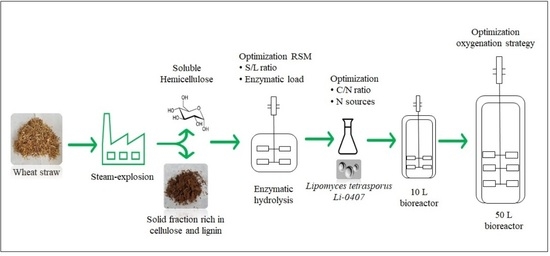
Optimization of Wheat Straw Conversion into Microbial Lipids by Lipomyces tetrasporus DSM 70314 from Bench to Pilot Scale
Abstract
1. Introduction
2. Materials and Methods
2.1. Feedstock Characterization
2.2. Feedstock and Pre-Treatment
2.3. Optimization of the Enzymatic Hydrolysis Process
2.4. Analytical Methods for Medium Characterization
2.5. Strain Cultivation
2.6. Fermentation Set-Up in Bench Scale
2.7. Fermentation in 10 L Bioreactor
2.8. Fermentation in 50 L Bioreactor
2.9. Lipid Extraction
2.10. Fatty Acid Methyl Esters (FAMEs)
3. Results and Discussion
3.1. Biomass Pre-Treatment and Enzymatic Hydrolysis
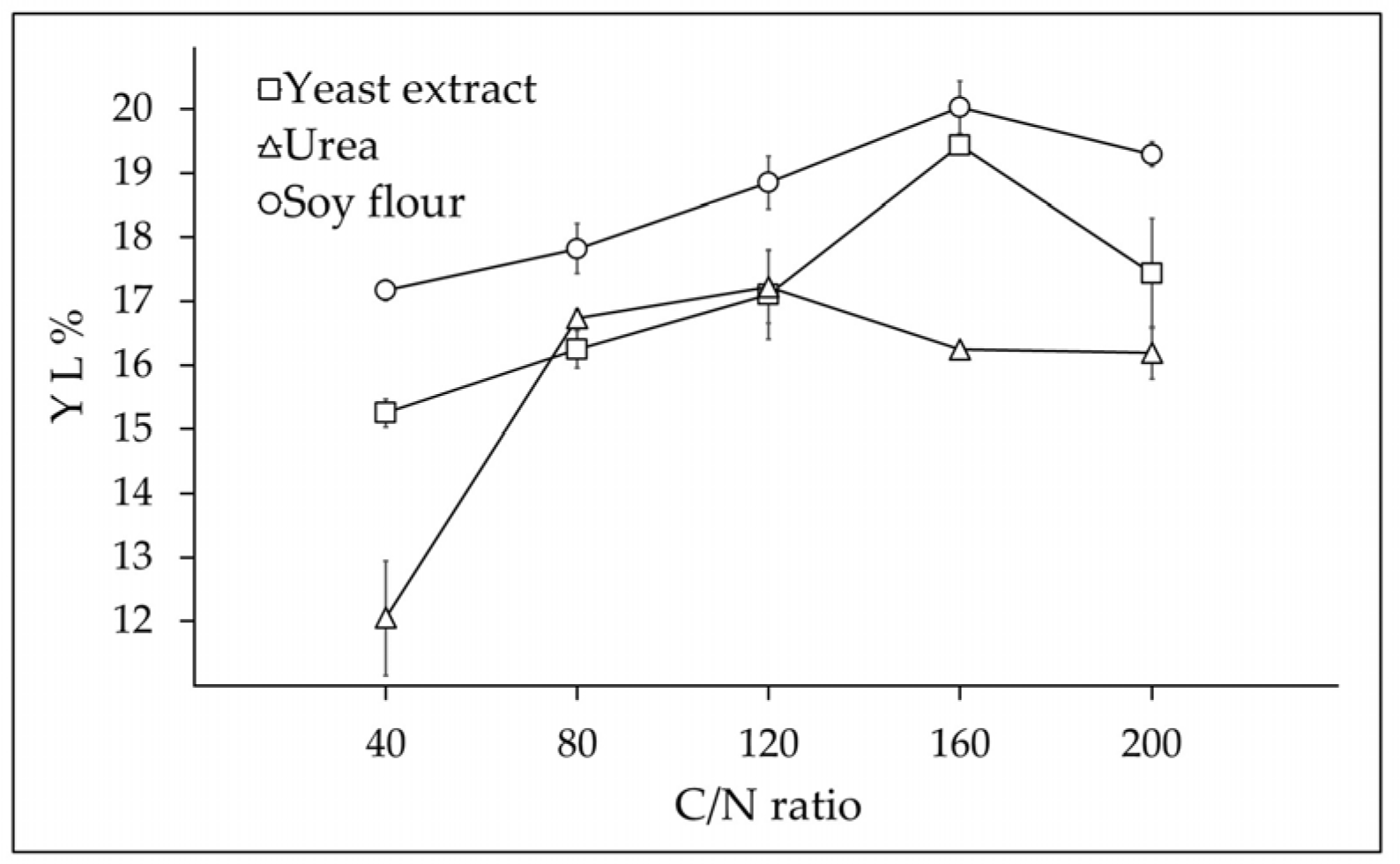
3.2. Optimization of Bench Scale Bioconversion with Three Nitrogen Sources and Five C/N Ratios
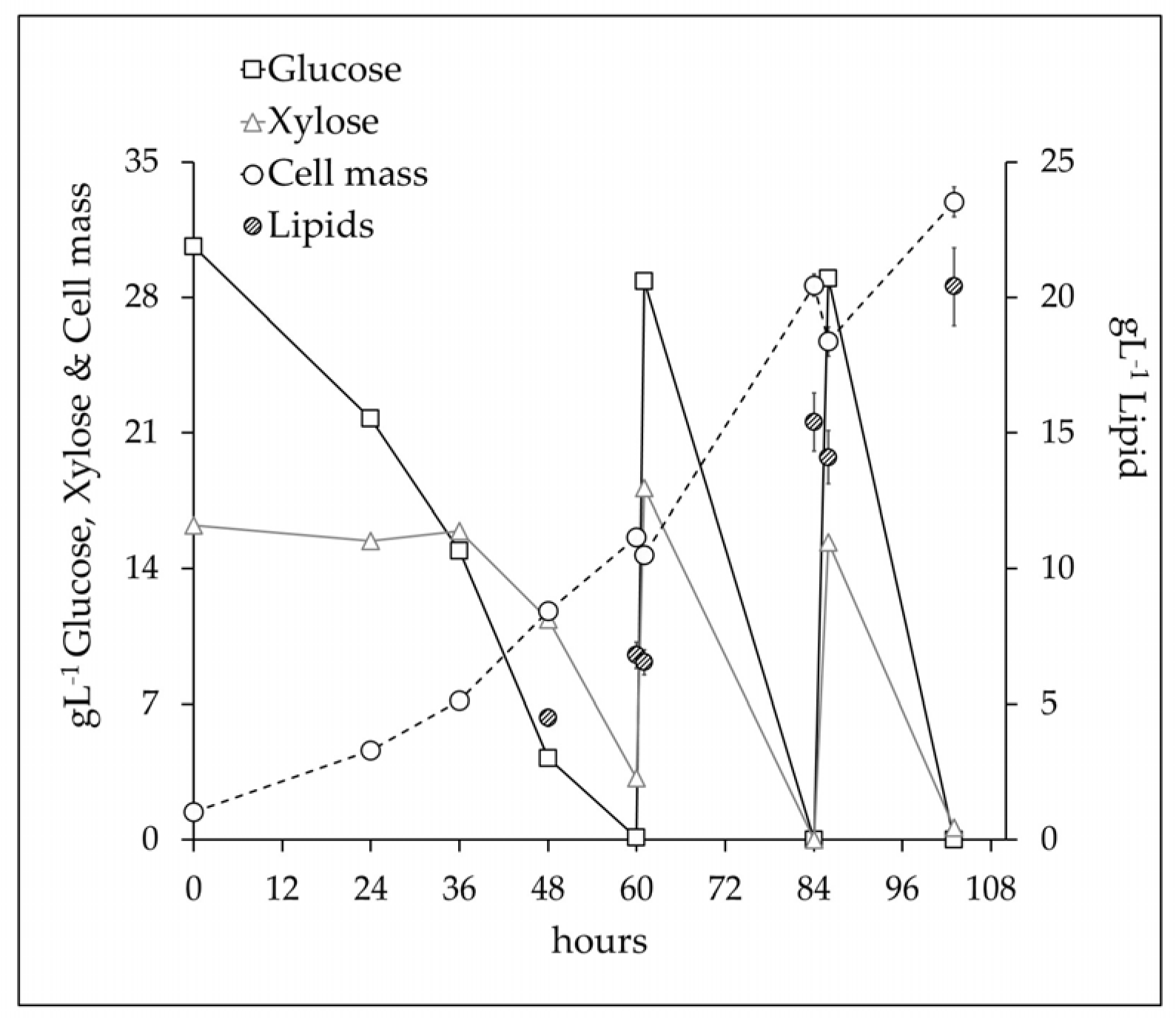
3.3. Scale Up at 10 L Bioreactor
3.4. Scale Up at 50 L Bioreactor
3.5. Lipid Profile
3.6. Mass Balance
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mercure, J.F.; Pollitt, H.; Viñuales, J.E.; Edwards, N.R.; Holden, P.B.; Chewpreecha, U.; Salas, P.; Sognnaes, I.; Lam, A.; Knobloch, F. Macroeconomic Impact of Stranded Fossil Fuel Assets. Nat. Clim. Change 2018, 8, 588–593. [Google Scholar] [CrossRef]
- Bórawski, P.; Bełdycka-Bórawska, A.; Szymańska, E.J.; Jankowski, K.J.; Dubis, B.; Dunn, J.W. Development of Renewable Energy Sources Market and Biofuels in The European Union. J. Clean. Prod. 2019, 228, 467–484. [Google Scholar] [CrossRef]
- Mayr, S.; Hollaus, B.; Madner, V. Palm Oil, the RED II and WTO Law: EU Sustainable Biofuel Policy Tangled up in Green? Rev. Eur. Comp. Int. Environ. Law 2020, 30, 233–248. [Google Scholar] [CrossRef]
- di Fidio, N.; Minonne, F.; Antonetti, C.; Galletti, A.M.R. Cutaneotrichosporon oleaginosus: A Versatile Whole-Cell Biocatalyst for the Production of Single-Cell Oil from Agro-Industrial Wastes. Catalysts 2021, 11, 1291. [Google Scholar] [CrossRef]
- Caporusso, A.; Capece, A.; de Bari, I. Oleaginous Yeasts as Cell Factories for the Sustainable Production of Microbial Lipids by the Valorization of Agri-Food Wastes. Fermentation 2021, 7, 50. [Google Scholar]
- Caporusso, A.; de Bari, I.; Valerio, V.; Albergo, R.; Liuzzi, F. Conversion of Cardoon Crop Residues into Single Cell Oils by Lipomyces tetrasporus and Cutaneotrichosporon curvatus: Process Optimizations to Overcome the Microbial Inhibition of Lignocellulosic Hydrolysates. Ind. Crop. Prod. 2020, 159, 113030. [Google Scholar] [CrossRef]
- Dien, B.S.; Zhu, J.Y.; Slininger, P.J.; Kurtzman, C.P.; Moser, B.R.; O’Bryan, P.J.; Gleisner, R.; Cotta, M.A. Conversion of SPORL Pretreated Douglas Fir Forest Residues into Microbial Lipids with Oleaginous Yeasts. RSC Adv. 2016, 6, 20695–20705. [Google Scholar] [CrossRef]
- Slininger, P.J.; Dien, B.S.; Kurtzman, C.P.; Moser, B.R.; Bakota, E.L.; Thompson, S.R.; O’Bryan, P.J.; Cotta, M.A.; Balan, V.; Jin, M.; et al. Comparative Lipid Production by Oleaginous Yeasts in Hydrolyzates of Lignocellulosic Biomass and Process Strategy for High Titers. Biotechnol. Bioeng. 2016, 113, 1676–1690. [Google Scholar] [CrossRef]
- Yuzbasheva, E.Y.; Scarcia, P.; Yuzbashev, T.V.; Messina, E.; Kosikhina, I.M.; Palmieri, L.; Shutov, A.V.; Taratynova, M.O.; Amaro, R.L.; Palmieri, F.; et al. Engineering Yarrowia lipolytica for the Selective and High-Level Production of Isocitric Acid through Manipulation of Mitochondrial Dicarboxylate–Tricarboxylate Carriers. Metab. Eng. 2020, 65, 156–166. [Google Scholar] [CrossRef]
- Caporusso, A.; Giuliano, A.; Liuzzi, F.; De Bari, I. Techno-Economic Analysis of a Lignocellulosic Biorefinery Producing Microbial Oils by Oleaginous Yeasts. Chem. Eng. Trans. 2022, 92, 637–642. [Google Scholar]
- Carpio, R.R.; Secchi, S.G.; Barros, R.O.; Oliveira, R.A.; Queiroz, S.; Teixeira, R.S.S.; Bon, E.P.S.; Secchi, A.R. Techno-Economic Evaluation of Second-Generation Ethanol from Sugarcane Bagasse: Commercial versus on-Site Produced Enzymes and Use of the Xylose Liquor. J. Clean. Prod. 2022, 369, 133340. [Google Scholar] [CrossRef]
