Abstract
In this study, Desertifilum tharense cyanobacteria, which has energy generation potential, was firstly isolated from the water sources from Denizli/Turkey, the culture-specific parameters were identified, characterization analyses were performed, and the production in photoreactors under laboratory conditions was performed. D. tharense cyanobacterium was subjected to a high temperature–pressure pretreatment process (HTPP) to increase methane production efficiency, and the pretreatment process was optimized for methane production. D. tharense had a total carbon (C) content of 50.2% and total organic carbon content (TOC) of 48.9%. The biochemical methane potential (BMP) of the raw D. tharense sample was measured as 261.8 mL methane (CH4) per gram of volatile solids (VS). In order to investigate the effects of HTPP and to determine the optimum process conditions, Central Composite Design (CCD) approach-based Response Surface Methodology (RSM) was used. BMP values of the samples treated with HTTP were measured in the range of 201.5–235 mLCH4 gVS−1 and lower than the raw sample. These results revealed that the HTPP is not suitable for the production of biofuel methane from D. tharense. The optimization of the HTPP was carried out by Design Expert software. For maximum BMP production, the software proposed a reaction temperature of 200 °C and a reaction time of 20 min as optimum conditions. With the proposed model, it was estimated that 227.1 mLCH4 g VS−1 methane could be produced under these conditions, and 211.4 mLCH4 g VS−1 methane was produced in the validation experiment. It was determined that D. tharense cyanobacterium could be used as a suitable biomass source for methane production. However, it was not necessary to use the HTTP as a pretreatment process prior to the methane production.
1. Introduction
Microalgae produce atmospheric oxygen through photosynthesis and constitute the first step in the aquatic food chain. They are used as a source of production of many useful products in a wide range of sectors, such as human nutrition, feed, agriculture, aquaculture, and cosmetics, considering their composition is rich in proteins, carbohydrates, and lipids [1]. Cyanobacteria (blue-green algae) are photosynthetic prokaryotes used as food by humans. They have also been recognized as an excellent source of vitamins and protein. They can also be used in the production of some chemicals, as well as have the potential to be used as a source of renewable fuel and bioactive compounds. There is still research on their use as antiviral, anti-tumour, antibacterial, anti-HIV, and food additives [2].
Biofuels such as biodiesel, bioethanol, biohydrogen, and biogas can be produced from microalgae due to their high fat content. Biogas production from microalgae has gained importance in recent years with the ability to use biogas produced by anaerobic degradation as an energy source [3]. The mineral composition of microalgae meets the requirements of anaerobic microflora. Microalgae, which contain the main components such as carbon, nitrogen, and phosphorus, as well as micronutrients such as iron, cobalt, and zinc, are suitable biomass for methanogenesis [4]. It has been determined that many different microalgae species (Scenedesmus spp., Chlorella spp., Euglena spp., Oscillatoria spp., Synechocystir sp., Spirulina sp., Euglena sp., Micractinium sp., Melosira sp., Oscillatoria sp.) can be potential substrates for methane production by anaerobic digestion if used together or alone [5].
Researchers noted that even among similar species, very different methane production potentials have been achieved, and the understanding of the cell wall structure was the main determining factor. The theoretical methane potential of microalgae was reported in the range between 480–800 mLCH4 gVS−1 of biomass [4]. Although the comparison is limited due to the different anaerobic degradation test conditions used in many different studies, the results obtained from experiments are quite wide-ranging. Considering the carbohydrate, fat, and protein values in the composition of microalgae, it is noteworthy that the theoretical methane potentials are significantly higher than those obtained in practice [6]. It is known that some microalgae are resistant to biodegradation. The cell walls of some microalgae are high in carbohydrates, which resist hydrolysis and are hardly broken down by bacteria. Although species such as Dunaliella salina and Chlamydomonas reinhardtii are easily degradable due to the lipidic double-layer and glycoprotein nature of their cell walls, the majority of microalgae contain resistant cell walls with many layers of cellulose, hemicellulose, algaenan, pectin, and peptide–glycan [7].
The application of pretreatment may be necessary if the decomposition of the raw materials used in the anaerobic digestion process is difficult. The biodegradability could be improved and the biogas production efficiency can be increased after pretreatment. Generally, pretreatment methods are based on the concept of lysis (cell destruction). Physical, chemical, and biological pretreatment methods and their combinations can be used to disrupt the cell wall. When pretreatment methods are applied, microbial cells are exposed to lysis or death by releasing the cell contents (lysate). The lysate produced by the dissolution of cell contents is rich in soluble chemical oxygen demand (sCOD). The new substrate formed by degradation offers the potential to be reused by microbial metabolism [8].
The applications of microwave [9], ultrasonication [10], thermal [9], thermo-chemical [11], high-pressure thermal hydrolysis [6], hydro-thermal [12], thermal-hydrolysis [13], alkaline [14], and hydrodynamic cavitation [15] draws attention in the literature for the pretreatment of microalgae. However, there are contradictory results on the effects of pretreatment that are also observed in the literature. Although there was no increase in the methane potential of Chlorella sp. and Spirulina maxima microalgae with thermal pretreatment [14], a significant increase in methane production was observed in Scenedesmus sp. [16], Nannochloropsis salina [17], Spirulina maxima [13], and microalgae culture growing in wastewater [9].
