Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Preparation and Identification of FCP Extract (E)
2.3. Cell Culture and Cell Viability Assay
2.4. Insulin-Resistance Induction
2.5. Glucose Consumption
2.6. Action Mechanism Analysis by Molecular Docking
2.7. Statistical Analysis
3. Results
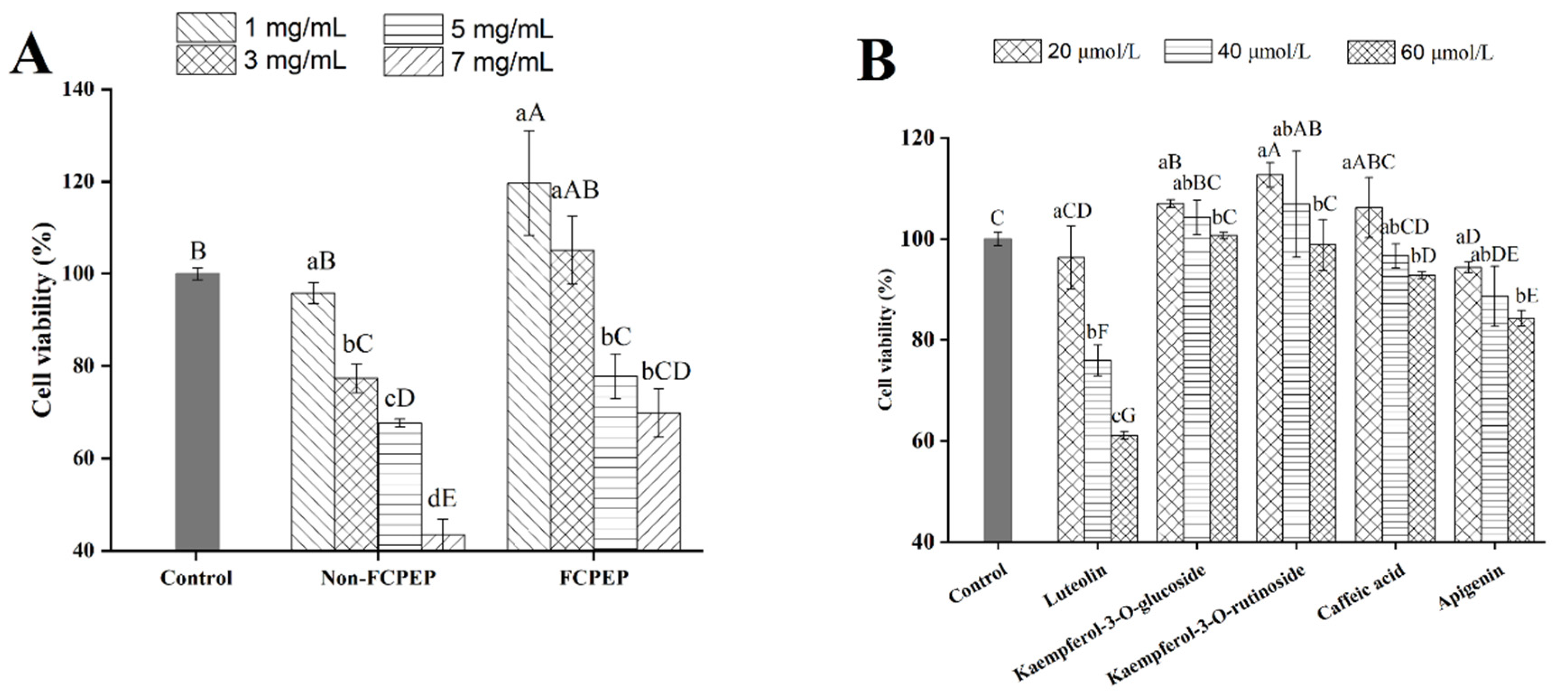
3.1. Effects of FCPE on Cell Viability of HepG2 Cells
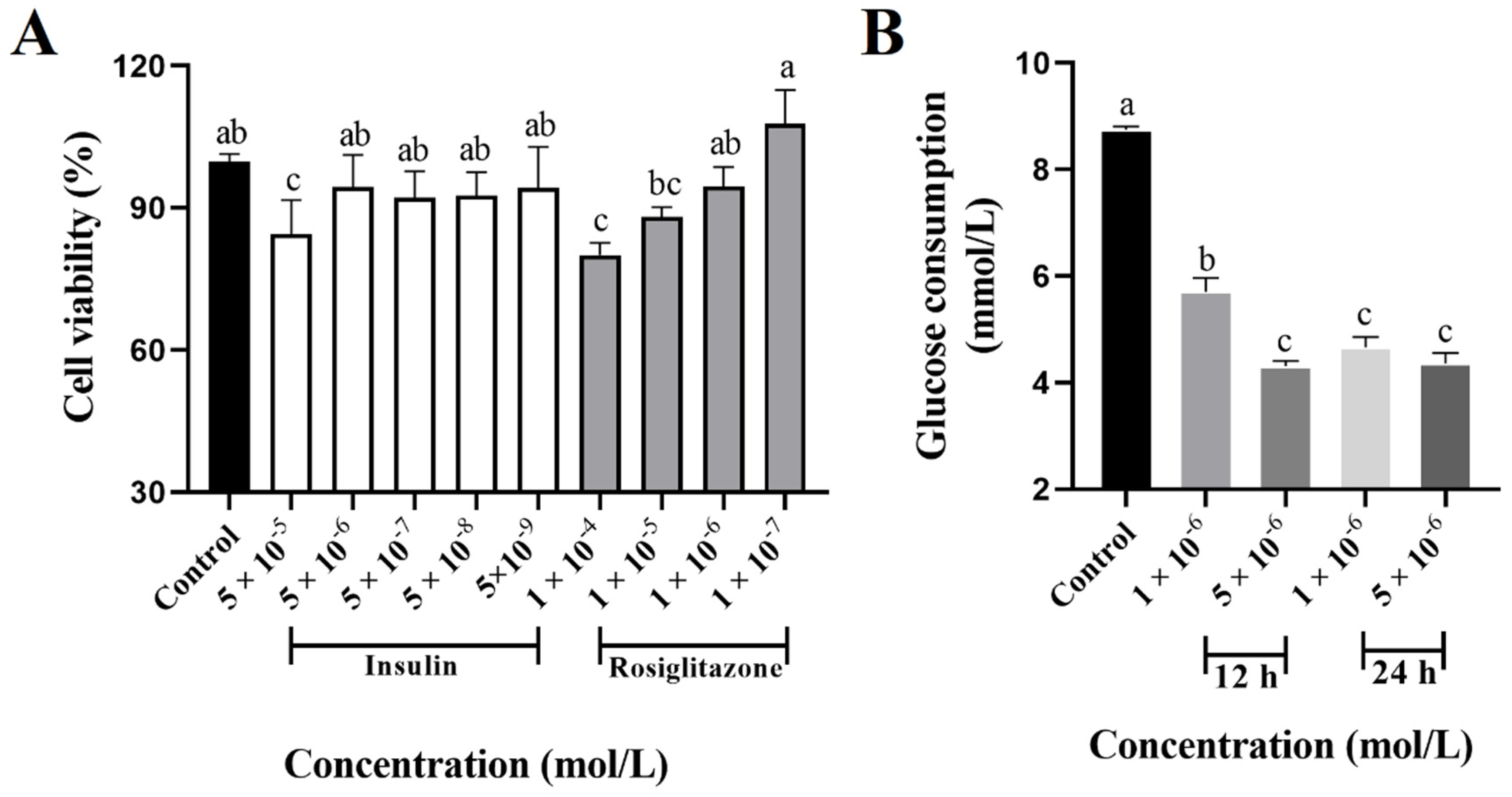
3.2. Insulin-Resistant HepG2 Cells Development
3.3. Effects of FCPE and Phenolic Compounds on Glucose Consumption in Insulin-Resistant HepG2 Cells
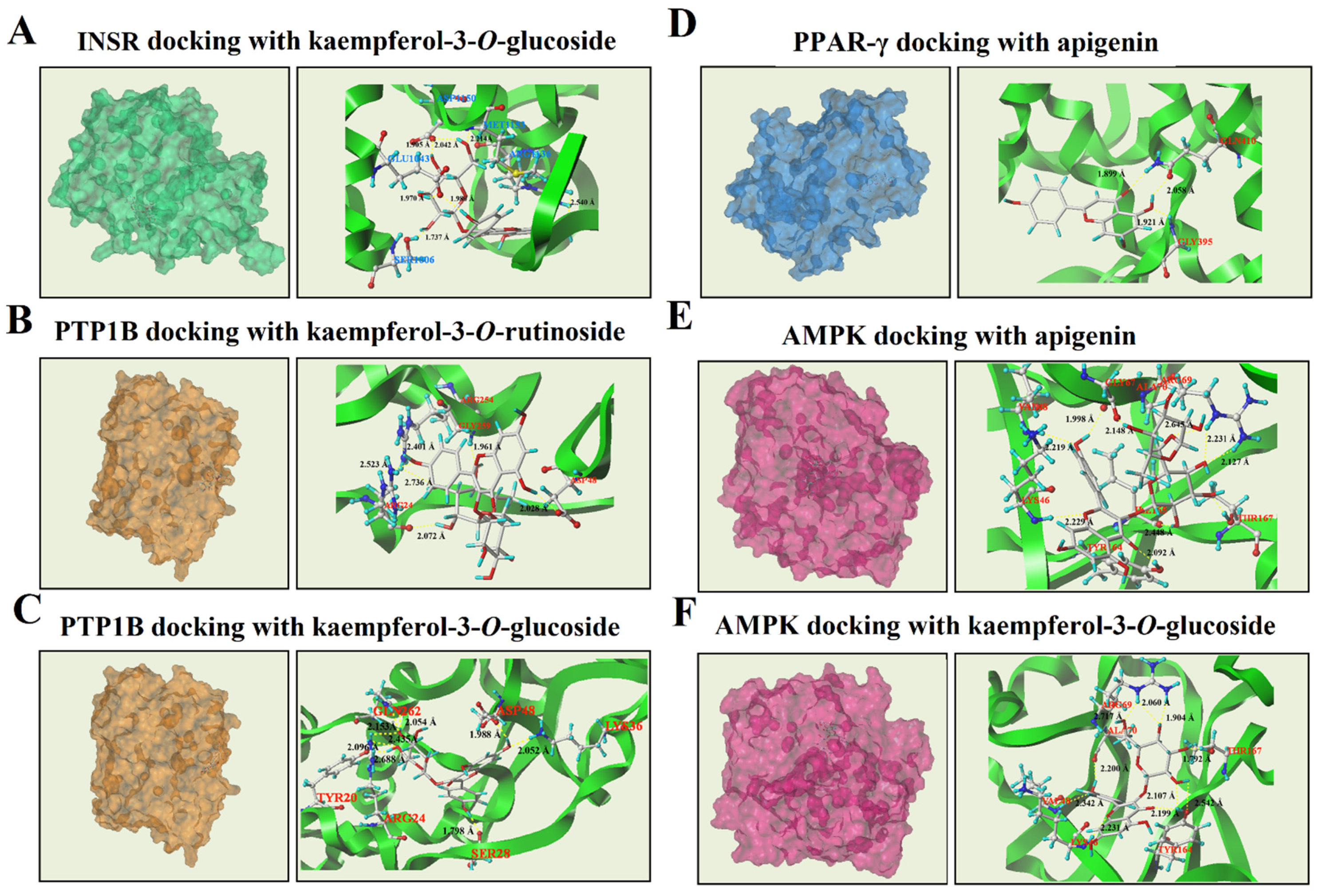
3.4. Molecular Docking Analysis of Phenolic Compounds with INSR, PTP1B, PPAR-γ, and AMPK
3.4.1. Molecular Docking Results of INSR
3.4.2. Molecular Docking Results of PTP1B
3.4.3. Molecular Docking Results of PPAR-γ
3.4.4. Molecular Docking Results of AMPK
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lee, S.H.; Park, S.Y.; Choi, C.S. Insulin resistance: From mechanisms to therapeutic strategies. Diabetes Metab. J. 2022, 46, 15–37. [Google Scholar]
- Park, S.Y.; Gautier, J.F.; Chon, S. Assessment of insulin secretion and insulin resistance in human. Diabetes Metab. J. 2021, 45, 641–654. [Google Scholar]
- Mlinar, B.; Marc, J.; Janez, A.; Pfeifer, M. Molecular mechanisms of insulin resistance and associated diseases. Clin. Chim. Acta 2007, 375, 20–35. [Google Scholar]
- Khalid, M.; Alkaabi, J.; Khan, M.A.B.; Adem, A. Insulin sgnal transduction perturbations in insulin resistance. Int. J. Mol. Sci. 2021, 22, 8590. [Google Scholar]
- Kumar, K.J.S.; Lin, C.; Tseng, Y.-H.; Wang, S.-Y. Fruits of Rosa laevigata and its bio-active principal sitostenone facilitate glucose uptake and insulin sensitivity in hepatic cells via AMPK/PPAR-γ activation. Phytomed. Plus 2021, 1, 100109. [Google Scholar]
