Evaluating the Potential of Newly Developed Energy Cane Clones for First- and Second-Generation Ethanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical and Chemical Compositions of Energy Cane Juice
2.3. Chemical Composition of Energy Cane Bagasse
2.4. Isolation and Screening of Yeasts for Energy Cane Juice Fermentation
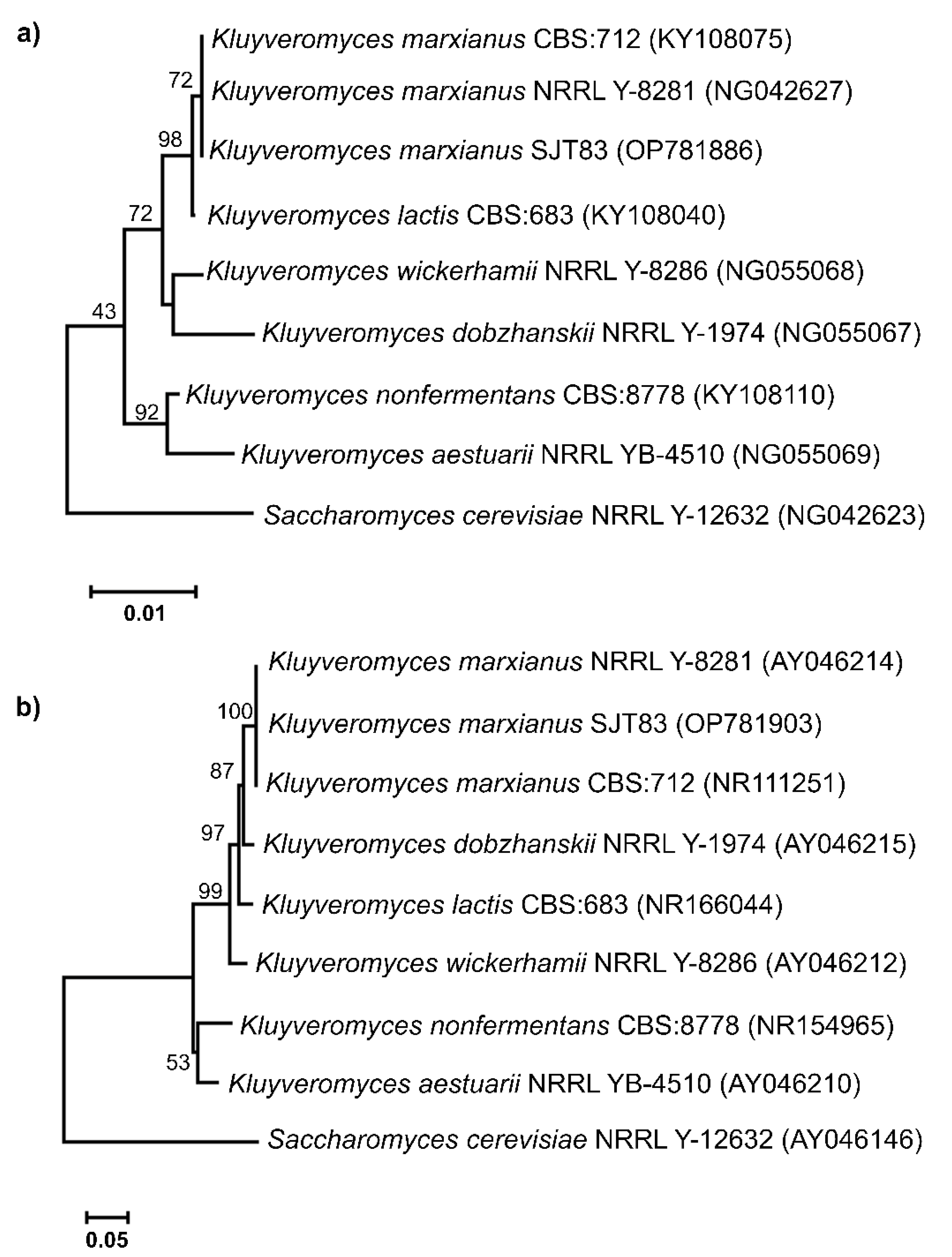
2.5. Yeast Identification and Phylogenetic Tree Analysis
2.6. Ethanol Production from Energy Cane Juice
2.7. Preparation of Energy Cane Bagasse Hemicellulosic Hydrolysate Using Dilute Acid Hydrolysis
2.8. Ethanol Production from Energy Cane Bagasse
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Energy Cane Juice and Bagasse
| Clone/Variety | TSS (°Brix) | Total Sugars (g/L) | FAN (mg/L) | Reference |
|---|---|---|---|---|
| TByEFC08-0035 | 18.0 ± 0.0 c | 180.15 ± 2.00 c | 127.23 ± 2.40 a | In this study |
| TByEFC10-0004 | 19.1 ± 0.3 b | 190.63 ± 1.20 b | 117.55 ± 1.22 b | In this study |
| Khon Kaen3 A | 21.1 ± 0.1 a | 224.00 ± 2.73 a | 99.07 ± 0.38 c | In this study |
| Biotec2 | 15.0 ± 0.0 d | 140.50 ± 0.27 d | 99.52 ± 0.41 c | In this study |
| TByEFC04-1208 | 18.4 ± 0.6 | 179.72 ± 3.60 | 120.24 ± 3.29 | [12] |
| TByEFC04-1155 | 16.3 ± 0.7 | 154.48 ± 0.83 | 74.90 ± 2.16 | [12] |
| VG11-X1 | 16.1 | NR | NR | [24] |
| Vx12-0015 | 16.3 | NR | NR | [25] |
| Vertix 1 | NR | 85 | NR | [26] |
| INTA05-3116 | 13.5 | NR | NR | [8] |
| NCo310 A | 15.6 | NR | NR | [25] |
