Abstract
Cellulosic sugars production for the valorization of lignocellulosic biomass residues in an industrial site has economic benefits and is promising if integrated into a biorefinery. Enzymatic hydrolysis (EH) of pretreated Eucalyptus globulus bark, an industrial residue of low-economic value widely available in Portuguese pulp and paper mills, could be an excellent approach to attain resource circularity and pulp mill profitability. This work evaluated the potential for improving cellulosic sugars concentrations by operating with high solids loading and introducing the additives Triton X-100, PEG 4000 and Tween 80 using a commercial enzymatic consortium with a dosage of 25 FPU gcarbohydrates−1. Additives did not improve enzymatic hydrolysis performance, but the effect of increasing solids loading to 14% (w/v) in batch operation was accomplished. The fed-batch operation strategy was investigated and, when starting with 11% (w/v) solids loading, allowed the feeding of 3% (w/v) fresh feedstock sequentially at 2, 4 and 6 h, attaining 20% (w/v) total solids loading. After 24 h of operation, the concentration of cellulosic sugars reached 161 g L−1, corresponding to an EH conversion efficiency of 76%. Finally, the fermentability of the fed-batch hydrolysate using the Ethanol Red® strain was evaluated in a 5 L bioreactor scale. The present results demonstrate that Eucalyptus globulus bark, previously pretreated by kraft pulping, is a promising feedstock for cellulosic sugars production, allowing it to become the raw material for feeding a wide range of bioprocesses.
1. Introduction
The pulp and paper sector is a leading consumer of woody biomass, generating a significant amount of forest residue daily [1,2]. Eucalyptus globulus is the most widely used wood source in Portuguese pulp mills, given its outstanding properties for producing high-quality paper [3,4,5,6,7]. Before wood pulping, debarking is required as a preparation step since the presence of bark negatively impacts the yield of the process [8,9]. Therefore, this industrial residue is highly available at the site, with about 0.5 Mton generated in Portugal in 2021 [10]. Most of the bark is burned for heat and power generation [5,11]. Nevertheless, other potential routes for upgrading bark into value-added products are emerging; one of these is the production of cellulosic sugars [7,12].
Given the complexity of the lignocellulosic matrix of E. globulus bark, pretreatment is a crucial step to improve access of the enzymes to the carbohydrate fraction [13]. Several pretreatments have been evaluated for lignocellulosic materials [14,15]. However, in the pulp and paper sector context, using the kraft process is beneficial since it is a leading delignification technology that is already well established. Compared to other chemical pretreatments, kraft pulping is advantageous since it is carried out at lower temperatures and pressure and presents lower sugar degradation, removing most of the lignin [16]. After the appropriate pretreatment, the hydrolysis step converts the polysaccharides into monomeric sugars for further microbial conversion to value-added products [17,18]. The hydrolysis process can either be catalyzed by acids or enzymes [13,19]. Enzymatic hydrolysis is preferred since it presents several advantages over acid hydrolysis, such as higher conversion efficiency, minimal by-product formation, lower equipment corrosion and lower energy requirements [14]. This method is also considered eco-friendlier due to the biodegradability of enzymes and milder operating conditions (45–55 °C and pH 4.5–5.5) [14]. Additionally, the great advantage of enzymatic over acid hydrolysis is the prevention of sugar degradation which considerably reduces ethanol conversion efficiency. The former enables only a few fermentation inhibitors, such as HMF and furfural, contrary to what happens in the latter which favors more extensive inhibition [18]. Enzymatic hydrolysis (EH) involves the cleavage of β-1,4-glycosidic bonds of cellulose and generates glucose using cellulases. Hemicelluloses are more susceptible to hydrolysis than cellulose due to their amorphous and branched nature [15,19]. However, the degradation of hemicelluloses requires complex systems of xylanases and accessory enzymes due to the different types of linkages in their chains [13,20].
Great research efforts have been made toward reducing the cost of the enzymatic consortium as it is still one of the main factors limiting the scaling-up of enzymatic hydrolytic processes [21,22]. Some authors have suggested using additives to reduce enzyme dosage [23,24,25,26]. Several additives, such as non-ionic surfactants, non-catalytic proteins and salts, have been evaluated for their ability to enhance the EH of several lignocellulosic materials [26,27]. The effect of these additives has been studied mainly for agricultural residues (such as sugarcane bagasse, wheat straw and corn straw) mainly pretreated by hydrothermal or dilute acid hydrolysis [24,28,29,30,31]. Some additives have allowed a reduction in the operational time and enzyme dosage (around 50%), while maintaining the overall performance [24,26,28]. However, the effects of these additives seem to be highly dependent on their concentration, the feedstock and the severity of the pretreatment [29].
Another limitation of enzymatic hydrolysis is related to the low concentration of sugars in the hydrolysate, compromising the techno-economic feasibility of this approach. Enzymatic hydrolysis should be performed using at least 15% (w/w) solids loading to overcome this issue [17]. This operational strategy offers significant economic benefits, including reduced capital investment, lower energy requirements and lower downstream processing costs [17,32,33]. The fed-batch operation strategy has been studied as a potential alternative to outsmart the difficulties of the high solids loading operations [17]. Pretreated biomass is fed in sequential additions, controlling the initial consistency of the slurry, solving some water constraints and promoting fast liquefaction and the mass transfer phenomena, particularly during the early saccharification stage [34].
The production of cellulosic sugars has been recognized as a promising platform for biochemicals, biofuels and biomaterials with the potential to replace fossil-based sources [35,36]. Advanced biofuels are considered a readily available potential renewable energy source to replace fossil fuels, enabling the transport sector to comply with decarbonization targets in the short–medium term. Currently, Portugal imports bioethanol to comply with gasoline-blending mandates [37,38]. Therefore, integrating this valorization pathway into a pulp and paper site using an abundant residue leads to the expansion of the portfolio of products and increases revenue, boosting market opportunities and contributing to an integrated biorefinery under the circular bioeconomy concept [35,36,39].
In this context, this work aimed to evaluate the production of cellulosic sugars from E. globulus bark previously pretreated by kraft pulping. In order to maximize sugar production, and in line with the above-described considerations, external and internal factors were checked. First, the effect of some additives referred to in the literature as being favorable to the enzymatic hydrolysis of other agricultural residues, pretreated by other methods different from kraft, was evaluated. Therefore, Triton X-100, PEG 4000 and Tween 80 were evaluated (0.350 L) at concentrations of 0, 2 and 6% (w/w). Secondly, internal factors such as substrate concentration, sequential feeding strategy and the first stage of scale-up (0.350 to 3.00 L) were tested. Batch EH experiments were carried out using 8, 11 and 14% (w/v) solids loading with a working volume of 1 L, aiming to select the optimal initial substrate concentration. Finally, a fed-batch operation strategy was followed to increase the final cellulosic sugars concentration of the hydrolysate. The fed-batch enzymatic hydrolysis experiment was initiated with 11% (w/v) solids loading, and 3% (w/v) was fed at 2, 4 and 6 h until a final substrate concentration of 20% (w/v) was achieved. The fermentability of the hydrolysate with the highest cellulosic sugars concentration was evaluated at a 5 L bioreactor scale. To our knowledge, this is the first time that E. globulus bark previously submitted to a kraft pulping pretreatment has been efficiently converted into cellulosic sugars and ethanol.
2. Materials and Methods
2.1. Raw Material and Pretreatment
Eucalyptus globulus bark was previously pretreated by kraft pulping at 170 °C for 75 min using an active alkali of 24%. The unbleached kraft pulp was kindly provided by RAIZ—Instituto de Investigação da Floresta e do Papel (Eixo, Portugal).
2.2. Chemical Characterization of Pretreated Eucalyptus globulus Bark
Pretreated Eucalyptus globulus bark was characterized following the laboratory analytical procedures (LAPs) from the National Renewable Energy Laboratory (NREL) [40].
The moisture content of the pretreated raw material was determined by drying at 105 °C for 24 h to a constant weight. Ash content was determined using a muffle furnace using a temperature ramping program [40].