- Zhao, Y.; Tang, Z.Q.; Shen, A.G.; Tao, T.; Wan, C.H.; Zhu, X.H.; Huang, J.R.; Zhang, W.L.; Xia, N.N.; Wang, S.X.; et al. The role of PTP1B O-GlcNAcylation in hepatic insulin resistance. Int. J. Mol. Sci. 2015, 16, 22856–22869. [Google Scholar]
- Bailey, C.J. Insulin resistance: Impact on therapeutic developments in diabetes. Diab. Vasc. Dis. Res. 2019, 16, 128–132. [Google Scholar]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: Advantages, limitations, and appropriate usage. Amer. Physiological. Soc. 2008, 294, E15–E26. [Google Scholar]
- Yan, H.; Huang, C.; Shen, X.; Li, J.; Zhou, S.; Li, W. GLP-1 RAs and SGLT-2 inhibitors for insulin resistance in nonalcoholic fatty liver disease: Systematic review and network meta-analysis. Front. Endocrinol. 2022, 13, 923606. [Google Scholar]
- Wondmkun, Y.T. Obesity, insulin resistance, and type 2 diabetes: Associations and therapeutic implications. Diabetes Metab. Syndr. Obes. 2020, 13, 3611–3616. [Google Scholar]
- Biobaku, F.; Ghanim, H.; Batra, M.; Dandona, P. Macronutrient-mediated inflammation and oxidative stress: Relevance to insulin resistance, obesity, and atherogenesis. J. Clin. Endocrinol. Metab. 2019, 104, 6118–6128. [Google Scholar]
- Incir, S.; Bolayirli, I.M.; Inan, O.; Aydin, M.S.; Bilgin, I.A.; Sayan, I.; Esrefoglu, M.; Seven, A. The effects of genistein supplementation on fructose induced insulin resistance, oxidative stress and inflammation. Life Sci. 2016, 158, 57–62. [Google Scholar]
- Salehi, B.; Hernandez-Alvarez, A.J.; Contreras, M.D.; Martorell, M.; Ramirez-Alarcon, K.; Melgar-Lalanne, G.; Matthews, K.R.; Sharifi-Rad, M.; Setzer, W.N.; Nadeem, M.; et al. Potential phytopharmacy and food applications of Capsicum spp.: A comprehensive review. Nat. Prod. Commun. 2018, 13, 1543–1556. [Google Scholar]
