Laccase Production from Local Biomass Using Solid State Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Selection of Microorganism
2.2. Media Maintenance
2.3. Preparation of the Fungal Spore
2.4. Inoculum Preparation
2.5. Lignocellulosic Substrates
2.6. Production of Enzymes
2.6.1. Sterilization of Biomass
2.6.2. Inoculation of Substrates
2.6.3. Extraction of Enzymes
2.7. Determination of Enzyme Activity
2.8. Optimization via Response Surface Methodology (RSM)
2.9. Coded and Non-Coded Values for RSM
2.10. Partial Purification via Ammonium Sulphate Precipitation
2.11. Dialysis
2.12. Column Chromatography
2.13. Characterization
2.13.1. Effect of Temperature
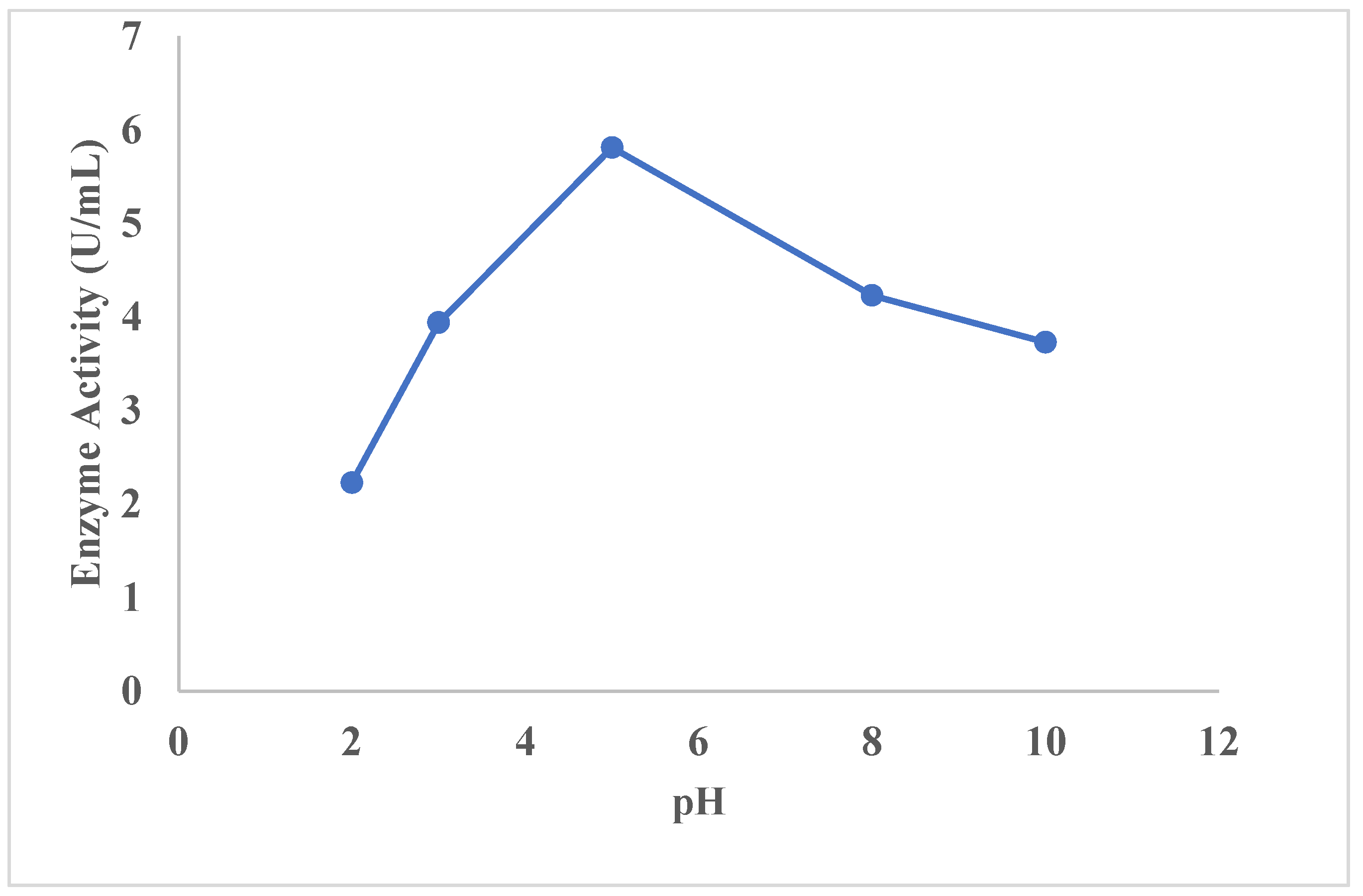
2.13.2. Effect of pH
2.14. Statistical Analysis
3. Results and Discussions
3.1. Screening of Biomass for Laccase Production
Composition of Biomass for Laccase Production
3.2. Optimization through Response Surface Methodology (RSM)
3.2.1. Optimization of Incubation Time
3.2.2. Optimization of Substrate Size and Moisture Level
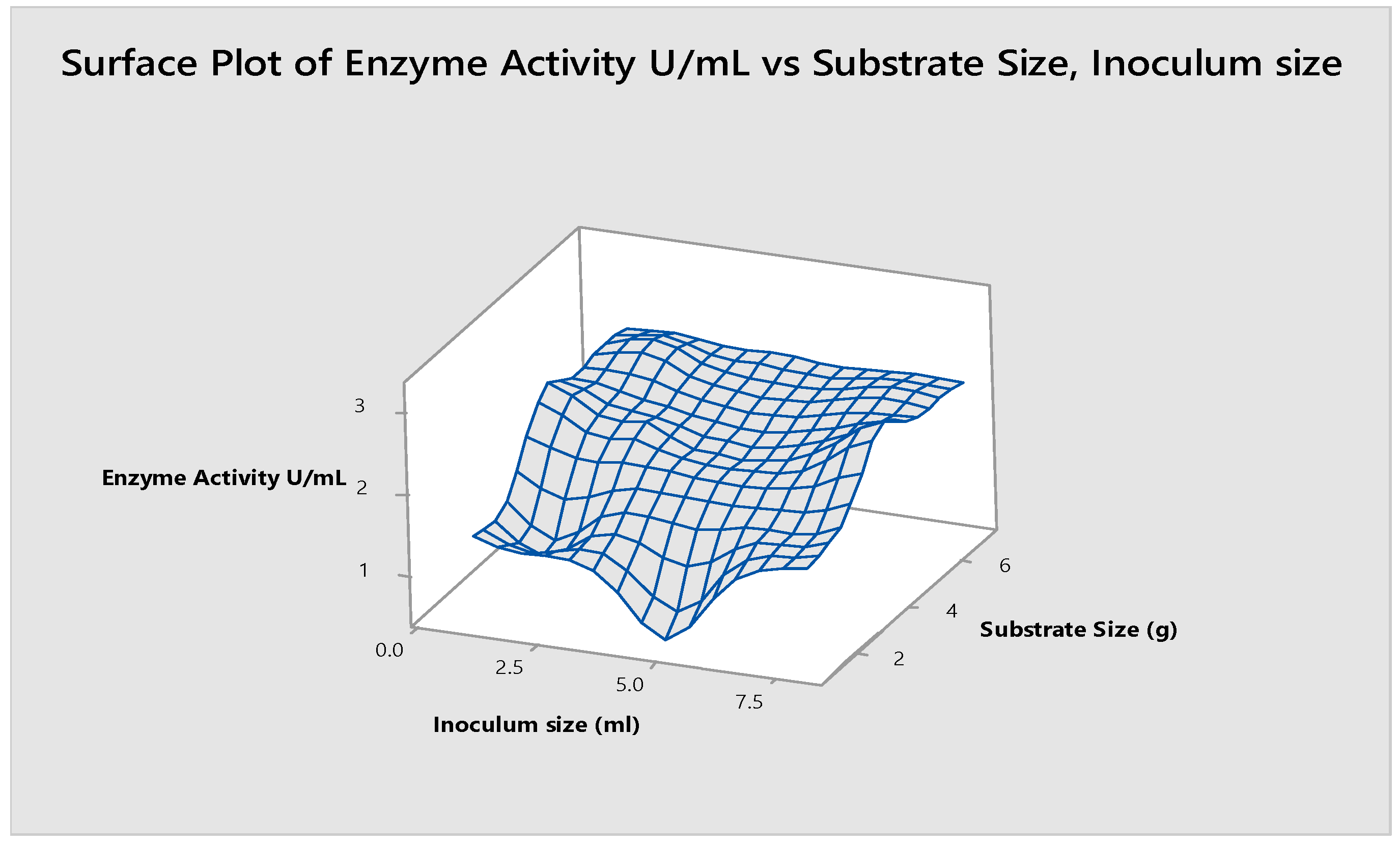
3.2.3. Optimization of Inoculum Size
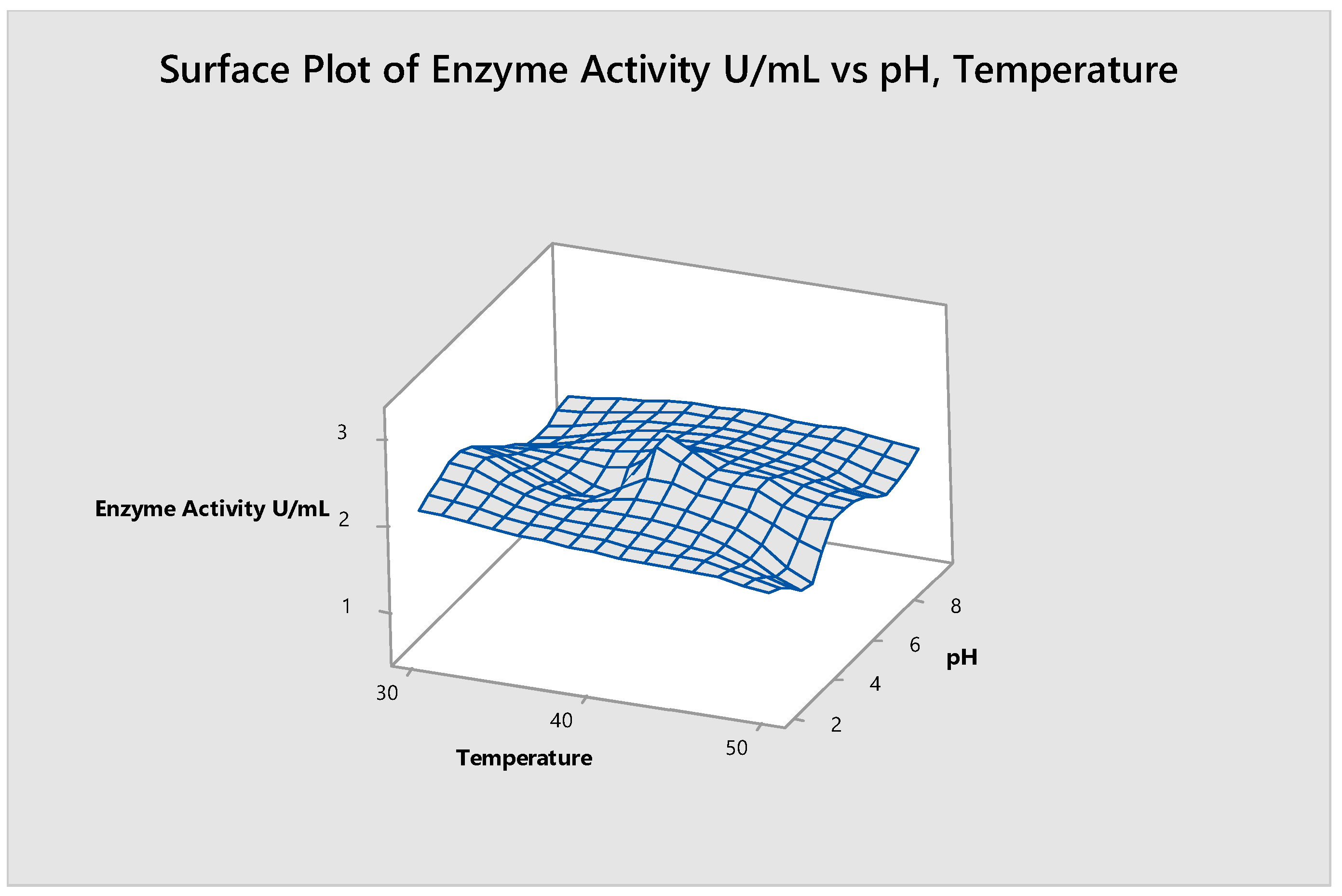
3.2.4. Optimization of Temperature and pH
3.3. Ammonium Sulphate Precipitation
3.4. Statistical Analysis
- (a)
