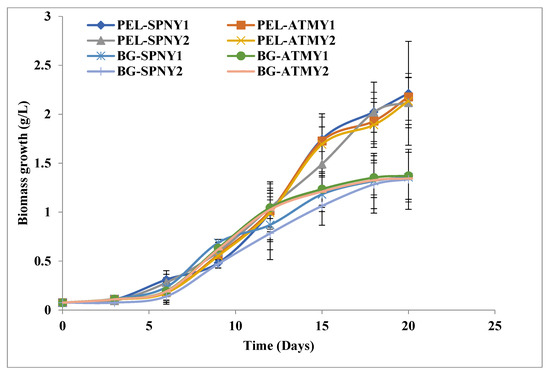
Figure 3.
Growth of C. pyrenoidosa at solar inclination angles of 32.9°.
C. pyrenoidosa showed the maximum biomass productivity, specific growth, and doubling time as 112.51 mg/L/D, 0.17/D, and 3.95/D, respectively, in PEL on the 20th day of cultivation. The maximum biomass productivity, specific growth, and doubling time were analyzed as 70.03 mg/L/D, 0.18/D, and 3.75/D with BG-11 at the 20th day of cultivation, which is listed in Table 1. It showed that the doubling time of C. pyrenoidosa was the highest in the BG-11 compared to the PEL. The highest biomass yield of C. pyrenoidosa was observed with PEL, which was 2.21 g/L PEL-SPNY1 on the 20th day of cultivation, followed by 2.17 g/L PEL-ATMY1, 2.11 g/L PEL-SPNY2, and 2.13 g/L PEL-ATMY2, whereas C. pyrenoidosa in BG-11 produced the highest biomass yield of 1.37 g/L BG-ATMY1 on the 20th day of cultivation, followed by 1.35 g/L BG-SPNY1 and 1.33 g/L BG-SPNY2, 1.34 g/L BG-SPNY2. C. pyrenoidosa achieved the highest biomass yield in PEL in comparison to BG-11, which was similar to our previous study [15]. The biomass yield of C. pyrenoidosa was recorded at 2.52 g/L biomass with PEL and 1.58 g/L with BG-11 in the controlled conditions of the laboratory. However, the outdoor cultivation of C. pyrenoidosa in FPPBR produced 2.17 g/L and 1.35 g/L biomass yields with PEL and BG-11, which were ≈16.12% and ≈17.03% lower than the laboratory control conditions. It could be low due to fluctuations of light and temperature in natural conditions, such as a low temperature in the morning and a high light intensity in the afternoon. Low temperature limits the enzyme activities of algal cells, and extreme temperature creates cell stress and consequently cell death. However, the rate of photosynthesis increases up to a certain limit of light saturation, and after that, photoinhibition starts, which leads to cell deaths, as the reason given by Wolf et al. [29]. Pruvost et al. [16] simulated a whole-year running solar rectangular photobioreactor with the cyanobacterium Arthospira plantensis, and the impact of the incidence angle of solar illumination on resultant productivities, which affect both light capture and light convey inside the bulk culture, was examined using a variety of parameters. They also suggested that by optimizing the residence duration as allowed by the model, near-maximal productivities might be reached for a given location.

Table 1.
Specific growth, doubling time, biomass productivity, and biomass yield of C. pyrenoidosa in PEL and BG-11 during the 20th day of the cultivation time.
The biomolecule profile of C. pyrenoidosa in BG-11 and PEL was analyzed at a solar inclination angle of 32.9° in which carbohydrate, protein, lipid and chlorophyll were estimated at regular intervals. Figure 4a–d illustrates the biomolecule profile of C. pyrenoidosa and Table 2 lists the highest biomolecule yield and productivity at selected solar inclination angles. At a solar inclination angle of 32.9°, the highest carbohydrate yield and productivity were 0.52 g/L and 27.49 mg/L/D with PEL (PEL-SPNY1) in February–March, and 0.47 g/L and 25.14 mg/L/D with BG-11 in October–November (BG-ATMY2) (Figure 4a). However, BG-11 was shared 34.57–36.25% of carbohydrate and PEL shared 22.04–36.25% of carbohydrate, which was lower than the BG-11, and it could be the high amount of nitrogen content in PEL because nitrogen starvation increases the biosynthesis of carbohydrates.
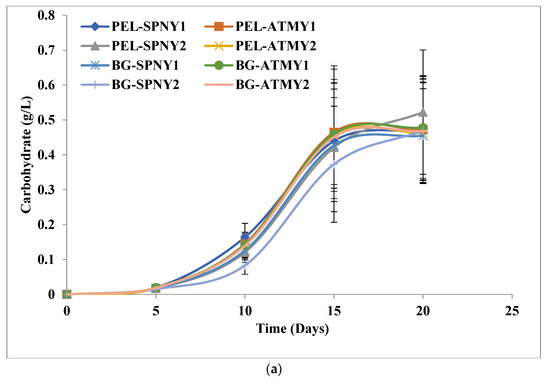
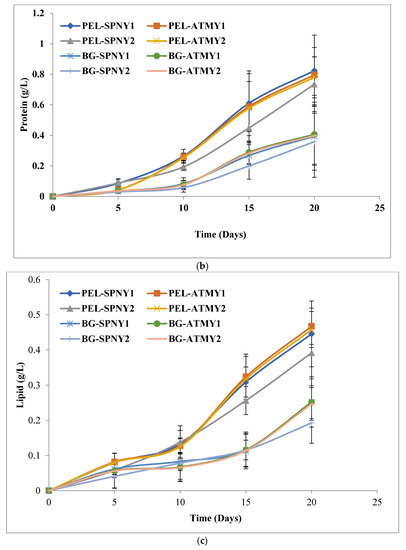
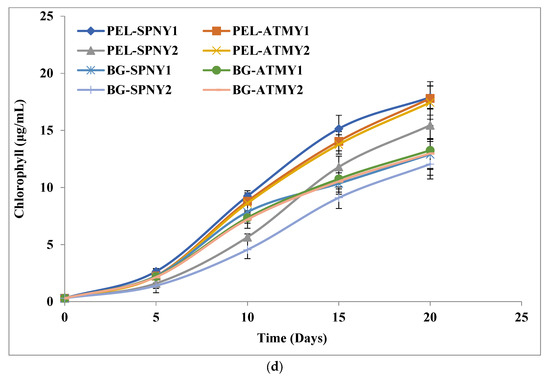
Figure 4.