- Donzella, S.; Fumagalli, A.; Arioli, S.; Pellegrino, L.; D’Incecco, P.; Molinari, F.; Speranza, G.; Ubiali, D.; Robescu, M.S.; Compagno, C. Recycling Food Waste and Saving Water: Optimization of the Fermentation Processes from Cheese Whey Permeate to Yeast Oil. Fermentation 2022, 8, 341. [Google Scholar] [CrossRef]
- Ciliberti, C.; Biundo, A.; Colacicco, M.; Agrimi, G.; Pisano, I. lla Physiological Characterisation of Yarrowia lipolytica Cultures Grown on Alternative Carbon Sources to Develop Microbial Platforms for Waste Cooking Oils Valorisation. Chem. Eng. Trans. 2022, 93, 241–246. [Google Scholar]
- Colacicco, M.; Ciliberti, C.; Agrimi, G.; Biundo, A.; Pisano, I. Towards the Physiological Understanding of Yarrowia lipolytica Growth and Lipase Production Using Waste Cooking Oils. Energies 2022, 15, 5217. [Google Scholar] [CrossRef]
- Sarris, D.; Rapti, A.; Papafotis, N.; Koutinas, A.A.; Papanikolaou, S. Production of Added-Value Chemical Compounds through Bioconversions of Olive-Mill Wastewaters Blended with Crude Glycerol by a Yarrowia lipolytica Strain. Molecules 2019, 24, 222. [Google Scholar] [CrossRef]
- di Fidio, N.; Dragoni, F.; Antonetti, C.; de Bari, I.; Raspolli Galletti, A.M.; Ragaglini, G. From Paper Mill Waste to Single Cell Oil: Enzymatic Hydrolysis to Sugars and Their Fermentation into Microbial Oil by the Yeast Lipomyces starkeyi. Bioresour. Technol. 2020, 315, 123790. [Google Scholar] [CrossRef]
- Giacomobono, R.; Albergo, R.; Valerio, V.; Caporusso, A.; de Bari, I. Modelling of the Citric Acid Production from Crude Glycerol by Wild-Type Yarrowia lipolytica DSM 8218 Using Response Surface Methodology (RSM). Life 2022, 12, 621. [Google Scholar]
- Huang, Q.; Kamal, R.; Shen, H.; Lu, H.; Song, J.; Chu, Y.; Xue, C.; Zhao, Z.K. Pilot-Scale Conversion of Corn Stover into Lipids by the Red Yeast Rhodosporidium toruloides. J. Environ. Chem. Eng. 2022, 10, 108858. [Google Scholar] [CrossRef]
- Tufail, T.; Saeed, F.; Afzaal, M.; Ain, H.B.U.; Gilani, S.A.; Hussain, M.; Anjum, F.M. Wheat Straw: A Natural Remedy against Different Maladies. Food Sci. Nutr. 2021, 9, 2335–2344. [Google Scholar] [CrossRef]
- de Bari, I.; Giuliano, A.; Petrone, M.T.; Stoppiello, G.; Fatta, V.; Giardi, C.; Razza, F.; Novelli, A. From Cardoon Lignocellulosic Biomass to Bio-1,4 Butanediol: An Integrated Biorefinery Model. Processes 2020, 8, 1585. [Google Scholar] [CrossRef]
- Bonatsos, N.; Marazioti, C.; Moutousidi, E.; Anagnostou, A.; Koutinas, A.; Kookos, I.K. Techno-Economic Analysis and Life Cycle Assessment of Heterotrophic Yeast-Derived Single Cell Oil Production Process. Fuel 2020, 264, 116839. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, J.; Bao, J. Cost Evaluation of Cellulase Enzyme for Industrial-Scale Cellulosic Ethanol Production Based on Rigorous Aspen Plus Modeling. Bioprocess Biosyst. Eng. 2016, 39, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Rosales-Calderon, O.; Arantes, V. A Review on Commercial-Scale High-Value Products That Can Be Produced alongside Cellulosic Ethanol. Biotechnol. Biofuels 2019, 12, 1–58. [Google Scholar]
- Duque, A.; Manzanares, P.; Ballesteros, I.; Ballesteros, M. Steam Explosion as Lignocellulosic Biomass Pretreatment. In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Guarneros-Flores, J.; Aguilar-Uscanga, M.G.; Morales-Martínez, J.L.; López-Zamora, L. Maximization of Fermentable Sugar Production from Sweet Sorghum Bagasse (Dry and Wet Bases) Using Response Surface Methodology (RSM). Biomass- Convers. Biorefinery 2019, 9, 633–639. [Google Scholar] [CrossRef]
- Hasan Ba Hamid, H.S.; Ku Ismail, K.S. Optimization of Enzymatic Hydrolysis for Acid Pretreated Date Seeds into Fermentable Sugars. Biocatal. Agric. Biotechnol. 2020, 24, 101530. [Google Scholar] [CrossRef]
- Chen, L.; Wei, Y.; Shi, M.; Li, Z.; Zhang, S.H. Statistical Optimization of a Cellulase from Aspergillus glaucus CCHA for Hydrolyzing Corn and Rice Straw by RSM to Enhance Yield of Reducing Sugar. Biotechnol. Lett. 2020, 42, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Sluiter, A.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass; NREL: Golden, CO, USA, 2004. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D.L.A.P. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; NREL: Golden, CO, USA, 2008. [Google Scholar]
