Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrates, Inocula, and Experimental Approach
- (1)
- The impact of co-digestion of SM + WS on the specific biogas yield (SBY) during 40 d of anaerobic digestion by using the Hohenheim Biogas Yield Test (HBT) procedure. Therefore, different variants with SM, WS, and SM + WS as co-digestion were conducted.
- (2)
- Two different pretreatment methods to enhance the substrate characteristics of the brown algae SM in co-digestion with WS in order to achieve increased SBY and SMY by reducing salinity and by enhancing disintegration. Therefore, a batch assay with increased volumes (compared to HBT) of 2 L glass vessels was carried out.
- (3)
- A second HBT series with the variants mentioned in (1) but with the inocula residues (digestates) of the 2 L batch assay (2), to analyze if the microbes have adapted to the new substrate to ultimately achieve higher SBY and SMY.
2.2. Pretreatment Methods for SM
2.2.1. Mechanical Pretreatment—Pressing
2.2.2. Thermal Pretreatment—Heating and Pressing
2.3. Digestion Systems
2.3.1. 2L-DE
2.3.2. Hohenheim Biogas Yield Test (HBT)
2.4. Laboratory and Statistical Analyses
2.4.1. Dry Matter Determination
2.4.2. Ash Content
2.4.3. Elementary Analysis (CHNO) and Theoretical (Stoichiometric) Biogas Potential
2.4.4. Multi-Elemental Determination
2.4.5. Gross Calorific Value (GCV) and Sulphur Chloride Determination
2.4.6. Statistical Analysis
3. Results and Discussion
3.1. Characteristics of Samples Used
3.2. Biogas Production
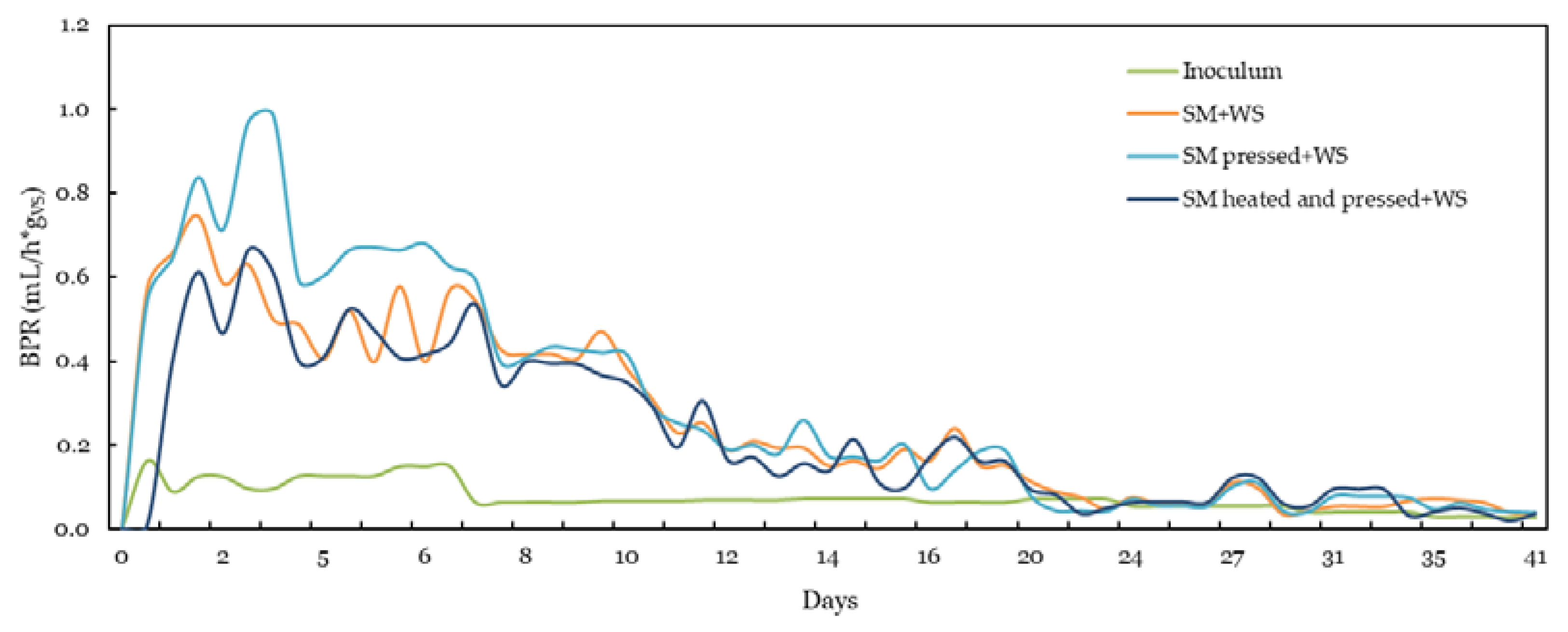
3.2.1. 2L-DE Results
3.2.2. HBT Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BPR | biogas production rate |
| DM | dry matter |
| EC | electrical conductivity |
| FM | fresh matter |
| GCV | gross calorific value |
| GPR | gas production rate |
| GOM | modified Gompertz model |
| HBT | Hohenheim biogas yield test |
| HRT | hydraulic retention time |
| SBY | specific biogas yield |