Biomolecules growth of C. pyrenoidosa at solar inclination angles of 32.9°: (a) carbohydrates; (b) protein; (c) lipid; (d) chlorophyll.
The protein content of C. pyrenoidosa was estimated at solar inclination angles of 32.9°, and Figure 4b represents the growth of protein in C. pyrenoidosa at a solar inclination angle of 32.9°. The highest protein yield and productivity were observed at 0.79 g/L and 43.35 mg/L/D with PEL (PEL-ATMY1) and 0.40 g/L and 21.39 mg/L/D with BG-11 (BG-ATMY1) in the months of October–November (Table 2). However, the highest range of protein was observed to be 35.98 to 38.53% with PEL, which was ≈7% higher than the BG-11. The intercellular lipid production of C. pyrenoidosa was investigated in FPPBR at both solar inclination angles, and the maximum lipid yield and productivity were found to be 0.46 g/L and 24.24 mg/L/D, respectively, with PEL in the month of October–November (PEL-ATMY1), whereas 0.25 g/L and 14.48 mg/L/D, yield and productivity, were recorded with BG-11 in the month of October–November (BG-ATMY1) (Figure 4c). The lipid production range was calculated to be 19.17–22.22% with PEL and 14.86–19.16% with BG-11. It showed that ≈3% more lipid accumulated with PEL compared to BG-11 at a solar inclination angle of 32.9°. A similar trend was reported by Singh et al. [15], in which PEL was shown to have higher lipid productivities compared to the BG-11 under lab-controlled conditions, and in this study, lipid productivity was recorded at 26.31 mg/L/D, which was higher than the present study. It could have optimized growth conditions in the lab in comparison to outdoor conditions. Chlorophyll is the main photosynthetic material that absorbs light and carbon dioxide from the atmosphere to form carbohydrates and oxygen. Figure 4d shows the chlorophyll growth in C. pyrenoidosa at a solar inclination angle of 32.9°, and the maximum chlorophyll yield was calculated at 17.9 µg/mL with PEL (PEL-SPNY1) in the month of February-March and 13.25 µg/mL with BG-11 (BG-SPNY1) in the same season. PEL was found to have 34.33% more chlorophyll than BG-11. The chlorophyll productivity with PEL and BG-11 was found to be 0.90 µg/mL/D and 0.67 µg/mL/D in the same season as the chlorophyll yield.
3.2. Biomass and Biomolecule Growth Pattern at a Solar Inclination Angle of 47.9°
In this experimental setup, inoculum amounts and all the arrangements were similar to the solar inclination angle of 32.9°, except for the solar inclination angle 47.9°. FPPBR at solar inclination angle of 47.9° received peak average solar irradiance of 924.42 Wm−2 (SPNY1), 654.5 W/m (ATMY1), 805.5 Wm−2 (SPNY2), and 625.87 W/m (ATMY2), which was ≈17.28 ± 2.23% higher than solar inclination angle 32.9°. The average temperature ranges of FPPBR were recorded 17.19–30.9 °C (SPNY1), 29.29–31.75 °C (ATMY1), 18.22–28.31 °C (SPNY2), and 28.25–30.03 °C (ATMY2) for 20 days (Figure 5), which was ≈3.9% higher than the solar inclination angle of 32.9°. FPPBR at solar inclination angle 47.9° had received a higher amount of solar irradiance; therefore, the average internal temperature of FPPBR at solar inclination angle 47.9° was recorded as being higher than that of FPPBR at solar inclination angle 32.9°. The maximum average energy received by FPPBR in the autumn season was 515.55 MJ, compared to 482.32 MJ in the spring season. In support of this, solar photovoltaic panels (or devices) that receive higher solar irradiance show higher temperatures compared to the solar panels (or devices) that receive lower irradiance, because solar irradiance is a form of energy that converts into heat and thus raises the temperature of solar photovoltaic panels (or devices) [30].
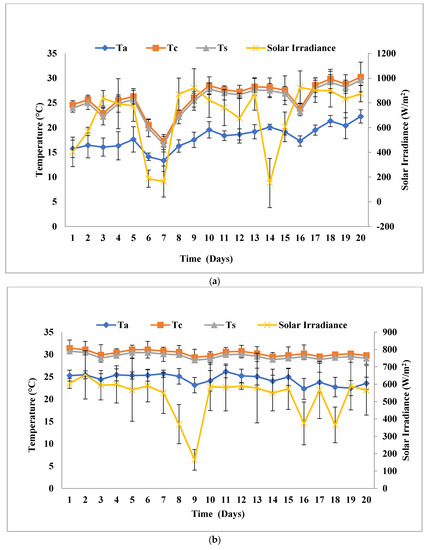
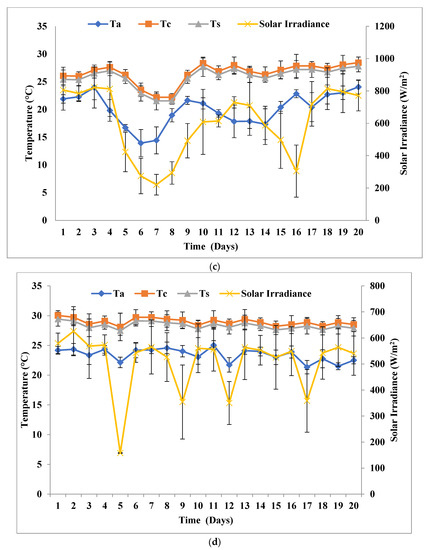
Figure 5.
Solar irradiance and temperature at solar inclination angle 47.9°: (a) SPNY1; (b) ATMY1; (c) SPNY2; (d) ATMY2.