Thermal pretreatment, which is one of the physical pretreatment methods, is among the most widely applied methods in the literature and has been shown to offer net energy contribution [9,17,18]. González-Fernández et al. [18] concluded that pretreatment at 70 °C resulted in a 22–24% increase in methane production, while a 48% increase in methane production was observed at 90 °C. Nannocloropsis salina was pretreated at 100–120 °C for 2 h, which resulted in a 108% increase in methane production [17]. It was stated that the thermal pretreatment of the Chlorella and Scenedesmus microalgae mixture at 120 °C for 30 min yielded a 20% increase in methane production [19], while the methane potential increased by 11–60% with the pretreatment of different microalgae mixtures at 110 and 140 °C for 15 min [13]. Passos et al. reported that thermal pretreatment of a mixture of Chlamydomona and Nitzchia microalgae at 75–95 °C resulted in a 12–61% increase in methane production; however, the energy balance was not met when the biomass was dilute (<1% VS) and the energy requirements could be met when the biomass concentration was high (>3% VS) [20].
In another study, Menendez et al. [21] investigated the effect of thermal hydrolysis at high pressure, tested reaction temperatures of 140, 160, and 180 °C, and found that the highest degradation and biogas production occurred at 160 °C. Mendez et al. [11] applied temperature and pressure pretreatment to Chlorella vulgaris microalgae biomass and determined that organic matter solubility was achieved and biogas production increased. They used 0.2 L of algal biomass in a 0.5 L laboratory-scale reactor. The reactor was operated at a constant stirring speed of 350 rpm at different reaction temperatures, pressures, and times (140 °C, 3 bar, 10–20 min; 160 °C, 6 bar, 10–20 min; 180 °C, 10 bar, 10–20 min) for examining the effect of pretreatment. The optimum conditions were determined at 160 °C, 6 bar, and 20 min, and pretreatment contributed 64% to methane yield. Keymer et al. [6] applied three different pretreatment methods (lipid extraction, high pressure thermal hydrolysis (HPTH), lipid extraction, and HPTH pretreatment together) to Scenedesmus microalgae biomass. The combination of HPTH and lipid extraction pretreatment method achieved a 33% increase in methane production yield.
Alzate et al. [13] assessed the potential of thermal hydrolysis, ultrasound, and a biological treatment (hydrolytic activity) to increase the biodegradability and biogas productivity from algal biomasses (microalgae A: Chlamydomonas, Scenedesmus, Nannochloropsis; microalgae B: Acutodesmus obliquus, Oocystis sp., Phormidium, Nitzschia sp.; microalgae C: Microspora). The highest increase in methane production (46–62%) was obtained for the thermal hydrolysis, but the biological pretreatment showed insignificant enhancements on methane production. The ultrasound pretreatment achieved methane increase ranging from 6% to 24%, and they did not observe much enhancement in energy input above 10,000 kJ/kg TS.
This study aims to determine the methane production potential of Desertifilum tharense and enhance the production efficiency by applying a high temperature and pressure pretreatment process. Correspondingly, this study focused on exploring the methane potential of cyanobacteria Desertifilum tharense with specific objectives of investigating for the first time in literature: (1) characterization of Desertifilum tharense and (2) optimization of high temperature and pressure pretreatment process.
2. Methods and Materials
2.1. Isolation, Identification, Culture Production, Harvesting and Determination of Culture Specific Parameters of Cyanobacteria
Native microalgae were isolated from a thermal source from the province of Denizli in southwestern of Turkey. The isolate has been identified as Desertifilum tharense through 16S rRNA amplification, DNA sequencing, and the NCBI online blast engine using RT-PCR. D. tharense was produced in 100-L Plexiglas tanks at room temperature (20–25 °C), using white fluorescent lamps with a constant brightness of 27 μmol/m2s (2000 lx) light power for a maximum of 30 days. Filtered air was pumped into the tanks at a flow rate of 12–15 L day−1. BG11 medium was used for the growth of D. tharense. Microalgae harvesting was carried out with sedimentation by centrifuging at 5000 rpm for 10 min. (Nüve, NF 800 model centrifuge, Ankara, Türkiye). The dry weight and chlorophyll content of D. tharense were determined according to the method proposed by Taştan et al. [22]. The specific growth rate, maximum productivity (Pmax), and CO2 fixation rate (FCO2) were calculated according to Perendeci et al. [23].
2.2. D. tharense Characterization Analyses
Concentrated biomass was used to determine the characterization of D. tharense, and the characterization analyses were performed in triplicate. Total solids (TS) and volatile solids (VS) analyses were measured according to Standard Method 2540-C [24]. Chemical oxygen demand (COD) analyses were performed according to ISO 6060-1989 method by Hach Lange DR5000 spectrophotometer (Hach, Düsseldorf, Germany) using Lange test kits. Organic carbon content of D. tharense was determined by TOC analyzer (Shimadzu 5500 model TOC and SSM5000 a solid sample module) (Shimadzu, Japan), and Total Kjeldahl nitrogen (TKN) was measured by TKN analyzer (Büchi Labortechnik AG, Flawil, Switzerland) (Büchi Digest, Automat K-438 and Büchi Auto Kjeldahl Unit K-370). The concentration of glucose was determined as carbohydrate concentration by the Anthrone method [25]. The protein content was measured by Lowry method [26] and the amount of lipid and extractable substances was measured by Soxhlet method [27]. Elemental analyzer (LECO, CHNS-932) (LECO, St. Joseph, MI, USA) was used for carbon, hydrogen, nitrogen, and sulfur (CHNS) analysis of samples. The total amount of phosphorus was made according to the phosphormolybdenum blue method (DIN 38405-D11-4, ISO 6878-1-1096) using Hach Lange PO4-P kits and a Hach Lange DR5000 spectrophotometer. The theoretical biochemical methane potential (TBMP) and higher heating value (HHV) of D. tharense were calculated with the help of Buswell and Dulong equations, respectively [28,29].
