Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium toruloides G17
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Inoculum Preparation
2.3. Optimization of Growth Conditions
2.4. Determination of Reducing Sugar Content
2.5. Determination of Astaxanthin Content
2.6. Astaxanthin Purification
2.7. Antioxidant Activity Determination
2.8. Cell Culture
2.9. MTT Assay
2.10. Statistical Analysis
3. Results and Discussion
3.1. RSM Model for The Optimization of Culture Conditions
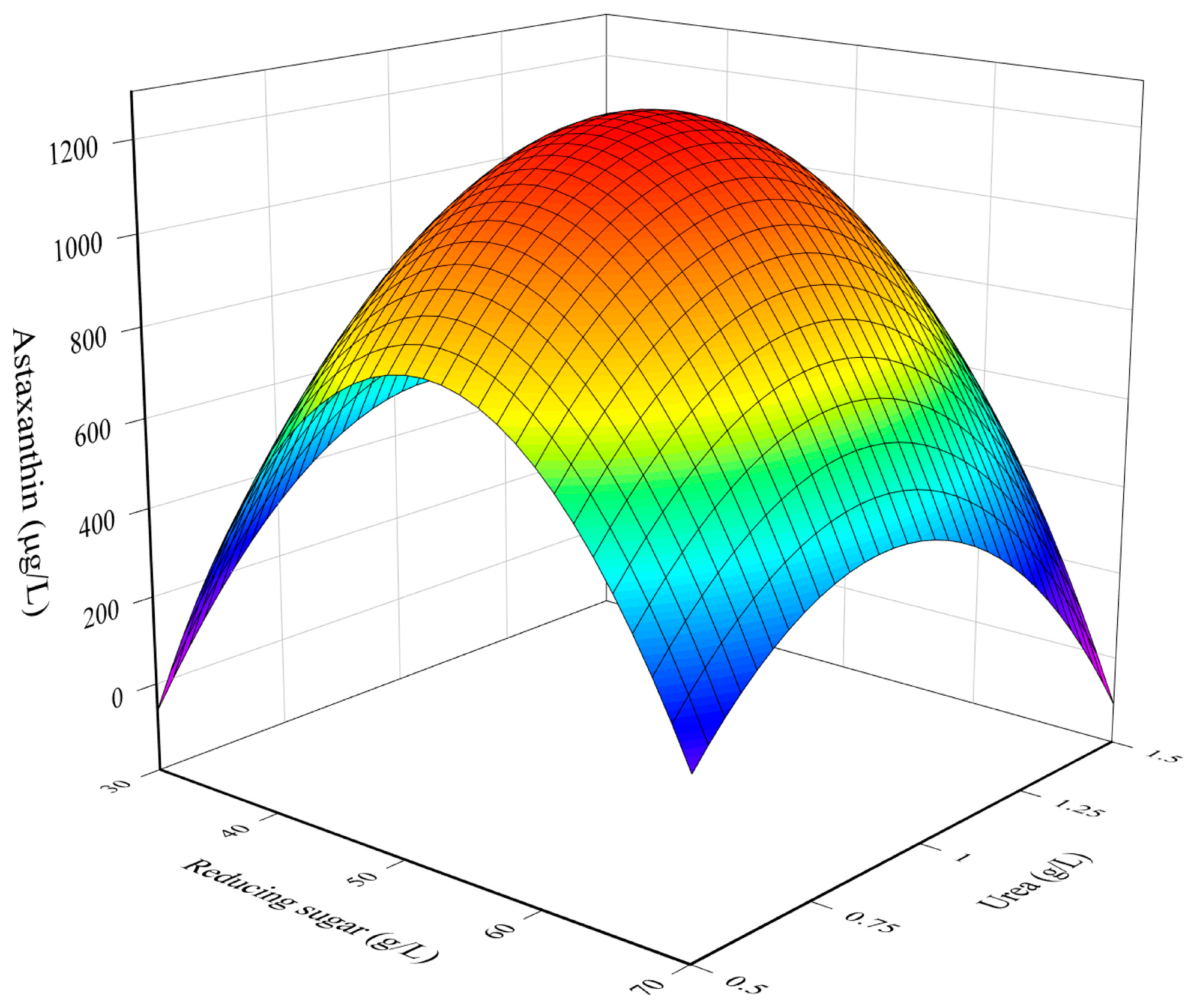
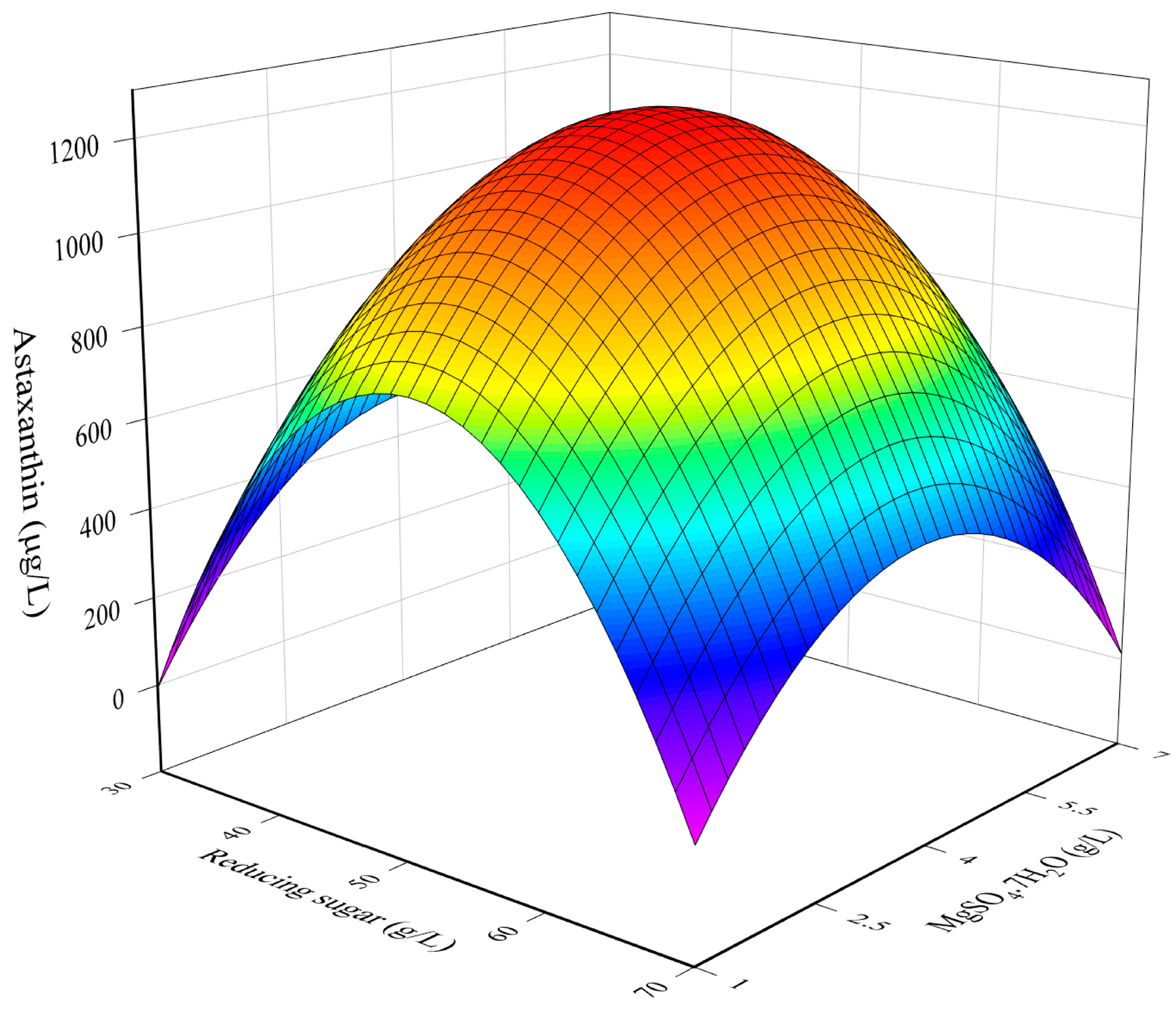
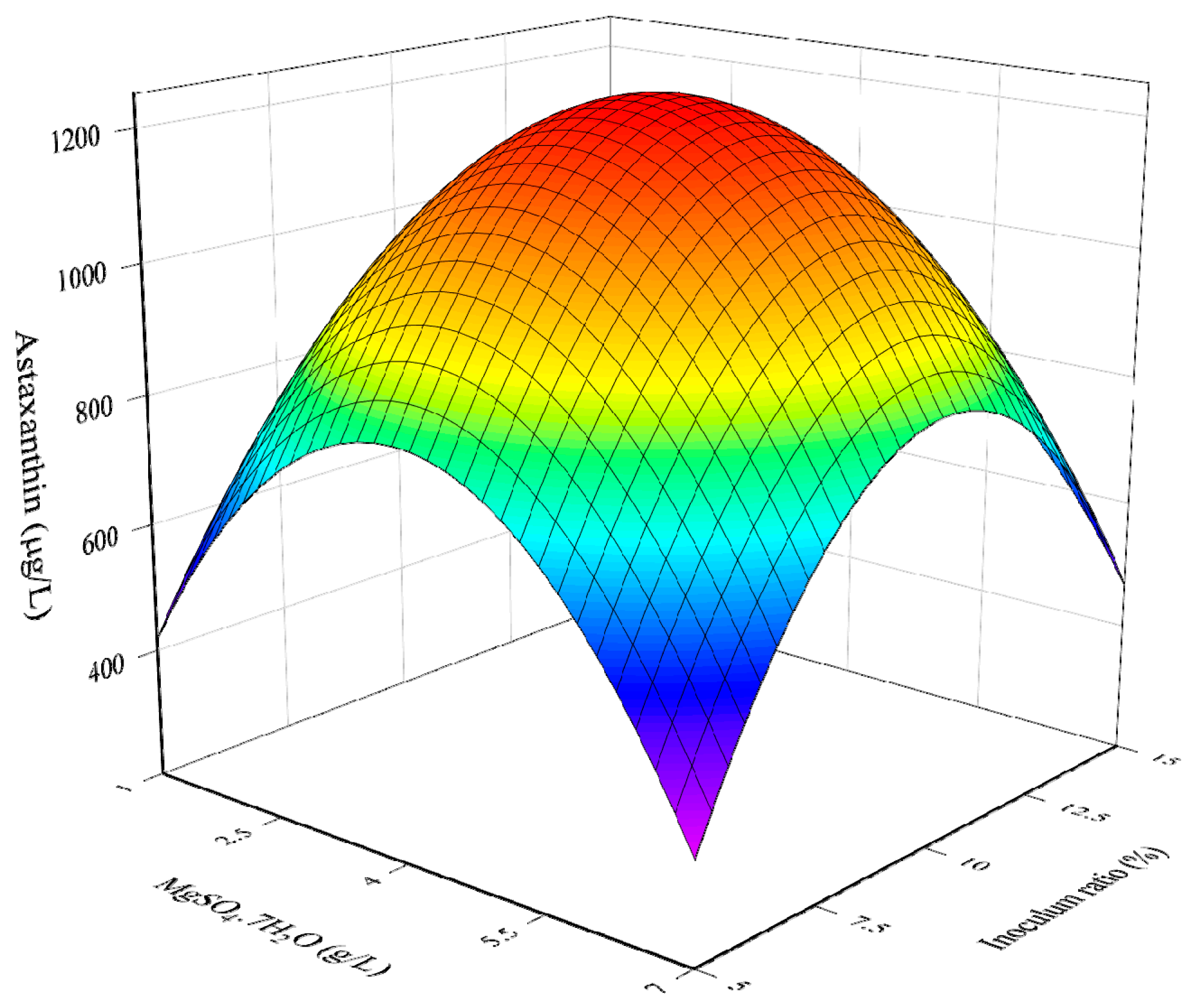
3.2. Combined Influence of Culture Conditions
3.3. Determination of Optimal Culture Conditions
3.4. Astaxanthin Purification
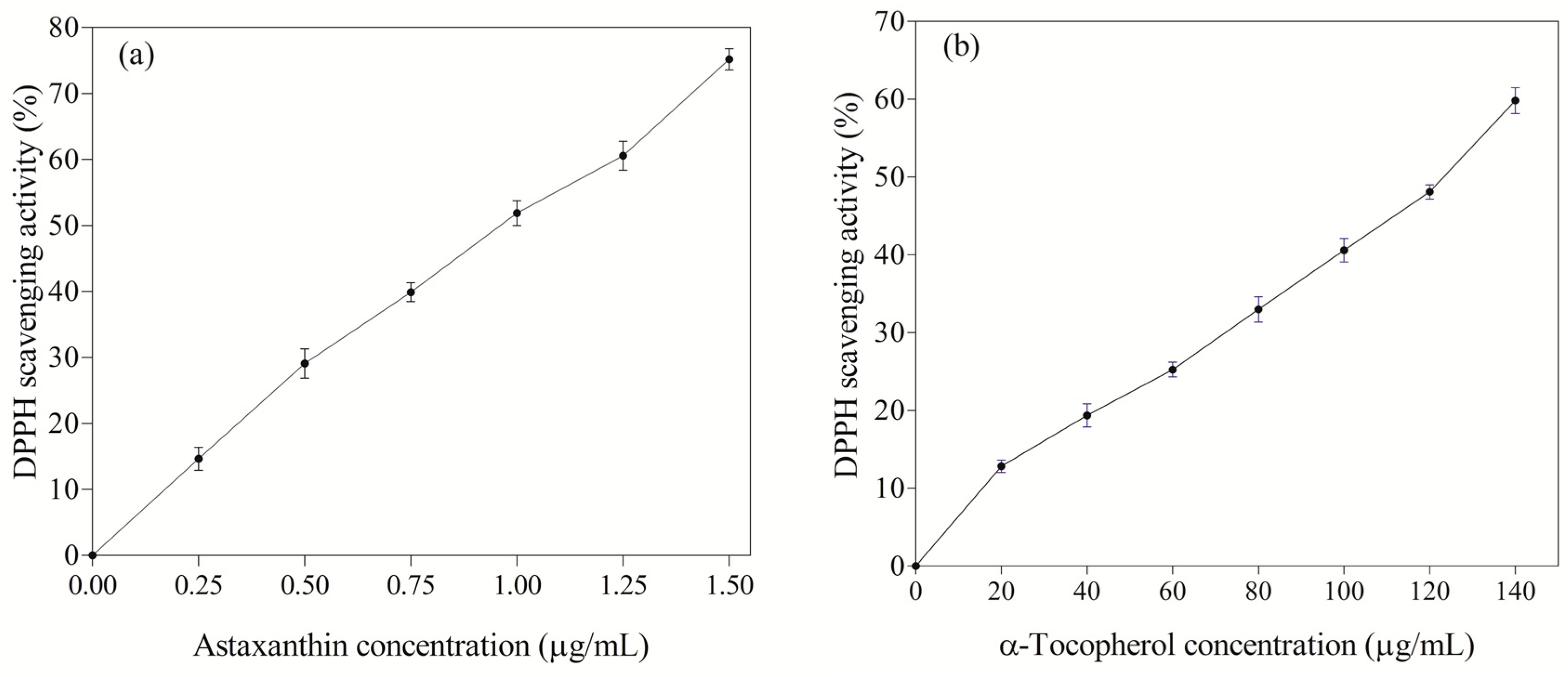
3.5. Antioxidant Activity of Astaxanthin