The biomass growth of C. pyrenoidosa was mentioned in Figure 6 with PEL and BG-11 in FPPBR. The highest biomass yield of C. pyrenoidosa in PEL was noticed at 2.40 g/L PEL-ATMY1 and subsequently at 2.35 g/L PEL-ATMY2, 2.32 g/L PEL-SPNY1, and 2.30 g/L PEL-SPNY2. The maximum biomass productivity and specific growth were observed at 122.37 mg/L/D and 0.18/D, respectively, with PEL-ATMY1, whereas the highest doubling time was analyzed at 3.86/D with PEL-SPNY2 in PEL. C. pyrenoidosa had the highest biomass yield with BG-11 at 1.45 g/L BG-ATMY1, followed by 1.41 g/L BG-SPNY1 and 1.40 g/L BG-SPNY2. The highest biomass productivity and doubling times were investigated at 74.17 mg/L/D and 3.68/D with BG-ATMY1, and specific growth was analyzed at 0.18/D in both seasons with BG-11 (Table 1). The relationship between solar irradiance and biomass productivity of algae was observed by Qiang et al. [31], and they suggested that higher solar irradiance could be able to augment the higher biomass productivity in FPPBR at various seasons. Hu et al. [32] conducted various investigations and suggested that there is an interrelation between the length of the light path and the biomass growth of algae. The smaller light path increases the cell density of algae compared to the large light path. They also suggested that the inclination of flat plate modular PBR at 30° provided better output compared to 60° and 90° because the inclined surface of PBR is able to capture maximum solar irradiance compared to vertical (90°) PBR. The photon flux density can be altered by altering the PBR and photon flux density. By inclining a tubular loop PBR at an angle to the horizontal during the day, the profile of photon flux density incident on the bioreactor can be changed. The photon flux density at midday declined as the angle of inclination increased, whereas the photon flux density early in the morning and late in the afternoon increased as the angle of inclination increased [33].
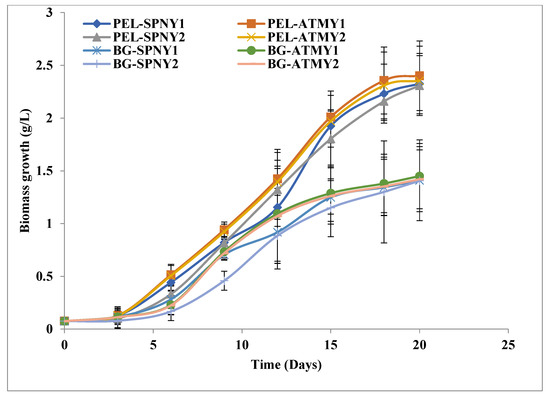
Figure 6.
Growth of C. pyrenoidosa at solar inclination angles of 47.9°.
Figure 7a–d illustrated the growth pattern biomolecules of C. pyrenoidosa at a solar inclination angle 47.9°, and Table 2 listed the highest biomolecule yields and productivities of PEL and BG-11. The biomolecule profile of C. pyrenoidosa was also analyzed at solar inclination angle 47.9°. Carbohydrate yield and productivity were analyzed at 0.54 g/L and 28.90 mg/L/D, respectively, with PEL in February–March (PEL-SPNY2) and 0.48 g/L and 25.22 mg/L/D, respectively, in BG-11 (BG-SPNY2) at solar inclination angle 47.9° (Table 2). The percentage of carbohydrates with BG-11 ranged from 33.37 to 34.40% and was more than ≈10% higher than PEL. Figure 7a shows the carbohydrate concentration of C. pyrenoidosa in both seasons in FPPBR. De Winter et al. [34] suggested that nitrogen limitation triggers carbohydrates biosynthesis in algal cells. Carbohydrates are considered an essential component for bioenergy production due to their easily fermentable ability into bioethanol and biobutanol [35]. Algae-derived carbohydrates have a significant role in pharmaceutical applications [36]. The accumulations of carbohydrates depend on the algal species and growth conditions.
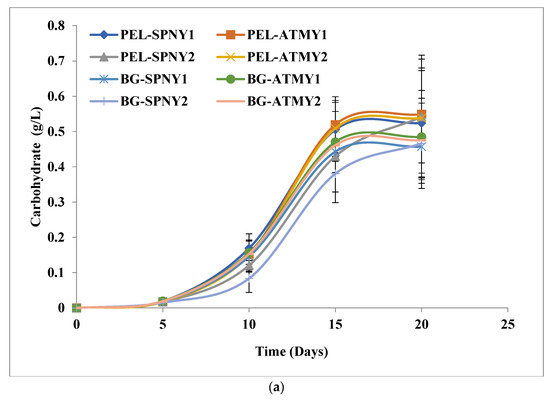
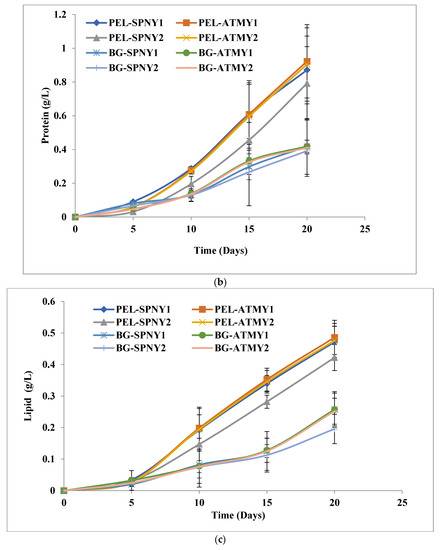
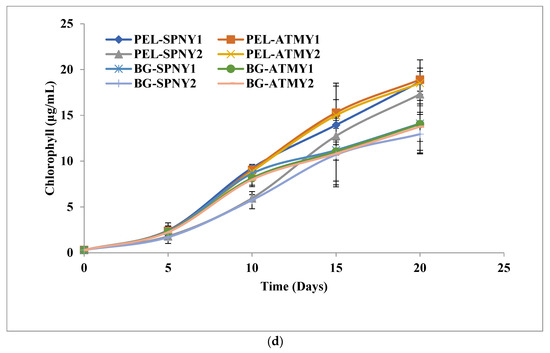
Figure 7.
Biomolecules growth of C. pyrenoidosa at solar inclination angles of 47.9°: (a) carbohydrates; (b) protein; (c) lipid; (d) chlorophyll.
A similar trend had occurred at solar inclination angle 47.9°, where the highest protein yield and productivity were analyzed at 0.92 g/L and 50 mg/L/D with PEL (PEL-ATMY1), whereas 33.99 g/L and 24.55 mg/L/D protein yield and productivity were recorded with BG-11 (BG-ATMY1) (Table 2) (Figure 7b). Here, PEL also shared ≈7% higher protein ranges (38.43–41.38%) than BG-11. It could have a higher nitrogen content in the PEL compared to BG-11. Under nitrogen-rich conditions, the protein content increased, whereas the carbohydrate content was produced in low quantities. Several parameters regulate protein biosynthesis, and these parameters vary from species to species [37,38]. The trend of protein production in C. pyrenoidosa was similar to our previous study, in which PEL was found superior to BG-11 in laboratory conditions because nitrate, nitrite, and ammonium were nitrogen sources that contributed a significant portion of poultry waste materials [15]. The protein synthesis in C. vulgaris was also affected by the solar irradiance, and Khalili et al. [39] reported that the increase in irradiance and its duration increased the growth rate as well as protein synthesis. Thirty-three percent of protein concentration was obtained at 37.5 µmol photons/m2/s with a 16:8 h photoperiod, which was increased to 46% at 100 µmol photons/m2/s with an 8:16 h photoperiod. Protein is an essential dietary molecule that is utilized in many nutraceutical products [40].