| LK92-11 A | 19.0 ± 0.8 | 187.40 ± 2.03 | 136.92 ± 1.25 | [12] |
3.2. Agronomic Characteristics of Energy Cane
3.3. Ethanol Production of Energy Cane
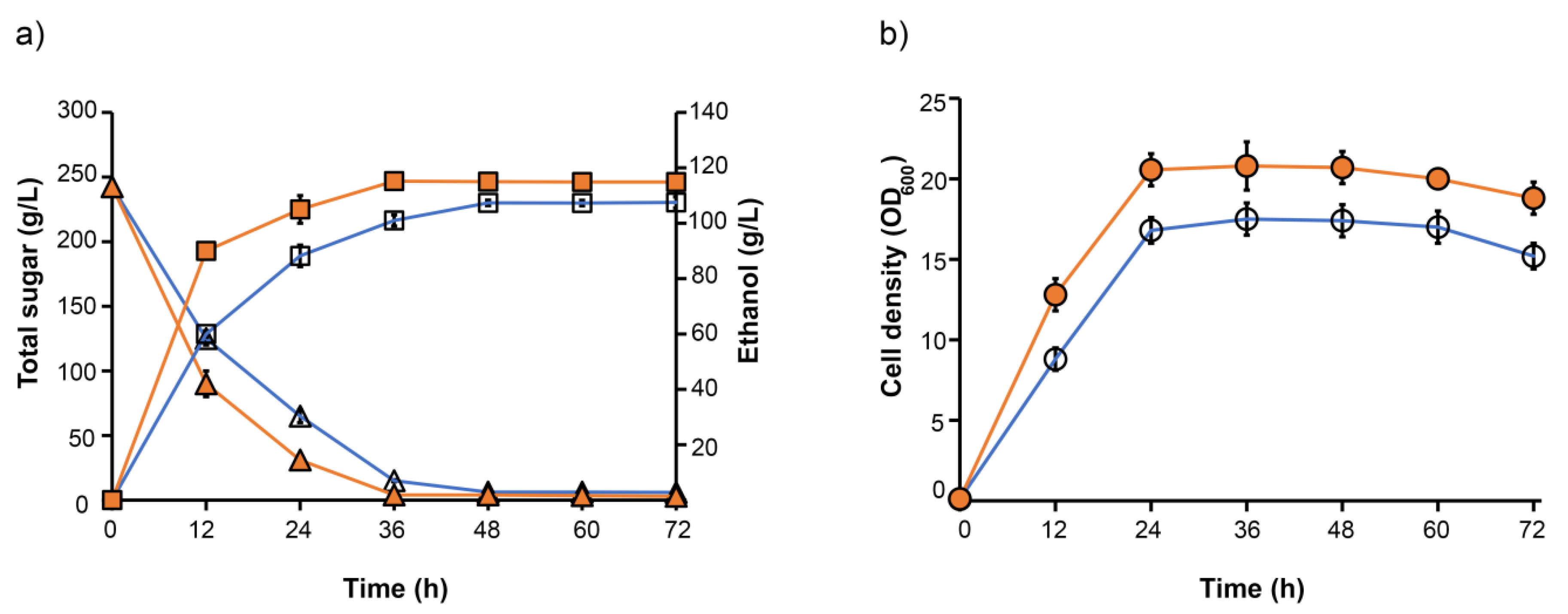
3.3.1. Selection of Yeast Strain for Ethanol Production from Juice
3.3.2. Ethanol Production of Energy Cane Juice and Bagasse
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diniz, A.L.; Ferreira, S.S.; ten-Caten, F.; Margarido, G.R.A.; dos Santos, J.M.; Barbosa, G.V.d.S.; Carneiro, M.S.; Souza, G.M. Genomic resources for energy cane breeding in the post genomics era. Comput. Struct. Biotechnol. J. 2019, 17, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807. [Google Scholar] [CrossRef]
- Chatwachirawong, P.; Thumkrasair, S.; Srisink, S. Sugarcane Breeding. Final Report: Research Development Design and Engineering Project BT-B-01-PG-11-4924; National Science and Technology Development Agency: Pathum Thani, Thailand, 2009.
- Hale, A.L.; Dufrene, E.O.; Tew, T.L.; Pan, Y.-B.; Viator, R.P.; White, P.M.; Veremis, J.C.; White, W.H.; Cobill, R.; Richard, E.P., Jr.; et al. Registration of ‘Ho 02-113’ sugarcane. J. Plant Regist. 2013, 7, 51–57. [Google Scholar] [CrossRef]
- Bischoff, K.P.; Gravois, K.A.; Reagan, T.E.; Hoy, J.W.; Kimbeng, C.A.; LaBorde, C.M.; Hawkins, G.L. Registration of ‘L 79-1002’ sugarcane. J. Plant Regist. 2008, 2, 211–217. [Google Scholar] [CrossRef]