| SM | sargassum muticum |
| SMY | specific methane yield |
| VS | volatile solids |
| WS | wheat straw |
| 2L-DE | 2L digestion experiment |
Appendix A
| Variant | SMY (Measured) (L/kgVS) | S (L/kgVS) | Rm (L/kgVS*d) | λ (d) | SMY (GOM) (L/kgVS) |
|---|---|---|---|---|---|
| Inoculum | 35.49 | 38.31 | 1.17 | 0.65 | 34.72 |
| SM + WS | 87.64 | 82.55 | 4.67 | 0.00 | 82.31 |
| SMpressed + WS | 100.86 | 93.93 | 6.59 | 0.12 | 93.82 |
| SMpressed and heated + WS | 74.75 | 71.24 | 3.61 | 0.30 | 70.51 |
References
- Broom, D. What Is the EU Doing to End Its Reliance on Russian Energy? 2022. Available online: https://www.weforum.org/agenda/2022/04/europe-russia-energy-alternatives/ (accessed on 8 December 2022).
- Pandey, A. Emerging Technologies and Biological Systems for Biogas Upgrading: Foreword. In Emerging Technologies and Biological Systems for Biogas Upgrading; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780128228081. [Google Scholar]
- Vivekanand, V.; Eijsink, V.G.H.; Horn, S.J. Biogas production from the brown seaweed Saccharina latissima: Thermal pretreatment and codigestion with wheat straw. J. Appl. Phycol. 2012, 24, 1295–1301. [Google Scholar] [CrossRef]
- Carlsson, A.S.; van Beilen, J.B.; Möller, R.; Clayton, D. Micro- and Macro-Algae: Utility for Industrial Applications: Outputs from the EPOBIO; CPL Press: Newbury, UK, 2007; pp. 1–2. ISBN 978-1-872691-29-9. [Google Scholar]
- Mendelsohn, R. What Causes Crop Failure? Clim. Chang. 2007, 81, 61–70. [Google Scholar] [CrossRef]
- Chislock, M.F.; Doster, E.; Zitomer, R.A.; Wilson, A.E. Eutrophication: Causes, Consequences, and Controls in Aquatic Ecosystems. Nat. Educ. Knowl. 2013, 4, 10. [Google Scholar]
- Kusek, K.M.; Hu, C.; Wang, M. Scientists Discover the Biggest Seaweed Bloom in the World 2019. Available online: https://www.usf.edu/marine-science/news/2019/scientists-discover-the-biggest-seaweed-bloom-in-the-world.aspx (accessed on 28 July 2022).