3.6. Anticancer Activity of Astaxanthin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faraone, I.; Sinisgalli, C.; Ostuni, A.; Armentano, M.F.; Carmosino, M.; Milella, L.; Russo, D.; Labanca, F.; Khan, H. Astaxanthin anticancer effects are mediated through multiple molecular mechanisms: A systematic review. Pharmacol. Res. 2020, 155, 104689. [Google Scholar] [CrossRef]
- Demirel, P.B.; Tuna, B.G. Anticancer properties of astaxanthin: A molecule of great promise. In Global Perspectives on Astaxanthin; Academic Press: Cambridge, MA, USA, 2021; pp. 427–445. [Google Scholar]
- Fakhri, S.; Abbaszadeh, F.; Dargahi, L.; Jorjani, M. Astaxanthin: A mechanistic review on its biological activities and health benefits. Pharmacol. Res. 2018, 136, 1–20. [Google Scholar] [CrossRef]
- Kurashige, M.; Okimasu, E.; Inoue, M.; Utsumi, K. Inhibition of oxidative injury of biological membranes by astaxanthin. Physiol. Chem. Phys. Med. NMR 1990, 22, 27–38. [Google Scholar]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in skin health, repair, and disease: A comprehensive review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Zhou, P.; Li, M.; Shen, B.; Yao, Z.; Bian, Q.; Ye, L.; Yu, H. Directed coevolution of β-carotene ketolase and hydroxylase and its application in temperature-regulated biosynthesis of astaxanthin. J. Agric. Food Chem. 2019, 67, 1072–1080. [Google Scholar] [CrossRef]
- Asker, D. Isolation and characterization of a novel, highly selective astaxanthin-producing marine bacterium. J. Agric. Food Chem. 2017, 65, 9101–9109. [Google Scholar] [CrossRef]
- Kamath, B.S.; Srikanta, B.M.; Dharmesh, S.M.; Sarada, R.; Ravishankar, G.A. Ulcer preventive and antioxidative properties of astaxanthin from Haematococcus pluvialis. Eur. J. Pharmacol. 2008, 590, 387–395. [Google Scholar] [CrossRef]
- Schmidt, I.; Schewe, H.; Gassel, S.; Jin, C.; Buckingham, J.; Hümbelin, M.; Sandmann, G.; Schrader, J. Biotechnological production of astaxanthin with Phaffia rhodozyma/Xanthophyllomyces dendrorhous. Appl. Microbiol. Biotechnol. 2011, 89, 555–571. [Google Scholar] [CrossRef]
- Chang, M.X.; Xiong, F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: Recent advances and future directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef]
- Nguyen, K.D. Astaxanthin: A Comparative Case of Synthetic vs. Natural Production. Chemical and Biomolecular Engineering Publications and Other Works. Available online: http://trace.tennessee.edu/utk_chembiopubs/94 (accessed on 5 January 2023).