- White, W.H.; Tew, T.L.; Cobill, R.M.; Burner, D.M.; Grisham, M.P.; Dufrene, E.O.; Pan, Y.-B.; Richard, E.P., Jr.; Legendre, B.L. Registration of ‘Ho 00-961’ sugarcane. J. Plant Regist. 2011, 5, 332–338. [Google Scholar] [CrossRef]
- Cursi, D.E.; Hoffmann, H.P.; Barbosa, G.V.S.; Bressiani, J.A.; Gazaffi, R.; Chapola, R.G.; Fernandes Junior, A.R.; Balsalobre, T.W.A.; Diniz, C.A.; Santos, J.M.; et al. History and current status of sugarcane breeding, germplasm development and molecular genetics in Brazil. Sugar Tech. 2022, 24, 112–133. [Google Scholar] [CrossRef]
- Kane, A.O.; Pellergini, V.O.A.; Espirito Santo, M.C.; Ngom, B.D.; García, J.M.; Acevedo, A.; Erazzú, L.E.; Polikarpov, I. Evaluating the potential of culms from sugarcane and energy cane varieties grown in Argentina for second-generation ethanol production. Waste Biomass Valorization 2022, 13, 329–343. [Google Scholar] [CrossRef]
- Govindaraj, P. SBIEC 14006—A high biomass energycane for power, alcohol and paper industries. J. Sugarcane Res. 2020, 10, 100–106. [Google Scholar] [CrossRef]
- Matsuoka, S.; Kennedy, A.J.; Santos, E.G.D.d.; Tomazela, A.L.; Rubio, L.C.S. Energy cane: Its concept, development, characteristics, and prospects. Adv. Bot. 2014, 2014, 597275. [Google Scholar] [CrossRef]
- Chatwachirawong, P.; Boonaek, K.; Raksopa, K. Yield Trial of Foragecane Varieties. Final Report: Research Development Design and Engineering Project BT-B-01-PM-11-5105; National Science and Technology Development Agency: Pathum Thani, Thailand, 2008.