The maximum lipid yield and productivity at solar inclination angle 47.9° were analyzed at 0.48 g/L and 28.03 mg/L/D, respectively, with PEL in the month of October-November (PEL-ATMY1) and 0.25 g/L and 14.39 mg/L/D, respectively, with BG-11 in the month of October-November (BG-ATMY1) (Figure 7c). PEL and BG-11 mediums showed similar patterns, such as a solar inclination angle of 32.9°, in the months of October and November (Table 2). Approximately 3% higher lipid range was recorded with PEL compared to BG-11 (14.33–18.77%). A rapid growth in lipid was observed between 15–20 days because most of the nitrogen was utilized between 1–15 days at both solar inclination angles. Carbohydrate content increased gradually with biomass growth (days 3–18), and there was no significant growth (Figure 4a, Figure 5a,e and Figure 7a). The accumulation of carbohydrates starts first, then it converts into higher fat molecules (lipids), which support the metabolic process in unfavorable environmental conditions for a longer duration. Lipid content increased with the cultivation time because carbohydrate content was high in algal cells initially, which converted into lipid under nitrogen-starved conditions in C. pyrenoidosa [41]. It is a well-established fact that adequate light intensity enhances the production of algal lipids, which can be because adequate light intensity promotes the storage of extra photoassimilates and then their transformation into chemical energy. Excess ATP and NADPH, which are created during photosynthesis, are required for lipid synthesis, particularly triacylglycerol synthesis. More lipids can be created when additional energy in the form of photons is supplied, and they use this surplus energy to defend the algal cells from photochemical damage [42]. Lipid content of C. vulgaris in an open bioreactor in a greenhouse increased from 19.5% to 25.6% when solar irradiance was increased from 25% to 100% in June, and lipid content increased from 17.3% to 24.7% when solar irradiance increased from 25% to 100% in September [43]. The FPPBR at solar inclination angle 47.9° receives more light energy compared to solar inclination angle 32.9°. It could be the reason behind the higher lipid content at solar inclination angles of 47.9° compared to solar inclination angles of 32.9°. After carbohydrate, lipid from algae is directly involved in biofuel applications.
The maximum yield of chlorophyll was recorded at 18.9 µg/ml with PEL (PEL-ATMY1) and 14.1 µg/mL (BG-ATMY1) with BG-11, which showed 34.04% higher chlorophyll in the months of October and November at a solar inclination angle of 47.9°. The chlorophyll productivities were 0.96 µg/mL/D and 0.72 µg/mL/D with PEL-ATMY1 and BG-ATMY1, respectively, which were similar to the trend of the solar inclination angle of 32.9° (Table 2) (Figure 7d). The chlorophyll content was also found to be high at a solar inclination angle of 47.9°, as were other biomolecules of C. pyrenoidosa. High solar irradiance and temperature could influence chlorophyll synthesis, which has a direct correlation with biomass growth in algal cells; however, lower temperatures negatively catalyze the photosynthetic rate [27]. Seyfabadi et al. [44] employed a variety of experimental studies to analyze the impact of irradiance on chlorophyll and carotene in C. vulgaris. They found that the chlorophyll-a content reduces under high light while β-carotene reverses to chlorophyll. Due to the adaptation mechanism of the environmental conditions of high light, smaller photosynthetic units were synthesized, whereas low light induces the synthesis of larger photosynthetic units to harvest light. Although high light intensity causes photoinhibition that reduces the cell density, the growth of C. vulgaris is found to be higher under warm white light as compared to red and blue light [39]. Chlorophyll is a pigment substance that is applied as a coloring agent for food substances.

Table 2.
Biomolecules yield and productivity of C. pyrenoidosa with PEL and BG-11 in FPPBR.
Table 2.
Biomolecules yield and productivity of C. pyrenoidosa with PEL and BG-11 in FPPBR.
| Samples | Carbohydrates | Protein | Lipid | Chlorophyll | ||||
|---|---|---|---|---|---|---|---|---|
| Yield (g/L) | Productivity (mg/L/D) | Yield (g/L) | Productivity (mg/L/D) | Yield (g/L) | Productivity (mg/L/D) | Yield (µg/mL) | Productivity (µg/mL/D) | |
| PEL-SPNY1 | 0.47± 0.15 | 24.80± 2.03 | 0.82 ± 0.23 | 43.35 ± 3.01 | 0.44 ± 0.09 | 23.90 ± 1.02 | 17.90 ± 0.97 | 0.90 ± 0.01 |
| PEL-ATMY1 | 0.47± 0.14 | 24.86 ± 1.91 | 0.79 ± 0.17 | 41.90 ± 1.16 | 0.46 ± 0.05 | 24.24 ± 2.16 | 17.80 ± 1.46 | 0.90 ± 0.01 |
| PEL-SPNY2 | 0.52± 0.17 | 27.49 ± 0.76 | 0.73 ± 0.13 | 38.66 ± 2.12 | 0.39 ± 0.07 | 21.93 ± 1.01 | 15.45 ± 1.42 | 0.78 ± 0.06 |
| PEL-ATMY2 | 0.46± 0.14 | 24.36 ± 1.23 | 0.78 ± 0.17 | 41.06 ± 1.06 | 0.45 ± 0.05 | 23.76 ± 1.91 | 17.44 ± 1.46 | 0.88 ± 0.03 |
| BG-SPNY1 | 0.45± 0.13 | 23.95 ± 1.52 | 0.39 ± 0.26 | 20.72 ± 0.93 | 0.25 ± 0.07 | 14.40 ± 0.12 | 12.90 ± 0.98 | 0.68 ± 0.01 |
| BG-ATMY1 | 0.47± 0.14 | 25.14 ± 1.96 | 0.40 ± 0.19 | 21.39 ± 1.30 | 0.25 ± 0.04 | 14.58 ± 0.03 | 13.25 ± 1.31 | 0.67 ± 0.08 |