- Asnin, L.; Park, S.W. Isolation and analysis of bioactive compounds in Capsicum peppers. Crit. Rev. Food Sci. Nutr. 2015, 55, 254–289. [Google Scholar]
- Mi, S.; Zhang, X.N.; Wang, Y.H.; Zheng, M.; Zhao, J.J.; Gong, H.Y.; Wang, X.H. Effect of different genotypes on the fruit volatile profiles, flavonoid composition and antioxidant activities of chilli peppers. Food Chem. 2022, 374, 131751. [Google Scholar]
- Watcharachaisoponsiri, T.; Sornchan, P.; Charoenkiatkul, S.; Suttisansanee, U. The alpha-glucosidase and alpha-amylase inhibitory activity from different chili pepper extracts. Int. Food Res. J. 2016, 23, 1439–1445. [Google Scholar]
- Cortes-Ferre, H.E.; Martinez-Avila, M.; Antunes-Ricardo, M.; Guerrero-Analco, J.A.; Monribot-Villanueva, J.L.; Gutierrez-Uribe, J.A. In vitro evaluation of anti-inflammatory activity of "Habanero" chili pepper (Capsicum chinense) seeds extracts pretreated with cellulase. Plant Foods Hum. Nutr. 2022. [Google Scholar] [CrossRef]
- Du, X.; Myracle, A.D. Fermentation alters the bioaccessible phenolic compounds and increases the alpha-glucosidase inhibitory effects of aronia juice in a dairy matrix following in vitro digestion. Food Funct. 2018, 9, 2998–3007. [Google Scholar]
- Li, M.Q.; Bao, X.; Zhang, X.T.; Ren, H.B.; Cai, S.B.; Hu, X.S.; Yi, J.J. Exploring the phytochemicals and inhibitory effects against alpha-glucosidase and dipeptidyl peptidase-IV in Chinese pickled chili pepper: Insights into mechanisms by molecular docking analysis. Lwt-Food Sci. Techol. 2022, 162, 113467. [Google Scholar]