For lignin and carbohydrate determination, pretreated E. globulus bark was submitted to acid hydrolysis [40]. About 300 mg of pretreated raw material (previously oven-dried at 40 °C) were hydrolyzed and incubated with 72% H2SO4 solution for 1 h at 30 °C. After that, hydrolysates were diluted with water to obtain a 4% (w/w) H2SO4 solution, autoclaved at 121°C for 1 h, and left to cool down to room temperature. The autoclaved solutions were vacuum-filtered using medium-porosity filtering crucibles (previously weighed). The acid-insoluble residue retained was considered as Klason lignin (after oven-drying at 105 °C to a constant weight). Acid-soluble lignin was quantified by absorbance at 205 nm using a UV–VIS spectrometer (Beckman, Brea, CA, USA, DU 650), using appropriately diluted aliquots of the primary filtrates. For sugar and degradation product analysis, about 15 mL of the same filtrate was neutralized using CaCO3 until a pH between 5 and 6 was reached; this was then filtered using a 0.2 µm nylon syringe filter (Clarify) before being injected into the high-performance liquid chromatography (HPLC) instrument for analysis.
These determinations were carried out in triplicate.
2.3. Enzymatic Hydrolysis
Commercial cellulase consortium (Cellic® CTec2), acquired from Sigma-Aldrich (St. Louis, MO, USA), was added at the beginning in all enzymatic hydrolysis experiments performed under either the batch or fed-batch operational mode. The filter paper unit (FPU) activity, whose analysis was based on the NREL standard procedure [41], was determined as 168.7 FPU mL−1.
All the experiments were run using citrate buffer (0.05 M, pH 4.8) and an enzyme dosage of 25 FPU gcarbohydrates−1. Enzymatic hydrolysis assays were performed for 24 h at 50 °C and 150 rpm, with a pH between 4.5 and 5.5 (adjusted by adding H2SO4 2 M and NaOH 2 M solutions) [42,43,44].
Samples were collected periodically to monitor the pH and concentration of sugars. The reaction was stopped by heating the samples in boiling water for 5 min to ensure the denaturation of enzymes. After cooling the samples on ice for 5 min, they were centrifuged at 13,000 rpm for 10 min at 4 °C. After proper dilution, the supernatant was analyzed for the determination of monomeric sugars.
2.3.1. Batch Enzymatic Hydrolysis—Effect of Additives
A series of batch enzymatic hydrolysis experiments were carried out in duplicate using 600 mL small jacketed glass bioreactors (Schott Duran®, Staffordshire, UK) with solids loading of 8% (w/v) and a working volume of 350 mL. An experiment without additives was used as a control assay. The effect of additives Triton X-100, PEG 4000 and Tween 80 was evaluated at concentrations of 2 and 6% (w/w). Additives were diluted in citrate buffer before its addition to the final mixture. The other enzymatic hydrolysis conditions were as described in Section 2.3.
2.3.2. Batch Enzymatic Hydrolysis—Optimal Initial Solids Loading and Scale-Up
A series of batch enzymatic hydrolysis experiments were carried out with 8, 11 and 14% (w/v) solids loading using a working volume of 1 L. After selecting the 11% (w/v) as the optimal initial solids loading concentration, the scale-up to a 3 L working volume was carried out, maintaining the remaining operating conditions.
2.3.3. Fed-Batch Enzymatic Hydrolysis
Fed-batch saccharification was started with 11% (w/v) pretreated biomass based on the total working volume (3 L). Further pretreated feedstock feedings of 3% (w/v) were fed at 2, 4 and 6 h, achieving a total solid loading of 20% (w/v).
2.4. Fermentation
The pre-inocula were prepared in duplicate by transferring 2–3 colonies from a maintenance YM Petri dish to a 100 mL Erlenmeyer flask containing 40 mL of liquid YM and then incubated for 24 h at 28 °C and 180 rpm. The inocula were prepared in duplicate by transferring a certain volume of pre-inoculum to 200 mL fresh liquid YM, which guaranteed an initial biomass concentration of about 1.5 g L−1. The inoculum was incubated for 14 h under the same conditions.
The fermentation assay was carried out in a 5 L BIOSTAT A plus bioreactor (Sartorius Stedim Biotech, Göttingen, Germany) with a working volume of 2 L, automatic control of stirring, temperature and pH by micro-DCU software and data acquisition by the MFCS/DA 3.0 system (Sartorius Stedim Systems, Göttingen, Germany). The pH was measured using an electrode EasyFerm Plus K8 325 (Hamilton, Bonaduz, Switzerland) and controlled to 5.50 ± 0.05 by adding KOH 5 M and H2SO4 1 M. The temperature was controlled at 28 °C, and the stirring was set at 180 rpm using two six-bladed Rushton turbines.
2.5. Analytical Methods
2.5.1. pH
The monitorization of the samples pH was performed using a benchtop meter (Hach sensION+ MM340, Loveland, CO, USA) with an InPro 3030/200 electrode (Mettler Toledo, Columbus, OH, USA).
2.5.2. Cell Concentration
The cell concentration was monitored spectrophotometrically (Shimadzu, UVmini-1240, Tokyo, Japan) by measuring the optical density at 620 nm. The cell concentration was determined from the optical density data using a calibration curve of optical density versus cell dry weight. The samples were appropriately diluted with NaCl 0.9% (w/v) to obtain an optical density value inside the validity range of the Beer–Lambert law.
2.5.3. Glucose, Xylose and Ethanol Quantification
HPLC was used to quantify glucose, xylose, ethanol and glycerol. After appropriate dilution, a total of 500 μL of each sample was filtered using centrifugal filters (VWR) with a membrane pore size of 0.2 μm (VWR) at 8000 rpm (Eppendorf, MiniSpin, Hamburg, Germany) for 8 min before HPLC injection. Samples were injected on a Rezex ROA-Organic Acid H+ (8%) 300 × 7.8 mm ion-exchange column (Phenomenex, Torrance, CA, USA) at 65 °C (oven Gecko 2000) connected to a refraction index detector L-2490 (Hitachi, Chiyoda, Japan). The injection volume was 10 µL and the eluent was H2SO4 0.005 N, with a flow rate of 0.500 mL min−1 (Hitachi, pump L-2130). Standard calibration curves were obtained frequently using freshly prepared standards in the range of 0–5 g L−1 for sugars and ethanol to ensure method linearity.
2.6. Calculations
2.6.1. Enzymatic Hydrolysis
The maximum concentration of glucose in the pretreated bark, [Glucose]pretreated bark (g L−1), was estimated from the kraft pulp compositionand calculated by Equation (1). It corresponds to the glucose concentration if the cellulose present in the kraft pulp were to be fully hydrolyzed to glucose.
where 1.11 is the mass conversion factor of cellulose to glucose (gglucose gcellulose−1); is the cellulose fraction in the dry weight kraft pulp (0.798 gcellulose gdry weight kraft pulp−1); is the dry weight of kraft pulp (gdry weight kraft pulp) and is the working volume (2 L).
Similarly, the maximum xylose concentration in the pretreated bark estimated from kraft pulp composition, [Xylose]pretreated bark (g L−1), was calculated based on Equation (2). It corresponds to the xylose concentration if the hemicelluloses present in the kraft pulp were to be fully hydrolyzed to xylose.
where 1.14 is the mass conversion factor of hemicelluloses to xylose (gxylose ghemicelluloses−1) and is the hemicelluloses fraction in the dry weight kraft pulp (0.155 ghemicelluloses gdry weight kraft pulp−1).
The enzymatic hydrolysis conversion efficiency, EH conversion efficiency (%), was calculated using Equation (3), based on the ratio between the sum of glucose and xylose concentration evaluated in the hydrolysate and the sum of respective maximum concentrations in the kraft pulp predicted by their chemical composition:
2.6.2. Fermentation
The maximum specific growth rate, µmax (h−1), was calculated from the slope of the linear regression obtained after plotting the natural logarithm of biomass concentration versus time during the exponential growth phase.
The volumetric glucose consumption rate, rglucose (g L−1 h−1), was calculated from the module of the linear regression slope of glucose concentration over time.