- Thammasittirong, S.N.-R.; Chatwachirawong, P.; Chamduang, T.; Thammasittirong, A. Evaluation of ethanol production from sugar and lignocellulosic part of energy cane. Ind. Crops Prod. 2017, 108, 598–603. [Google Scholar] [CrossRef]
- Senatham, S.; Chamduang, T.; Kaewchingduang, Y.; Thammasittirong, A.; Srisodsuk, M.; Elliston, A.; Roberts, I.N.; Waldron, K.W.; Thammasittirong, S.N.-R. Enhanced xylose fermentation and hydrolysate inhibitor tolerance of Scheffersomyces shehatae for efficient ethanol production from non-detoxified lignocellulosic hydrolysate. SpringerPlus 2016, 5, 1040. [Google Scholar] [CrossRef]
- AOAC. Official Methods of the Association of Official Analytical Chemists; AOAC: Washington, DC, USA, 1980. [Google Scholar]
- Sripodok, C.; Thammasittirong, A.; Thammasittirong, S.N.-R. Antifungal activity of soil yeast (Lachancea kluyveri SP132) against rice pathogenic fungi and its plant growth promoting activity. J. Int. Soc. Southeast Asian Agric. Sci. 2019, 25, 55–65. [Google Scholar]
- Ohara, S.; Fukushima, Y.; Sugimoto, A.; Terajima, Y.; Ishida, T.; Sakoda, A. Rethinking the cane sugar mill by using selective fermentation of reducing sugars by Saccharomyces dairenensis, prior to sugar crystallization. Biomass Bioener. 2012, 42, 78–85. [Google Scholar] [CrossRef]
- González-Mendoza, D.; Argumedo-Delira, R.; Morales-Trejo, A.; Pulido-Herrera, A.; Cervantes-Díaz, L.; Grimaldo-Juarez, O.; Alarcón, A. A rapid method for isolation of total DNA from pathogenic filamentous plant fungi. Genet. Mol. Res. 2010, 9, 162–166. [Google Scholar] [CrossRef]
- Namnuch, N.; Thammasittirong, A.; Thammasittirong, S.N.-R. Lignocellulose hydrolytic enzymes production by Aspergillus flavus KUB2 using submerged fermentation of sugarcane bagasse waste. Mycology 2021, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Fanelli, A.; Reinhardt, L.; Matsuoka, S.; Ferraz, A.; da Franca Silva, T.; Hatfield, R.D.; Romanel, E. Biomass composition of two new energy cane cultivars compared with their ancestral Saccharum spontaneum during internode development. Biomass Bioener. 2020, 141, 105696. [Google Scholar] [CrossRef]
- Aragon, D.; Suhr, M.; Kochergin, V. Evaluation of energy cane and sweet sorghum as feedstocks for conversion into fuels and chemicals. Sugar Ind. 2013, 138, 651–655. [Google Scholar] [CrossRef]
- Hill, A.E.; Stewart, G.G. Free Amino Nitrogen in Brewing. Fermentation 2019, 5, 22. [Google Scholar] [CrossRef]
- Davila-Gomez, F.J.; Chuck-Hernandez, C.; Perez-Carrillo, E.; Rooney, W.L.; Serna-Saldivar, S.O. Evaluation of bioethanol production from five different varieties of sweet and forage sorghums (Sorghum bicolor (L.) Moench). Ind. Crops Prod 2011, 33, 611–616. [Google Scholar] [CrossRef]