- Xia, T.; Duan, W.H.; Zhang, Z.J.; Fang, B.; Zhang, B.; Xu, B.C.; de la Cruz, C.B.V.; El-Seedi, H.; Simal-Gandara, J.; Wang, S.Y.; et al. Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNK-IRS-1 and PI3K/Akt signaling pathways. Food Chem. 2021, 335, 127513. [Google Scholar]
- Farias, D.D.; de Araujo, F.F.; Neri-Numa, I.A.; Pastore, G.M. Antidiabetic potential of dietary polyphenols: A mechanistic review. Food Res. Int. 2021, 145, 110383. [Google Scholar]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Tudoreanu, L.; Stefan, G. Plant polyphenols mechanisms of action on insulin resistance and against the loss of pancreatic beta cells. Crit. Rev. Food Sci. Nutr. 2022, 62, 325–352. [Google Scholar]
- Kheirollahzadeh, F.; Eftekhari, E.; Ghollasi, M.; Behzadi, P. Anti-hyperglycemic effects of Eryngium billardierei F. Delaroche extract on insulin-resistance HepG2 cells in vitro. Mol. Biol. Rep. 2022, 49, 3401–3411. [Google Scholar]
- Fu, Y.S.; Liu, X.J.; Ma, Q.; Yi, J.J.; Cai, S.B. Phytochemical bioaccessibility and in vitro antidiabetic effects of Chinese sumac (Rhus chinensis Mill.) fruits after a simulated digestion: Insights into the mechanisms with molecular docking analysis. Int. J. Food Sci. Technol. 2022, 57, 2656–2669. [Google Scholar]
- Alaaeldin, R.; Abdel-Rahman, I.A.M.; Hassan, H.A.; Youssef, N.; Allam, A.E.; Abdelwahab, S.F.; Zhao, Q.L.; Fathy, M. Carpachromene ameliorates insulin resistance in HepG2 cells via modulating IR/IRS1/PI3k/Akt/GSK3/FoxO1 pathway. Molecules 2021, 26, 7629. [Google Scholar]
- Manzano, M.; Giron, M.D.; Vilchez, J.D.; Sevillano, N.; El-Azem, N.; Rueda, R.; Salto, R.; Lopez-Pedrosa, J.M. Apple polyphenol extract improves insulin sensitivity in vitro and in vivo in animal models of insulin resistance. Nutr. Metab. 2016, 13, 1–10. [Google Scholar]