- Jard, G.; Marfaing, H.; Carrère, H.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. French Brittany macroalgae screening: Composition and methane potential for potential alternative sources of energy and products. Bioresour. Technol. 2013, 144, 492–498. [Google Scholar] [CrossRef]
- Soto, M.; Vázquez, M.A.; de Vega, A.; Vilariño, J.M.; Fernández, G.; de Vicente, M.E.S. Methane potential and anaerobic treatment feasibility of Sargassum muticum. Bioresour. Technol. 2015, 189, 53–61. [Google Scholar] [CrossRef]
- McHugh, D.J. A Guide to the Seaweed Industry; Food and Agriculture Organization of the United Nations: Rome, Italy, 2003; ISBN 92-5-104958-0. [Google Scholar]
- Murphy, J.D.; Drosg, B.; Allen, E.; Jerney, J.; Xia, A.; Herrmann, C. A Perspective on Algal Biogas; IEA Bioenergy: Paris, France, 2015; ISBN 978-1-910154-18-2. [Google Scholar]
- Sargassum: Online Article on the Webpage of the Reef Resilience Network 2020. Available online: https://reefresilience.org/de/management-strategies/managing-local-threats/sargassum/#:~:text=Es%20gibt%20jedoch%20im%20Allgemeinen,zu%20ihrer%20Bew%C3%A4ltigung%20erforderlich%20sind.&text=Schwimmende%20Sargassum%20Arten%20bilden%20dicke%20Matten%20an%20der%20Wasseroberfl%C3%A4che (accessed on 1 August 2022).
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2016, 15, 67–88. [Google Scholar] [CrossRef]
- González-López, N.; Moure, A.; Domínguez, H. Hydrothermal fractionation of Sargassum muticum biomass. J. Appl. Phycol. 2012, 24, 1569–1578. [Google Scholar] [CrossRef]
- Maneein, S.; Milledge, J.J.; Harvey, P.J.; Nielsen, B.V. Methane production from Sargassum muticum: Effects of seasonality and of freshwater washes. Energy Built Environ. 2021, 2, 235–242. [Google Scholar] [CrossRef]
- Namvar, F.; Mohamad, R.; Baharara, J.; Zafar-Balanejad, S.; Fargahi, F.; Rahman, H.S. Antioxidant, antiproliferative, and antiangiogenesis effects of polyphenol-rich seaweed (Sargassum muticum). Biomed. Res. Int. 2013, 2013, 604787. [Google Scholar] [CrossRef]
- Moen, E.; Horn, S.; Østgaard, K. Biological degradation of Ascophyllum nodosum. J. Appl. Phycol. 1997, 9, 347–357. [Google Scholar] [CrossRef]
- Milledge, J.J.; Nielsen, B.V.; Harvey, P.J. The inhibition of anaerobic digestion by model phenolic compounds representative of those from Sargassum muticum. J. Appl. Phycol. 2019, 31, 779–786. [Google Scholar] [CrossRef]