- Chen, J.; Wang, X.; Zhang, B.; Yang, Y.; Song, Y.; Zhang, F.; Liu, B.; Zhou, Y.; Yi, Y.; Shan, Y.; et al. Integrating Enzymatic Hydrolysis into Subcritical Water Pretreatment Optimization for Bioethanol Production from Wheat Straw. Sci. Total. Environ. 2021, 770, 145321. [Google Scholar] [CrossRef]
- de Bari, I.; Liuzzi, F.; Villone, A.; Braccio, G. Hydrolysis of Concentrated Suspensions of Steam Pretreated Arundo Donax. Appl. Energy 2013, 102, 179–189. [Google Scholar] [CrossRef]
- Jakobsson, E. Optimization of the Pretreatment of Wheat Straw for Production of Bioethanol; Lund University: Lund, Sweden, 2002. [Google Scholar]
- Ballesteros, I.; Negro, M.J.; Oliva, J.M.; Cabañas, A.; Manzanares, P.; Ballesteros, M. Ethanol Production from Steam-Explosion Pretreated Wheat Straw. Proc. Appl. Biochem. Biotechnol. 2006, 130, 496–508. [Google Scholar] [CrossRef]
- Irfan, M.; Gulsher, M.; Abbas, S.; Syed, Q.; Nadeem, M.; Baig, S. Effect of Various Pretreatment Conditions on Enzymatic Saccharification. Songklanakarin J. Sci. Technol. 2011, 33, 397–404. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride–Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef]
- Liuzzi, F.; Mastrolitti, S.; de Bari, I. Hydrolysis of Corn Stover by Talaromyces cellulolyticus Enzymes: Evaluation of the Residual Enzymes Activities Through the Process. Appl. Biochem. Biotechnol. 2019, 188, 690–705. [Google Scholar] [CrossRef] [PubMed]
- de Bari, I.; Liuzzi, F.; Ambrico, A.; Trupo, M. Arundo Donax Refining to Second Generation Bioethanol and Furfural. Processes 2020, 8, 1591. [Google Scholar] [CrossRef]
- Crognale, S.; Liuzzi, F.; D’Annibale, A.; de Bari, I.; Petruccioli, M. Cynara Cardunculus a Novel Substrate for Solid-State Production of Aspergillus tubingensis Cellulases and Sugar Hydrolysates. Biomass Bioenergy 2019, 127, 105276. [Google Scholar] [CrossRef]
- Rodrigues, A.C.; Haven, M.Ø.; Lindedam, J.; Felby, C.; Gama, M. Celluclast and Cellic® CTec2: Saccharification/Fermentation of Wheat Straw, Solid-Liquid Partition and Potential of Enzyme Recycling by Alkaline Washing. Enzym. Microb. Technol. 2015, 79–80, 70–77. [Google Scholar] [CrossRef]
- Horn, S.J.; Nguyen, Q.D.; Westereng, B.; Nilsen, P.J.; Eijsink, V.G.H. Screening of Steam Explosion Conditions for Glucose Production from Non-Impregnated Wheat Straw. Biomass Bioenergy 2011, 35, 4879–4886. [Google Scholar] [CrossRef]
- Alvira, P.; Negro, M.J.; Ballesteros, I.; González, A.; Ballesteros, M. Steam Explosion for Wheat Straw Pretreatment for Sugars Production. Bioethanol 2016, 2, 3. [Google Scholar] [CrossRef]
- Macrelli, S.; Zacchi, G.; Mogensen, J. Techno-Economic Evaluation of 2nd Generation Bioethanol Production from Sugar Cane Bagasse and Leaves Integrated with the Sugar-Based Ethanol Process [Electronic Resource]. Biotechnol. Biofuels 2012, 5, 22. [Google Scholar] [CrossRef]
- Weiss, N.D.; Felby, C.; Thygesen, L.G. Enzymatic Hydrolysis Is Limited by Biomass–Water Interactions at High-Solids: Improved Performance through Substrate Modifications. Biotechnol. Biofuels 2019, 12, 3. [Google Scholar] [CrossRef]
- Du, J.; Liang, J.; Gao, X.; Liu, G.; Qu, Y. Optimization of an Artificial Cellulase Cocktail for High-Solids Enzymatic Hydrolysis of Cellulosic Materials with Different Pretreatment Methods. Bioresour. Technol. 2020, 295, 122272. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kim, J.E.; Kim, J.K.; Lee, S.H.; Yu, J.H.; Kim, K.H. Evaluation of Commercial Cellulase Preparations for the Efficient Hydrolysis of Hydrothermally Pretreated Empty Fruit Bunches. Bioresources 2017, 12, 7840. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Aggelis, G. Lipids of Oleaginous Yeasts. Part I: Biochemistry of Single Cell Oil Production. Eur. J. Lipid Sci. Technol. 2011, 113, 1031–1051. [Google Scholar]
- Madani, M.; Enshaeieh, M.; Abdoli, A. Single Cell Oil and Its Application for Biodiesel Production. Process Saf. Environ. Prot. 2017, 111, 747–756. [Google Scholar] [CrossRef]
- Hansen, M.A.T.; Jørgensen, H.; Laursen, K.H.; Schjoerring, J.K.; Felby, C. Structural and Chemical Analysis of Process Residue from Biochemical Conversion of Wheat Straw (Triticum Aestivum L.) to Ethanol. Biomass- Bioenergy 2013, 56, 572–581. [Google Scholar] [CrossRef]