- Substrate Size and pH
- (b)
- Substrate Size and Temperature
- (c)
- Substrate Size and Inoculum Size
- (d)
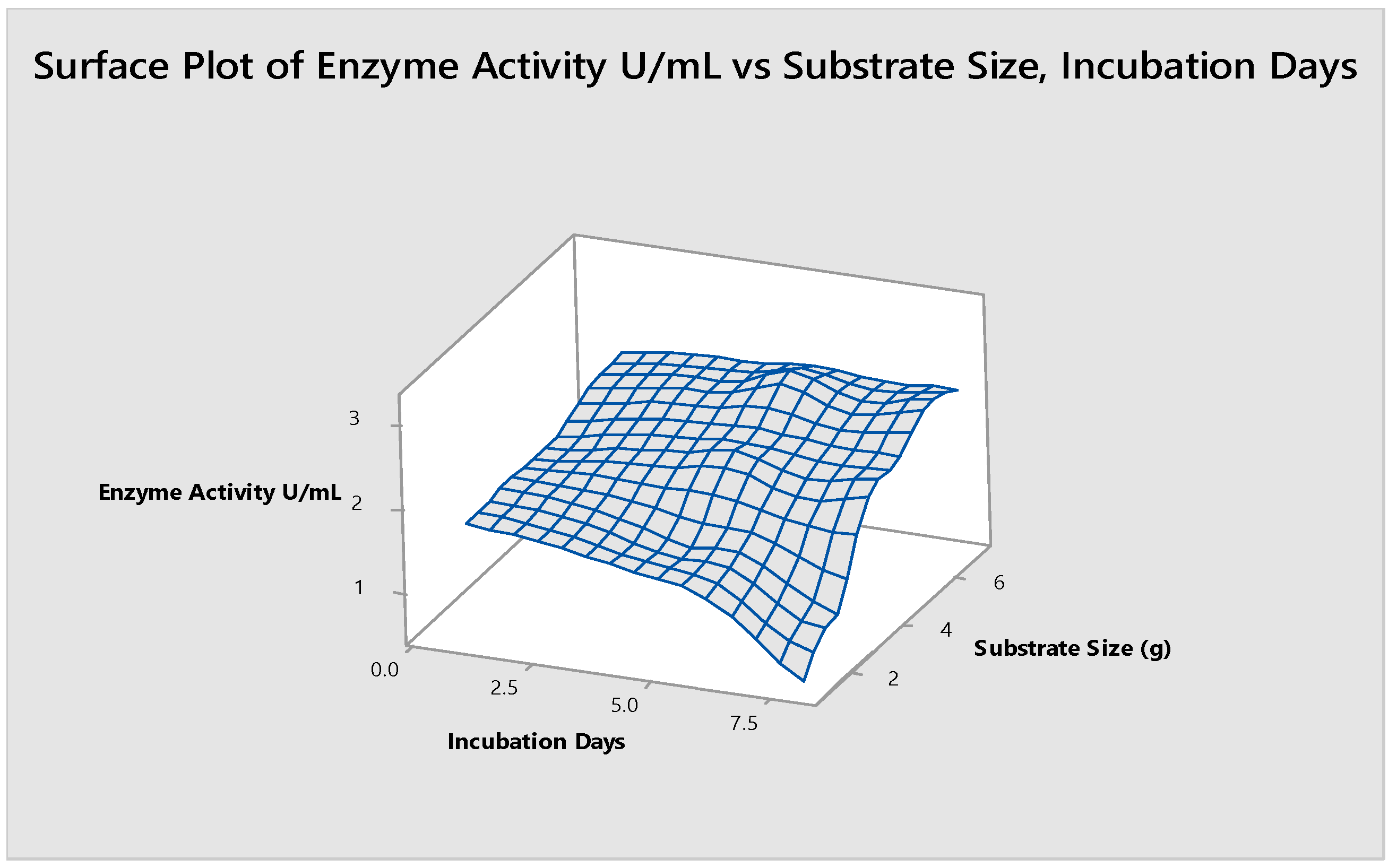
- Substrate Size and Incubation Days
- (e)
- Substrate Size and Moisture Level
- (f)
- pH and Temperature
- (g)
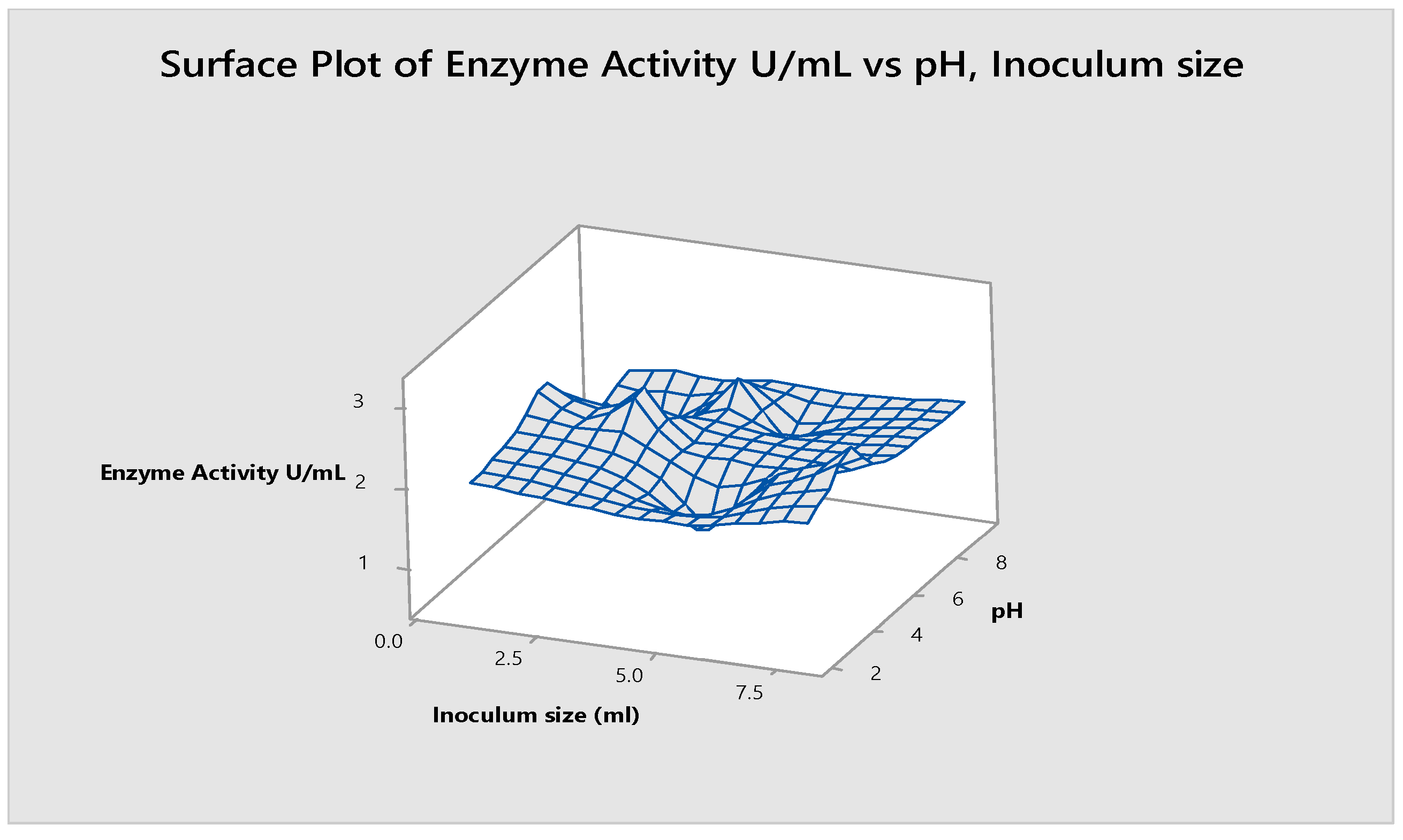
- pH and Inoculum Size
3.5. Substrate Size and pH
3.6. Substrate Size and Temperature
3.7. Substrate Size and Inoculum Size
3.8. Substrate Size and Moisture Level
3.9. Substrate Size and Incubation Days
3.10. pH and Temperature
3.11. pH and Inoculum Size
3.12. Interpretations of Regression Surface Analysis
3.13. Characterization of the Laccase Enzyme
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baldrian, P. Fungal laccases-occurrence and properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, L.; Zhao, L.; Ding, Z.; Ma, H.; Terry, N. Fungal Laccase Production from Lignocellulosic Agricultural Wastes by Solid-State Fermentation: A Review. Microorganisms 2019, 7, 665. [Google Scholar] [CrossRef] [PubMed]
- Statistics Sweden. Design Your Questions Right: How to Develop, Test, Evaluate and Improve Questionnaires 2004. Available online: http://www.scb.se/statistik/_publikationer/OV9999_2004A01_BR_X97OP0402.pdf (accessed on 5 June 2020).