- Marquez, G.P.B.; Santiañez, W.J.E.; Trono, G.C.; Montaño, M.N.E.; Araki, H.; Takeuchi, H.; Hasegawa, T. Seaweed biomass of the Philippines: Sustainable feedstock for biogas production. Renew. Sustain. Energy Rev. 2014, 38, 1056–1068. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Barakat, A.; Quéméneur, M.; Trably, E.; Steyer, J.-P.; Carrère, H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014, 32, 934–951. [Google Scholar] [CrossRef] [PubMed]
- Borines, M.G.; de Leon, R.L.; Cuello, J.L. Bioethanol production from the macroalgae Sargassum spp. Bioresour. Technol. 2013, 138, 22–29. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, R.M.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing, as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef]
- Wang, X.; Yang, G.; Feng, Y.; Ren, G.; Han, X. Optimizing feeding composition and carbon-nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour. Technol. 2012, 120, 78–83. [Google Scholar] [CrossRef]
- Paul, R.; Suhartini, S.; Sulu, M.; Melville, L. Feasibility of seaweed and agricultural crop waste residues as codigestion feedstock. In Proceedings of the 15th IWA World Conference on Anaerobic Digestion, Beijing, China, 17–20 October 2017. [Google Scholar]
- Rivera-Hernández, Y.; Hernández-Eugenio, G.; Balagurusamy, N.; Espinosa-Solares, T. Sargassum-pig manure co-digestion: An alternative for bioenergy production and treating a polluting coastal waste. Renew. Energy 2022, 199, 1336–1344. [Google Scholar] [CrossRef]
- Hülsemann, B.; Zhou, L.; Merkle, W.; Hassa, J.; Müller, J.; Oechsner, H. Biomethane Potential Test: Influence of Inoculum and the Digestion System. Appl. Sci. 2020, 10, 2589. [Google Scholar] [CrossRef]
- Achinas, S.; Euverink, G.J.W. Theoretical analysis of biogas potential prediction from agricultural waste. Resour. Effic. Technol. 2016, 2, 143–147. [Google Scholar] [CrossRef]
- Kum, K.Y.; Kirchhof, R.; Luick, R.; Heinrich, M. Danshen (Salvia miltiorrhiza) on the Global Market: What Are the Implications for Products’ Quality? Front. Pharmacol. 2021, 12, 621169. [Google Scholar] [CrossRef]
- Amon, T.; Behrendt, U.; Daniel-Gromke, J.; Denysenko, V.; Döhler, H.; Falke, I.; Fischer, E.; Friehe, J.; Gattermann, H.; Grebe, S.; et al. Leitfaden Biogas: Von der Gewinnung zur Nutzung, Gülzow. 2016. Available online: https://mediathek.fnr.de/leitfaden-biogas.html (accessed on 31 July 2022).