- Zhang, X.T.; Du, L.; Zhang, W.M.; Yang, M.; Chen, L.; Hou, C.; Li, J.K. Pomegranate peel polyphenols alleviate insulin resistance through the promotion of insulin signaling pathway in skeletal muscle of metabolic syndrome rats. Food Sci. Hum. Well. 2022, 11, 1076–1085. [Google Scholar]
- Vyas, P.; Kalidindi, S.; Chibrikova, L.; Igamberdiev, A.U.; Weber, J.T. Chemical analysis and effect of blueberry and Lingonberry fruits and leaves against glutamate-mediated excitotoxicity. J. Agric. Food Chem. 2013, 61, 7769–7776. [Google Scholar]
- Chen, L.; Teng, H.; Cao, H. Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol. 2019, 127, 182–187. [Google Scholar]
- Huang, Q.; Chen, L.; Teng, H.; Song, H.B.; Wu, X.Q.; Xu, M.Y. Phenolic compounds ameliorate the glucose uptake in HepG2 cells' insulin resistance via activating AMPK anti-diabetic effect of phenolic compounds in HepG2 cells. J. Funct. Foods 2015, 19, 487–494. [Google Scholar]
- Rath, P.; Ranjan, A.; Ghosh, A.; Chauhan, A.; Gurnani, M.; Tuli, H.S.; Habeeballah, H.; Alkhanani, M.F.; Haque, S.; Dhama, K.; et al. Potential therapeutic target protein tyrosine phosphatase-1B for modulation of insulin resistance with polyphenols and its quantitative structure-activity relationship. Molecules 2022, 27, 2212. [Google Scholar]
- Morris, C.J.; Della Corte, D. Using molecular docking and molecular dynamics to investigate protein-ligand interactions. Mod. Phys. Lett. B 2021, 35, 2130002. [Google Scholar]
- Tsuji-Hosokawa, A.; Takasawa, K.; Nomura, R.; Miyakawa, Y.; Numakura, C.; Hijikata, A.; Shirai, T.; Ogawa, Y.; Kashimada, K.; Morio, T. Molecular mechanisms of insulin resistance in 2 cases of primary insulin receptor defect-associated diseases. Pediatr. Diabetes 2017, 18, 917–924. [Google Scholar]