- Kemppainen, K.; Rommi, K.; Holopainen, U.; Kruus, K. Steam Explosion of Brewer’s Spent Grain Improves Enzymatic Digestibility of Carbohydrates and Affects Solubility and Stability of Proteins. Appl. Biochem. Biotechnol. 2016, 180, 94–108. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Shi, H.; Ding, K.; Zhou, X.; Zhao, G.; Hadiatullah, H. Impact of Steam Explosion Pretreatment of Defatted Soybean Meal on the Flavor of Soy Sauce. LWT 2022, 156, 113034. [Google Scholar] [CrossRef]
- Caporusso, A.; de Bari, I.; Liuzzi, F.; Albergo, R.; Valerio, V.; Viola, E.; Pietrafesa, R.; Siesto, G.; Capece, A. Optimized Conversion of Wheat Straw into Single Cell Oils by Yarrowia lipolytica and Lipomyces tetrasporus and Synthesis of Advanced Biofuels. Renew. Energy 2023, 202, 184–195. [Google Scholar] [CrossRef]
- Zhang, L.; Li, X.; Yong, Q.; Yang, S.T.; Ouyang, J.; Yu, S. Impacts of Lignocellulose-Derived Inhibitors on l-Lactic Acid Fermentation by Rhizopus oryzae. Renew. Energy 2023, 202, 184–195. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, D.H.; Wu, H.; Lou, W.Y.; Zong, M.H. Evaluating the Influence of Inhibitors Present in Lignocellulosic Hydrolysates on the Cell Membrane Integrity of Oleaginous Yeast Trichosporon fermentans by Flow Cytometry. Renew. Energy 2023, 202, 184–195. [Google Scholar] [CrossRef]
- Gong, Z.; Wang, Q.; Shen, H.; Hu, C.; Jin, G.; Zhao, Z.K. Co-Fermentation of Cellobiose and Xylose by Lipomyces starkeyi for Lipid Production. Bioresour. Technol. 2012, 117, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Probst, K.V.; Vadlani, P.v. Single Cell Oil Production by Lipomyces starkeyi: Biphasic Fed-Batch Fermentation Strategy Providing Glucose for Growth and Xylose for Oil Production. Bioresour. Technol. 2012, 117, 20–24. [Google Scholar] [CrossRef]
- Vorapreeda, T.; Thammarongtham, C.; Cheevadhanarak, S.; Laoteng, K. Alternative Routes of Acetyl-CoA Synthesis Identified by Comparative Genomic Analysis: Involvement in the Lipid Production of Oleaginous Yeast and Fungi. Microbiology 2012, 158, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Z.; Zhang, X.; Hu, F.; Ryu, D.D.Y.; Bao, J. Screening of Oleaginous Yeast Strains Tolerant to Lignocellulose Degradation Compounds. Appl. Biochem. Biotechnol. 2009, 159, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Peng, F.; Du, W.; Liu, C.; Liu, D. Effects of Some Inhibitors on the Growth and Lipid Accumulation of Oleaginous Yeast Rhodosporidium toruloides and Preparation of Biodiesel by Enzymatic Transesterification of the Lipid. Bioprocess Biosyst. Eng. 2012, 35, 993–1004. [Google Scholar] [CrossRef]
- Xavier, M.C.A.; Coradini, A.L.V.; Deckmann, A.C.; Franco, T.T. Lipid Production from Hemicellulose Hydrolysate and Acetic Acid by Lipomyces starkeyi and the Ability of Yeast to Metabolize Inhibitors. Biochem. Eng. J. 2017, 118, 11–19. [Google Scholar] [CrossRef]
- Ge, C.; Chen, H.; Mei, T.; Tang, X.; Chang, L.; Gu, Z.; Zhang, H.; Chen, W.; Chen, Y.Q. Application of a ω-3 Desaturase with an Arachidonic Acid Preference to Eicosapentaenoic Acid Production in Mortierella alpina. Front. Bioeng. Biotechnol. 2018, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.I.; Lim, D.H.; Baek, K.S.; Jang, S.S.; Park, B.Y.; Mayakrishnan, V. Production of chitinase from Escherichia fergusonii, chitosanase from Chryseobacterium indologenes, Comamonas koreensis and its application in N-acetylglucosamine production. Int. J. Biol. Macromol. 2018, 112, 1115–1121. [Google Scholar] [CrossRef]
- Mitrović, I.; Tančić-Živanov, S.; Purar, B.; Trivunović, Z.; Mitrović, B. Effect of different carbon and nitrogen sources combination in medium for production of biocontrol agent Trichoderma harzianum. Ratar. Povrt. 2021, 58, 1–6. [Google Scholar] [CrossRef]
- Li, S.; Jin, Z.; Hu, D.; Yang, W.; Yan, Y.; Nie, X.; Lin, J.; Zhang, Q.; Gai, D.; Ji, Y.; et al. Effect of solid-state fermentation with Lactobacillus casei on the nutritional value, isoflavones, phenolic acids and antioxidant activity of whole soybean flour. LWT Food Sci. Technol. 2020, 125, 109264. [Google Scholar] [CrossRef]