2.3. High Temperature-Pressure Pretreatment Process
The response surface method (RSM), which is one of the statistical experimental design methods, based on central composite design (CCD) was preferred to determine the optimum process conditions of high temperature–pressure pretreatment, and also the effects of HTPP on the biogas production efficiency. The CCD is a useful statistical tool for evaluating the relationships between independent and dependent variables and determining the effect of each variable [30].
Design-Expert® software (Version 7.0) was used for the experimental plan. The central composite was applied as two-factor and face-centered. The ranges for each independent variable were determined based on the literature and previous experimental experiences. The levels of the independent variables are coded in the range of −1 and +1. The coded levels and ranges of the independent variables used in the HTPP experiments are given in Table 1.

Table 1.
Selected independent variables and their ranges for HTPP.
The independent variables, levels, and dependent variables were transferred to the program for the determination of the experimental sets. The experiments of HTPP have been proposed by the Design Expert® program in order to increase the access of enzymes to the substrate and enrich biogas production by disrupting the structure of the D. tharense cell membrane. An HTPP reactor system (PARR 5500 Series Compact Reactor–PARR 4848 Reactor Controller) (PARR, Moline, IL, USA) was used to perform the proposed experiments. The HTPP reactor system was operated to investigate the effect of variables on the pretreatment of D. tharense at different reaction temperatures (100–200 °C) and different reaction times (10–20 min). D. tharense sample was added to the reactor, and the reactor content was filled to 120 mL with distilled water in the pretreatment experiments. The initial pH values of the reactor contents were measured, and pretreatment was performed by placing the reactor in the experimental system. After the specified reaction time was reached, the reactor chamber was cooled to room temperature. At the end of cooling, the output pH values were measured. All the pretreatment experiments were carried out in parallel.
2.4. High Temperature-Pressure Pretreatment Process Efficiency Analysis
The effects of independent variables on the system were performed through the responses of dependent variables. Therefore, determination of the dependent variables was important. The soluble chemical oxygen demand (sCOD), extractables and lipids, and biochemical methane potential (BMP) parameters were used as response variables in determining the efficiency of HTPP.
The pH values were measured at the beginning and end of the pretreatment tests with the WTW Inolab® Multi 9310 Set C pH meter (WTW, Weilheim, Germany). Soxhlet method was used for extractables and lipid analysis. For the analysis of the extractable substances present in the samples, the sample was extracted with petroleum ether in the Soxhlet cartridge and the petroleum ether and the sample were collected in the Soxhlet balloon. A Heidolph (Heidolph, Schwabach, Germany) 4000 rotary evaporator at 70–80 °C was used to remove petroleum ether. The evaporated samples were dried (105 °C) for 24 h, and the weights of the extraction flasks were measured to determine the amount of lipid present and the amount of substance that could be extracted [27,28]. The solid–liquid mixture in the reactor was centrifuged at 4400 rpm for 5 min to separate the solid and liquid fraction, and the dissolved chemical oxygen demand (sCOD) analysis was performed according to ISO 6060-1989 method by Hach Lange DR5000 spectrophotometer using Hach Lange test kits (Hach, Berlin, Germany).
2.5. Biochemical Methane Potential (BMP)
Standard BMP test was used to determine biogas potentials of the raw and pretreated D. tharense samples with HTPP. This method is based on the principle of incubating a certain amount of biomass mixed with anaerobic inoculum at a certain temperature, measuring the volume of biogas produced and the biogas composition at mesophilic conditions. The BMP test was performed according to the method applied by Mottet et al. [31] and Perendeci et al. [32]. According to the proposed method, the active inoculum concentration in the BMP reactor (500 mL capacity) was ensured to be 3–5 g VS L−1 and the substrate to inoculum ratio was 0.5 (gVS gVS−1 for solid samples, gCOD gVS−1 for liquid samples). The appropriate amounts of macro and micronutrients were added to maintain the inoculum sludge activity during the experiment. NaHCO3 was also added to buffer the pH change in the reactor. A gas mixture of N2/CO2 (70%/30%) was used to remove oxygen from the reactors. The reactors were closed with butyl rubber stoppers and placed in the Daihan WIS-150 incubator (35 °C) (Daihan Scientific, Gangwon, South Korea). The volume of biogas formed in the reactor was measured by water displacement principle and recorded during the 80-day incubation period. The gas components (methane and carbon dioxide) in the biogas were determined by Varian CP 4900 (Varian Inc., Palo Alto, CA, USA) micro gas chromatography with PPQ column (10 m). The temperature of the injector port and column oven was selected as 110 and 50 °C, respectively, and helium as the carrier gas was used at a flow rate of 25 mL min−1 in micro gas chromatography. A gas standard consisting of 60% (v/v) CH4 and 40% CO2 was used for calibration. The BMP tests were conducted in triplicate. The activated anaerobic inoculum used in the BMP tests was taken from the anaerobic sludge digestion reactor located at Hurma wastewater treatment plant in Antalya city. The gas production of the anaerobic sludge was considered in calculating the biogas production of the samples. The methane production was calculated as mL of methane per gram of volatile solids (mLCH4 gVS−1) added to the reactor.
2.6. Scanning Electron Microscopy (SEM)
SEM examination was made to observe surface properties D. tharense. Samples were lyophilized and coated with gold palladium for 120 s under an 18-mA vacuum (Polaron, SC7620 Sputter Coater). After coating, the samples were examined at different magnifications and images were captured by SEM (Zeis, Leo 1430) (Jena, Germany) at 15 kV voltage.
3. Results and Discussion
3.1. D. tharense Characterization Results
The studies on microalgae and cyanobacteria show that dry matter weight (X), specific growth rate (μ), maximum productivity (Pmax), and CO2 fixation rate (FCO2) parameters vary from species to species, and these parameters are important for methane production efficiency. Table 2 shows the measured and calculated growth kinetics and specific properties of D. tharense.