- Wen, Z.; Zhang, S.; Odoh, C.K.; Jin, M.; Zhao, Z.K. Rhodosporidium toruloides—A potential red yeast chassis for lipids and beyond. FEMS Yeast Res. 2020, 20, foaa038. [Google Scholar] [CrossRef]
- Tran, T.N.; Ngo, D.H.; Tran, Q.T.; Nguyen, H.C.; Su, C.H.; Ngo, D.N. Enhancing astaxanthin biosynthesis by Rhodosporidium toruloides mutants and optimization of medium compositions using response surface methodology. Processes 2020, 8, 497. [Google Scholar] [CrossRef]
- Wu, Y.H.; Yang, J.; Hu, H.Y.; Yu, Y. Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol. Eng. 2013, 60, 155–159. [Google Scholar] [CrossRef]
- Tran, T.N.; Quang-Vinh, T.; Huynh, H.T.; Hoang, N.S.; Nguyen, H.C.; Dai-Nghiep, N. Astaxanthin production by newly isolated Rhodosporidium toruloides: Optimization of medium compositions by response surface methodology. Not. Bot. Horti Agrobot. 2019, 47, 320–327. [Google Scholar] [CrossRef]
- Dalsasso, R.R.; Pavan, F.A.; Bordignon, S.E.; de Aragão, G.M.F.; Poletto, P. Polyhydroxybutyrate (PHB) production by Cupriavidus necator from sugarcane vinasse and molasses as mixed substrate. Process Biochem. 2019, 85, 12–18. [Google Scholar] [CrossRef]
- Yew, G.Y.; Khoo, K.S.; Chia, W.Y.; Ho, Y.C.; Law, C.L.; Leong, H.Y.; Show, P.L. A novel lipids recovery strategy for biofuels generation on microalgae Chlorella cultivation with waste molasses. J. Water Process Eng. 2020, 38, 101665. [Google Scholar] [CrossRef]
- Yew, G.Y.; Puah, B.K.; Chew, K.W.; Teng, S.Y.; Show, P.L.; Nguyen, T.H.P. Chlorella vulgaris FSP-E cultivation in waste molasses: Photo-to-property estimation by artificial intelligence. Chem. Eng. J. 2020, 402, 126230. [Google Scholar] [CrossRef]
- Kundu, S.; Panda, T.; Majumdar, S.; Guha, B.; Bandyopadhyay, K. Pretreatment of Indian cane molasses for increased production of citric acid. Biotechnol. Bioeng. 1984, 26, 1114–1121. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Prasertsung, I.; Aroonraj, K.; Kamwilaisak, K.; Saito, N.; Damrongsakkul, S. Production of reducing sugar from cassava starch waste (CSW) using solution plasma process (SPP). Carbohydr. Polym. 2019, 205, 472–479. [Google Scholar] [CrossRef]
- An, G.H.; Bielich, J.; Auerbach, R.; Johnson, E.A. Isolation and characterization of carotenoid hyperproducing mutants of yeast by flow cytometry and cell sorting. Nat. Biotechnol. 1991, 9, 70–73. [Google Scholar] [CrossRef]
- Fang, T.J.; Cheng, Y.S. Improvement of astaxanthin production by Phaffia rhodozyma through mutation and optimization of culture conditions. J. Ferment. Bioeng. 1993, 75, 466–469. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci. Technol. Inter. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Pham, D.C.; Chang, Y.C.; Lin, S.R.; Fuh, Y.M.; Tsai, M.J.; Weng, C.F. FAK and S6K1 inhibitor, neferine, dually induces autophagy and apoptosis in human neuroblastoma cells. Molecules 2018, 23, 3110. [Google Scholar] [CrossRef]
- Koschwanez, J.H.; Foster, K.R.; Murray, A.W. Sucrose utilization in budding yeast as a model for the origin of undifferentiated multicellularity. PLoS Biol. 2011, 9, e1001122. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Su, C.H.; Yu, Y.K.; Huong, D.T.M. Sugarcane bagasse as a novel carbon source for heterotrophic cultivation of oleaginous microalga Schizochytrium sp. Ind. Crop. Prod. 2018, 121, 99–105. [Google Scholar] [CrossRef]