- Antonopoulou, I.; Spanopoulos, A.; Matsakas, L. Single cell oil and ethanol production by the oleaginous yeast Trichosporon fermentans utilizing dried sweet sorghum stalks. Renew. Energy 2019, 146, 1609–1617. [Google Scholar] [CrossRef]
- Huang, C.; Wu, H.; Li, R.-F.; Zong, M.-H. Improving lipid production from bagasse hydrolysate with Trichosporon fermentans by response surface methodology. New Biotechnol. 2011, 29, 372–378. [Google Scholar] [CrossRef]
- Annamalai, N.; Sivakumar, N.; Oleskowicz-Popiel, P. Enhanced production of microbial lipids from waste office paper by the oleaginous yeast Cryptococcus curvatus. Fuel 2018, 217, 420–426. [Google Scholar] [CrossRef]
- Lopes, H.J.S.; Bonturi, N.; Kerkhoven, E.J.; Miranda, E.A.; Lahtvee, P.-J. C/N ratio and carbon source-dependent lipid production profiling in Rhodotorula toruloides. Appl. Microbiol. Biotechnol. 2020, 104, 2639–2649. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of Brewers’ Spent Grain for the Production of Lipids by Oleaginous Yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Bohnen, F.; Mehlmer, N.; Brueck, T. Multi-Factorial-Guided Media Optimization for Enhanced Biomass and Lipid Formation by the Oleaginous Yeast Cutaneotrichosporon oleaginosus. Front. Bioeng. Biotechnol. 2019, 7, 54. [Google Scholar] [CrossRef]
- Sitepu, I.R.; Sestric, R.; Ignatia, L.; Levin, D.; German, J.B.; Gillies, L.A.; Almada, L.A.; Boundy-Mills, K.L. Manipulation of culture conditions alters lipid content and fatty acid profiles of a wide variety of known and new oleaginous yeast species. Bioresour. Technol. 2013, 144, 360–369. [Google Scholar] [CrossRef]
- Chopra, J.; Sen, R. Process optimization involving critical evaluation of oxygen transfer, oxygen uptake and nitrogen limitation for enhanced biomass and lipid production by oleaginous yeast for biofuel application. Bioprocess Biosyst. Eng. 2018, 41, 1103–1113. [Google Scholar] [CrossRef]
- Bouchedja, D.N.; Danthine, S.; Kar, T.; Fickers, P.; Sassi, H.; Boudjellal, A.; Blecker, C.; Delvigne, F. pH level has a strong impact on population dynamics of the yeast Yarrowia lipolytica and oil micro-droplets in multiphasic bioreactor. FEMS Microbiol. Lett. 2018, 365, fny173. [Google Scholar] [CrossRef]
- Routledge, S.J. Beyond De-Foaming: The Effects of Antifoams on Bioprocess Productivity. Comput. Struct. Biotechnol. J. 2012, 3, e201210001. [Google Scholar] [CrossRef]
- Calvey, C.H.; Su, Y.-K.; Willis, L.B.; McGee, M.; Jeffries, T.W. Nitrogen limitation, oxygen limitation, and lipid accumulation in Lipomyces starkeyi. Bioresour. Technol. 2016, 200, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Guerreiro, F.; Constantino, A.; Lima-Costa, E.; Raposo, S. A new combined approach to improved lipid production using a strictly aerobic and oleaginous yeast. Eng. Life Sci. 2018, 19, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, J.; Wu, D.; Li, J.; Tyagi, R.D.; Surampalli, R.Y. Economical lipid production from Trichosporon oleaginosus via dissolved oxygen adjustment and crude glycerol addition. Bioresour. Technol. 2018, 273, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Kitcha, S.; Cheirsilp, B. Enhancing Lipid Production from Crude Glycerol by Newly Isolated Oleaginous Yeasts: Strain Selection, Process Optimization, and Fed-Batch Strategy. BioEnergy Res. 2012, 6, 300–310. [Google Scholar] [CrossRef]
- Yen, H.-W.; Liu, Y.X. Application of airlift bioreactor for the cultivation of aerobic oleaginous yeast Rhodotorula glutinis with different aeration rates. J. Biosci. Bioeng. 2014, 118, 195–198. [Google Scholar] [CrossRef] [PubMed]
- Yen, H.-W.; Zhang, Z. Effects of dissolved oxygen level on cell growth and total lipid accumulation in the cultivation of Rhodotorula glutinis. J. Biosci. Bioeng. 2011, 112, 71–74. [Google Scholar] [CrossRef]
- Unrean, P.; Khajeeram, S.; Champreda, V. Combining metabolic evolution and systematic fed-batch optimization for efficient single-cell oil production from sugarcane bagasse. Renew. Energy 2017, 111, 295–306. [Google Scholar] [CrossRef]