Table 2.
Average growth kinetics and specific characteristics of D. tharense.
As can be seen from Table 2, D. tharense dry weight and specific growth rate values were found as 3.61 g L−1 and 0.36 L day−1, respectively. The specific growth rate (μ) of D. tharense is within the limits determined for microalgae. There is no study in the literature that calculated the specific growth rate for D. tharense. However, the specific growth rate for Synechocystis was found to be 1.033 ± 0.027 L day−1 in the study performed by Patel et al. [33].
In a study conducted by Assunção et al. [34] with S. obliquus, C. vulgaris, and C. protothecoides species, the highest specific growth rate, maximum productivity, and theoretical CO2 fixation rate at 10% CO2 were found as 1.28 day−1, 0.28 g L−1.day−1, and 0.56 g L−1.day−1 for S. obliquus, respectively. In this study, D. tharense maximum productivity and CO2 fixation rate were found 0.35 g L−1.day−1 and 0.06 g day−1, respectively.
The results of the characterization analyses of D. tharense are given in Table 3. Detail comparison of characterization analysis results can be found in Perendeci et al. [23].

Table 3.
The characterization analysis results of the D. tharense.
The total solids and volatile solids values of D. tharense were measured as 97.4 g kg−1 and 91 g kg−1, respectively. Since the samples were centrifuged before the characterization analyses, TS and VS contents were higher than the values determined for other species in the literature. The extractable substance and lipid content of D. tharense was measured as 1.2%. In the literature, the amount of extractable matter and lipids for different species has been reported in the range of 7–23% [4] and 2–22% [35] based on dry matter weight. The amount of extractive matter and lipid measured for D. tharense is lower than the values given in the literature for algae. In this study, the protein content of D. tharense was measured as 28.1 mg L−1, while the protein contents of different microalgae species in the literature are observed in a wide range of 6–71% [4,35], and the protein content of Chlorella spp. was found as 20.2 mg L−1 by Gimenez et al. [36]. Detailed comparisons of protein, carbohydrate, and lipid contents of different microalgae species were discussed in Al hattab and Ghaly [4,37].
As can be seen from the elemental analysis results, the amount of total carbon was measured as 50.2%. Total organic carbon content (TOC) was determined as 48.9%. This suggests that the D. tharense carbon content originates from organic carbon. Specifically, the elemental analysis results of D. tharense are at the same level detected for different algal species [14]. The total nitrogen content of D. tharense was measured as 7.07%. The calorific value of D.tharense was determined as 5220 kcal/kg. Although there is no study on the calorific values of cyanobacteria in the literature, the calorific value calculated for D. tharense is higher than commercial fuels such as lignite (2500–4800 kcal/kg) and wood (3400 kcal/kg).
SEM examination was made to observe the surface properties of D. tharense. As seen from SEM images in Figure 1a,b, the raw D. tharense obtained from the growth reactor has a compact, smooth, and continuous surface.
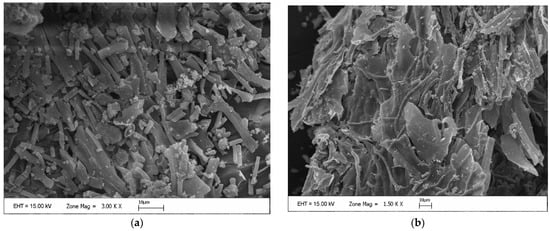
Figure 1.
(a,b) SEM images of raw D. tharense.
3.2. Effects of HTPP Process on pH, sCOD, Extractables with Lipids and BMP
In the process of HTPP experiments carried out in a closed reactor, the vapor pressure in the reactor increased and was measured as 1, 2, and 13 bar at 100, 150, and 200 °C, respectively. Figure 2 demonstrates the values of pH (a), sCOD (b), extractive matter and lipids (c), and BMP (d) measured under different high temperature–pressure pretreatment process conditions. As can be seen from Figure 2a, the pH values measured before and after the HTPP process ranged between 5.2–6.0 and 5.3–6.1, respectively. There was no significant change observed in pH values with pretreatment application. The pH value decreased slightly after HTPP application under the conditions of 200 °C reaction temperature and 10-, 15-, and 20-min reaction time.
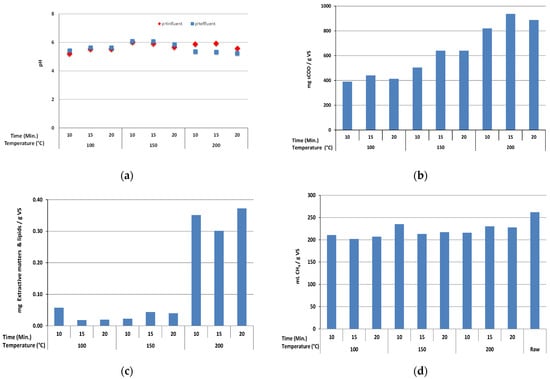
Figure 2.
Effects of high temperature–pressure pretreatment on pH (a), sCOD (b), extractive matter and lipids (c), and BMP (d).