- Ganugapati, J.; Baldwa, A.; Lalani, S. Molecular docking studies of banana flower flavonoids as insulin receptor tyrosine kinase activators as a cure for diabetes mellitus. Bioinformation 2012, 8, 216–220. [Google Scholar]
- Paoli, P.; Cirri, P.; Caselli, A.; Ranaldi, F.; Bruschi, G.; Santi, A.; Camici, G. The insulin-mimetic effect of Morin: A promising molecule in diabetes treatment. Biochim. Biophys. Acta. Gen. Subj. 2013, 1830, 3102–3111. [Google Scholar]
- Zheng, T.; Yang, X.; Wu, D.; Xing, S.; Bian, F.; Li, W.; Chi, J.; Bai, X.; Wu, G.; Chen, X.; et al. Salidroside ameliorates insulin resistance through activation of a mitochondria-associated AMPK/PI3K/Akt/GSK3β pathway. Br. J. Pharmacol. 2015, 172, 3284–3301. [Google Scholar]
- Sancho, R.A.S.; Pastore, G.M. Evaluation of the effects of anthocyanins in type 2 diabetes. Food Res. Int. 2012, 46, 378–386. [Google Scholar]
- Dayarathne, L.A.; Ranaweera, S.S.; Natraj, P.; Rajan, P.; Lee, Y.J.; Han, C.-H. The effects of naringenin and naringin on the glucose uptake and AMPK phosphorylation in high glucose treated HepG2 cells. J. Vet. Sci. 2021, 22, e92. [Google Scholar]
- Kim, H.-i.; Ahn, Y.-h. Role of peroxisome proliferator-activated receptor-γ in the glucose-sensing apparatus of liver and β-cells. Diabetes 2004, 53, S60–S65. [Google Scholar]
- Feng, X.J.; Weng, D.; Zhou, F.F.; Owen, Y.D.; Qin, H.H.; Zhao, J.F.; Yu, W.; Huang, Y.H.; Chen, J.J.; Fu, H.J.; et al. Activation of PPAR gamma by a natural flavonoid modulator, apigenin ameliorates obesity-related inflammation via regulation of macrophage polarization. Ebiomedicine 2016, 9, 61–76. [Google Scholar]

| Phenolic Compounds | INSR | PTP1B | PPAR-γ | AMPK |
|---|---|---|---|---|
| Luteolin | 3.2183 | 3.7325 | 3.5430 | 4.3724 |
| Kaempferol-3-O-glucoside | 5.8579 | 5.3547 | - | 7.0769 |