| BG-SPNY2 | 0.46± 0.13 | 24.70 ± 2.02 | 0.35 ± 0.18 | 18.86 ± 0.38 | 0.19 ± 0.05 | 10.86 ± 0.01 | 12.05 ± 2.17 | 0.61 ± 0.01 |
| BG-ATMY2 | 0.46± 0.14 | 24.64 ± 0.86 | 0.39 ± 0.19 | 20.96 ± 1.02 | 0.24 ± 0.04 | 14.28 ± 0.81 | 12.98 ± 1.30 | 0.66 ± 0.06 |
3.3. Biomass Growth Pattern at a Solar Inclination Angle of 90°
The initial three solar inclination angles were selected for the experimental observation of biomass growth and the biomolecule profile of C. pyrenoidosa. The three solar inclined angles were 32.9° (latitudinal location), 47.9°, and 90°, respectively. In this pre-test experiment, the average biomass yield of C. pyrenoidosa was obtained at 1.12 g/L with BG-11 at the solar inclination angle of 90° in the spring and autumn seasons. In FPPBR, C. pyrenoidosa did not show much biomass yield in comparison to solar inclination angles of 32.9° and 47.9°. The solar inclination angle of 90° was vertical to the earth’s horizontal surface, where the peak solar irradiance was very low in comparison to that at 32.9° and 47.9° (500 W/m2) because striking solar irradiance covers the minimum area of FPPBR at a tilted surface. Due to this, the internal average temperature range of FPPBR was also recorded low in the spring and autumn seasons (18–22 °C), which was low as compared to the optimum growth temperature. Therefore, only 32.9° and 47.9° solar inclination angles were selected for the further analysis of biomolecules. The solar irradiance on the surface of 90° did not completely irradiate in the FPPBR, and it could have caused a shading effect in the culture; consequently, algae did not grow properly. Hu et al. [32] obtained lower biomass productivity in comparison to 30° and 60°. Sukačová et al. [45] investigated helical-tubular, multi-tubular, and flat-panel PBRs for biomass and lipid production. They found that the multi-tubular PBR had the lowest energy consumption with the highest sensitivity to temperature, and the multi-tubular PBR is a suitable design for colder climate regions. They conceptualized a hybrid system for the cultivation of algae, merging the advantages of open pond systems in the summer and closed PBR systems in the winter.
3.4. Comparison of Solar Inclination Angles of 32.9° and 47.9°
The yield and productivity of biomass, protein, and chlorophyll were calculated to be higher at the solar inclination angle of 47.9° as compared to the solar inclination angle of 32.9°. The growth characteristics of C. pyrenoidosa at both solar inclination angles were summarized in Table 2. Figure 8 illustrates the comparative yield and productivity of biomass and biomolecules of C. pyrenoidosa with PEL and BG-11 at both solar inclination angles. The biomass yield and productivity were calculated with PEL and BG-11 in the range of 5.09–10.26%, 3.94–5.72%, and 5.27–10.63%, 4.06–5.90%, respectively. Protein yield and productivity with PEL and BG-11 were noticed at 5.78–15.58%, 3.00–9.23%, and 13.06–20.87%, 14.80–22.15%, respectively. Chlorophyll yield and productivity with PEL and BG-11 were determined 4.18–11.97%, 6.03–9.30%, and 4.36–12.31%, 6.21–9.43%, respectively. However, the yield and productivity of carbohydrates and lipids with PEL at solar inclination angle 47.9° were shown to be high percentages as compared to solar inclination angle 32.9°, but the yield and productivity of carbohydrates and lipids with BG-11 were shown to be low percentage ranges (Figure 8). Both culture mediums (PEL and BG-11) showed nitrogen deficiency after the 15th day, which could be the reason behind the lipid stimulation in the algal cells. Ansari et al. [25] suggested that nitrogen deficiency stimulates lipid synthesis in the algal cells. Whereas high percentages of carbohydrate and lipid content in PEL as compared to BG-11 were noticed, it may be due to the presence of high organic content in PEL. The initial high concentration of total organic carbon and total nitrogen increased the lipid productivity in the mixotrophic cultivation of Chlorella sp. in wastewater [46]. This may be the reason for high lipid synthesis.
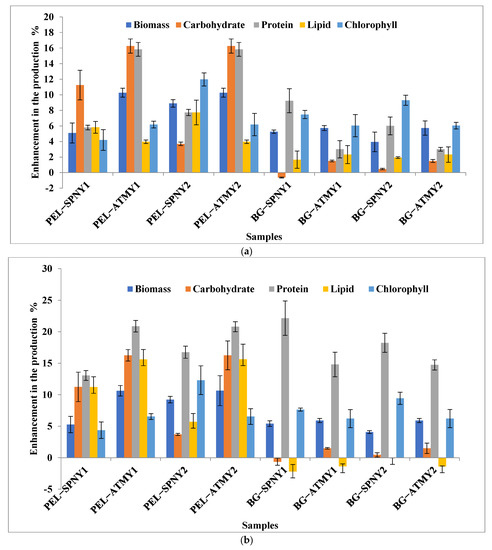
Figure 8.
Enhancement in the production of biomass and biomolecules at 47.9° with respect to 32.9°: (a) yield; (b) productivity.
Solar inclination angles and algal biomass production were based on modeling and theoretical studies. In these studies, various locations and parameters, such as PBR design, algal species, solar irradiance, and solar inclination angles, have been selected for the investigation. These theoretical estimation of algal biomass production was supported by various researcher in their studies [10,18], but the actual experimental studies were very difficult to match theoretical results. According to Qiang et al. [31], each latitude has an optimal surface solar inclination angle for FPPBR. The solar inclination angle of a FPPBR and harvesting of solar irradiance have a correlation, and algal biomass productivity depends upon the solar irradiance captured by algae up to a certain limit, and the higher solar irradiance causes a heating effect in the reactor because around 50% of the energy in solar irradiance is near and mid-infrared above 750 nm that is able to create a heating effect in the culture medium [28].