The sCOD value of the raw D. tharense sample was measured as 35.3 mg gVS−1. The sCOD values of the samples treated with a high temperature–pressure process ranged between 390.7–937.1 mg gVS−1. The sCOD values increased with the increase of the reaction temperature from 100 °C to 150 °C and 200 °C. It was determined that high reaction temperature increased the solubility by disintegrating the D. tharense cells and caused an increase in the sCOD values. The maximum sCOD concentration was determined with a value of 937.1 mg gVS−1 at 200 °C reaction temperature and 15 min reaction time. The minimum sCOD concentration as 390.7 mg gVS−1 was observed under the pretreatment conditions of 100 °C reaction temperature and 10 min reaction time. In a study conducted by Keymer et al. (2013), the sCOD value for raw algae biomass was found to be 51 mg gVS−1, and after the application of pretreatment, the sCOD value increased and was found as 580 mg gVS−1 [6].
The extractive matter and lipids value of the raw D. tharense sample was measured as 0.082 mg gVS−1. As can be seen from Figure 2c, the extractive matter and lipids values of the samples treated with high temperature–pressure varied between 0.0185–0.3725 mg gVS−1. The extractive matter and lipids values increased with increasing the reaction temperature from 100 °C to 200 °C. It was determined that high reaction temperature (200 °C) caused an increase in extractive matter and lipids values by disintegrating D. tharense cells.
There are a limited number of studies investigating methane potentials of cyanobacteria in the literature. The comparison of the BMP values of some cyanobacteria in the literature and the methane potential value of D. tharense used in this study are presented in Table 4.

Table 4.
BMP values of some Cyanobacteria.
The BMP value of the raw D. tharense sample was measured as 261.8 mLCH4 gVS−1. The BMP value of D. tharense is in a similar range compared with the cyanobacteria in the literature. On the other hand, Spirulina maxima and Spirulina platensis have higher methane yields than D. tharanse. The BMP values of the samples treated with high temperature-pressure were measured in the range of 201.5–235 mLCH4 gVS−1 and lower than the raw D. tharense sample. The BMP results obtained in this study indicate that the HTPP process is not suitable for biofuel methane production from D. tharense. Cumulative methane production results from the pretreated and raw D. tharanse samples during the 80-day incubation period of BMP tests are given in Figure 3. All BMP tests of pretreated and raw samples showed a rapid increase in methane yield from 156 to 190 mL CH4 g VS−1 between days 5 to 9. This rapid increase in methane yields indicated that D. tharanse has high biodegradability, proving that pretreatment is unnecessary.
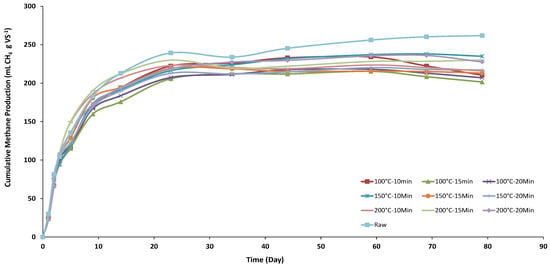
Figure 3.
Cumulative methane production results from the pretreated and raw D. tharanse samples during the BMP test.
Furthermore, the methane yield of anaerobic digestion is influenced by the biomass composition, specifically, the content of proteins, carbohydrates, and lipids in the biomass. For instance, biomass with high protein content releases ammonia during anaerobic digestion, inhibiting methanogens. Using carbohydrate-rich biomass can result in the accumulation of volatile fatty acids, decreasing the pH in the digester and, thus, inhibiting methanogens. Both situations would destabilize the anaerobic digestion microbiome resulting in low methane yields. While lipids are easily degradable, they cause microbial inhibition at high concentrations, resulting in mass transfer problems in the digesters. Therefore, biomass composition is essential for methane yields [39]. The composition of microalgal and cyanobacterial biomass is highly variable [37].
Soluble COD is generally the preferred parameter for evaluating the pretreatment effect on biomass biodegradability. As seen in Figure 2b, the solubilization of D. tharense was increased parallel with the increased severity of the high temperature–pressure pretreatment conditions (time and process temperature). As seen in Table 3, the sugar content of D.tharanse was measured as 1099.8 ± 28.93 mg/gVS. Related to carbohydrate content, sCOD increased with the increasing severity of the high temperature–pressure pretreatment. On the other hand, the high temperature–pressure pretreatment process did not increase methane production appreciably. The produced recalcitrant by-products cannot be converted into methane and may create inhibition. Similar results indicating the formation of recalcitrant compounds were reported in the literature [5,15,40]. Furthermore, Bermudez et al. (2019) found that the methane yields from cyanobacteria Pseudanabaena sp. CY14-1 decreased while carbohydrate content in biomass increased. Therefore, they concluded that biomass composition does affect the methane yield [39].
3.3. Modeling of HTPP Process
Models for sCOD, extractive matter with lipids, and BMP were developed by RSM by using obtained values from the experiments of HTPP process. Model equations for sCOD, extractive matter with lipids, and BMP, model statistics, and information are presented in Table 5.

Table 5.
Model equations, statistics, and information.
The Design Expert program proposed a modified model with high predictive power to determine the increase amount of sCOD values of the samples treated with HTPP. The coefficient of determination, denoted as R2, expresses the ratio of the explained variation to the total variation and reveals the model’s predictive power. The R2 value of the sCOD model was calculated as 0.9888. The p-value of the model was found as 0.0004 and the model was statistically significant. It is seen that the p-values of the reaction temperature and reaction time variables were calculated as <0.0001 and 0.0427, respectively. The effects of these two variables on the sCOD model were statistically significant since the p-values were less than 0.05. When the second-order effects were examined, the p-values for reaction temperature and reaction time were calculated as 0.0718 and 0.0470, respectively. It was determined that the reaction temperature was not statistically significant on the sCOD model since its p-value was greater than 0.05, and the reaction time was statistically effective on the sCOD model since it was less than 0.05. It was seen that 98.88% of the sCOD model responses could be explained by the variables used in this model according to the calculated R2 value. Since there is no major difference between the terms R2 (0.9888) and the Add–R2 (0.9776), the terms in the model are sufficient.