The volumetric ethanol productivity, Prodvol (gethanol L−1 h−1), was calculated based on Equation (4), considering the differences (∆) between the maximum ethanol concentration reached and its concentration at the beginning of the fermentation.
The ethanol yield, Yethanol/substrate (gethanol gsubstrate−1), was calculated according to Equation (5), considering both glucose and xylose as the substrates and the maximum ethanol concentration. Substrate concentration differences (∆) were calculated from the beginning of the fermentation until the maximum ethanol concentration was reached.
The fermentation conversion efficiency was calculated by Equation (6), considering the maximum theoretical ethanol yield of 0.511 gethanol gsugars−1 [45]:
3. Results and Discussion
3.1. Chemical Characterization of Raw Material
The major components of the unbleached kraft pulp from E. globulus bark were cellulose (79.8 wt %) and hemicelluloses (15.5 wt %), representing a fraction of carbohydrates of about 95 wt %. Therefore, kraft pretreatment allowed the retention of most of the cellulose and hemicelluloses in the solid fraction, a valuable source of cellulosic sugars. Table 1 shows the chemical characterization of the unbleached kraft pulp of E. globulus bark used in the present work.

Table 1.
Chemical characterization of the unbleached kraft pulp from Eucalyptus globulus bark.
According to Oliveira et al. [46], the Klason and acid-soluble lignin content of E. globulus bark ranged from 14.97–26.6 wt % and 2.48–7.50 wt %, respectively. Similarly, Pinto et al. [47] reported a Klason and acid-soluble lignin content varying between 26.0 and 2.9 wt %. Therefore, kraft pretreatment proved to be an effective delignification method by drastically reducing the total lignin to about 2.6 wt %. This reduction is crucial to improve enzymatic digestion and ensure a satisfactory EH conversion efficiency by enhancing cellulose accessibility and limiting non-productive lignin-enzyme bindings [18]. Compared with other chemical pretreatments, the kraft process has some benefits, namely, operation at lower temperature and pressure, lower sugar degradation and removal of most of the lignin [48]. For all these reasons, the kraft process may be considered a promising chemical pretreatment for bark [49]. Several studies have reported the feasibility of kraft pulping as a pretreatment for different lignocellulosic materials, including acacia [50], birch [51], beech [51], eucalyptus [52], wheat straw [53], pine [51], poplar [53] and spruce [54]. Using this conventional pulping process as a pretreatment step for bark residues can signify a new paradigm for the pulp and paper sector. Besides being a well-established technology to produce pulp, this is an alternative with lower investment costs and risks since the required process units are already available in an existing pulp mill [55].
3.2. Enzymatic Hydrolysis of Kraft Pulp from Eucalyptus globulus Bark
3.2.1. Effect of Additives on Enzymatic Hydrolysis
The enzymatic hydrolysis assay with 8% (w/v) solids loading carried out as a control experiment without additives resulted in a concentration of cellulosic sugars of 82.8 g L−1, accounting for 70.0 and 12.8 g L−1 of glucose and xylose, respectively. This experiment reached an outstanding enzymatic hydrolysis conversion efficiency (> 95%) since a high enzymatic dosage was used (25 FPU gCH−1). There is a great interest in minimizing the enzyme load used, given its associated high cost. Some authors have suggested using additives to reduce enzymatic dosages and/or decrease hydrolysis time while maintaining the overall performance [24,26,28]. Accordingly, the addition of Triton X-100, PEG 4000 and Tween 80 were tested at 2 and 6% (w/w) concentrations. The time course for cellulosic sugars in the first 6 h of enzymatic hydrolysis, the final concentration of cellulosic sugars and the enzymatic hydrolysis conversion efficiency obtained after 24 h of operation are represented in Figure 1A–C), respectively.
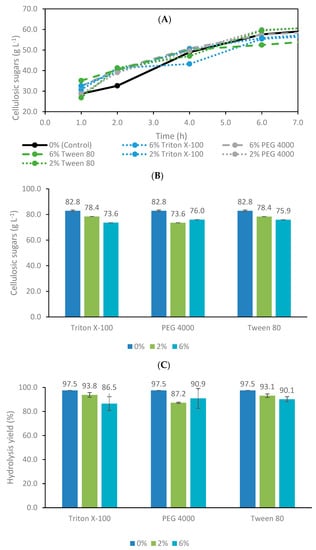
Figure 1.
(A) Time course for cellulosic sugars concentration at the initial stage of the enzymatic hydrolysis experiments. (B) Concentration of cellulosic sugars after 24 h of enzymatic hydrolysis. (C) Hydrolysis conversion efficiency of additive experiments after 24 h of operation.
Although in the first 4 h almost all the additives provided a slightly higher sugar concentration than the control, at about 6 h of hydrolysis the latter was very similar to the others (Figure 1A). Despite the similarities concerning the sugar concentrations in all the experiments, the highest sugar concentration after 24 h was obtained in the control test without any additive.
For the range of operational conditions tested, none of the additives were able to improve the sugar concentration after 24 h compared with the control assay. The hydrolysis conversion efficiency was very similar, regardless of the concentration of the additive evaluated. On one hand, the enzymatic hydrolysis conversion efficiency of the control assay was already excellent (>95%). Therefore, the eventual benefit could be related to decreased enzymatic dosage or hydrolysis time. However, there was no evidence that additives significantly benefit the enzymatic hydrolysis rate, at least at the early stage. On the other hand, these results may be related to the presence of residual lignin which was only about 2% (w/w). The effect of additives depends on the nature and severity of the lignocellulosic biomass pretreatment. The lignin content might show a significant impact on the improving action of the surfactant. Non-ionic surfactants (such as Tween 20 and Triton X-100) have a hydrophobic fraction that can bind to the residual lignin of the pretreated feedstock through hydrophobic interactions, avoiding the adsorption of cellulases to the lignin, which is responsible for reducing enzymatic activity. These surfactants also have a protective effect on cellulases from denaturing by heat and shear force [56]. Moreover, some authors have already stated that the effect of additives seems to be more evident under harsher conditions, such as low enzymatic dosage, high solids loading, high agitation rate and non-optimal pH [56]. In fact, this was noticed in some preliminary experiments using E. globulus bark subjected to combined pretreatment of steam explosion and organosolv. These assays showed that additives benefit the final concentration of cellulosic sugars, improving hydrolysis conversion efficiency after 24 h (data not shown). These additives were evaluated in the same concentrations and following the same operational conditions, but lignin content in raw material was higher than 30% (w/w), in contrast to 2% (w/w) in kraft pulp. Similar conclusions were stated by Chen et al. [56], who found that the effect of non-ionic surfactant Tween 20 is highly dependent on pretreatment, hydrolysis conditions and lignin. The enzymatic conversion was significantly improved by non-ionic surfactants when using samples pretreated by dilute acid or steam explosion, which had a much higher lignin content [56]. Another promising strategy consists of combining several additives and optimizing their concentrations. Xu et al. [57] accomplished the best enzymatic saccharification of alkali-pretreated sugarcane bagasse by combining whey protein powder, sophorolipid, Tween 80 and calcium lignosulfonates. The synergistic effect between various additives was evident since the conversion efficiency was considerably enhanced over the addition of a single additive [57]. Several authors recently reviewed the main effects of additives in enzymatic hydrolysis using lignocellulosic materials [26,58,59,60].
In conclusion, these experiments demonstrate the techno-feasibility of producing cellulosic sugars from E. globulus bark previously pretreated by kraft pulping. However, the potential benefit of these additives may have been compromised due to the low solids loading, reduced residual lignin content and operating conditions adopted. With this in mind, no additives were used in the following experiments. Even if additives improve hydrolysis performance, it is crucial to evaluate their cost–benefit. For example, Rocha-Martín et al. [24] estimated PEG 4000’s cost contribution to 2G bioethanol production from lignocellulosic hydrolysate and concluded that the price must be reduced to avoid compromising overall economic feasibility. The use of additives represents an additional cost and could compromise downstream processing. Furthermore, it is crucial to evaluate the impact of these additives on the fermentation step since they might inhibit the fermenting microorganisms. However, in the case of surfactants, depending on the concentration and agitation rate, the foam may be considerable and require antifoam use, representing another additional cost. In future, additives should be evaluated under more severe hydrolysis conditions, for example, operating at high solids loading, high stirring rate and/or under low enzymatic dosage. Furthermore, a wide range of concentrations should be assessed, and this should be done by designing experiments to minimize the number of assays required. Last, but not least, a deep understanding of the interactions between enzymes and additives and the effect of additives on hydrolysate bioprocessing are crucial to take advantage of this approach.