- Kantharaj, P.; Boobalan, B.; Sooriamuthu, S.; Mani, R. Lignocellulose degrading enzymes from fungi and their industrial applications. Int. J. Curr. Res. Rev. 2017, 9, 1. [Google Scholar]
- Hoegger, P.J.; Kilaru, S.; James, T.Y.; Thacker, J.R.; Kües, U. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006, 273, 2308–2326. [Google Scholar] [CrossRef]
- Messerschmidt, A.; Huber, R. The blue oxidases, ascorbate oxidase, laccase and ceruloplasmin. Modelling and structural relationships. Eur. J. Biochem. 1990, 187, 341–352. [Google Scholar] [CrossRef]
- Kunamneni, A.; Ballesteros, A.; Plou, F.J.; Alcade, M. Fungal laccases—A versatile enzyme for biotechnological applications. In Communicating Current Research and Educational Topics and Trends in Applied Microbiology; Mendez-Vilas, A., Ed.; Formatex: Badajoz, Spain, 2007; pp. 233–244. [Google Scholar]
- Bourbonnais, R.; Paice, M.G.; Reid, I.D.; Lanthier, P.; Yaguchi, M. Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azinobis(3-ethylbenzthiazoline-6-sulfonate) in kraft lignin depolymerization. Appl. Environ. Microbiol. 1995, 61, 1876–1880. [Google Scholar] [CrossRef]
- Leontievsky, A.; Myasoedova, N.; Pozdnyakova, N.; Golovleva, L. ‘Yellow’ laccase of Panus tigrinus oxidizes non-phenolic sub-strates without electron-transfer mediators. FEBS Lett. 1997, 413, 446–448. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–267. [Google Scholar] [CrossRef]
- Li, K.; Xu, F.; Eriksson, K.-E.L. Comparison of fungal laccases and redox mediators in oxidation of a nonphenolic lignin model compound. Appl. Environ. Microbiol. 1999, 65, 2654–2660. [Google Scholar] [CrossRef]
- Lenz, J.; Hölker, U.; Höfer, M. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef]
- Castilho, L.R.; Polato, C.M.; Baruque, E.A.; Sant’Anna, G.L., Jr.; Freire, D.M. Economic analysis of lipase production by Penicillium restrictum in solid-state and submerged fermentations. Biochem. Eng. J. 2000, 4, 239–247. [Google Scholar] [CrossRef]
- Viniegra-González, G.; Favela-Torres, E.; Aguilar, C.N.; de Jesus Rómero-Gomez, S.; Dıaz-Godınez, G.; Augur, C. Advantages of fungal enzyme production in solid state over liquid fermentation systems. Biochem. Eng. J. 2003, 13, 157–167. [Google Scholar] [CrossRef]
- Roy, R.V.; Das, M.; Banerjee, R.; Bhowmick, A.K. Comparative studies on rubber biodegradation through solid-state and submerged fermentation. Process. Biochem. 2006, 41, 181–186. [Google Scholar] [CrossRef]
- Forootanfar, H.; Faramarzi, M.A. Insights into laccase producing organisms, fermentation states, purification strategies, and biotechnological applications. Biotechnol. Prog. 2015, 31, 1443–1463. [Google Scholar] [CrossRef] [PubMed]
- Dittmer, N.T.; Suderman, R.J.; Jiang, H.; Zhu, Y.-C.; Gorman, M.J.; Kramer, K.J.; Kanost, M.R. Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 2003, 34, 29–41. [Google Scholar] [CrossRef]
- Palacios-Cabrera, H.; Taniwaki, M.H.; Hashimoto, J.M.; De Menezes, H.C. Growth of Aspergillus ochraceus, A. carbonarius and A. niger on culture media at different water activities and temperatures. Braz. J. Microbiol. 2005, 36, 24–28. [Google Scholar] [CrossRef]
- Arora, D.S.; Sharma, R.K. Ligninolytic Fungal Laccases and Their Biotechnological Applications. Appl. Biochem. Biotechnol. 2009, 160, 1760–1788. [Google Scholar] [CrossRef]
- Majeau, J.-A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef]
- El Monssef, R.A.A.; Hassan, E.; Ramadan, E.M. Production of laccase enzyme for their potential application to decolorize fungal pigments on aging paper and parchment. Ann. Agric. Sci. 2016, 61, 145–154. [Google Scholar] [CrossRef]
- Afreen, S.; Anwer, R.; Singh, R.; Fatma, T. Extracellular laccase production and its optimization from Arthrospira maxima catalyzed decolorization of synthetic dyes. Saudi J. Biol. Sci. 2018, 25, 1446–1453. [Google Scholar] [CrossRef]