The Design Expert program suggested a quadratic model for estimating the amount of extractive matter and lipids measured in samples treated by the HTPP process. However, a modified model was preferred to increase model predictive power by removing variables that reduce model prediction capability. The R2 value of the model established for the dependent variable extractive matter and lipids was calculated as 0.9798. The p-value for the obtained model was less than 0.0001 indicates that the model was statistically significant at 99.999% confidence interval. The p-values of the reaction temperature variable (<0.0001) were found to be statistically significant, since its p-value was less than 0.05. The second-order effect of the reaction temperature on the extractive matter and lipids was also statistically effective with the low p-value (0.0002). The adjusted R2 value of the model was found as 0.9731.
A modified model for forecasting the amount of BMP values of the treated samples by the HTPP was chosen to enhance the model prediction capacity. Even if a statistically significant model was obtained with a low p-value of 0.0285, a moderate R2 value of 0.5193 for the BMP model was found. R2 value shows that the model could explain 51.93% of the BMP values. Reaction temperature was statistically significant since the p-value was less than 0.05.
The response surface plots expressing the interaction effects of reaction time and reaction temperature, as independent variables in HTPP, on the amount of sCOD, extractive matter with lipids, and BMP are given in Figure 4a–c, respectively. Although there is no change in sCOD by increasing the reaction time at the constant value of reaction temperature, a significant increase is observed by increasing the reaction temperature while the reaction time is constant. Compared to raw D. tharanse sCOD value, the maximum sCOD increase was observed as 2262% at 200 °C reaction temperature and 15 min reaction HTPP conditions. As detected in sCOD, a similar trend was also observed for extractive matter and lipids (Figure 4b). Increasing the reaction temperature resulted in a significant increase in the amount of extractive matter and lipids. A maximum value of extractable matter with lipid was detected as 0.2840 g lipid gVS−1 under the same HTPP conditions (200 °C–15 min). As can be seen from Figure 4c, like the acquired identical results for sCOD and extractive and lipid, the same observation was attained for BMP. The maximum BMP value was obtained as 223 mL CH4 gVS−1 under HTPP conditions at 200 °C and 10–20 min. In conclusion, the reaction temperature was detected as an influential variable in HTPP.
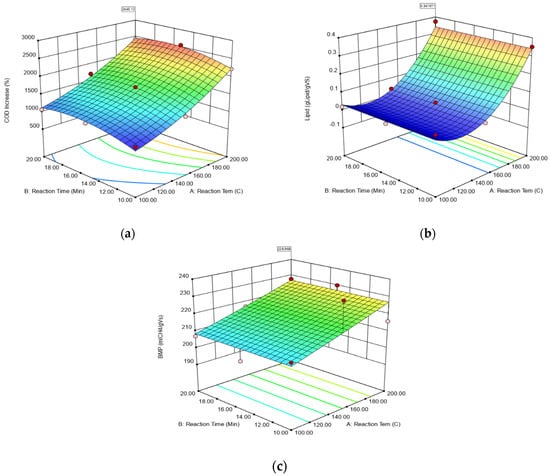
Figure 4.
The response surface graphs of impacts of independent variables on reaction time and reaction temperature. (a) sCOD; (b) extractive matter and lipids; (c) BMP.
3.4. Optimization of HTPP Process for Methane Production
Two different approaches were considered in the optimization for the high temperature and pressure pretreatment process to produce methane from D. tharense cyanobacteria. The first approach took into account maximum methane production at a minimum cost. The reaction temperature and reaction time were kept in range and minimum level, respectively, in the optimization process since they are cost-increasing factors in pretreatment. In the second approach, the reaction temperature and reaction time are left in the range to ensure maximum methane production not regarding the cost. The optimization conditions, model prediction results, and validation experiment results for achieving maximum methane production at minimum cost and for maximum methane production are presented in Table 6.

Table 6.
Optimization conditions for maximum methane production at minimum cost and for maximum methane production, model prediction results, and validation experiment results.
In the validation experiment, the values estimated by the model as a result of the optimization are compared with the values obtained from the validation experiments, and the confidence in the models is determined. For maximum methane production with minimum cost, a reaction temperature of 106 °C and a reaction time of 11 min were recommended as optimum conditions. The BMP value was predicted by the model as 209.5 mL CH4 gVS−1. In the validation experiment, a BMP value of 205.1 mL CH4 gVS−1 was obtained. The percentage of error between the predicted and measured values for maximum methane production at minimum cost was calculated as 2.1%. The small error between the validation result and the prediction of the methane production model shows that the model’s reliability is high. For maximum methane production, 200 °C reaction temperature and 20 min reaction time were proposed as optimum conditions. The result predicted by the model under these conditions was realized as 227.1 mL CH4 gVS−1. In the validation experiment, 211.4 mL CH4 gVS−1 were obtained. Even high temperature and pressure pretreatment is unnecessary for the D. tharanse having high biodegradability and low cell wall thickness; the developed model will give insight into this current design space.
4. Conclusions
The application of the HTPP process to increase methane production efficiency and optimization of the pretreatment process were carried out for D. tharense isolated from Denizli/Turkey region, for the first time in the literature. It was determined that methane production increased with increasing the reaction temperature applied in the HTPP. However, methane production from raw D. tharense was found to be higher than those treated with HTTP. It has been determined that D. tharense cyanobacteria are suitable for use as a biomass source for methane production, but it is not necessary to apply an HTPP before methane production. Microalgae can be a source for different fields, such as food additives, blue-colored dye for natural products, fertilizer and/or soil amendment, and input for biofuel production. Microalgae are considered a magic product in recent decades; however, life cycle analysis indicated that they do not yet offer the desired level of positive contributions because of the energy requirements for growing, harvesting, and cell wall breakdown. In addition, microalgae and cyanobacteria need to be examined on a species basis to produce different products.