| Kaempferol-3-O-rutinoside | 4.0451 | 6.3401 | - | - |
| Caffeic acid | - | 4.0343 | - | 3.3657 |
| Apigenin | 3.6774 | 3.4906 | 4.6780 | 6.293 |
| Insulin Resistance-Related Proteins | Phenolic Compounds | T-Score | Number of Hydrogen Bonds | Amino Acid Residue | Average Hydrogen Bond Distance (Å) |
|---|---|---|---|---|---|
| INSR | kaempferol-3-O-glucoside | 5.8579 | 7 | Ser1006, Glu1043, Arg1136, Met1153, Asp1150 | 2.055 |
| PTP1B | kaempferol-3-O-rutinoside | 6.3401 | 6 | Arg24, Asp48, Arg254, Gly259 | 2.287 |
| kaempferol-3-O-glucoside | 5.3547 | 8 | Tyr20, Arg24, Ser28, Lys36, Asp48, Gln262 | 2.158 | |
| PPAR-γ | Apigenin | 4.6780 | 3 | Gln410, Gly395 | 1.96 |
| AMPK | Apigenin | 6.2390 | 10 | Lys46, Val48, Gly67, Arg69, Ala70, Tyr164, Ile165, Thr167 | 2.131 |
| kaempferol-3-O-glucoside | 7.0769 | 10 | Lys46, Val48, Ala70, Arg69, Tyr164, Thr167 | 1.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Li, M.; Cai, S.; Zhou, L.; Hu, X.; Yi, J. Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways. Fermentation 2023, 9, 84. https://doi.org/10.3390/fermentation9020084
Wang T, Li M, Cai S, Zhou L, Hu X, Yi J. Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways. Fermentation. 2023; 9(2):84. https://doi.org/10.3390/fermentation9020084
Chicago/Turabian StyleWang, Tao, Meiqi Li, Shengbao Cai, Linyan Zhou, Xiaosong Hu, and Junjie Yi. 2023. "Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways" Fermentation 9, no. 2: 84. https://doi.org/10.3390/fermentation9020084
APA StyleWang, T., Li, M., Cai, S., Zhou, L., Hu, X., & Yi, J. (2023). Polyphenol-Rich Extract of Fermented Chili Pepper Alleviates Insulin Resistance in HepG2 Cells via Regulating INSR, PTP1B, PPAR-γ, and AMPK Pathways. Fermentation, 9(2), 84. https://doi.org/10.3390/fermentation9020084