3.2.2. Optimal Initial Solids Loading and Scale-Up
Once the excellent results obtained from the hydrolysis of pretreated E. globulus bark using a solids loading of 8% (w/v) were proven, even without feed additives, the following experiments aimed to maximize the sugar concentration of hydrolysates. For this purpose, solids loadings of 8, 11 and 14% (w/v) were evaluated, increasing the working volume from 350 mL to 1 L.
According to the results presented in Figure 2, it was observed that the increase in the solids loading improves the final concentration of sugars. The higher the solids loading, the higher the content of dry pulp mass per suspension, leading to an increase in the content of polysaccharides susceptible to enzymatic attack. For the experiment with the lowest solids loading, it would probably not even be necessary to have extended the hydrolysis to 24 h. Increasing total solids loading from 8 to 14% (w/v) resulted in a boost of sugars concentration of about 40 g L−1. However, this increase negatively impacts the enzymatic hydrolysis conversion efficiency, determining a considerable decrease of about 13 %. The 14% (w/v) solids loading experiment significantly extended the liquefaction time. This increase in liquefaction time impacts energy requirements, affecting not only the operating time and costs but also the size and design of equipment [24]. Certainly, this observation may be related to rheological problems [32,61]. This possibility had already been reported in the literature as one of the technical challenges hindering the implementation of a high solids operation mode. In fact, lignocellulosic materials typically have a low density and a hygroscopic nature [62], leading to a high consistency of the slurry due to a lack of available water and higher mixing energy requirements [32,63]. Indeed, this mechanical behaviour is related to mass and heat transfer phenomena, hindering temperature control and proper enzyme–substrate contact and prejudicing the conversion of polysaccharides into monomeric sugars. All these issues may decrease hydrolysis conversion efficiency [32,63,64].
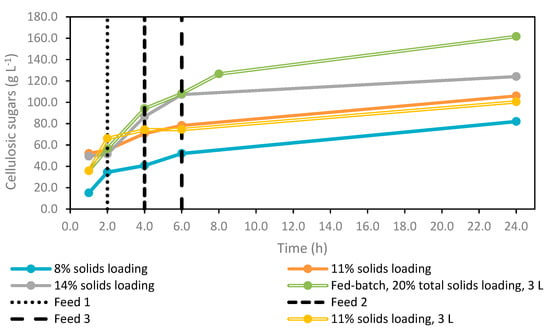
Figure 2.
Profile of cellulosic sugars concentrations (g L−1) for enzymatic hydrolysis experiments with 8, 11, 14 and 20% (w/v) solids loading. Enzymatic hydrolysis experiments using a 3 L working volume are represented with a double line, whereas the remaining experiments were carried out using a 1 L working volume. Intermittent feedings of kraft pulp during the fed-batch assay are signalled by dashed lines.
Based on previous considerations, an initial solids loading of 11% (w/v) was selected as optimal to start the fed-batch operation. This assay accomplished a cellulosic sugars concentration and enzymatic hydrolysis conversion efficiency of 106.0 g L−1 and 90.7%, respectively (Table 2). These results were similar to the data reported by Gao et al. [44], who observed a delay in liquefaction time and hydrolysis rate operating at 14% (w/v) solids loading of sugarcane bagasse previously pretreated by NaOH. Indeed, glucose concentration and hydrolysis conversion efficiency decreased over 12% (w/v) solids loading. Therefore, these authors started the fed-batch enzymatic hydrolysis with 12% (w/v) solids loading, where 7% (w/v) fresh solids were added sequentially until the final solids loading of 33% (w/v) was achieved [44]. Using the same raw material but submitted to a different pretreatment (aqueous ammonia), Raj and Krishnan [43] concluded that the maximum glucose release rate in the first 6 h was achieved for 14% (w/v) solids loading. The solids loading range of 6–16% (w/v) was evaluated, and authors observed a slowdown in the hydrolysis rate and liquefaction for the highest solids loading of 16% (w/v). Therefore, optimal initial solids loading seems to depend not only on the feedstock but also on the pretreatment, enzyme loading and hydrolysis operating conditions. For this reason, it should be optimized for each specific situation, namely, feedstock, its pretreatment, the enzymes used with their temperature and pH requirements, and the geometry of the bioreactor, among others. Most studies reported in the literature selected an initial solids loading ranging between 8 and 15% (w/v), aiming to ensure a low consistency media and fast liquefaction [60]. Based on these considerations, 11% (w/v) initial solids loading was selected in the present work, corresponding to a trade-off between final sugar concentration, hydrolysis conversion efficiency and liquefaction time.

Table 2.
Sugars concentration and conversion efficiency after 24 h for the enzymatic hydrolysis assays.
Considering these preliminary studies, the scale-up was evaluated from 1 to 3 L using 11% (w/v) solids loading. These experiments followed a similar trend, with a slight decrease of about 5 g L−1 in sugar concentration (Table 2), resulting in a slight drop of enzymatic hydrolysis conversion efficiency from 90.7 to 85.8%. These slight discrepancies could be related to the scaling of the process, mainly due to the agitation system. Both experiments were carried out with the same impeller; thus, it is expectable that a smaller working volume results in the best performance. An impeller similar to an anchor was used (Figure 3A), as this design was proven in previous work to be suitable for this type of slurry mixture. Figure 3B corresponds to the initial liquefaction stage of the reaction mixture. Before testing on a pilot scale, it is crucial to adapt the impeller design not only to the kind of feedstock, but also to vessel dimensions, working volume, and rheology, among others [17,65].
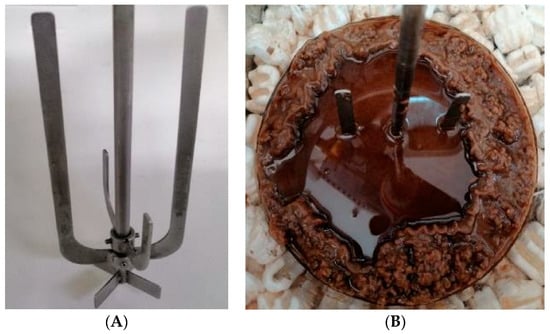
Figure 3.
(A) Impeller configuration used in enzymatic hydrolysis experiments of the present work. (B) Initial liquefaction stage of the reaction medium during enzymatic hydrolysis of kraft pulp from Eucalyptus globulus bark.
3.2.3. Fed-Batch Enzymatic Hydrolysis
The optimal initial solids loading determined in previous batch experiments was taken as the initial condition for the fed-batch experiment. The fed-batch operation strategy was initiated with 11% (w/v) solids loading, and doses of 3% (w/v) pretreated feedstock were added sequentially at 2, 4 and 6 h until 20% (w/v) total solids loading was reached. The enzymatic consortium was fed all at once at the beginning with the initial solids loading of 11% (w/v). Most authors have indicated that the one-time addition benefits enzymatic hydrolysis since it promotes rapid liquefaction and a higher initial hydrolysis rate resulting from a higher enzymes/substrate ratio. Gao et al. [44] observed that whole addition favours the initial enzymatic hydrolysis stage with more sugars produced. After 72 h of enzymatic hydrolysis, they did not observe such a difference between whole or split addition since the loss of enzyme activity was more evident for the cellulase added earlier [44].