- Sossa, K.; Alarcón, M.; Aspé, E.; Urrutia, H. Effect of ammonia on the methanogenic activity of methylaminotrophic methane producing Archaea enriched biofilm. Anaerobe 2004, 10, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Mercado, I.D.; Weber, B.; Durán-García, M.D. Use of Hydrothermal Pretreatment to Enhance Biogas Production from Pelagic Sargassum. Bioenergy Res. 2022, 15, 1639–1648. [Google Scholar] [CrossRef]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef]
- Jackowiak, D.; Bassard, D.; Pauss, A.; Ribeiro, T. Optimisation of a microwave pretreatment of wheat straw for methane production. Bioresour. Technol. 2011, 102, 6750–6756. [Google Scholar] [CrossRef]
- Jard, G.; Dumas, C.; Delgenes, J.P.; Marfaing, H.; Sialve, B.; Steyer, J.P.; Carrère, H. Effect of thermochemical pretreatment on the solubilization and anaerobic biodegradability of the red macroalga Palmaria palmata. Biochem. Eng. J. 2013, 79, 253–258. [Google Scholar] [CrossRef]
- Montingelli, M.E.; Benyounis, K.Y.; Stokes, J.; Olabi, A.G. Pretreatment of macroalgal biomass for biogas production. Energy Convers. Manag. 2016, 108, 202–209. [Google Scholar] [CrossRef]
- Kristahn, R.; Negele, J.; Benkowitsch, E.; Mairhofer, D.; Weil, P.; Warth, B. Biogas aus Makroalgen: Verfahrenstechnische und ökonomische Untersuchung in Bezug auf die Bretagne; University of Hohenheim: Stuttgart, Germany, 2011. [Google Scholar]
- Kaltschmitt, M.; Hartmann, H.; Hofbauer, H. Energie aus Biomasse: Grundlagen, Techniken und Verfahren; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–1867. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P.J. Ensilage and anaerobic digestion of Sargassum muticum. J. Appl. Phycol. 2016, 28, 3021–3030. [Google Scholar] [CrossRef]
- Bird, K.T.; Chynoweth, D.P.; Jerger, D.E. Effects of marine algal proximate composition on methane yields. J. Appl. Phycol. 1990, 2, 207–213. [Google Scholar] [CrossRef]
- Gunaseelan, N.V. Anaerobic digestion of biomass for methane production: A review. Biomass Bioenergy 1997, 13, 83–114. [Google Scholar] [CrossRef]
- Leong, Y.K.; Chang, J.-S. Integrated role of algae in the closed-loop circular economy of anaerobic digestion. Bioresour. Technol. 2022, 360, 127618. [Google Scholar] [CrossRef]
- Caxiano, I.N.; Mello, P.A.; Alijó, P.H.R.; Teixeira, L.V.; Cano, R.F.; Maia, J.G.S.S.; Bastos, J.B.V.; Pavão, M.S.G. Continuous design and economic analysis of a Sargassum muticum biorefinery process. Bioresour. Technol. 2022, 343, 126152. [Google Scholar] [CrossRef]