- Zeng, Y.; Bian, D.; Xie, Y.; Jiang, X.; Li, X.; Li, P.; Zhang, Y.; Xie, T. Utilization of food waste hydrolysate for microbial lipid and protein production by Rhodosporidium toruloides Y2. J. Chem. Technol. Biotechnol. 2017, 92, 666–673. [Google Scholar] [CrossRef]
- Howard, J.; Rackemann, D.W.; Bartley, J.P.; Samori, C.; Doherty, W.O. Conversion of sugar cane molasses to 5-hydroxymethylfurfural using molasses and bagasse-derived catalysts. ACS Sustain. Chem. Eng. 2018, 6, 4531–4538. [Google Scholar] [CrossRef]
- Fang, C.J.; Ku, K.L.; Lee, M.H.; Su, N.W. Influence of nutritive factors on C50 carotenoids production by Haloferax mediterranei ATCC 33500 with two-stage cultivation. Bioresour. Technol. 2010, 101, 6487–6493. [Google Scholar] [CrossRef]
- Varmira, K.; Habibi, A.; Bahramian, E.; Jamshidpour, S. Progressive agents for improvement of carotenogenesis in Rhodotorula rubra. J. Adv. Food Sci. Technol. 2016, 3, 70–78. [Google Scholar]
- Narayanaswamy, S. Plant Cell and Tissue Culture; Tata McGraw-Hill Education: New York, NY, USA, 1994. [Google Scholar]
- Ling, O.S.; Kiong, A.L.P.; Hussein, S. Establishment and optimisation of growth parameters for cell suspension cultures of Ficus deltoidea. Am. Eurasian J. Sustain. Agric. 2008, 2, 38–49. [Google Scholar]
- Xie, H.; Zhou, Y.; Hu, J.; Chen, Y.; Liang, J. Production of astaxanthin by a mutant strain of Phaffia rhodozyma and optimization of culture conditions using response surface methodology. Ann. Microbiol. 2014, 64, 1473–1481. [Google Scholar] [CrossRef]
- An, J.; Gao, F.; Ma, Q.; Xiang, Y.; Ren, D.; Lu, J. Screening for enhanced astaxanthin accumulation among Spirulina platensis mutants generated by atmospheric and room temperature plasmas. Algal Res. 2017, 25, 464–472. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Nguyen, H.N.T.; Huang, M.Y.; Lin, K.H.; Pham, D.C.; Tran, Y.B.; Su, C.H. Optimization of aqueous enzyme-assisted extraction of rosmarinic acid from rosemary (Rosmarinus officinalis L.) leaves and the antioxidant activity of the extract. J. Food Process. Preserv. 2021, 45, e15221. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Kuan-Hung, L.; Huang, M.Y.; Chi-Ming, Y.; Tin-Han, S.; Hsiung, T.C.; Yen-Chang, L.; Fun-Chi, T. Antioxidant activities of the methanol extracts of various parts of Phalaenopsis orchids with white, yellow, and purple flowers. Not. Bot. Horti Agrobot. 2018, 46, 457–465. [Google Scholar] [CrossRef]
- Do, T.H.; Huynh, T.D.; Vo, K.A.; Nguyen, K.A.; Cao, T.S.; Nguyen, K.N.; Nguyen, H.A.H.; Nguyen, T.T.T.; Le, N.P.N.; Truong, D.H. Saponin-rich fractions from Codonopsis javanica root extract and their in vitro antioxidant and anti-enzymatic efficacy. J. Food Process. Preserv. 2022, 46, e16113. [Google Scholar] [CrossRef]
- Truong, D.H.; Nguyen, D.H.; Ta, N.T.A.; Bui, A.V.; Do, T.H.; Nguyen, H.C. Evaluation of the use of different solvents for phytochemical constituents, antioxidants, and in vitro anti-inflammatory activities of Severinia buxifolia. J. Food Qual. 2019, 2019, 8178294. [Google Scholar] [CrossRef]
- Chintong, S.; Phatvej, W.; Rerk-Am, U.; Waiprib, Y.; Klaypradit, W. In vitro antioxidant, antityrosinase, and cytotoxic activities of astaxanthin from shrimp waste. Antioxidants 2019, 8, 128. [Google Scholar] [CrossRef]
- Santos-Sánchez, N.F.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. In Antioxidants; IntechOpen: London, UK, 2019; Volume 10, pp. 1–29. [Google Scholar]