- Surwase, S.V.; Patil, S.A.; Srinivas, S.; Jadhav, J.P. Interaction of small molecules with fungal laccase: A Surface Plasmon Resonance based study. Enzym. Microb. Technol. 2016, 82, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Sethi, B.; Satpathy, A.; Tripathy, S.; Parida, S.; Singdevsachan, S.K.; Behera, B. Production of ethanol and clarification of apple juice by pectinase enzyme produced from Aspergillus terreus NCFT 4269.10. Int. J. Biol. Res. 2016, 4, 67. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Biotechnological Transformation of Lignocellulosic Biomass in to Industrial Products: An Overview. Adv. Biosci. Biotechnol. 2016, 07, 149–168. [Google Scholar] [CrossRef]
- Habib, N.; Niaz, M.; Akhtar, S.; Shahid, S. In silico phylogenetic analysis of fungal lipase genes and harnessing the inherent potential of Aspergillus niger IBP2013 for extracellular triglycerol acyl-hydrolase production under solid state fermentation. Pak. J. Bot. 2018, 50, 2019–2029. [Google Scholar]
- Powell, K.A.; Renwick, A.; Peberdy, J.F. (Eds.) The Genus Aspergillus: From Taxonomy and Genetics to Industrial Application; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; Volume 69. [Google Scholar]
- Kalra, K.; Chauhan, R.; Shavez, M.; Sachdeva, S. Isolation of laccase producing Trichoderma spp. and effect of pH and temperature on its activity. Int. J. Chemtech. Res. 2013, 5, 2229–2235. [Google Scholar]
- Yang, J.-T.; Wu, C.-E.; Li, Y.-Y.; Jia, S.-Q.; Fan, G.-J.; Peng, F.-R. Identification and Purification of an Allergic Glycoprotein from Ginkgo biloba Kernel. Agric. Sci. China 2011, 10, 631–641. [Google Scholar] [CrossRef]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Purification and immobilization of laccase from Trichoderma harzianum strain HZN10 and its application in dye decolorization. J. Genet. Eng. Biotechnol. 2017, 15, 139–150. [Google Scholar] [CrossRef]
- Koser, S.; Anwar, Z.; Iqbal, Z.; Anjum, A.; Aqil, T.; Mehmood, S.; Irshad, M. Utilization of Aspergillus oryzae to produce pectin lyase from various agro-industrial residues. J. Radiat. Res. Appl. Sci. 2014, 7, 327–332. [Google Scholar] [CrossRef]
- Seth, M.; Chand, S. Biosynthesis of tannase and hydrolysis of tannins to gallicacid by Aspergillus awamori-optimisation of process parameter. Process Biochem. 2000, 36, 39–44. [Google Scholar] [CrossRef]
- Znidarsic, P.; Pavko, A. The morphology of filamentous fungi in submerged cultivations as a bioprocess parameter. Food Technol Biotechnol. 2001, 39, 237–252. [Google Scholar]
- El-Batal, A.I.; Abd-Algawad, M.H.; Abdelbaky, N.M. Enhancement of some natural antioxidants activity via microbial bio-conversion process using gamma irradiation and incorporation into gold nanoparticles. World Appl. Sci. J. 2012, 19, 1–11. [Google Scholar]
- Revankar, M.S.; Desai, K.M.; Lele, S.S. Solid-state Fermentation for Enhanced Production of Laccase using Indigenously Isolated Ganoderma sp. Appl. Biochem. Biotechnol. 2007, 143, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Carvalheiro, F.; Fernandes, T.; Duarte, L.C.; Gírio, F.M. Wheat Straw Autohydrolysis: Process Optimization and Products Characterization. Appl. Biochem. Biotechnol. 2008, 153, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.X.; Zhao, X.F.S.H.; Sun, R.C. Isolation and characterization of cellulose from sugarcane bagasse. Polym. Degrad. Stab. 2004, 84, 331–339. [Google Scholar] [CrossRef]
- Rubin, E.M. Genomics of cellulosic biofuels. Nature 2008, 454, 841–845. [Google Scholar] [CrossRef]
- Mendes, C.A.D.C.; Adnet, F.A.D.O.; Leite, M.C.A.M.; Furtado, C.R.G.; Sousa, A.M.F.D. Chemical, physical, me-chanical, thermal and morphological characterization of corn husk residue. Cellul. Chem. Technol. 2015, 49, 727–735. [Google Scholar]
- Hemery, Y.; Rouau, X.; Lullien-Pellerin, V.; Barron, C.; Abecassis, J. Dry processes to develop wheat fractions and products with enhanced nutritional quality. J. Cereal Sci. 2007, 46, 327–347. [Google Scholar] [CrossRef]
- Shabbir, H.; Kausar, T.; Noreen, S.; Rehman, H.U.; Hussain, A.; Huang, Q.; Gani, A.; Su, S.; Nawaz, A. In Vivo Screening and Antidiabetic Potential of Polyphenol Extracts from Guava Pulp, Seeds and Leaves. Animals 2020, 10, 1714. [Google Scholar] [CrossRef]