- Ceccato-Antonini, S.R.; Bassi, A.P.G.; Paraluppi, A.L.; Sandos, E.G.D.; Matsuoka, S. Deterioration and fermentability of energy cane juice. Ciência Rural 2017, 47, 1–7. [Google Scholar] [CrossRef]
- Yanagui, K.; Camargo, E.L.O.; Abreu, L.G.F.d.; Nagamatsu, S.T.; Fiamenghi, M.B.; Silva, N.V.; Carazzolle, M.F.; Nascimento, L.C.; Franco, S.F.; Bressiani, J.A.; et al. Internode elongation in energy cane shows remarkable clues on lignocellulosic biomass biosynthesis in Saccharum hybrids. Gene 2022, 828, 146476. [Google Scholar] [CrossRef]
- Grassi, M.C.B.; Pereira, G.A.G. Energy-cane and RenovaBio: Brazilian vectors to boost the development of biofuels. Ind. Crops Prod. 2019, 129, 201–205. [Google Scholar] [CrossRef]
- Hesam, F.; Tarzi, B.G.; Honarvar, M.; Jahadi, M. Valorization of sugarcane bagasse to high value-added xylooligosaccharides and evaluation of their prebiotic function in a synbiotic pomegranate juice. Biomass Conv. Bioref. 2023, 13, 787–799. [Google Scholar] [CrossRef]
- Viator, R.P.; Richard, E.P. Sugar and energy cane date of planting effects on cane, sucrose, and fiber yields. Biomass Bioener. 2012, 40, 82–85. [Google Scholar] [CrossRef]
- Cruz, L.P.; Pacheco, V.S.; Silva, L.M.; Almeida, R.L.; Miranda, M.T.; Pissolato, M.D.; Machado, E.C.; Ribeiro, R.V. Morpho-physiological bases of biomass production by energy cane and sugarcane: A comparative study. Ind. Crops Prod. 2021, 171, 113884. [Google Scholar] [CrossRef]
- Zhao, D.; Momotaz, A.; LaBorde, C.; Irey, M. Biomass yield and carbohydrate composition in sugarcane and energy cane grown on mineral soils. Sugar Tech 2020, 22, 630–640. [Google Scholar] [CrossRef]
- Dalen, M.S.; Tubana, B.S.; Kwakye, S.; Han, K.-J. Nitrogen rate and harvest date effects on energy cane yield, quality parameters, nutrient uptake and biomass chemical composition. Agrosystems Geosci. Environ. 2022, 5, e20302. [Google Scholar] [CrossRef]
- Knoll, J.E.; Anderson, W.F.; Missaoui, A.; Hale, A.; Hanna, W.W. Biomass production and stability of five energycane cultivars at two latitudes in Georgia. Agrosystems Geosci. Environ. 2021, 4, e20146. [Google Scholar] [CrossRef]
- Limtong, S.; Sringiew, C.; Yongmanitchai, W. Production of fuel ethanol at high temperature from sugar cane juice by a newly isolated Kluyveromyces marxianus. Bioresour. Technol. 2007, 98, 3367–3374. [Google Scholar] [CrossRef]
- Baptista, M.; Domingues, L. Kluyveromyces marxianus as a microbial cell factory for lignocellulosic biomass valorisation. Biotechnol. Adv. 2022, 60, 108027. [Google Scholar] [CrossRef] [PubMed]