- Grillo, G.; Tabasso, S.; Solarino, R.; Cravotto, G.; Toson, C.; Ghedini, E.; Menegazzo, F.; Signoretto, M. From Seaweeds to Cosmeceutics: A Multidisciplinar Approach. Sustainability 2021, 13, 13443. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, C.; Garcia-Oliveira, P.; Lourenço-Lopes, C.; Silva, S.A.; Garcia-Perez, P.; Carvalho, A.P.; Domingues, V.F.; Barroso, M.F.; Delerue-Matos, C.; et al. Screening of Bioactive Properties in Brown Algae from the Northwest Iberian Peninsula. Foods 2021, 10, 1915. [Google Scholar] [CrossRef] [PubMed]
- Rocha, C.P.; Pacheco, D.; Cotas, J.; Marques, J.C.; Pereira, L.; Gonçalves, A.M.M. Seaweeds as Valuable Sources of Essential Fatty Acids for Human Nutrition. Int. J. Environ. Res. Public Health 2021, 18, 4968. [Google Scholar] [CrossRef] [PubMed]
- Gasausbeute in Landwirtschaftlichen Biogasanlagen: Potenziale, Erträge, Einflussfaktoren; Dandikas, V.; Herrmann, C.; Hülsemann, B.; Oechsner, H. (Eds.) Kuratorium für Technik und Bauwesen in der Landwirtschaft e.V. (KTBL): Darmstadt, Germany, 2021; ISBN 978-3-945088-85-2. [Google Scholar]
- Gaserträge und Nährstoffgehalte—NawaRo. Eindhoven/Hessisch Oldendorf [Online]. Available online: https://www.archea-biogas.de/BiogasAZ/Substrate/NachwachsendeRohstoffe/NachwachsendeRohstoffe.html (accessed on 28 May 2023).
- Chala, B.; Oechsner, H.; Müller, J. Introducing Temperature as Variable Parameter into Kinetic Models for Anaerobic Fermentation of Coffee Husk, Pulp and Mucilage. Appl. Sci. 2019, 9, 412. [Google Scholar] [CrossRef]


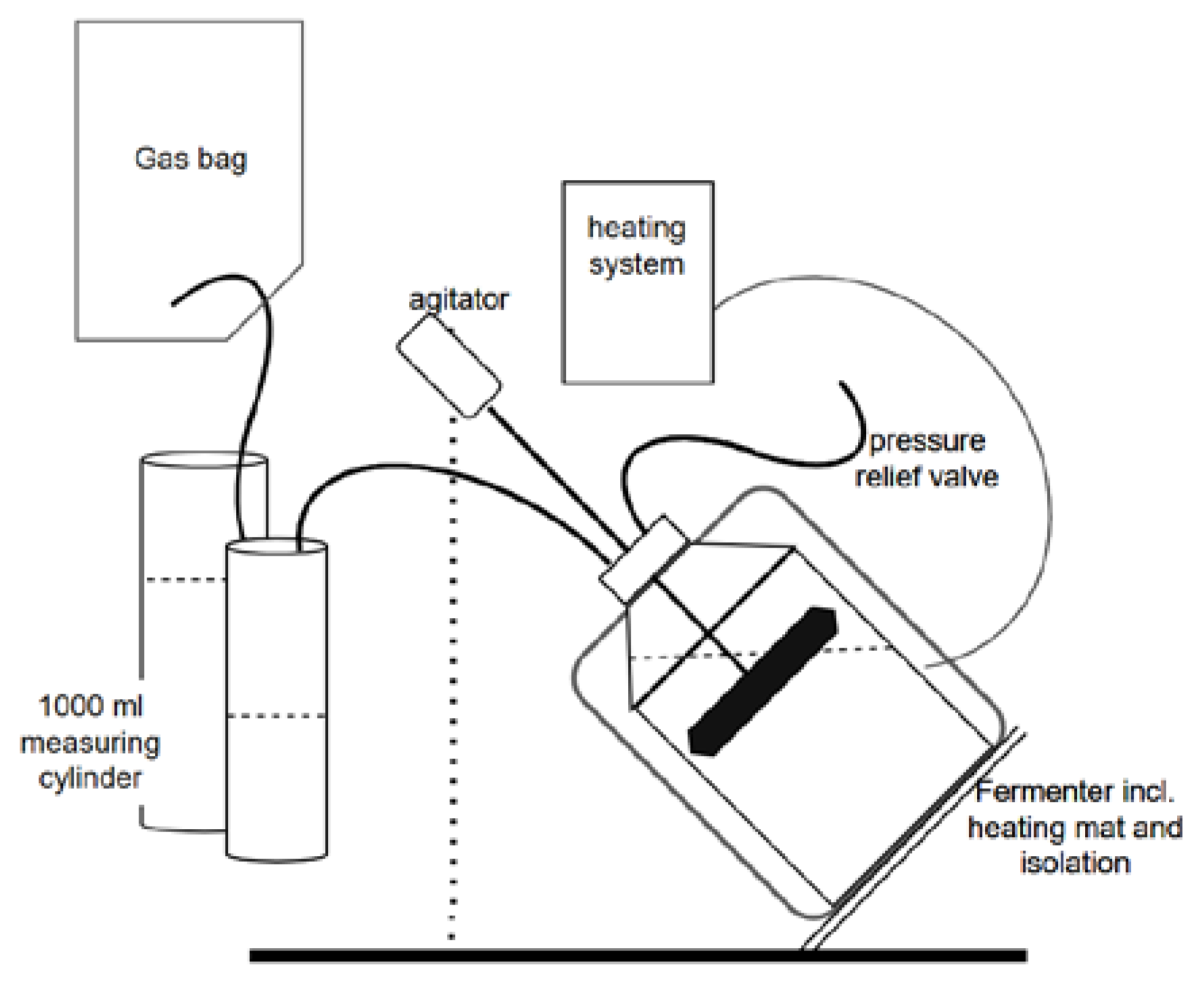


| Substrate | FM2 | DM | VS | Digester No. |
|---|---|---|---|---|
| g | g | g | (-) | |
| Inoculum | - | 84.8 ± 1.9 | 54.7 ± 1.9 | 1–12 |
| WS | - | 47.1 ± 0.3 | 44.05 ± 0.3 | 4–12 |
| SM | 265 ± 0.12 | 61.3 ± 0.1 | 41.35 ± 0.1 | 4–6 |
| SMpressed | 226 ± 6.3 | 58.9 ± 6.3 | 39.7 ± 6.3 | 7–9 |
| SMheated and pressed | 230 ± 3.9 | 57 ± 3.9 | 39.3 ± 3.9 | 10–12 |
| Variants | Unit | Blanks | SM | WS | SM + WS |
|---|---|---|---|---|---|
| S 1–3 | S 4–6 | S 7–9 | S 10–12 | ||
| SM | g | - | 0.5 ± 0.01 | - | 0.3 ± 0.01 |
| WS | g | - | - | 0.51 | 0.2 ± 0.01 |
| Inoculum | mL | 30 | 30 | 30 | 30 |
| Parameter | Unit | SM (Untreated) | WS | Inoculum Thermophilic | Inoculum Mesophilic 1 | Inoculum Mixture D8 |
|---|---|---|---|---|---|---|
| DM | %FM | 11.82 ± 0.47 | - | 6.73 ± 0.45 | 4.4 ± 0.0 | NA |
| VS | %DM | 67.45 ± 0.42 | 93.59 ± 0.33 | 65.89 ± 0.47 | 61.7 ± 0.1 | NA |
| Residual moisture | %DM | 12.34 ± 0.47 | 11.19 ± 0.59 | - | - | - |
| Ash | %DM | 32.55 ± 0.41 | 6.41 ± 0.08 | NA | 1.7 ± 0.0 (%FM) | NA |