- Saunders, R.M. Rice bran: Composition and potential food uses. Food Rev. Int. 1985, 1, 465–495. [Google Scholar] [CrossRef]
- Sivakumar, R.; Rajendran, R.; Balakumar, C.; Tamilvendan, M. Isolation, screening and optimization of production medium for thermostable laccase production from Ganoderma sp. Int. J. Eng. Sci. Technol. 2010, 2, 7133–7141. [Google Scholar]
- Srinivasan, C.; Dsouza, T.M.; Boominathan, K.; Reddy, C.A. Demonstration of laccase in the white rot basidiomycete Phanerochaetechrysosporium BKM-F1767. Appl. Environ. Microbiol. 1995, 61, 4274–4277. [Google Scholar] [CrossRef] [PubMed]
- Schlosser, D.; Grey, R.; Fritsche, W. Patterns of ligninolytic enzymes in Trametes versicolor. Distribution of extra-and in-tracellular enzyme activities during cultivation on glucose, wheat straw and beech wood. Appl. Microbiol. Biotechnol. 1997, 47, 412–418. [Google Scholar] [CrossRef]
- Elshafei, A.M.; Hassan, M.M.; Haroun, B.M.; Elsayed, M.A.; Othman, A.M. Optimization of Laccase Production from Penicillium martensii NRC 345. Adv. Life Sci. 2012, 2, 31–37. [Google Scholar] [CrossRef]
- Ranimol, G.; Venugopal, T.; Gopalakrishnan, S.; Sunkar, S. Production of laccase from Trichoderma harzianum and its application in dye decolourisation. Biocatal. Agric. Biotechnol. 2018, 16, 400–404. [Google Scholar]
- Shrestha, P.; Joshi, B.; Joshi, J.; Malla, R.; Sreerama, L. Isolation and physicochemical characterization of laccase from Ganoderma lucidum-CDBT1 isolated from its native habitat in Nepal. Biomed. Res. Int. 2016, 2016, 3238909. [Google Scholar] [CrossRef]
- Sumathi, T.; Viswanath, B.; Lakshmi, A.S.; SaiGopal, D.V.R. Production of Laccase by Cochliobolus sp. Isolated from Plastic Dumped Soils and Their Ability to Degrade Low Molecular Weight PVC. Biochem. Res. Int. 2016, 2016, 9519527. [Google Scholar] [CrossRef]
- Khammuang, S.; Sarnthima, R. Laccase activity from fresh fruiting bodies of Ganoderma sp. MK05: Purification and Remazol Brilliant Blue R decolorization. J. Biol. Sci. 2009, 9, 83–87. [Google Scholar] [CrossRef]
- Dhakar, K.; Pandey, A. Laccase Production from a Temperature and pH Tolerant Fungal Strain of Trametes hirsute (MTCC 11397). Enzym. Res. 2013, 2013, 869062. [Google Scholar] [CrossRef]
- Nishizawa, Y.; Nakabayashi, K.; Shinagawa, E. Purification and characterization of laccase from white rot fungus Trametes sanguinea M85-2. J. Ferment. Bioeng. 1995, 80, 91–93. [Google Scholar] [CrossRef]
- Edae, T.; Alemu, M. Selection and optimization of lignocellulosic substrate for laccase production from Pleurotus species. Int. J. Biotechnol. 2017, 8, 38–48. [Google Scholar]
- Pointing, S.B.; Jones, E.B.G.; Vrijmoed, L.L.P. Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 2000, 92, 139–144. [Google Scholar] [CrossRef]
- Lang, E.; Gonser, A.; Zadrazil, F. Influence of incubation temperature on activity of ligninolytic enzymes in sterile soil by Pleurotus sp. and Dichomitus squalens. J. Basic Microb. Int. J. Biochem. Physiol. Genet. Morphol. Ecol. Microorg. 2000, 40, 33–39. [Google Scholar]
- Kumar, R.; Kaur, J.; Jain, S.; Kumar, A. Optimization of laccase production from Aspergillus flavus by design of experiment technique: Partial purification and characterization. J. Genet. Eng. Biotechnol. 2016, 14, 125–131. [Google Scholar] [CrossRef] [PubMed]






| Value Type | Variables | Values | ||||
|---|---|---|---|---|---|---|
| Coded | All Variables | −α | −1 | 0 | +1 | +α |
| Non-coded | pH | 2 | 4 | 5.5 | 8 | 9.5 |
| Temperature (°C) | 30 | 37 | 40 | 45 | 50 | |
| Moisture Level | 20 | 40 | 60 | 80 | 100 | |
| Inoculum Size (mL) | 2 | 4 | 6 | 7 | 8 | |
| Incubation Time | 1 | 3 | 5 | 7 | 9 | |
| Sr # | Substrate Size (g) | pH | Temperature (°C) | Inoculum Size (mL) | Moisture Level (%) | Incubation Time (Days) | Enzyme Activity U/mL Observed | Predicted |
|---|---|---|---|---|---|---|---|---|
| 1 | 4 | 2 | 45 | 5 | 60 | 5 | 1.926 | 2.021 |
| 2 | 4 | 5.5 | 37 | 5 | 60 | 5 | 1.634 | 1.564 |