Biomass production of algae in FPPBR was enhanced by optimization of solar inclination angles. A vertical solar inclination angle of 90° resulted in the highest biomass production in three latitudes, i.e., the Netherlands, France, and Algeria. Among these latitudinal locations, Algeria (latitudes 28.0339° N, 1.6596° E) had the highest biomass production [18]. Qiang et al. [31] analyzed algal biomass productivity at four different solar inclination angles, which were 10°, 30°, 60°, and 90°, respectively, in Israel (31.0461° N, 34.8516° E). Small solar inclination angles of 10° and 30°had maximum biomass productivity in summers, and large angles in the vicinity of 60° had maximum biomass productivity in winters because, in the winter, sun irradiance covered a large surface area of FPPBR, which created a thermal comfort for algal growth, whereas in the summer, small angles prevented overheating of the reactor. Posten [47] suggested that vertical placement (90°) of PBR in the sunlight scattered the solar radiation to a large surface area in order to capture maximum solar irradiance, and therefore more algal cells were exposed to lower solar intensities. Pruvost et al. [11] investigated a building-integrated FPPBR in Nantes (France) (47.2184° N, 1.33° W) at 45° and 90°solar inclination angles, with expected biomass productivity of Chlorella sp. of 9.43 gm2/D and 7.68 gm2/D, respectively.
The solar inclination angles were potential parameters to alter algal biomass productivity not only in laboratory-scale PBRs but also at the commercial level because large-scale algal biomass was grown in open solar PBRs to reduce energy load. Temperature and light management may be specific requirements for algal species and their products. The fluctuation of solar irradiance caused trouble for algae because fluctuating irradiance could also directly alter the temperature of the PBR, whereas adjustments in solar inclination can control temperature. The frequent adjustment in inclination angles increases the biomass productivity in high-altitude areas [31]. The monthly optimal inclination is more suitable for solar energy gain than the seasonal and yearly inclined angles for any location [48]. The cultivation of algae in an outdoor environment reduces energy and cost loads through the utilization of natural sunlight and increases the energy performance of FPPBR.
The protein, fiber, and ash content of Chlorella sp. is not affected by solar irradiance, whereas the lipid and nitrogen-free extractable content are dependent on the light intensity and environmental conditions [43]. The composition of algal biomass (carbohydrates, proteins, and lipids) depends on the environmental conditions, which include light intensity, temperature, pH, and the availability of nutrients in the culture medium. In comparison to lab-controlled conditions, biomolecule production in outdoor cultivation under closed PBR is still developing. In the contemporary era, seasonal optimization of the solar inclination angle is required to help create reliable cultivation systems all around the year. The carbon fixation rate and biomass production for bioenergy applications can be explored to determine the sustainability of the cultivation of algae in outdoor environmental conditions. Therefore, there is a need for such a type of energy-self-sustaining PBR in the near future to increase the share of the bioeconomy in the global renewable energy scenario.
3.5. FTIR Analysis of Algal Biomass
The biomass of C. pyrenoidosa was harvested at both solar inclination angles, i.e., 32.9° and 47.9°. The FTIR spectra of harvested biomass from algae were analyzed. The FTIR spectra were recorded over the wavelength region 4000–400 cm−1 and each peak of the spectra was assigned a specific functional group. The FTIR spectra at both solar inclination angles (i.e., 32.9° and 47.9°) were identical, and major variations were not shown in the positions of functional groups. The biomass of C. pyrenoidosa exhibited strong peaks in the range of 3400–3500 cm−1 and 2800–3000 cm−1, indicating the presence of protein–carbohydrate and lipid–carbohydrate functional groups. Other strong groups of peaks in the range of 700–1250 cm−1 indicated the presence of carbohydrates, nucleic acids, and antioxidant enzymes. The presence of lipid in biomass at solar inclination angle 47.9° showed 3411 cm−1, 2920 cm−1, 2600 cm−1, and 2493 cm−1. Biomass showed 3361 cm−1, 3236 cm−1 stretching bands of O–H group and N–H amide of proteins, respectively, while 2924 cm−1 characterizes the methyl (–CH) and methylene (–CH2) groups of lipids. The –C=O stretching vibration of the ester group of lipids and fatty acyl long chains is represented at 1722 cm−1 and the 1636 cm−1 stretching band probably represents the protein amide groups (–N–H, C–N). The presence of 1161 cm−1, 1010 cm−1, and 875 cm−1 bands denotes stretching of C–O and –C–O–C– type polysaccharide compounds that represent various forms of carbohydrates. The FTIR spectra of the algae Scenedesmus sp., C. vulgaris, and C. pyrenoidosa were analyzed by Duygu et al. [49], Bajwa and Bishnoi [50], Ansari et al. [51], and Sudhakar and Premalatha [52]. The results are very similar to the present study.
4. Conclusions
The FPPBR is an effective cultivation system for high biomass productivity in outdoor climatic conditions. This study revealed that outdoor cultivation of Chlorella pyrenoidosa under solar radiation plays a significant role in the production of biomass and biomolecules. The maximum productivities of biomass were achieved at 122.37 mg/L/D with PEL and 74.17 mg/L/D with BG-11 medium in the autumn season at a solar inclination angle of 47.9° as compared to 32.9° and 90°. The outdoor cultivation at an optimized solar inclination angle to scale-up algal biomass and biomolecule production for commercial applications and the low risk of contamination in PBR are an advantage that maintains the purity of the biomass. The uncertain outdoor climatic conditions also require more investigation for algal biomass growth and biomolecular composition.