This experiment resulted in a cellulosic sugars concentration of 161.6 g L−1, corresponding to about 136.4 g L−1 of glucose and 25.1 g L−1 of xylose. Among all the experiments, this strategy resulted in the highest sugar concentration (Figure 2). However, the conversion efficiency declined from 85.8 to 76.0% compared with the previous assay using 11% (w/v) solids loading, which can be related to the short hydrolysis time (only 24 h). Most of the enzymatic hydrolysis works reported in the literature lasted between 48 and 96 h, and some of them exceeded 120 h. Accordingly, another experiment was performed under the same conditions, but the hydrolysis time was extended to 48 h. This prolongation attained a maximum sugar concentration of about 180 g L−1 (data not shown). Indeed, double the time was required to enhance the cellulosic sugars titer by about 20 g L−1, corresponding to an increase of about 10% in conversion efficiency. Additional operating costs will not offset this slight increase in sugar concentration. Besides hydrolysis time, the efficiency decline can also be related to technical issues, namely, the inhibition of enzymatic activity due to the high sugar concentration since it is known that accumulation of glucose and cellobiose causes end-product enzyme inhibition [66,67,68]. The benefits of fed-batch over batch mode for enzymatic hydrolysis are more pronounced when operating with solids loadings above 15% (w/v) [60].
Despite the high cellulosic sugars concentration accomplished, there is still potential for improvement. There are several important parameters to investigate, namely, the solids and/or enzyme feeding strategy (whole vs. split addition), impeller design, agitation rate, optimization of enzyme dosage and fed-batch strategy on the simultaneous saccharification and fermentation (SSF) configuration [34,63]. There is no consensus in the literature concerning solids loading intervals, but rather the opposite, as some authors have evaluated intervals in the range of 12–24 h [69,70] and others as short as 5–10 min [71]. On the other hand, some works have mentioned that feed periodicity does not significantly influence enzymatic hydrolysis efficiency and final sugar concentration but mainly impacts the time required for liquefaction and hydrolysis operating conditions [60].
Rheological studies of enzymatic hydrolysis using a high feedstock concentration are crucial to minimizing enzyme inactivation through improved reactor and agitation systems designs [59,60]. Computational fluid dynamics is a valuable tool for understanding how mixing speed and impeller configuration impact the distribution of insoluble solids [49]. The impeller design assumes a key role, mainly when operating using high solids loading at a larger scale. The plate-and-frame [72], helical ribbon [33] and peg mixer [61] impellers have already proved to be efficient for this type of lignocellulosic slurry with non-Newtonian behaviour.
Compared to the works reported in the literature, which are summarized in Table 3, it can be concluded that our results are promising ones. In particular, the fed-batch approach adopted in the present work allowed e a high glucose concentration (>130 g L−1) to be achieved within only 24 h whereas most of the studies reported an enzymatic hydrolysis time 2.5–6 times longer. The shorter feeding strategy adopted in the present work, with the feeding of 3% (w/v) of the pretreated feedstock at 2, 4 and 6 h, probably contributed to this effect. In the literature, feedings intervals are longer (6–12 h) and contain a higher solids loading, ranging between 5–10% (w/v). Overall, the works reported in the literature achieved a higher total solids concentration (≥30% (w/v)) than the present work. However, this did not always translate into a significant boost in the cellulosic sugars concentration obtained and hydrolysis conversion efficiency. The present work obtained a relatively high cellulosic sugars concentration, about 160 g L−1, in a short time (24 h), maintaining a conversion efficiency similar to or even higher than those typically reported in the literature for other simpler raw materials, such as sugarcane bagasse. Still, there is potential for optimization, particularly concerning the enzymatic dosage, which significantly contributes to production costs and could favor economic viability.

Table 3.
Fed-batch enzymatic hydrolysis experiments of lignocellulosic materials at high solids loading.
3.3. Fermentability of Hydrolysate Derived from Fed-Batch Strategy
The fermentability of the produced fed-batch hydrolysate was assayed at the bioreactor scale since it has the highest potential to be converted into a high ethanol titer as well as other bioproducts. The Ethanol Red® industrial strain was used since it was already proven to be tolerant to high ethanol concentrations, having an outstanding fermentation performance [74,75], also with other types of lignocellulosic biomass [76].
As shown in Figure 4, this experiment was initiated with a glucose and xylose concentration of 124.7 and 23.1 g L−1, respectively. The lag phase for cell adaptation lasted ca. 3 h, when glucose consumption for biomass growth and ethanol production became evident. Biomass concentration increased exponentially between 3 and 8 h with a specific growth rate of 0.117 ± 0.010 h−1. The profile of biomass and ethanol concentrations followed a similar trend. Most of the glucose was consumed between 8 h and 18 h, while the xylose remained in the medium at the end of fermentation, and its contribution to ethanol production was not remarked. The maximum ethanol concentration of 51.2 g L−1 was achieved after 22 h of fermentation, corresponding to a conversion efficiency of 75.9 % and volumetric productivity of 2.33 g L−1 h−1. This range of ethanol concentration is much higher than the minimum titer (>40 g L−1), which ensures the economic feasibility of the distillation process for product recovery [34,63]. This performance corresponds to a yield of 290 kg of ethanol per ton of pretreated bark (also referred to as kraft pulp). Considering a pulping yield of 44.6%, this also translates into a yield of 129 kg of ethanol per ton of Eucalyptus globulus bark. Considering the estimated availability of about 0.5 Mton of Eucalyptus bark in Portugal in 2021 [10], about 64 kton of cellulosic ethanol could potentially be produced annually from this abundant residue already existing in the site, which is typically burnt. Cellulosic ethanol can be employed not only to blend with gasoline to comply with mandates but also as a solvent and intermediate for the chemistry industry. In Portugal, apart from forest waste, there are no other raw materials in sufficient quantity that do not compete with the food chain and which are without seasonal restrictions to make the dedicated production of bioethanol a viable and feasible project. Therefore, the present approach has high potential since it valorizes a residue already existing on-site that until now is only used for direct burning.
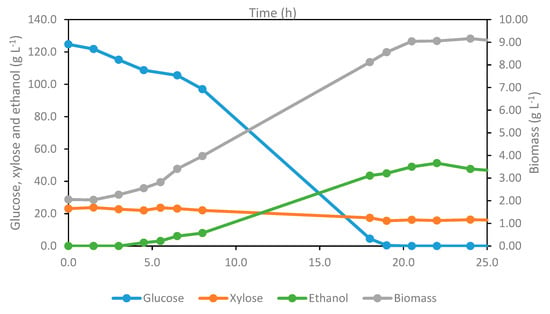
Figure 4.
Profile of glucose, xylose, ethanol and biomass for Ethanol Red® fermentation (2 L, 180 rpm, and 28 °C, pH 5.5).
This fermentation performance proved at the bioreactor scale is comparable to, or even higher than, values reported in the literature for other lignocellulosic materials [42,77,78]. Despite the outstanding fermentation performance, the consumption of xylose was negligible, accounting for about 15% (w/w) of the total cellulosic sugars in pretreated Eucalyptus globulus bark. If xylose could be converted, an even higher yield would be obtained. Aiming to promote this, in our previous work [77], Spathaspora passalidarum, a naturally xylose-fermenting yeast, was evaluated following either mono- and simultaneous co-culture with Ethanol Red®. Nevertheless, this strain did not improve fermentation performance, while seeming unsuitable for the range of cellulosic sugars concentrations present in this hydrolysate [77]. This strain showed low tolerance to ethanol and lignocellulosic-derived inhibitors, requiring strict control of operating conditions, such as pH and dissolved oxygen level [79]. Specific strains resulting from approaches such as genetic modification or evolutionary engineering have already been assessed as strategies to maximize conversion efficiency by co-fermenting glucose and xylose [79,80].
Some authors have already reported the cellulosic ethanol production from bark derived from different wood species. Spruce bark previously pretreated by hot water extraction resulted in 18.3 g L−1 of ethanol and a conversion efficiency of 62.3% following an integrated setup [81]. Frankó et al. [82] also demonstrated that simultaneous saccharification and fermentation (SSF) of spruce wood and bark mixtures resulted in the highest overall efficiency, regardless of the bark content. The range of ethanol concentrations reported varied from 20.9 to 45.8 g L−1 for the proportion of spruce bark from 100% to 0%, respectively. Therefore, an increase in the bark content negatively affected the ethanol conversion efficiency [82]. To complement our work, pretreated Eucalyptus globulus bark was also studied in the SSF configuration and new publications are currently being prepared.