Author Contributions
Conceptualization, N.A.P.; methodology, M.Ş.; software, F.Y.; validation, M.F.; formal analysis, M.Ş.; investigation, M.F. and N.A.P.; data curation, F.Y.; writing—original draft, V.Y.; writing—review and editing, N.A.P.; supervision, N.A.P.; project administration, V.Y.; funding acquisition, V.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Scientific and Technological Research Council of Turkey, Grant Number Tubitak 115Y334. This study was supported by the Project Management Unit of Akdeniz University, Turkey under Grant FLY-2018-3427.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to a Non-Disclosure Agreement.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jankowska, E.; Sahu, A.K.; Oleskowicz-Popiel, P. Biogas from Microalgae: Review on Microalgae’s Cultivation, Harvesting and Pretreatment for Anaerobic Digestion. Renew. Sustain. Energy Rev. 2017, 75, 692–709. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive Compounds from Cyanobacteria and Microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Ehimen, E.A.; Holm-Nielsen, J.B.; Poulsen, M.; Boelsmand, J.E. Influence of Different Pre-Treatment Routes on the Anaerobic Digestion of a Filamentous Algae. Renew. Energy 2013, 50, 476–480. [Google Scholar] [CrossRef]
- Sialve, B.; Bernet, N.; Bernard, O. Anaerobic Digestion of Microalgae as a Necessary Step to Make Microalgal Biodiesel Sustainable. Biotechnol. Adv. 2009, 27, 409–416. [Google Scholar] [CrossRef]
- Mussgnug, J.H.; Klassen, V.; Schlüter, A.; Kruse, O. Microalgae as Substrates for Fermentative Biogas Production in a Combined Biorefinery Concept. J. Biotechnol. 2010, 150, 51–56. [Google Scholar] [CrossRef]
- Keymer, P.; Ruffell, I.; Pratt, S.; Lant, P. High Pressure Thermal Hydrolysis as Pre-Treatment to Increase the Methane Yield during Anaerobic Digestion of Microalgae. Bioresour. Technol. 2013, 131, 128–133. [Google Scholar] [CrossRef]
- Diltz, R.; Pullammanappallil, P. Biofuels from Algae. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; IntechOpen: London, UK, 2013; p. 348. [Google Scholar]
- Low, E.W.; Chase, H.A. Reducing Production of Excess Biomass during Wastewater Treatment. Water Res. 1999, 33, 1119–1132. [Google Scholar] [CrossRef]
- Passos, F.; Hernández-Mariné, M.; García, J.; Ferrer, I. Long-Term Anaerobic Digestion of Microalgae Grown in HRAP for Wastewater Treatment. Effect of Microwave Pretreatment. Water Res. 2014, 49, 351–359. [Google Scholar] [CrossRef]
- Lee, J.; Cho, D.H.; Ramanan, R.; Kim, B.H.; Oh, H.M.; Kim, H.S. Microalgae-Associated Bacteria Play a Key Role in the Flocculation of Chlorella Vulgaris. Bioresour. Technol. 2013, 131, 195–201. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Timmers, R.A.; Ballesteros, M.; González-Fernández, C. Enhancing Methane Production of Chlorella vulgaris via Thermochemical Pretreatments. Bioresour. Technol. 2013, 149, 136–141. [Google Scholar] [CrossRef]
- Passos, F.; Ferrer, I. Influence of Hydrothermal Pretreatment on Microalgal Biomass Anaerobic Digestion and Bioenergy Production. Water Res. 2015, 68, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Alzate, M.E.; Muñoz, R.; Rogalla, F.; Fdz-Polanco, F.; Pérez-Elvira, S.I. Biochemical Methane Potential of Microalgae: Influence of Substrate to Inoculum Ratio, Biomass Concentration and Pretreatment. Bioresour. Technol. 2012, 123, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Bohutskyi, P.; Kula, T.; Kessler, B.A.; Hong, Y.; Bouwer, E.J.; Betenbaugh, M.J.; Allnutt, F.C.T. Mixed Trophic State Production Process for Microalgal Biomass with High Lipid Content for Generating Biodiesel and Biogas. Bioenergy Res. 2014, 7, 1174–1185. [Google Scholar] [CrossRef]
- Fardinpoor, M.; Perendeci, N.A.; Yılmaz, V.; Taştan, B.E.; Yılmaz, F. Effects of Hydrodynamic Cavitation-Assisted NaOH Pretreatment on Biofuel Production from Cyanobacteria: Promising Approach. Bioenergy Res. 2022, 15, 289–302. [Google Scholar] [CrossRef]
- González-Fernández, C.; Ballesteros, M. Microalgae Autoflocculation: An Alternative to High-Energy Consuming Harvesting Methods. J. Appl. Phycol. 2013, 25, 991–999. [Google Scholar] [CrossRef]
- Schwede, S.; Kowalczyk, A.; Gerber, M.; Span, R. Anaerobic Co-Digestion of the Marine Microalga Nannochloropsis Salina with Energy Crops. Bioresour. Technol. 2013, 148, 428–435. [Google Scholar] [CrossRef]
- González-Fernández, C.; Sialve, B.; Bernet, N.; Steyer, J.P. Thermal Pretreatment to Improve Methane Production of Scenedesmus Biomass. Biomass Bioenergy 2012, 40, 105–111. [Google Scholar] [CrossRef]