- Ha-Tran, D.M.; Nguyen, T.T.M.; Huang, C.-C. Kluyveromyces marxianus: Current state of omics studies, strain improvement strategy and potential industrial implementation. Fermentation 2020, 6, 124. [Google Scholar] [CrossRef]
- Sirohi, R.; Pandey, J.P. Dilute acid hydrolysis of spoiled wheat grains: Analysis of chemical, rheological and spectral characteristics. Bioresour. Technol. 2019, 283, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, Y.; Cheng, H.; Görgens, J.F. Evaluation of bagasse from different varieties of sugarcane by dilute acid pretreatment and enzymatic hydrolysis. Ind. Crops Prod. 2013, 51, 7–18. [Google Scholar] [CrossRef]
- Pu, Y.; Hu, F.; Huang, F.; Davison, B.H.; Ragauskas, A.J. Assessing the molecular structure basis for biomass recalcitrance during dilute acid and hydrothermal pretreatments. Biotechnol. Biofuels 2013, 6, 15. [Google Scholar] [CrossRef]
- Wu, S.; Lan, Y.; Wu, Z.; Peng, Y.; Chen, S.; Huang, Z.; Xu, L.; Gelbič, I.; Guan, X.; Zhang, L.; et al. Pretreatment of spent mushroom substrate for enhancing the conversion of fermentable sugar. Bioresour. Technol. 2013, 148, 596–600. [Google Scholar] [CrossRef] [PubMed]

| Clone/Variety | Cellulose (%) | Hemicellulose (%) | Lignin (%) | Fiber (%) |
|---|---|---|---|---|
| TByEFC08-0035 | 44.25 ± 0.90 b | 23.30 ± 0.75 c | 13.10 ± 0.20 d | 24.16 ± 1.79 b |
| TByEFC10-0004 | 37.15 ± 0.49 c | 28.11 ± 0.36 a | 15.10 ± 0.47 b | 13.44 ± 1.25 c |
| Khon Kaen3 | 37.78 ± 0.32 c | 28.63 ± 0.72 a | 13.85 ± 0.43 c | 13.78 ± 1.17 c |
| Biotec2 | 48.05 ± 1.10 a | 25.81 ± 0.70 b | 16.09 ± 0.18 a | 31.30 ± 0.40 a |
| Clone/Variety | Juice Yield (L/ha) | Sugar Yield (t/ha) | Fiber Yield (t/ha) | Cane Yield (t/ha) |
|---|---|---|---|---|
| TByEFC08-0035 | 6213.00 ± 115.20 c | 1.12 ± 0.02 c | 9.04 ± 0.03 b | 34.51 ± 1.31 a |
| TByEFC10-0004 | 13,912.50 ± 298.00 a | 2.65 ± 0.02 a | 3.56 ± 0.12 c | 18.34 ± 0.40 c |
| Khon Kaen3 | 9347.00 ± 102.55 b | 2.10 ± 0.04 b | 2.90 ± 0.27 d | 14.95 ± 0.61 d |
| Biotec2 | 5585.00 ± 43.00 d | 0.79 ± 0.01 d | 11.56 ± 0.56 a | 30.40 ± 1.21 b |
| Clone/Variety | Ethanol A (g/L) | Productivity (g/L/h) | Ethanol Yield B (gp/gs) | Theoretical Yield C (%) |
|---|---|---|---|---|
| K. marxianus SJT83 | ||||
| TByEFC08-0035 | 81.05 ± 1.78 c | 3.37 ± 0.07 b | 0.46 ± 0.01 a | 91.68 ± 2.00 ab |
| TByEFC10-0004 | 87.10 ± 1.27 b | 3.63 ± 0.05 a | 0.48 ± 0.00 a | 94.69 ± 1.38 a |
| Khon Kaen3 | 99.15 ± 4.03 a | 2.75 ± 0.11 c | 0.48 ± 0.02 a | 93.28 ± 3.79 ab |
| Biotec2 | 62.20 ± 1.41 d | 2.60 ± 0.06 c | 0.45 ± 0.01 a | 88.85 ± 2.02 b |
| S. cerevisiae ND48 | ||||
| TByEFC08-0035 | 76.50 ± 2.83 b | 2.12 ± 0.08 b | 0.45 ± 0.02 a | 87.55 ± 3.24 ab |