Author Contributions
H.M.S.: Writing—original draft, Investigation, Methodology, Acquisition of Data, V.V.T.: Conceptualization, Methodology, Supervision, R.K.: Supervision, Methodology, Review and Editing, A.S.: Supervision and review. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
Har Mohan Singh is grateful to MNRE (Govt. of India), India, for providing a Senior Research Fellowship from the National Renewable Energy Fellowship Programme at the School of Energy Management, Shri Mata Vaishno Devi University, Katra (J and K), India.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Benemann, J. Microalgae for Biofuels and Animal Feeds. Energies 2013, 6, 5869–5886. [Google Scholar] [CrossRef]
- De Vree, J.H.; Bosma, R.; Janssen, M.; Barbosa, M.J.; Wijffels, R.H. Comparison of four outdoor pilot-scale photobioreactors. Biotechnol. Biofuels 2015, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.M.; Kothari, R.; Gupta, R.; Tyagi, V. Bio-fixation of flue gas from thermal power plants with algal biomass: Overview and research perspectives. J. Environ. Manag. 2019, 245, 519–539. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.C.; Bassin, I.D.; Cammarota, M.C. Microalgae and Cyanobacteria Biomass Pretreatment Methods: A Comparative Analysis of Chemical and Thermochemical Pretreatment Methods Aimed at Methane Production. Fermentation 2022, 8, 497. [Google Scholar] [CrossRef]
- Tropea, A. Biofuels Production and Processing Technology. Fermentation 2022, 8, 319. [Google Scholar] [CrossRef]
- Mahata, C.; Das, P.; Khan, S.; Thaher, M.I.A.; Quadir, M.A.; Annamalai, S.N.; Al Jabri, H. The Potential of Marine Microalgae for the Production of Food, Feed, and Fuel (3F). Fermentation 2022, 8, 316. [Google Scholar] [CrossRef]
- Ahmad, S.; Iqbal, K.; Kothari, R.; Singh, H.M.; Sari, A.; Tyagi, V. A critical overview of upstream cultivation and downstream processing of algae-based biofuels: Opportunity, technological barriers and future perspective. J. Biotechnol. 2022, 351, 74–98. [Google Scholar] [CrossRef]
- Shareefdeen, Z.; Elkamel, A.; Babar, Z.B. Recent Developments on the Performance of Algal Bioreactors for CO2 Removal: Focusing on the Light Intensity and Photoperiods. BioTech 2023, 12, 10. [Google Scholar] [CrossRef]
- Fuchs, T.; Arnold, N.D.; Garbe, D.; Deimel, S.; Lorenzen, J.; Masri, M.; Mehlmer, N.; Weuster-Botz, D.; Brück, T.B. A Newly Designed Automatically Controlled, Sterilizable Flat Panel Photobioreactor for Axenic Algae Culture. Front. Bioeng. Biotechnol. 2021, 9, 697354. [Google Scholar] [CrossRef]
- Goetz, V.; Le Borgne, F.; Pruvost, J.; Plantard, G.; Legrand, J. A generic temperature model for solar photobioreactors. Chem. Eng. J. 2011, 175, 443–449. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Gouic, B.; Lepine, O.; Legrand, J.; Le Borgne, F. Microalgae culture in building-integrated photobioreactors: Biomass production modelling and energetic analysis. Chem. Eng. J. 2016, 284, 850–861. [Google Scholar] [CrossRef]
- Sharma, M.K.; Kumar, D.; Dhundhara, S.; Gaur, D.; Verma, Y.P. Optimal Tilt Angle Determination for PV Panels Using Real Time Data Acquisition. Glob. Chall. 2020, 4, 1900109. [Google Scholar] [CrossRef]
- Akhlaghi, S.; Sangrody, H.; Sarailoo, M.; Rezaeiahari, M. Efficient operation of residential solar panels with determination of the optimal tilt angle and optimal intervals based on forecasting model. IET Renew. Power Gener. 2017, 11, 1261–1267. [Google Scholar] [CrossRef]
- Xu, L.; Weathers, P.J.; Xiong, X.-R.; Liu, C.-Z. Microalgal bioreactors: Challenges and opportunities. Eng. Life Sci. 2009, 9, 178–189. [Google Scholar] [CrossRef]
- Singh, H.M.; Tyagi, V.; Kothari, R.; Azam, R.; Slathia, P.S.; Singh, B. Bioprocessing of cultivated Chlorella pyrenoidosa on poultry excreta leachate to enhance algal biomolecule profile for resource recovery. Bioresour. Technol. 2020, 316, 123850. [Google Scholar] [CrossRef]
- Pruvost, J.; Cornet, J.F.; Goetz, V.; Legrand, J. Theoretical investigation of biomass productivities achievable in solar rectangular photobioreactors for the cyanobacterium Arthrospira platensis. Biotechnol. Prog. 2012, 28, 699–714. [Google Scholar] [CrossRef]
- Beckman, W.A.D. Solar Engineering of Thermal Processes; John Wiley & Sons, Inc.: Hobken, NJ, USA, 2013. [Google Scholar]
- Slegers, P.M.; Wijffels, R.H.; Van Straten, G.; Van Boxtel, A.J.B. Design scenarios for flat panel photobioreactors. Appl. Energy 2011, 88, 3342–3353. [Google Scholar] [CrossRef]
- Pathak, A.K.; Tyagi, V.V.; Anand, S.; Pandey, A.K.; Kothari, R. Advancement in solar still integration with phase change materials-based TES systems and nanofluid for water and wastewater treatment applications. J. Therm. Anal. Calorim. 2022, 147, 9181–9227. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biolog. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biolog. Chem. 1941, 140, 315–322. [Google Scholar] [CrossRef]
- Anschau, A.; Caruso, C.S.; Kuhn, R.C.; Franco, T.T. Validation of the sulfophosphovanillin (spv) method for the determination of lipid content in oleaginous microorganisms. Braz. J. Chem. Engin. 2017, 34, 19–27. [Google Scholar] [CrossRef]
- Xia, A.; Hu, Z.; Liao, Q.; Huang, Y.; Zhu, X.; Ye, W.; Sun, Y. Enhancement of CO2 transfer and microalgae growth by perforated inverted arc trough internals in a flat-plate photobioreactor. J. Bioresour. Technol. 2018, 269, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Ansari, F.A.; Singh, P.; Guldhe, A.; Bux, F. Microalgal cultivation using aquaculture wastewater: Integrated biomass generation and nutrient remediation. Algal Res. 2017, 21, 169–177. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. From piggery wastewater nutrients to biogas: Microalgae biomass revalorization through anaerobic digestion. Renew. Energy 2016, 96, 1103–1110. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, P. Effect of temperature and light on the growth of algae species: A review. Renew. Sustain. Energy Rev. 2015, 50, 431–444. [Google Scholar] [CrossRef]