| C | %DM | 31.94 ± 0.27 | 46.95 ± 0.16 | NA | NA | NA |
| H | %DM | 5.17 ± 0.11 | 6.52 ± 0.03 | NA | NA | NA |
| N | %DM | 2.34 ± 0.07 | 0.66 ± 0.05 | NA | NA | NA |
| GCV | kJ/gDM | 12.53 ± 12 | 18.79 ± 18.9 | NA | NA | NA |
| Ca | g/kgDM | 10.39 ± 0.5 | 2.97 ± 0.17 | 54.90 ± 0.54 | NM | 28.33 ± 1.67 |
| K | g/kgDM | 75.78 ± 1.94 | 15.59 ± 0.24 | 0.02 ± 0.87 | NM | 116.28 ± 5.25 |
| P | g/kgDM | 1.26 ± 0.04 | 0.92 ± 0.03 | 1.79 ± 3.98 | NM | 5.54 ± 0.26 |
| S | g/kgDM | 7.91 ± 0.12 | 0.67 ± 0.02 | 0.99 ± 2.2 | NM | 10.00 ± 0.6 * |
| Co | g/kgDM | 0.001 ± 0.00 | <0.001 | 0.001 ± 0.03 | 0.003 | 0.002 ± 0.00 |
| Fe | g/kgDM | 2.17 ± 0.2 | 0.15 ± 0.02 | 8.98 ± 0.17 | 3.24 | 6.00 ± 0.37 |
| Mn | g/kgDM | 0.25 ± 0.01 | 0.04 ± 0.00 | 0.40 ± 4.8 | 0.316 | 0.37 ± 0.02 |
| Mg | g/kgDM | 12.95 * ± 0.31 | 0.68 ± 0.03 | 7.36 ± 0.29 | NM | 7.47 ± 0.36 |
| Mo | g/kgDM | <0.001 | <0.001 | 0.003 ± 0.09 | 0.007 | 0.004 ± 0.00 |
| Na | g/kgDM | 25.65 ± 0.68 | 0.14 ± 0.03 | 1.89 ± 0.09 | NM | 12.48 ± 0.65 |
| Ni | g/kgDM | <0.001 | <0.001 | 0.008 ± 0.14 | 0.015 | 11.04 ± 0.00 |
| Se | g/kgDM | 0.003 ± 0.00 | <0.001 | <0.001 | 0.001 | <0.001 |
| Cu | g/kgDM | 0.005 ± 0.00 | <0.001 | 0.06 ± 1.84 | 0.091 | 0.065 ± 0.00 |
| Zn | g/kgDM | 0.023 ± 0.00 | 0.011 ± 0.00 | 0.26 ± 5.71 | 0.378 | 0.400 ± 0.02 |
| Cl | g/kgDM | 104.7 ± 12.3 | 4.04 ± 0.18 | NA | NM | NA |
| Substrate | SBY a | SMY b | Methane Concentration in % Week | |||
|---|---|---|---|---|---|---|
| mL/gVS | mL/gVS | 1 | 3 | 5 | 6 | |
| Inoculum | 52.8 ± 3.6 | 35.5 ± 0.45 | 59.59 ± 1.4 | - | 69.9 ± 0.5 | 72.2 ± 0.6 |
| SM + WS | 201.37 ± 30.38 | 87.64 ± 8.72 | 42.8 ± 6.3 | 61.9 ± 3.4 | 61.4 ± 0.9 | 62.6 ± 1.5 |
| SMpressed + WS | 220.65 ± 8.87 | 100.86 ± 11.37 | 45.9 ± 5.9 | 59.1 ± 2.4 | 61.1 ± 1.8 | 60.9 ± 2.3 |
| SMheated and pressed + WS | 178.67 ± 8.16 | 74.75 ± 0.94 | 39.9 ± 6.4 | 61.9 ± 4.7 | 61.3 ± 2.5 | 61.5 ± 1.4 |
| Parameter | Unit | Soaking Water (Untreated) | Press Water (SMpressed) | Press Water (SMheated and pressed) |
|---|---|---|---|---|
| EC | mS | 39.9 | 41.3 | 42.1 |
| Ca | mg/L | 95.89 ± 1.65 * | 565.15 ± 27.85 * | 341.89 ± 3.72 * |
| K | mg/L | 1880.04 ± 25.75 * | 2238.87 ± 20 * | 2349.44 ± 41.46 * |
| P | mg/L | 69.58 ± 0.23 * | 82.80 ± 1.28 * | 99.78 ± 2.71 * |
| S | mg/L | 234.83 ± 1.70 * | 337.41 ± 3.76 * | 843.75 ± 20.92 * |
| Co | mg/L | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.04 ± 0.00 |