- Qiao, K.; Wasylenko, T.M.; Zhou, K.; Xu, P.; Stephanopoulos, G. Lipid production in Yarrowia lipolytica is maximized by engineering cytosolic redox metabolism. Nat. Biotechnol. 2017, 35, 173–177. [Google Scholar] [CrossRef]
- Soccol, C.R.; Neto, C.J.D.; Soccol, V.T.; Sydney, E.B.; da Costa, E.S.F.; Medeiros, A.B.P.; Vandenberghe, L.P.D.S. Pilot scale biodiesel production from microbial oil of Rhodosporidium toruloides DEBB 5533 using sugarcane juice: Performance in diesel engine and preliminary economic study. Bioresour. Technol. 2017, 223, 259–268. [Google Scholar] [CrossRef]
- Saran, S.; Mathur, A.; Dalal, J.; Saxena, R. Process optimization for cultivation and oil accumulation in an oleaginous yeast Rhodosporidium toruloides A29. Fuel 2017, 188, 324–331. [Google Scholar] [CrossRef]
- Banerjee, A.; Sharma, T.; Nautiyal, A.K.; Dasgupta, D.; Hazra, S.; Bhaskar, T.; Ghosh, D. Scale-up strategy for yeast single cell oil production for Rhodotorula mucilagenosa IIPL32 from corn cob derived pentosan. Bioresour. Technol. 2020, 309, 123329. [Google Scholar] [CrossRef] [PubMed]
- Ageitos, J.M.; Vallejo, J.; Veiga-Crespo, P.; Villa, T.G. Oily yeasts as oleaginous cell factories. Appl. Microbiol. Biotechnol. 2011, 90, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Qiao, K.; Abidi, S.H.I.; Liu, H.; Zhang, H.; Chakraborty, S.; Watson, N.; Ajikumar, P.K.; Stephanopoulos, G. Engineering lipid overproduction in the oleaginous yeast Yarrowia lipolytica. Metab. Eng. 2015, 29, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Chi, Z.; Liu, Y.; Frear, C.; Chen, S. Study of a two-stage growth of DHA-producing marine algae Schizochytrium limacinum SR21 with shifting dissolved oxygen level. Appl. Microbiol. Biotechnol. 2009, 81, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Geng, L.J.; Ren, L.J.; Ji, X.J.; Hao, N.; Chen, K.Q.; Huang, H. Influence of Oxygen on the Biosynthesis of Polyun-saturated Fatty Acids in Microalgae. Bioresour. Technol. 2018, 250, 868–876. [Google Scholar] [CrossRef]
- Lu, H.; Chen, H.; Tang, X.; Yang, Q.; Zhang, H.; Chen, Y.Q.; Chen, W. Metabolomics analysis reveals the role of oxygen control in the nitrogen limitation induced lipid accumulation in Mortierella alpina. J. Biotechnol. 2020, 325, 325–333. [Google Scholar] [CrossRef]
- Yu, O.; Kim, K.H. Lignin to Materials: A Focused Review on Recent Novel Lignin Applications. Appl. Sci. 2020, 10, 4626. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Olmedo-Martínez, J.L.; Vega-Rios, A.; Flores-Gallardo, S.; Zaragoza-Contreras, E.A. Lignin in storage and renewable energy applications: A review. J. Energy Chem. 2018, 27, 1422–1438. [Google Scholar] [CrossRef]
- Borsella, E.; De Bari, I.; Colucci, P.; Mastrolitti, S.; Liuzzi, F.; De Stefanis, A.; Valentini, V.; Gallese, F.; Perez, G. Lignin Depolymerization by Catalytic Hydrodeoxygenation Performed with Smectitic Clay-Based Materials. Energy Technol. 2020, 8, 633. [Google Scholar] [CrossRef]
- Hasanly, A.; Talkhoncheh, M.K.; Alavijeh, M.K. Techno-economic assessment of bioethanol production from wheat straw: A case study of Iran. Clean Technol. Environ. Policy 2017, 20, 357–377. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Vick, B.A.; Ebelhar, M.W.; Buehring, N.; Baldwin, B.S.; Astatkie, T.; Miller, J.F. Yield, Oil Content, and Composition of Sunflower Grown at Multiple Locations in Mississippi. Agron. J. 2008, 100, 635–642. [Google Scholar] [CrossRef]
- Fore, S.R.; Porter, P.; Lazarus, W. Net energy balance of small-scale on-farm biodiesel production from canola and soybean. Biomass Bioenergy 2011, 35, 2234–2244. [Google Scholar] [CrossRef]
- Felten, D.; Fröba, N.; Fries, J.; Emmerling, C. Energy balances and greenhouse gas-mitigation potentials of bioenergy cropping systems (Miscanthus, rapeseed, and maize) based on farming conditions in Western Germany. Renew. Energy 2013, 55, 160–174. [Google Scholar] [CrossRef]
- Ozturk, H.H. Energy Analysis for Biodiesel Production from Rapeseed Oil. Energy Explor. Exploit. 2014, 32, 1005–1031. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Kumar, R.R.; Yuvaraj, D.; Jayamuthunagai, J.; Kumar, R.P.; Palani, S. Biodiesel Production Using Chemical and Biological Methods—A Review of Process, Catalyst, Acyl Acceptor, Source and Process Variables. Re-Newable Sustain. Energy Rev. 2014, 38, 368–382. [Google Scholar] [CrossRef]