4. Conclusions
Enzymatic hydrolysis of Eucalyptus globulus bark, previously pretreated by kraft pulping, was investigated for its ability to achieve a high concentration of cellulosic sugars. The additives Triton X-100, PEG 4000 and Tween 80 did not benefit sugar production or hydrolysis conversion efficiency under the operating conditions evaluated. Batch saccharification experiments with 8, 11 and 14% (w/v) solids loading performed at a small-bench scale with 1 L of working volume demonstrated a decreased hydrolysis conversion efficiency with increased solids loading. Cellulosic sugars ranged from 82 to 124 g L−1, with the solids loading of 11% (w/v) selected as the optimal initial solids loading. Aiming to boost cellulosic sugars concentration, fed-batch enzymatic hydrolysis was performed, starting with 11% (w/v) solids loading, based on a total working volume of 3 L, and 3% (w/v) pretreated feedstock was fed at 2, 4 and 6 h, reaching a total solids loading of 205 (w/v). After 24 h, cellulosic sugars reached 161 g L−1, accounting for 136 g L−1 of the glucose concentration and corresponding to an enzymatic hydrolysis conversion efficiency of 76%. Furthermore, an excellent cellulosic sugars concentration was accomplished in a short period of only 24 h. Even so, the conversion efficiency was similar or even higher than that reported in the literature for other pretreated raw materials. The fed-batch hydrolysate proved to be a promising source for cellulosic ethanol production, resulting in a concentration above 50 g L−1 at the bioreactor scale. Considering the present results, this work demonstrates that E. globulus bark pretreated by kraft pulping has a high potential to be converted into cellulosic sugars. Integrating a biorefinery into a pulp and paper mill using Eucalyptus globulus bark as feedstock could be an excellent opportunity for this sector, taking advantage of an abundant residue and using kraft pulping as a pretreatment. Cellulosic sugars produced by enzymatic hydrolysis could feed several bioprocesses providing bioethanol or other high value-added products based on resource circularity and improve the profitability of this industrial sector.
Author Contributions
M.S.T.A. carried out the experiments, collected and analyzed the data and wrote the manuscript; J.M.S.R. and A.M.R.B.X. developed the concept, supervised the work and reviewed the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was carried out under the Project InPaCTus—Innovative Products and Technologies from Eucalyptus. Project N.º 21874 funded by Portugal 2020 through European Regional Development Fund (ERDF) in the frame of COMPETE 2020 nº246/AXIS II/2017. This work was developed within the scope of the project CICECO-Aveiro Institute of Materials, UIDB/50011/2020, UIDP/50011/2020 & LA/P/0006/2020, financed by national funds through the FCT/MEC (PIDDAC). Authors would like to thank the CIEPQPF—Strategic Research Centre Project UIDB/00102/2020, funded by the Fundação para a Ciência e Tecnologia (FCT).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors are thankful to RAIZ—Instituto de Investigação da Floresta e do Papel for supplying the pretreated Eucalyptus globulus bark and all the equipment required for the enzymatic hydrolysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Branco, R.; Serafim, L.; Xavier, A. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef]
- Stoklosa, R.J.; Hodge, D.B. Integration of (Hemi)-Cellulosic Biofuels Technologies with Chemical Pulp Production. In Biorefineries; Qureshi, N., Hodge, D., Vertes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 73–100. ISBN 9780444595041. [Google Scholar]
- Pinto, F.; Gominho, J.; André, R.N.; Gonçalves, D.; Miranda, M.; Varela, F.; Neves, D.; Santos, J.; Lourenço, A.; Pereira, H. Improvement of gasification performance of Eucalyptus globulus stumps with torrefaction and densification pre-treatments. Fuel 2017, 206, 289–299. [Google Scholar] [CrossRef]
- Souza, A.G.; Lima, G.F.; Rodrigues, R.C.L.B.; Cesarino, I.; Leão, A.L.; Rosa, D.S. A New Approach for Conversion of Eucalyptus Lignocellulosic Biomass into Cellulose Nanostructures: A Method that Can Be Applied in Industry. J. Nat. Fibers 2019, 18, 1501–1511. [Google Scholar] [CrossRef]
- Neiva, D.M.; Araújo, S.; Gominho, J.; Carneiro, A.C.; Pereira, H. Potential of Eucalyptus globulus industrial bark as a biorefinery feedstock: Chemical and fuel characterization. Ind. Crop. Prod. 2018, 123, 262–270. [Google Scholar] [CrossRef]
- Resquin, F.; Barrichelo, L.E.G.; da Silva, F.G.; Brito, J.O.; Sansigolo, C.A. Wood quality for kraft pulping of Eucalyptus globulus origins planted in Uruguay. Sci. For./For. Sci. 2006, 72, 57–66. [Google Scholar]
- Rodrigues, A.E.; Pinto, P.C.; Barreiro, M.F.; da Costa, C.E.; da Mota, M.F.; Fernandes, I. An Integrated Approach for Added-Value Products from Lignocellulosic Biorefineries: Vanillin, Syringaldehyde, Polyphenols and Polyurethane, 1st ed.; Springer: Cham, Switzerland, 2018; ISBN 978-3-319-99312-6. [Google Scholar]
- Ressel, J.B. Wood Yard Operations. In Handbook of Pulp; Sixta, H., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2006; Volume 1, pp. 69–107. ISBN 3527309993. [Google Scholar]
- Ek, M.; Gellerstedt, G.; Henriksson, G. Pulping Chemistry and Technology. In Pulp and Paper Chemistry and Technology; Ek, M., Gellerstedt, G., Henriksson, G., Eds.; Walter de Gruyter GmbH & Co. KG: Berlin, Germany, 2009; Volume 2. [Google Scholar]
- Biond-Forest Fibers from Portugal. Boletim Estatístico Biond 2021. Available online: https://www.biond.pt/publicacoes/boletim-estatistico-2021/ (accessed on 28 November 2022).