| Fe | mg/L | 0.88 ± 0.25 | 40.47 ± 3.30 | 39.02 ± 0.99 |
| Mn | mg/L | 13.23 ± 0.07 | 16.39 ± 0.22 | 23.16 ± 0.23 |
| Mg | mg/L | 1051.28 ± 6.71 * | 1050.09 ± 9.50 * | 1196.42 ± 39.93 * |
| Mo | mg/L | <0.001 | <0.001 | <0.001 |
| Na | mg/L | 1599.52 ± 8.28 | 2011.38 ± 12.4 | 2139.08 ± 36.77 |
| Ni | mg/L | 0.01 ± 0.00 | 0.14 ± 0.01 | 0.06 ± 0.00 |
| Se | mg/L | 0.04 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 |
| Cu | mg/L | <0.001 | 0.07 ± 0.01 | <0.001 |
| Zn | mg/L | 0.13 ± 0.02 | 0.76 ± 0.03 | 0.91 ± 0.01 |
| Cl | mg/L | NA | NA | NA |
| Parameter | Inoculum | SM | WS | SM + WS |
|---|---|---|---|---|
| SBY HBT 1 a (mL/gVS) | 55.3 ± 3.06 | 117.2 ± 0.95 | 211.33 ± 19.9 | 175 ± 7.97 |
| SBY HBT 2 b (mL/gVS) | 42.3 ± 0.34 | 109.01 ± 5.43 | 243.63 ± 6.64 | 179.65 ± 4.69 |
| C:N of substrate without inoculum | NA | 14:1 | 71:1 | 38:1 |
| Contained VS (g) | 1.09 | 0.042 | 0.052 | 0.047 |
| VS ratio of substrate to inoculum | - | 0.038 | 0.047 | 0.043 |
| GPR HBT 1 (%) | 2.26% | 0.70% | 0.49% | 0.58% |
| GPR HBT 2 (%) | 1.33% | 0.85% | 0.36% | 0.55% |
| Macroalgal Species | SMY | Reference |
|---|---|---|
| mL/gVS | ||
| SMpressed + WS | 101 ± 11 | this study |
| S. muticum untreated | 130 ± 1 | [8] |
| S. muticum untreated | 100 ± 50 | [38] |
| S. muticum ensiled (whole) | 110 ± 80 | |
| S. muticum chopped prior to ensiling | 60 ± 10 | |
| S. muticum untreated | 166–208 | [9] |
| S. fluitans untreated | 180 | [39] |
| S. pteropleuron untreated | 150 | |
| S. fluitans untreated | 165 ± 8 | [40] |
| S. pteropleuron untreated | 145 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hütter, M.; Sailer, G.; Hülsemann, B.; Müller, J.; Poetsch, J. Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw. Fermentation 2023, 9, 820. https://doi.org/10.3390/fermentation9090820
Hütter M, Sailer G, Hülsemann B, Müller J, Poetsch J. Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw. Fermentation. 2023; 9(9):820. https://doi.org/10.3390/fermentation9090820
Chicago/Turabian StyleHütter, Miriam, Gregor Sailer, Benedikt Hülsemann, Joachim Müller, and Jens Poetsch. 2023. "Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw" Fermentation 9, no. 9: 820. https://doi.org/10.3390/fermentation9090820
APA StyleHütter, M., Sailer, G., Hülsemann, B., Müller, J., & Poetsch, J. (2023). Impact of Thermo-Mechanical Pretreatment of Sargassum muticum on Anaerobic Co-Digestion with Wheat Straw. Fermentation, 9(9), 820. https://doi.org/10.3390/fermentation9090820