| Feedstock Characterization % | |
|---|---|
| Glucan | 38.4 ± 3.2 |
| Xylan | 16.7 ± 1.1 |
| Arabinan | 3.1 ± 0.2 |
| Galactan | 1.2 ± 0.1 |
| Lignin | 20.6 ± 1.1 |
| Extractive | 4.3 ± 0.3 |
| Ash | 6.2 ± 0.1 |
| Other | 9.5 ± 3.4 |
| Carbon | 43.6 |
| Hydrogen | 6.4 |
| Nitrogen | 0.2 |
| Oxygen | 49.8 |
| Glucan | Xylan | Arabinan | Galactan | Lignin | Ashes | |
|---|---|---|---|---|---|---|
| Internal composition of the water-insoluble fraction (%) | 47.6 | 3.4 | 0.1 | 0.3 | 29.5 | 5.6 |
| Sugar recovery in the liquid fraction (%) | 2.2 | 87.5 | 37.9 | 64.5 | / | / |
| Run | % S/L | FPU | Glucose Y% |
|---|---|---|---|
| 1 | 20 | 15 | 87.3 |
| 2 | 20 | 10 | 70.1 |
| 3 | 25 | 15 | 73.3 |
| 4 | 25 | 20 | 76.5 |
| 5 | 25 | 10 | 61.3 |
| 6 | 20 | 15 | 86.7 |
| 7 | 15 | 10 | 73.5 |
| 8 | 20 | 15 | 88.0 |
| 9 | 15 | 20 | 90.8 |
| 10 | 15 | 15 | 88.4 |
| 11 | 20 | 20 | 89.4 |
| Source | DF | F-Value | p-Value (Prob > F) | |
|---|---|---|---|---|
| Model | 5 | 117.21 | <0.0001 | significant |
| A-SL | 1 | 173.55 | <0.0001 | |
| B-FPU | 1 | 269.10 | <0.0001 | |
| AB | 1 | 0.66 | 0.4524 | |
| A2 | 1 | 42.73 | 0.0013 | |
| B2 | 1 | 62.34 | 0.0005 | |
| Residual | 5 | |||
| Lack of Fit | 3 | 5.88 | 0.1489 | not significant |
| Pure Error | 2 | |||
| Cor Total | 10 |
| Glucose gL−1 | Xylose gL−1 | Galactose gL−1 | Arabinose gL−1 | A.a gL−1 | 5-HMF gL−1 | Furfural gL−1 | Phenols gL−1 | |
|---|---|---|---|---|---|---|---|---|
| WSH | 86.4 ± 0.9 | 16.2 ± 0.7 | 0.9 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.2 | 0.14 | 0.71 | 5.0 |
| HEM | 0.98 ± 0.1 | 16.4 ± 0.9 | 0.7 ± 0.1 | 1.4 ± 0.1 | 1.9 ± 0.3 | 0.15 | 0.72 | 5.3 |
| WSH/HEM | 30.3 ± 0.7 | 16.3 ± 0.9 | 0.9 ± 0.1 | 1.7 ± 0.1 | 2.3 ± 0.2 | 0.15 | 0.72 | 5.2 |
| 1X gL−1 | 2Y X % w/w | X/h gL−1h−1 | 1L gL−1 | 2Y L % w/w | 3I L/X % w/w | |
|---|---|---|---|---|---|---|
| Y 40 | 16.6 ± 0.6 a | 41.3 ± 0.9 a | 0.13 ± 0.01 a | 6.1 ± 0.2 a | 15.3 ± 0.2 a | 36.9 ± 0.1 |
| S 40 | 18.3 ± 0.6 a | 43.1 ± 2.0 a | 0.11 ± 0.01 a | 7.3 ± 0.1 a | 17.2 ± 0.2 a | 39.8 ± 1.0 |
| U 40 | 13.4 ± 0.6 b | 33.2 ± 0.9 b | 0.04 ± 0.01 b | 4.9 ± 0.3 b | 12.1 ± 0.9 b | 36.3 ± 3.0 |
| Y 80 | 15.0 ± 0.3 a | 36.9 ± 0.7 a | 0.08 ± 0.01 a | 6.6 ± 0.1 a | 16.3 ± 0.3 a | 44.0 ± 0.1 a |
| S 80 | 16.8 ± 0.5 b | 40.2 ± 0.2 b | 0.10 ± 0.01 b | 7.5 ± 0.1 b | 17.8 ± 0.4 b | 44.3 ± 1.0 a |
| U 80 | 11.8 ± 0.3 c | 28.8 ± 0.9 c | 0.05 ± 0.01 c | 6.9 ± 0.1 a | 16.7 ± 0.1 ab | 58.1 ± 1.0 b |
| Y 120 | 14.7 ± 0.1 a | 34.7 ± 0.4 a | 0.08 ± 0.01 a | 7.2 ± 0.2 | 17.1 ± 0.7 | 49.3 ± 1.0 a |
| S 120 | 15.6 ± 0.6 a | 37.0 ± 1.0 a | 0.08 ± 0.01 a | 7.9 ± 0.2 | 18.9 ± 0.4 | 50.9 ± 0.7 ab |
| U 120 | 12.5 ± 0.4 b | 29.3 ± 0.5 b | 0.06 ± 0.01 b | 7.3 ± 0.2 | 17.2 ± 0.6 | 58.8 ± 3.0 b |
| Y 160 | 13.8 ± 0.3 a | 33.9 ± 1.0 a | 0.10 ± 0.01 a | 7.9 ± 0.1 a | 19.4 ± 0.1 a | 57.3 ± 2.0 a |
| S 160 | 15.3 ± 0.4 a | 36.9 ± 3.0 ab | 0.08 ± 0.01 b | 8.3 ± 0.2 a | 20.0 ± 0.4 ab | 54.3 ± 0.1 ab |
| U 160 | 10.7 ± 0.2 b | 26.1 ± 1.0 ac | 0.05 ± 0.01 c | 6.7 ± 0.1 b | 16.3 ± 0.1 ac | 62.4 ± 2.0 ac |
| Y 200 | 13.6 ± 0.1 a | 32.9 ± 0.9 a | 0.09 ± 0.01 a | 7.2 ± 0.1 a | 17.4 ± 0.9 a | 53.0 ± 1.0 |
| S 200 | 15.5 ± 0.4 b | 36.4 ± 0.2 b | 0.07 ± 0.01 b | 8.2 ± 0.2 b | 19.3 ± 0.2 ab | 53.1 ± 0.2 |
| U 200 | 12.3 ± 0.3 c | 29.8 ± 0.4 c | 0.06 ± 0.01 c | 6.7 ± 0.3 a | 16.2 ± 0.4 ac | 54.3 ± 0.7 |
| F Value | ||||||
|---|---|---|---|---|---|---|
| 1 df | X | YX | L | YL | IL/X | |
| N source: | 2 | 273.5 *** | 152.2 *** | 187 *** | 62.19 ** | 39.02 ** |
| C/N ratio: | 4 | 42.07 ** | 30.48 ** | 90.69 *** | 34.69 ** | 128.2 *** |
| Interaction: | 8 | 3.371 * | 2.177 * | 15.3 ** | 5.706 * | 10.33 ** |
| C12:0 | C14:0 | C16:0 | C16:1 | C18:0 | C18:1 | C18:2 | C18:3 | C20:0 | C22:0 | C24:0 | 1 Sat | 2 Unsat | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Y 40 | 0.49 ± 0.03 | 0.66 ± 0.01 | 29.60 ± 0.90 | 2.36 ± 0.02 | 20.15 ± 0.70 | 43.72 ± 0.80 | 1.10 ± 0.02 | 0.04 ± 0.01 | 0.59 ± 0.10 | 0.41 ± 0.07 | 0.88 ± 0.07 | 52.78 ± 1.00 | 47.22 ± 0.80 |
| Y 80 | 0.55 ± 0.02 | 0.68 ± 0.02 | 33.13 ± 0.90 | 1.59 ± 0.01 | 23.67 ± 0.20 | 37.50 ± 0.90 | 0.90 ± 0.01 | - | 0.83 ± 0.07 | 1.15 ± 0.15 | - | 60.01 ± 0.90 | 39.99 ± 0.90 |