- Domingues, R.M.A.; Sousa, G.D.A.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Eucalyptus globulus biomass residues from pulping industry as a source of high value triterpenic compounds. Ind. Crop. Prod. 2010, 31, 65–70. [Google Scholar] [CrossRef]
- Neiva, D.M.; Luís, Â.; Gominho, J.; Domingues, F.; Duarte, A.P.; Pereira, H. Bark residues valorization potential regarding antioxidant and antimicrobial extracts. Wood Sci. Technol. 2020, 54, 559–585. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Suely, A.; Boyce, A.N.; Faruq, G. Bioethanol production from renewable sources: Current perspectives and technological progress. Renew. Sustain. Energy Rev. 2017, 71, 475–501. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Review of Second Generation Bioethanol Production from Residual Biomass. Food Technol. Biotechnol. 2018, 56, 174–187. [Google Scholar] [CrossRef]
- Aditiya, H.B.; Mahlia, T.M.I.; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Fatma, S.; Hameed, A.; Noman, M.; Ahmed, T.; Sohail, I.; Shahid, M.; Tariq, M.; Tabassum, R. Lignocellulosic Biomass: A Sustainable Bioenergy Source for Future. Protein Pept. Lett. 2018, 25, 148–163. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef]
- Huang, C.; Li, R.; Tang, W.; Zheng, Y.; Meng, X. Improve Enzymatic Hydrolysis of Lignocellulosic Biomass by Modifying Lignin Structure via Sulfite Pretreatment and Using Lignin Blockers. Fermentation 2022, 8, 558. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy. Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Kumar, A.; Naraian, R. Differential Expression of the Microbial β-1, 4-Xylanase, and β-1, 4-Endoglucanase Genes. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, H.B., Gupta, V.K., Jogaiah, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 95–111. ISBN 9780444635037. [Google Scholar]
- Padella, M.; O’Connell, A.; Prussi, M. What is still Limiting the Deployment of Cellulosic Ethanol? Analysis of the Current Status of the Sector. Appl. Sci. 2019, 9, 4523. [Google Scholar] [CrossRef]
- Qiao, J.; Cui, H.; Wang, M.; Fu, X.; Wang, X.; Li, X.; Huang, H. Integrated biorefinery approaches for the industrialization of cellulosic ethanol fuel. Bioresour. Technol. 2022, 360, 127516. [Google Scholar] [CrossRef]
- Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioprocess Biosyst. Eng. 2018, 41, 1133–1142. [Google Scholar] [CrossRef]
- Rocha-Martín, J.; Martinez-Bernal, C.; Pérez-Cobas, Y.; Reyes-Sosa, F.M.; García, B.D. Additives enhancing enzymatic hydrolysis of lignocellulosic biomass. Bioresour. Technol. 2017, 244, 48–56. [Google Scholar] [CrossRef]
- Börjesson, J.; Peterson, R.; Tjerneld, F. Enhanced enzymatic conversion of softwood lignocellulose by poly(ethylene glycol) addition. Enzyme Microb. Technol. 2007, 40, 754–762. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Libardi Junior, N.; Bento, H.B.S.; de Carvalho, A.K.F.; de Souza Vandenberghe, L.P.; Soccol, C.R.; Aminabhavi, T.M.; Chandel, A.K. Process strategies to reduce cellulase enzyme loading for renewable sugar production in biorefineries. Chem. Eng. J. 2023, 451, 138690. [Google Scholar] [CrossRef]
- Li, X.; Zheng, Y. Lignin-enzyme interaction: Mechanism, mitigation approach, modeling, and research prospects. Biotechnol. Adv. 2017, 35, 466–489. [Google Scholar] [CrossRef]
- Florencio, C.; Badino, A.C.; Farinas, C.S. Addition of Soybean Protein Improves Saccharification and Ethanol Production from Hydrothermally Pretreated Sugarcane Bagasse. Bioenergy Res. 2019, 12, 81–93. [Google Scholar] [CrossRef]
- Brondi, M.G.; Vasconcellos, V.M.; Giordano, R.C.; Farinas, C.S. Alternative Low-Cost Additives to Improve the Saccharification of Lignocellulosic Biomass. Appl. Biochem. Biotechnol. 2019, 187, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, J.F.; Ferraz, A.; Aguiar, A. Alkaline-sulfite pretreatment and use of surfactants during enzymatic hydrolysis to enhance ethanol production from sugarcane bagasse. Bioprocess Biosyst. Eng. 2016, 39, 441–448. [Google Scholar] [CrossRef]
- Cheng, J.; Yu, Y.; Zhu, M. Enhanced biodegradation of sugarcane bagasse by Clostridium thermocellum with surfactant addition. Green Chem. 2014, 16, 2689–2695. [Google Scholar] [CrossRef]
- He, L.; Han, Q.; Jameel, H.; Chang, H.M.; Phillips, R.; Wang, Z. Comparison of One-Stage Batch and Fed-Batch Enzymatic Hydrolysis of Pretreated Hardwood for the Production of Biosugar. Appl. Biochem. Biotechnol. 2018, 184, 1441–1452. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, D.; Huang, J.; Yu, Z.; Dai, G.; Bao, J. Simultaneous saccharification and ethanol fermentation at high corn stover solids loading in a helical stirring bioreactor. Biotechnol. Bioeng. 2010, 105, 718–728. [Google Scholar] [CrossRef]
- Gomes, A.C.; Moysés, D.N.; Santa Anna, L.M.M.; Castro, A.M. Fed-batch strategies for saccharification of pilot-scale mild-acid and alkali pretreated sugarcane bagasse: Effects of solid loading and surfactant addition. Ind. Crop. Prod. 2018, 119, 283–289. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- He, Y.; Bagley, D.M.; Leung, K.T.; Liss, S.N.; Liao, B.Q. Recent advances in membrane technologies for biorefining and bioenergy production. Biotechnol. Adv. 2012, 30, 817–858. [Google Scholar] [CrossRef]
- Guerrero, M. Portugal Biofuel Market Outlook 2017; USDA Foreign Agricultural Service: Global Agricultural Information Network: Madrid, Spain, 2017. [Google Scholar]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trends Biotechnol. 2019, 37, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Guigou, M.; Cabrera, M.N.; Vique, M.; Bariani, M.; Guarino, J.; Ferrari, M.D.; Lareo, C. Combined pretreatments of eucalyptus sawdust for ethanol production within a biorefinery approach. Biomass Convers. Biorefinery 2019, 9, 293–304. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Laboratory Analytical Procedure (LAP): Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 27 October 2021).
- Adney, B.; Baker, J. Laboratory Analytical Procedure (LAP): Measurement of Cellulase Activities. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 30 June 2019).
- Branco, R.H.R.; Amândio, M.S.T.; Serafim, L.S.; Xavier, A.M.R.B. Ethanol production from hydrolyzed kraft pulp by mono- and co-cultures of yeasts: The challenge of C6 and C5 sugars consumption. Energies 2020, 13, 744. [Google Scholar] [CrossRef]
- Raj, K.; Krishnan, C. Improved high solid loading enzymatic hydrolysis of low-temperature aqueous ammonia soaked sugarcane bagasse using laccase-mediator system and high concentration ethanol production. Ind. Crop. Prod. 2019, 131, 32–40. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, J.; Yuan, Z.; Zhang, Y.; Liu, Y.; Liang, C. Optimization of fed-batch enzymatic hydrolysis from alkali-pretreated sugarcane bagasse for high-concentration sugar production. Bioresour. Technol. 2014, 167, 41–45. [Google Scholar] [CrossRef]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef]
- Oliveira, R.J.; Santos, B.; Mota, M.J.; Pereira, S.R.; Branco, P.C.; Pinto, P.C.R. The impact of acid hydrolysis conditions on carbohydrate determination in lignocellulosic materials: A case study with Eucalyptus globulus bark. Holzforschung 2021, 75, 957–967. [Google Scholar] [CrossRef]
- Pinto, P.C.R.; Oliveira, C.; Costa, C.A.E.; Rodrigues, A.E. Performance of Side-Streams from Eucalyptus Processing as Sources of Polysaccharides and Lignins by Kraft Delignification. Ind. Eng. Chem. Res. 2016, 55, 516–526. [Google Scholar] [CrossRef]
- Monrroy, M.; García, J.-R.; Mendonça, R.T.; Baeza, J.; Freer, J. Kraft pulping of Eucalyptus globulus as a pretreatment for bioethanol production by simultaneous saccharification and fermentation. J. Chil. Chem. Soc. 2012, 57, 1113–1117. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Ko, C.H.; Wang, Y.N.; Chang, F.C.; Chen, J.J.; Chen, W.H.; Hwang, W.S. Potentials of lignocellulosic bioethanols produced from hardwood in Taiwan. Energy 2012, 44, 329–334. [Google Scholar] [CrossRef]