- Sun, J.; Yan, J.; Dong, H.; Gao, K.; Yu, K.; He, C.; Mao, X. Astaxanthin with different configurations: Sources, activity, post-modification and application in foods. Curr. Opin. Food Sci. 2023, 49, 100955. [Google Scholar] [CrossRef]
- Ambati, R.R.; Moi, P.S.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef]
- Naguib, Y.M. Antioxidant activities of astaxanthin and related carotenoids. J. Agric. Food Chem. 2000, 48, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Abdelmalek, B.E.; Sila, A.; Ghlissi, Z.; Taktak, M.A.; Ayadi, M.A.; Bougatef, A. The influence of natural astaxanthin on the formulation and storage of marinated chicken steaks. J. Food Biochem. 2016, 40, 393–403. [Google Scholar] [CrossRef]
- Karnila, R.; Edison; Ramadhani, N.R. Antioxidant activity of astaxanthin flour extract of mud crab (Scylla serrata) with different acetone concentrations. In Proceedings of the IOP Conference Series: Earth and Environmental Science 2021, Pekanbaru, Indonesia, 10–11 September 2020; p. 012047. [Google Scholar]
- Tan, Y.; Ye, Z.; Wang, M.; Manzoor, M.F.; Aadil, R.M.; Tan, X.; Liu, Z. Comparison of different methods for extracting the astaxanthin from Haematococcus pluvialis: Chemical composition and biological activity. Molecules 2021, 26, 3569. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Xiong, Y.J.; Yang, M.M.; Zhu, M.J. Preparation of astaxanthin mask from Phaffia rhodozyma and its evaluation. Process Biochem. 2019, 79, 195–202. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Zeng, X.; Huangfu, J.; Jiang, Y.; Wang, M.; Chen, F. Astaxanthin is responsible for antiglycoxidative properties of microalga Chlorella zofingiensis. Food Chem. 2011, 126, 1629–1635. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.J.; Lee, B.J.; Joo, M.K.; Chun, H.J.; Lee, S.W.; Bak, Y.T. Astaxanthin inhibits proliferation of human gastric cancer cell lines by interrupting cell cycle progression. Gut Liver 2016, 10, 369. [Google Scholar] [CrossRef]
- Ramamoorthy, K.; Raghunandhakumar, S.; Anand, R.; Paramasivam, A.; Kamaraj, S.; Nagaraj, S.; Ezhilarasan, D.; Lakshmi, T.; Dua, K.; Chellappan, D.K. Anticancer effects and lysosomal acidification in A549 cells by astaxanthin from Haematococcus lacustris. Bioinformation 2020, 16, 965. [Google Scholar] [CrossRef] [PubMed]

| Variables | Symbols | Variable Levels | ||||
|---|---|---|---|---|---|---|
| −2 | −1 | 0 | 1 | 2 | ||
| Reducing sugar concentration (g/L) | X1 | 30 | 40 | 50 | 60 | 70 |
| Urea concentration (g/L) | X2 | 0.5 | 0.75 | 1.0 | 1.25 | 1.5 |
| MgSO4·7H2O concentration (g/L) | X3 | 1.0 | 2.5 | 4.0 | 5.5 | 7.0 |
| Inoculum ratio (%) | X4 | 5.0 | 7.5 | 10.0 | 12.5 | 15.0 |
| Run | Variables | Astaxanthin Yield (μg/L) | ||||
|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Experimental | Predicted | |
| 1 | −1 | −1 | −1 | −1 | 761.27 | 739.10 |
| 2 | 1 | −1 | −1 | −1 | 792.65 | 780.25 |
| 3 | −1 | 1 | −1 | −1 | 784.20 | 759.78 |
| 4 | 1 | 1 | −1 | −1 | 665.33 | 646.53 |
| 5 | −1 | −1 | 1 | −1 | 825.50 | 794.65 |
| 6 | 1 | −1 | 1 | −1 | 783.58 | 778.95 |
| 7 | −1 | 1 | 1 | −1 | 782.38 | 787.09 |
| 8 | 1 | 1 | 1 | −1 | 639.37 | 616.99 |
| 9 | −1 | −1 | −1 | 1 | 651.44 | 618.02 |
| 10 | 1 | −1 | −1 | 1 | 734.84 | 725.67 |
| 11 | −1 | 1 | −1 | 1 | 779.57 | 779.74 |
| 12 | 1 | 1 | −1 | 1 | 757.95 | 732.99 |
| 13 | −1 | −1 | 1 | 1 | 707.63 | 721.97 |