| TByEFC10-0004 | 81.44 ± 2.20 b | 2.26 ± 0.11 b | 0.45 ± 0.02 a | 88.54 ± 4.41 ab |
| Khon Kaen3 | 95.05 ± 4.17 a | 2.64 ± 0.11 a | 0.46 ± 0.02 a | 90.74 ± 3.98 a |
| Biotec2 | 59.00 ± 2.55 c | 2.46 ± 0.10 a | 0.43 ± 0.02 a | 82.28 ± 2.64 b |
| Clone/Variety | Ethanol A (g/L) | Productivity (g/L/h) | Ethanol yield B (gp/gs) | Theoretical Yield C (%) |
|---|---|---|---|---|
| TByEFC08-0035 | 9.81 ± 0.27 a | 0.27 ± 0.00 a | 0.46 ± 0.01 a | 90.98 ± 2.49 a |
| TByEFC10-0004 | 9.25 ± 0.64 ab | 0.26 ± 0.02 a | 0.44 ± 0.03 a | 86.12 ± 5.92 a |
| Khon Kaen3 | 9.85 ± 0.92 a | 0.27 ± 0.02 a | 0.45 ± 0.04 a | 87.62 ± 8.18 a |
| Biotec2 | 8.10 ± 0.50 b | 0.23 ± 0.01 a | 0.42 ± 0.03 a | 82.99 ± 5.07 a |
| Clone | Ethanol Production on Laboratory Scale | Ethanol Production per Cultivation Area | ||||
|---|---|---|---|---|---|---|
| Juice (g/L) | Bagasse (g/L) | Total (g/L) | Juice (kg/ha) | Bagasse (kg/ha) | Total (kg/ha) | |
| TByEFC08-0035 | 81.05 ± 1.41 c | 9.81 ± 0.43 a | 90.85 ± 0.98 c | 503.56 ± 10.54 c | 790.68 ± 7.81 a | 1294.23 ± 18.34 b |
| TByEFC10-0004 | 87.10 ± 2.83 b | 9.25 ± 1.06 ab | 96.35 ± 3.89 b | 1211.76 ± 33.74 a | 257.38 ± 5.06 d | 1469.14 ± 28.68 a |
| Khon Kaen3 | 99.15 ± 3.26 a | 9.85 ± 0.92 a | 109.00 ± 4.17 a | 926.80 ± 6.02 b | 265.98 ± 1.12 c | 1192.72 ± 7.14 c |
| Biotec2 | 62.20 ± 2.83 d | 8.10 ± 0.71 b | 70.30 ± 3.54 d | 347.41 ± 3.41 d | 413.64 ± 6.56 b | 761.05 ± 3.51 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thammasittirong, S.N.-R.; Chatwachirawong, P.; Khemdee, K.; Thammasittirong, A. Evaluating the Potential of Newly Developed Energy Cane Clones for First- and Second-Generation Ethanol Production. Fermentation 2023, 9, 267. https://doi.org/10.3390/fermentation9030267
Thammasittirong SN-R, Chatwachirawong P, Khemdee K, Thammasittirong A. Evaluating the Potential of Newly Developed Energy Cane Clones for First- and Second-Generation Ethanol Production. Fermentation. 2023; 9(3):267. https://doi.org/10.3390/fermentation9030267
Chicago/Turabian StyleThammasittirong, Sutticha Na-Ranong, Prasert Chatwachirawong, Kedwarin Khemdee, and Anon Thammasittirong. 2023. "Evaluating the Potential of Newly Developed Energy Cane Clones for First- and Second-Generation Ethanol Production" Fermentation 9, no. 3: 267. https://doi.org/10.3390/fermentation9030267
APA StyleThammasittirong, S. N.-R., Chatwachirawong, P., Khemdee, K., & Thammasittirong, A. (2023). Evaluating the Potential of Newly Developed Energy Cane Clones for First- and Second-Generation Ethanol Production. Fermentation, 9(3), 267. https://doi.org/10.3390/fermentation9030267