| 3 | 2 | 5.5 | 45 | 2 | 100 | 5 | 1.218 | 1.224 |
| 4 | 2 | 5.5 | 45 | 7 | 20% | 5 | 1.319 | 1.311 |
| 5 | 6 | 5.5 | 45 | 7 | 100 | 8 | 2.454 | 2.254 |
| 6 | 6 | 5.5 | 45 | 2 | 20 | 5 | 3.206 | 3.328 |
| 7 | 4 | 5.5 | 50 | 4 | 60 | 5 | 1.954 | 1.955 |
| 8 | 6 | 9.5 | 45 | 2 | 100 | 5 | 1.858 | 1.785 |
| 9 | 2 | 8 | 50 | 2 | 20 | 8 | 0.932 | 1.011 |
| 10 | 6 | 4 | 50 | 7 | 20 | 3 | 2.274 | 2.255 |
| 11 | 4 | 5.5 | 45 | 5 | 60 | 5 | 2.336 | 2.345 |
| 12 | 2 | 5.5 | 45 | 7 | 100 | 5 | 1.959 | 1.975 |
| 13 | 4 | 4 | 30 | 8 | 60 | 8 | 2.628 | 2.644 |
| 14 | 4 | 8 | 30 | 3 | 125 | 1 | 1.696 | 1.664 |
| 15 | 4 | 5.5 | 40 | 1 | 60 | 5 | 2.521 | 2.445 |
| 16 | 7 | 8 | 50 | 4 | 60 | 3 | 2.285 | 2.221 |
| 17 | 4 | 8 | 30 | 5 | 50 | 8 | 1.566 | 1.550 |
| 18 | 4 | 4 | 30 | 5 | 60 | 1 | 2.049 | 2.110 |
| 19 | 1 | 4 | 50 | 5 | 60 | 8 | 0.555 | 0.996 |
| 20 | 4 | 5.5 | 45 | 3 | 60 | 5 | 2.701 | 2.785 |
| Purification Step | Volume (mL) | Total Enzyme Activity (U) | Yield (%) | Purification Fold |
|---|---|---|---|---|
| Cell-free Supernatant | 250 | 801.5 | 100 | 1 |
| (NH4)2SO4 Precipitation | 100 | 544.8 | 69.5 | 1.34 |
| Dialysis | 25 | 224.3 | 48.03 | 1.83 |
| Column Chromatography | 10 | 112.4 | 30.72 | 4.07 |
| Analysis of Variance | |||||
|---|---|---|---|---|---|
| Source | DF | Adj SS | Adj MS | F-Value | p-Value |
| Model | 19 | 7.79432 | 0.410228 | 0.000 | 0.000 |
| Linear | 6 | 0.40646 | 0.067744 | 0.000 | 0.000 |
| Substrate Size | 1 | 0.05847 | 0.058473 | 0.000 | 0.000 |
| pH | 1 | 0.01027 | 0.010266 | 0.000 | 0.000 |
| Temperature | 1 | 0.09015 | 0.090151 | 0.000 | 0.000 |
| Inoculum Size | 1 | 0.03451 | 0.034515 | 0.000 | 0.000 |
| Moisture Level | 1 | 0.00796 | 0.007958 | 0.000 | 0.000 |
| Incubation Days | 1 | 0.29379 | 0.293788 | 0.000 | 0.000 |
| Square | 6 | 0.89527 | 0.149212 | 0.000 | 0.000 |
| Substrate Size × Substrate Size | 1 | 0.04984 | 0.049838 | 0.000 | 0.000 |
| pH × pH | 1 | 0.07747 | 0.077475 | 0.000 | 0.000 |
| Temperature × Temperature | 1 | 0.37892 | 0.378921 | 0.000 | 0.000 |
| Inoculum size × Inoculum size | 1 | 0.01497 | 0.014965 | 0.000 | 0.000 |
| Moisture level × Moisture level | 1 | 0.00000 | 0.000000 | 0.000 | 0.000 |
| Incubation Days × Incubation Days | 1 | 0.51024 | 0.510241 | 0.000 | 0.000 |
| 2-Way Interaction | 7 | 1.34118 | 0.191597 | 0.000 | 0.000 |
| Substrate Size × pH | 1 | 0.00540 | 0.005404 | 0.000 | 0.000 |
| Substrate Size × Temperature | 1 | 0.17158 | 0.171578 | 0.000 | 0.000 |
| Substrate Size × Inoculum size | 1 | 0.12129 | 0.121290 | 0.000 | 0.000 |
| Substrate Size × Moisture level | 1 | 0.08448 | 0.084481 | 0.000 | 0.000 |
| Substrate Size × Incubation Days | 1 | 0.00440 | 0.004400 | 0.000 | 0.000 |
| pH × Temperature | 1 | 0.00071 | 0.000711 | 0.000 | 0.000 |
| pH × Inoculum size | 1 | 0.02710 | 0.027096 | 0.000 | 0.000 |
| Error | 0 | ||||
| Total | 19 | 7.79432 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasan, S.; Anwar, Z.; Khalid, W.; Afzal, F.; Zafar, M.; Ali, U.; Refai, M.Y.; Afifi, M.; AL-Farga, A.; Aljobair, M.O. Laccase Production from Local Biomass Using Solid State Fermentation. Fermentation 2023, 9, 179. https://doi.org/10.3390/fermentation9020179
Hasan S, Anwar Z, Khalid W, Afzal F, Zafar M, Ali U, Refai MY, Afifi M, AL-Farga A, Aljobair MO. Laccase Production from Local Biomass Using Solid State Fermentation. Fermentation. 2023; 9(2):179. https://doi.org/10.3390/fermentation9020179
Chicago/Turabian StyleHasan, Shoaib, Zahid Anwar, Waseem Khalid, Fareed Afzal, Muddassar Zafar, Usman Ali, Mohammed Y. Refai, Mohamed Afifi, Ammar AL-Farga, and Moneera O. Aljobair. 2023. "Laccase Production from Local Biomass Using Solid State Fermentation" Fermentation 9, no. 2: 179. https://doi.org/10.3390/fermentation9020179
APA StyleHasan, S., Anwar, Z., Khalid, W., Afzal, F., Zafar, M., Ali, U., Refai, M. Y., Afifi, M., AL-Farga, A., & Aljobair, M. O. (2023). Laccase Production from Local Biomass Using Solid State Fermentation. Fermentation, 9(2), 179. https://doi.org/10.3390/fermentation9020179