| 14 | 1 | −1 | 1 | 1 | 804.15 | 772.77 |
| 15 | −1 | 1 | 1 | 1 | 898.87 | 855.46 |
| 16 | 1 | 1 | 1 | 1 | 734.14 | 751.85 |
| 17 | −2 | 0 | 0 | 0 | 506.90 | 544.28 |
| 18 | 2 | 0 | 0 | 0 | 458.95 | 481.83 |
| 19 | 0 | −2 | 0 | 0 | 790.36 | 825.07 |
| 20 | 0 | 2 | 0 | 0 | 799.28 | 824.83 |
| 21 | 0 | 0 | −2 | 0 | 731.66 | 774.11 |
| 22 | 0 | 0 | 2 | 0 | 830.71 | 848.52 |
| 23 | 0 | 0 | 0 | −2 | 773.91 | 809.24 |
| 24 | 0 | 0 | 0 | 2 | 798.10 | 823.03 |
| 25 | 0 | 0 | 0 | 0 | 1221.52 | 1245.03 |
| 26 | 0 | 0 | 0 | 0 | 1295.59 | 1245.03 |
| 27 | 0 | 0 | 0 | 0 | 1282.52 | 1245.03 |
| 28 | 0 | 0 | 0 | 0 | 1241.34 | 1245.03 |
| 29 | 0 | 0 | 0 | 0 | 1201.03 | 1245.03 |
| 30 | 0 | 0 | 0 | 0 | 1214.55 | 1245.03 |
| 31 | 0 | 0 | 0 | 0 | 1258.63 | 1245.03 |
| Source | DF b | SS b | MS b | F-Value | p-Value |
|---|---|---|---|---|---|
| Model a | 14 | 1,582,917 | 113,066 | 76.49 | <0.001 |
| Error | 16 | 23,649 | 1478 | ||
| Lack-of-fit | 10 | 16,071 | 1607 | 1.27 | 0.4 |
| Pure Error | 6 | 7578 | 1263 | ||
| Total | 30 | 1,606,567 |
| Term | Parameter Estimate | Standard Error | t-Value | p-Value a |
|---|---|---|---|---|
| β0 | 1245.0 | 14.5 | 85.68 | 0.000 a |
| β1 | −15.61 | 7.85 | −1.99 | 0.064 |
| β2 | −0.06 | 7.85 | −0.01 | 0.994 |
| β3 | 18.60 | 7.85 | 2.37 | 0.03 a |
| β4 | 3.45 | 7.85 | 0.44 | 0.667 |
| β11 | −182.99 | 7.19 | −25.45 | 0.000 a |
| β22 | −105.02 | 7.19 | −14.61 | 0.000 a |
| β33 | −108.43 | 7.19 | −15.08 | 0.000 a |
| β44 | −107.22 | 7.19 | −14.91 | 0.000 a |
| β12 | −38.60 | 9.61 | −4.02 | 0.001 a |
| β13 | −14.21 | 9.61 | −1.48 | 0.159 |
| β14 | 16.62 | 9.61 | 1.73 | 0.103 |
| β23 | −7.06 | 9.61 | −0.73 | 0.473 |
| β24 | 35.26 | 9.61 | 3.67 | 0.002 a |
| β34 | 12.10 | 9.61 | 1.26 | 0.226 |
| Sources | IC50 Value (μg/mL) | References |
|---|---|---|
| R. toruloides | 0.97 | This study |
| Shrimp waste | 17.50 | [41] |
| P. longirostris | 6.30 | [46] |
| Scylla serrata | 805.84 | [47] |
| H. pluvialis | 15.39–56.25 | [48] |
| Phaffia rhodozyma | 31.79 | [49] |
| Chlorella zofingiensis | 1040–2930 | [50] |
| Sample | IC50 Value (µg/mL) | |||
|---|---|---|---|---|
| MCF7 | A549 | HeLa | HK2 | |
| Astaxanthin | 55.60 ± 2.6 | 56.38 ± 4.1 | 69.07 ± 2.4 | 111.34 ± 1.4 |
| Cisplatin | 21.68 ± 0.8 | 2.4 ± 0.4 | 6.8 ± 0.9 | 1.8 ± 0.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.N.; Tran, N.-T.; Tran, T.-A.; Pham, D.-C.; Su, C.-H.; Nguyen, H.C.; Barrow, C.J.; Ngo, D.-N. Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium toruloides G17. Fermentation 2023, 9, 148. https://doi.org/10.3390/fermentation9020148
Tran TN, Tran N-T, Tran T-A, Pham D-C, Su C-H, Nguyen HC, Barrow CJ, Ngo D-N. Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium toruloides G17. Fermentation. 2023; 9(2):148. https://doi.org/10.3390/fermentation9020148
Chicago/Turabian StyleTran, Tuyet Nhung, Ngoc-Tri Tran, Thu-Anh Tran, Dinh-Chuong Pham, Chia-Hung Su, Hoang Chinh Nguyen, Colin J. Barrow, and Dai-Nghiep Ngo. 2023. "Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium toruloides G17" Fermentation 9, no. 2: 148. https://doi.org/10.3390/fermentation9020148
APA StyleTran, T. N., Tran, N.-T., Tran, T.-A., Pham, D.-C., Su, C.-H., Nguyen, H. C., Barrow, C. J., & Ngo, D.-N. (2023). Highly Active Astaxanthin Production from Waste Molasses by Mutated Rhodosporidium toruloides G17. Fermentation, 9(2), 148. https://doi.org/10.3390/fermentation9020148