- Cho, S.; Park, S.; Seon, J.; Yu, J.; Lee, T. Evaluation of Thermal, Ultrasonic and Alkali Pretreatments on Mixed-Microalgal Biomass to Enhance Anaerobic Methane Production. Bioresour. Technol. 2013, 143, 330–336. [Google Scholar] [CrossRef]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Pretreatment of Microalgae to Improve Biogas Production: A Review. Bioresour. Technol. 2014, 172, 403–412. [Google Scholar] [CrossRef]
- Bermúdez Menéndez, J.M.; Arenillas, A.; Menéndez Díaz, J.Á.; Boffa, L.; Mantegna, S.; Binello, A.; Cravotto, G. Optimization of Microalgae Oil Extraction under Ultrasound and Microwave Irradiation. J. Chem. Technol. Biotechnol. 2014, 89, 1779–1784. [Google Scholar] [CrossRef]
- Ertit Taştan, B.; Duygu, E.; Dönmez, G. Boron Bioremoval by a Newly Isolated Chlorella sp. and Its Stimulation by Growth Stimulators. Water Res. 2012, 46, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Perendeci, N.A.; Yılmaz, V.; Ertit Taştan, B.; Gökgöl, S.; Fardinpoor, M.; Namlı, A.; Steyer, J.P. Correlations between Biochemical Composition and Biogas Production during Anaerobic Digestion of Microalgae and Cyanobacteria Isolated from Different Sources of Turkey. Bioresour. Technol. 2019, 281, 209–216. [Google Scholar] [CrossRef] [PubMed]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Loewus, F.A. Improvement in Anthrone Method for Determination of Carbohydrates Errors in Volumetric Analysis Arising from Adsorption. Anal. Chem. 1952, 24, 219. [Google Scholar] [CrossRef]
- Waterborg, J.H.; Matthews, H.R. The Lowry Method for Protein Quantitation. Protein Protoc. Handb. 1984, 1, 7–9. [Google Scholar]
- Bridoux, G.; Dhulster, P.; Manem, J. Analyse des Graisses Dans les Stations d’épuration. Tech. Sci. Méthodes Génie Urbain Génie Rural 1994, 5, 257–262. [Google Scholar]
- Lesteur, M.; Bellon-Maurel, V.; Gonzalez, C.; Latrille, E.; Roger, J.M.; Junqua, G.; Steyer, J.P. Alternative Methods for Determining Anaerobic Biodegradability: A Review. Process Biochem. 2010, 45, 431–440. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Theisen, H.; Vigil, S. Integrated Solid Waste Management. Engineering Principles and Management Issues; McGraw-Hill: New York, NY, USA, 1993; ISBN 0070632375. [Google Scholar]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Mottet, A.; Steyer, J.P.; Déléris, S.; Vedrenne, F.; Chauzy, J.; Carrère, H. Kinetics of Thermophilic Batch Anaerobic Digestion of Thermal Hydrolysed Waste Activated Sludge. Biochem. Eng. J. 2009, 46, 169–175. [Google Scholar] [CrossRef]
- Perendeci, N.A.; Gökgöl, S.; Orhon, D. Impact of Alkaline H2O2 Pretreatment on Methane Generation Potential of Greenhouse Crop Waste under Anaerobic Conditions. Molecules 2018, 23, 1794. [Google Scholar] [CrossRef]
- Patel, A.; Gami, B.; Patel, P.; Patel, B. Microalgae: Antiquity to Era of Integrated Technology. Renew. Sustain. Energy Rev. 2017, 71, 535–547. [Google Scholar] [CrossRef]
- Assunção, M.F.G.; Amaral, R.; Martins, C.B.; Ferreira, J.D.; Ressurreição, S.; Santos, S.D.; Varejão, J.M.T.B.; Santos, L.M.A. Screening Microalgae as Potential Sources of Antioxidants. J. Appl. Phycol. 2017, 29, 865–877. [Google Scholar] [CrossRef]
- Becker, E.W. Micro-Algae as a Source of Protein. Biotechnol. Adv. 2007, 25, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Giménez, J.B.; Bouzas, A.; Carrere, H.; Steyer, J.P.; Ferrer, J.; Seco, A. Assessment of Cross-Flow Filtration as Microalgae Harvesting Technique Prior to Anaerobic Digestion: Evaluation of Biomass Integrity and Energy Demand. Bioresour. Technol. 2018, 269, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Al hattab, M.; Ghaly, A. Microalgae Oil Extraction Pre-Treatment Methods: Critical Review and Comparative Analysis Fundamentals of Renewable Energy and Applications. J. Fundam. Renew. Energy Appl. 2015, 5, 1–26. [Google Scholar] [CrossRef]
- Mendez, L.; Mahdy, A.; Ballesteros, M.; González-Fernández, C. Chlorella vulgaris vs Cyanobacterial Biomasses: Comparison in Terms of Biomass Productivity and Biogas Yield. Energy Convers. Manag. 2015, 92, 137–142. [Google Scholar] [CrossRef]
- Cuellar-Bermudez, S.P.; Magdalena, J.A.; Muylaert, K.; Gonzalez-Fernandez, C. High methane yields in anaerobic digestion of the cyanobacterium Pseudanabaena sp. Algal Res. 2019, 44, 101689. [Google Scholar] [CrossRef]
- Choudhary, P.; Assemany, P.P.; Naaz, F.; Bhattacharya, A.; De Siqueira Castro, J.; do Couto Couto, E.D.A.; Calijuri, M.L.; Pant, K.K.; Malik, A. A review of biochemical and thermochemical energy; onversion routes of wastewater grown algal biomass. Sci. Total Environ. 2020, 726, 137961. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).