- Pruvost, J.; Le Borgne, F.; Artu, A.; Cornet, J.F.; Legrand, J. Industrial photobioreactors and scale-up concepts. Adv. Chem. Eng. 2016, 48, 257–310. [Google Scholar]
- Wolf, J.; Stephens, E.; Steinbusch, S.; Yarnold, J.; Ross, I.L.; Steinweg, C.; Doebbe, A.; Krolovitsch, C.; Müller, S.; Jakob, G.; et al. Multifactorial comparison of photobioreactor geometries in parallel microalgae cultivations. Algal Res. 2016, 15, 187–201. [Google Scholar] [CrossRef]
- Perraki, V.; Kounavis, P. Effect of temperature and radiation on the parameters of photovoltaic modules. J. Renew. Sustain. Energy 2016, 8, 013102. [Google Scholar] [CrossRef]
- Qiang, H.; Faiman, D.; Richmond, A. Optimal tilt angles of enclosed reactors for growing photoautotrophic microorganisms outdoors. J. Fermen. Bioeng. 1998, 85, 230–236. [Google Scholar] [CrossRef]
- Hu, Q.; Guterman, H.; Richmond, A. A flat inclined modular photobioreactor for outdoor mass cultivation of photoautotrophs. Biotechnol. Bioeng. 1996, 51, 51–60. [Google Scholar] [CrossRef]
- Lee, Y.K.; Low, C.S. Effect of photobioreactor inclination on the biomass productivity of an outdoor algal culture. Biotechnol. Bioeng. 1991, 38, 995–1000. [Google Scholar] [CrossRef]
- De Winter, L.; Schepers, L.W.; Cuaresma, M.; Barbosa, M.J.; Martens, D.E.; Wijffels, R.H. Circadian rhythms in the cell cycle and biomass composition of Neochlorisole oabundans under nitrogen limitation. J. Biotechnol. 2014, 187, 25–33. [Google Scholar] [CrossRef]
- Moenaert, A.; López-Contreras, A.M.; Budde, M.; Allahgholi, L.; Hou, X.; Bjerre, A.B.; Örlygsson, J.; Karlsson, E.N.; Friðjónsson, Ó.H.; Hreggviðsson, G.Ó. Evaluation of Laminaria Digitata Hydrolysate for the Production of Bioethanol and Butanol by Fermentation. Fermentation 2023, 9, 59. [Google Scholar] [CrossRef]
- Chia, S.R.; Ong, H.C.; Chew, K.W.; Show, P.L.; Phang, S.M.; Ling, T.C.; Nagarajan, D.; Lee, D.J.; Chang, J.S. Sustainable approaches for algae utilisation in bioenergy production. Renew. Energy 2018, 129, 838–852. [Google Scholar] [CrossRef]
- Guccione, A.; Biondi, N.; Sampietro, G.; Rodolfi, L.; Bassi, N.; Tredici, M.R. Chlorella for protein and biofuels: From strain selection to outdoor cultivation in a Green Wall Panel photobioreactor. Biotechnol. Biofuels Bioprod. 2014, 7, 84. [Google Scholar] [CrossRef]
- Amorim, M.L.; Soares, J.; Coimbra, J.S.D.R.; Leite, M.D.O.; Albino, L.F.T.; Martins, M.A. Microalgae proteins: Production, separation, isolation, quantification, and application in food and feed. Crit. Rev. Food Sci. Nutr. 2020, 61, 1976–2002. [Google Scholar] [CrossRef]
- Khalili, A.; Najafpour, G.D.; Amini, G.; Samkhaniyani, F. Influence of nutrients and LED light intensities on biomass production of microalgae Chlorella vulgaris. Biotechnol. Bioprocess Eng. 2015, 20, 284–290. [Google Scholar] [CrossRef]
- Tropea, A.; Ferracane, A.; Albergamo, A.; Potortì, A.G.; Lo Turco, V.; Di Bella, G. Single cell protein production through multi food-waste substrate fermentation. Fermentation 2022, 8, 91. [Google Scholar] [CrossRef]
- Dong, L.; Li, D.; Li, C. Characteristics of lipid biosynthesis of Chlorella pyrenoidosa under stress conditions. Bioprocess Biosyst Eng. 2020, 43, 877–884. [Google Scholar] [CrossRef]
- Solovchenko, A.E.; Khozin-Goldberg, I.; Didi-Cohen, S.; Cohen, Z.; Merzlyak, M.N. Effects of light intensity and nitrogen starvation on growth, total fatty acids and arachidonic acid in the green microalga Parietochloris incisa. J. Phycol. 2008, 20, 245–251. [Google Scholar] [CrossRef]
- Metsoviti, M.N.; Papapolymerou, G.; Karapanagiotidis, I.T.; Katsoulas, N. Effect of light intensity and quality on growth rate and composition of Chlorella vulgaris. Plants 2019, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Seyfabadi, J.; Ramezanpour, Z.; AminiKhoeyi, Z. Protein, fatty acid, and pigment content of Chlorella vulgaris under different light regimes. J. Appl. Phycol. 2011, 23, 721–726. [Google Scholar] [CrossRef]
- Sukačová, K.; Lošák, P.; Brummer, V.; Máša, V.; Vícha, D.; Zavřel, T. Perspective design of algae photobioreactor for greenhouses—A comparative study. Energies 2021, 14, 1338. [Google Scholar] [CrossRef]
- Gao, F.; Yang, H.L.; Li, C.; Peng, Y.Y.; Lu, M.M.; Jin, W.H.; Bao, J.J.; Guo, Y.M. Effect of organic carbon to nitrogen ratio in wastewater on growth, nutrient uptake and lipid accumulation of a mixotrophic microalgae Chlorella sp. Biores. Technol. 2019, 282, 118–124. [Google Scholar] [CrossRef]
- Posten, C. Review: Design principles of photo-bioreactors for cultivation of microalgae. Eng. Life Sci. 2009, 9, 165–177. [Google Scholar] [CrossRef]
- Yadav, A.K.; Chandel, S.S. Tilt angle optimization to maximize incident solar radiation: A review. Renew. Sustain. Energy Rev. 2013, 23, 503–513. [Google Scholar] [CrossRef]
- Duygu, D.Y.; Udoh, A.U.; Ozer, T.B.; Akbulut, A.; Erkaya, I.A.; Yildiz, K.; Guler, D. Fourier transform infrared (FTIR) spectroscopy for identification of Chlorella vulgaris Beijerinck 1890 and Scenedesmus obliquus (Turpin) Kützing 1833. Afr. J. Biotechnol. 2012, 11, 3817–3824. [Google Scholar]
- Bajwa, K.; Bishnoi, N.R. Osmotic stress induced by salinity for lipid overproduction in batch culture of Chlorella pyrenoidosa and effect on others physiological as well as physicochemical attributes. J. Algal Biomass Util. 2015, 6, 26–34. [Google Scholar]
- Ansari, A.A.; Khoja, A.H.; Nawar, A.; Qayyum, M.; Ali, E. Wastewater treatment by local microalgae strains for CO2 sequestration and biofuel production. Appl. Water Sci. 2017, 7, 4151–4158. [Google Scholar] [CrossRef]
- Sudhakar, K.; Premalatha, M. Characterization of micro algal biomass through FTIR/TGA/CHN analysis: Application to Scenedesmus sp. Energy Sources Part A Recovery Utili. Environ. Eff. 2015, 37, 2330–2337. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).