- Buzała, K.P.; Kalinowska, H.; Małachowska, E.; Przybysz, P. The utility of selected kraft hardwood and softwood pulps for fuel ethanol production. Ind. Crop. Prod. 2017, 108, 824–830. [Google Scholar] [CrossRef]
- Troncoso, E.; Castillo, R.; Valenzuela, R.; Reyes, P.; Freer, J.; Norambuena, M.; Rodríguez, J.; Parra, C. Chemical and microstructural changes in Eucalyptus globulus fibers subjected to four different pretreatments and their influence on the enzymatic hydrolysis. J. Chil. Chem. Soc. 2017, 62, 3442–3446. [Google Scholar] [CrossRef]
- Buzała, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Production of glucose-rich enzymatic hydrolysates from cellulosic pulps. Cellulose 2015, 22, 663–674. [Google Scholar] [CrossRef]
- Novozhilov, E.V.; Sinel’nikov, I.G.; Aksenov, A.S.; Chukhchin, D.G.; Tyshkunova, I.V.; Rozhkova, A.M.; Osipov, D.O.; Zorov, I.N.; Sinitsyn, A.P. Biocatalytic conversion of kraft pulp using cellulase complex of Penicillium verruculosum. Catal. Ind. 2016, 8, 95–100. [Google Scholar] [CrossRef]
- Pettersson, K.; Mahmoudkhani, M.; Schenck, A. Opportunities for Biorefineries in the Pulping Industry. In Systems Perspectives on Biorefineries; Sandén, B., Pettersson, K., Eds.; Chalmers University of Technology: Göteborg, Sweden, 2014; pp. 68–80. [Google Scholar]
- Chen, Y.-A.; Zhou, Y.; Qin, Y.; Liu, D.; Zhao, X. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Zhang, J.; Zhang, Y.; Guo, Y.; Xu, H.; Xu, J.; Wang, Z. Enhancement of high-solids enzymatic hydrolysis efficiency of alkali pretreated sugarcane bagasse at low cellulase dosage by fed-batch strategy based on optimized accessory enzymes and additives. Bioresour. Technol. 2019, 292, 121993. [Google Scholar] [CrossRef]
- Madadi, M.; Song, G.; Sun, F.; Sun, C.; Xia, C.; Zhang, E.; Karimi, K.; Tu, M. Positive role of non-catalytic proteins on mitigating inhibitory effects of lignin and enhancing cellulase activity in enzymatic hydrolysis: Application, mechanism, and prospective. Environ. Res. 2022, 215, 114291. [Google Scholar] [CrossRef]
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to enhance enzymatic hydrolysis of lignocellulosic biomass for biorefinery applications: A review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- da Silva, A.S.; Espinheira, R.P.; Teixeira, R.S.S.; de Souza, M.F.; Ferreira-Leitão, V.; Bon, E.P.S. Constraints and advances in high-solids enzymatic hydrolysis of lignocellulosic biomass: A critical review. Biotechnol. Biofuels 2020, 13, 58. [Google Scholar] [CrossRef]
- Zhang, X.; Qin, W.; Paice, M.G.; Saddler, J.N. High consistency enzymatic hydrolysis of hardwood substrates. Bioresour. Technol. 2009, 100, 5890–5897. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.; Patel, A.; Raj, T.; Tsai, M.-L.; Chen, C.-W.; Dong, C.-D. Advances and Challenges in Biocatalysts Application for High Solid-Loading of Biomass for 2nd Generation Bio-Ethanol Production. Catalysts 2022, 12, 615. [Google Scholar] [CrossRef]
- Chen, H.Z.; Liu, Z.H. Enzymatic hydrolysis of lignocellulosic biomass from low to high solids loading. Eng. Life Sci. 2017, 17, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.-Y.; Xu, J.-L.; Yuan, Z.-H.; Qi, W.; Zhuang, X.-S.; He, M.-C. High solid and low enzyme loading based saccharification of agricultural biomass. Bioresources 2012, 7, 345–353. [Google Scholar] [CrossRef]
- Shiva; Climent Barba, F.; Rodríguez-Jasso, R.M.; Sukumaran, R.K.; Ruiz, H.A. High-solids loading processing for an integrated lignocellulosic biorefinery: Effects of transport phenomena and rheology—A review. Bioresour. Technol. 2022, 351, 127044. [Google Scholar] [CrossRef]
- Hsieh, C.W.C.; Cannella, D.; Jørgensen, H.; Felby, C.; Thygesen, L.G. Cellulase inhibition by high concentrations of monosaccharides. J. Agric. Food Chem. 2014, 62, 3800–3805. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, X.; Gregg, D.J.; Saddler, J.N. Effects of Sugar Inhibition on Cellulases and β-Glucosidase During Enzymatic Hydrolysis of Softwood Substrates. In Proceedings of the Twenty-Fifth Symposium on Biotechnology for Fuels and Chemicals, Applied Biochemistry and Biotechnology, Breckenridge, CO, USA, 4–7 May 2003; Humana Press: Totowa, NJ, USA, 2004; Volume 113–116, pp. 1115–1126. [Google Scholar]
- Rastogi, M.; Shrivastava, S. Recent advances in second generation bioethanol production: An insight to pretreatment, saccharification and fermentation processes. Renew. Sustain. Energy Rev. 2017, 80, 330–340. [Google Scholar] [CrossRef]
- Yang, M.; Li, W.; Liu, B.; Li, Q.; Xing, J. High-concentration sugars production from corn stover based on combined pretreatments and fed-batch process. Bioresour. Technol. 2010, 101, 4884–4888. [Google Scholar] [CrossRef]
- Liu, Z.H.; Chen, H.Z. Periodic peristalsis releasing constrained water in high solids enzymatic hydrolysis of steam exploded corn stover. Bioresour. Technol. 2016, 205, 142–152. [Google Scholar] [CrossRef]
- Hernández-Beltrán, J.U.; Hernández-Escoto, H. Enzymatic hydrolysis of biomass at high-solids loadings through fed-batch operation. Biomass Bioenergy 2018, 119, 191–197. [Google Scholar] [CrossRef]
- Wang, W.; Zhuang, X.; Yuan, Z.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X. High consistency enzymatic saccharification of sweet sorghum bagasse pretreated with liquid hot water. Bioresour. Technol. 2012, 108, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.H.; Park, H.M.; Kim, D.H.; Yang, J.; Kim, K.H. Fed-Batch Enzymatic Saccharification of High Solids Pretreated Lignocellulose for Obtaining High Titers and High Yields of Glucose. Appl. Biochem. Biotechnol. 2017, 182, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, H.L.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process. Biochem. 2017, 53, 10–16. [Google Scholar] [CrossRef]
- Demeke, M.M.; Dietz, H.; Li, Y.; Foulquié-Moreno, M.R.; Mutturi, S.; Deprez, S.; Den Abt, T.; Bonini, B.M.; Liden, G.; Dumortier, F.; et al. Development of a D-xylose fermenting and inhibitor tolerant industrial Saccharomyces cerevisiae strain with high performance in lignocellulose hydrolysates using metabolic and evolutionary engineering. Biotechnol. Biofuels 2013, 6, 89. [Google Scholar] [CrossRef]
- Fernandes-Klajn, F.; Romero-García, J.M.; Díaz, M.J.; Castro, E. Comparison of fermentation strategies for ethanol production from olive tree pruning biomass. Ind. Crop. Prod. 2018, 122, 98–106. [Google Scholar] [CrossRef]
- Amândio, M.S.T.; Rocha, J.M.S.; Serafim, L.S.; Xavier, A.M.R.B. Cellulosic Bioethanol from Industrial Eucalyptus globulus Bark Residues Using Kraft Pulping as a Pretreatment. Energies 2021, 14, 2185. [Google Scholar] [CrossRef]
- Malik, K.; Sharma, P.; Yang, Y.; Zhang, P.; Zhang, L.; Xing, X.; Yue, J.; Song, Z.; Nan, L.; Yujun, S.; et al. Lignocellulosic biomass for bioethanol: Insight into the advanced pretreatment and fermentation approaches. Ind. Crop. Prod. 2022, 188, 115569. [Google Scholar] [CrossRef]
- Cunha, J.T.; Soares, P.O.; Baptista, S.L.; Costa, C.E.; Domingues, L. Engineered Saccharomyces cerevisiae for lignocellulosic valorization: A review and perspectives on bioethanol production. Bioengineered 2020, 11, 883–903. [Google Scholar] [CrossRef]
- Díaz, M.J.; Moya, M.; Castro, E. Bioethanol Production from Steam-Exploded Barley Straw by Co-Fermentation with Escherichia coli SL100. Agronomy 2022, 12, 874. [Google Scholar] [CrossRef]
- Kemppainen, K.; Inkinen, J.; Uusitalo, J.; Nakari-Setälä, T.; Siika-aho, M. Hot water extraction and steam explosion as pretreatments for ethanol production from spruce bark. Bioresour. Technol. 2012, 117, 131–139. [Google Scholar] [CrossRef]
- Frankó, B.; Galbe, M.; Wallberg, O. Influence of bark on fuel ethanol production from steam-pretreated spruce. Biotechnol. Biofuels 2015, 8, 15–25. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).