| Y 120 | - | - | 31.10 ± 1.00 | 2.07 ± 0.03 | 21.50 ± 0.50 | 43.41 ± 0.70 | 1.04 ± 0.01 | - | - | - | 0.89 ± 0.08 | 53.49 ± 1.00 | 46.51 ± 0.70 |
| Y 160 | 0.39 ± 0.01 | 0.64 ± 0.01 | 31.88 ± 1.00 | 2.15 ± 0.01 | 19.60 ± 0.70 | 41.98 ± 0.90 | 1.11 ± 0.02 | - | 0.71 ± 0.15 | 0.49 ± 0.04 | 1.05 ± 0.09 | 54.76 ± 1.00 | 45.24 ± 0.90 |
| Y 200 | 0.39 ± 0.01 | 0.63 ± 0.02 | 31.60 ± 1.00 | 2.06 ± 0.01 | 20.66 ± 0.20 | 42.49 ± 1.00 | 1.03 ± 0.01 | - | 0.72 ± 0.10 | 0.42 ± 0.05 | - | 54.42 ± 1.00 | 45.58 ± 1.00 |
| U 40 | - | - | 32.54 ± 0.70 | 2.04 ± 0.01 | 19.90 ± 0.40 | 44.10 ± 0.70 | 1.40 ± 0.01 | 0.03 ± 0.01 | - | - | - | 52.43 ± 0.80 | 47.57 ± 0.70 |
| U 80 | 0.41 ± 0.01 | 0.60 ± 0.01 | 30.92 ± 0.70 | 2.14 ± 0.01 | 19.77 ± 0.60 | 43.22 ± 0.90 | 0.88 ± 0.01 | - | 0.68 ± 0.09 | 0.42 ± 0.06 | 0.94 ± 0.05 | 52.81 ± 0.90 | 47.19 ± 0.90 |
| U 120 | 0.35 ± 0.03 | 0.49 ± 0.01 | 31.77 ± 0.50 | 2.23 ± 0.01 | 19.61 ± 0.90 | 42.34 ± 1.00 | 0.91 ± 0.02 | - | 0.67 ± 0.05 | 0.50 ± 0.05 | 1.12 ± 0.06 | 54.51 ± 1.00 | 45.49 ± 1.00 |
| U 160 | 0.44 ± 0.01 | 0.69 ± 0.01 | 32.08 ± 0.30 | 2.19 ± 0.01 | 19.76 ± 0.60 | 41.72 ± 0.50 | 0.92 ± 0.01 | - | 0.65 ± 0.07 | 0.44 ± 0.04 | 1.10 ± 0.06 | 55.17 ± 0.70 | 44.83 ± 0.50 |
| U 200 | 0.41 ± 0.01 | 0.65 ± 0.01 | 31.99 ± 0.80 | 2.27 ± 0.01 | 19.89 ± 0.30 | 41.67 ± 0.60 | 0.92 ± 0.01 | - | 0.71 ± 0.04 | 0.43 ± 0.04 | 1.05 ± 0.06 | 55.14 ± 0.90 | 44.86 ± 0.60 |
| S 40 | 0.41 ± 0.02 | 0.56 ± 0.01 | 21.89 ± 0.10 | 5.56 ± 0.03 | 18.83 ± 0.90 | 45.09 ± 0.50 | 2.29 ± 0.01 | 0.11 ± 0.01 | 4.27 ± 0.03 | 0.39 ± 0.04 | 0.59 ± 0.04 | 46.95 ± 0.90 | 53.05 ± 0.50 |
| S 80 | 0.38 ± 0.01 | 0.66 ± 0.02 | 24.98 ± 0.50 | 5.93 ± 0.02 | 18.09 ± 0.50 | 45.81 ± 0.80 | 2.42 ± 0.02 | - | 0.73 ± 0.03 | 0.43 ± 0.05 | 0.55 ± 0.07 | 45.84 ± 0.70 | 54.16 ± 0.80 |
| S 120 | 0.50 ± 0.02 | 0.68 ± 0.01 | 20.73 ± 0.40 | 7.12 ± 0.03 | 16.80 ± 0.60 | 49.72 ± 0.90 | 2.59 ± 0.01 | - | 0.70 ± 0.09 | 0.40 ± 0.04 | 0.75 ± 0.04 | 40.57 ± 0.80 | 59.43 ± 0.90 |
| S 160 | 0.49 ± 0.01 | 0.72 ± 0.02 | 20.80 ± 1.00 | 6.43 ± 0.03 | 19.58 ± 0.20 | 47.70 ± 1.00 | 2.40 ± 0.01 | - | 0.62 ± 0.12 | 0.40 ± 0.03 | 0.84 ± 0.05 | 43.47 ± 1.00 | 56.53 ± 1.00 |
| S 200 | 0.39 ± 0.01 | 0.70 ± 0.01 | 20.49 ± 1.00 | 5.56 ± 0.03 | 20.53 ± 0.90 | 47.83 ± 1.00 | 2.44 ± 0.02 | - | 0.64 ± 0.10 | 0.46 ± 0.04 | 0.94 ± 0.09 | 44.17 ± 1.00 | 55.83 ± 1.00 |
| 10 L | 0.80 ± 0.01 | 0.34 ± 0.01 | 26.95 ± 0.70 | 4.14 ± 0.01 | 6.59 ± 0.60 | 55.93 ± 0.80 | 5.24 ± 0.01 | - | - | - | - | 34.69 ± 0.90 | 65.31 ± 0.80 |
| 50 L | - | - | 26.70 ± 0.80 | 3.38 ± 0.02 | 7.65 ± 0.90 | 57.20 ± 0.70 | 3.42 ± 0.01 | - | 0.71 ± 0.01 | 0.49 ± 0.04 | 0.45 ± 0.09 | 35.99 ± 1.00 | 64.01 ± 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caporusso, A.; De Bari, I.; Giuliano, A.; Liuzzi, F.; Albergo, R.; Pietrafesa, R.; Siesto, G.; Romanelli, A.; Braccio, G.; Capece, A. Optimization of Wheat Straw Conversion into Microbial Lipids by Lipomyces tetrasporus DSM 70314 from Bench to Pilot Scale. Fermentation 2023, 9, 180. https://doi.org/10.3390/fermentation9020180
Caporusso A, De Bari I, Giuliano A, Liuzzi F, Albergo R, Pietrafesa R, Siesto G, Romanelli A, Braccio G, Capece A. Optimization of Wheat Straw Conversion into Microbial Lipids by Lipomyces tetrasporus DSM 70314 from Bench to Pilot Scale. Fermentation. 2023; 9(2):180. https://doi.org/10.3390/fermentation9020180
Chicago/Turabian StyleCaporusso, Antonio, Isabella De Bari, Aristide Giuliano, Federico Liuzzi, Roberto Albergo, Rocchina Pietrafesa, Gabriella Siesto, Assunta Romanelli, Giacobbe Braccio, and Angela Capece. 2023. "Optimization of Wheat Straw Conversion into Microbial Lipids by Lipomyces tetrasporus DSM 70314 from Bench to Pilot Scale" Fermentation 9, no. 2: 180. https://doi.org/10.3390/fermentation9020180
APA StyleCaporusso, A., De Bari, I., Giuliano, A., Liuzzi, F., Albergo, R., Pietrafesa, R., Siesto, G., Romanelli, A., Braccio, G., & Capece, A. (2023). Optimization of Wheat Straw Conversion into Microbial Lipids by Lipomyces tetrasporus DSM 70314 from Bench to Pilot Scale. Fermentation, 9(2), 180. https://doi.org/10.3390/fermentation9020180