Development of a Molasses-Based Medium for Agrobacterium tumefaciens Fermentation for Application in Plant-Based Recombinant Protein Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Plasmids and Culture Conditions
2.2. Cane Molasses Pretreatment
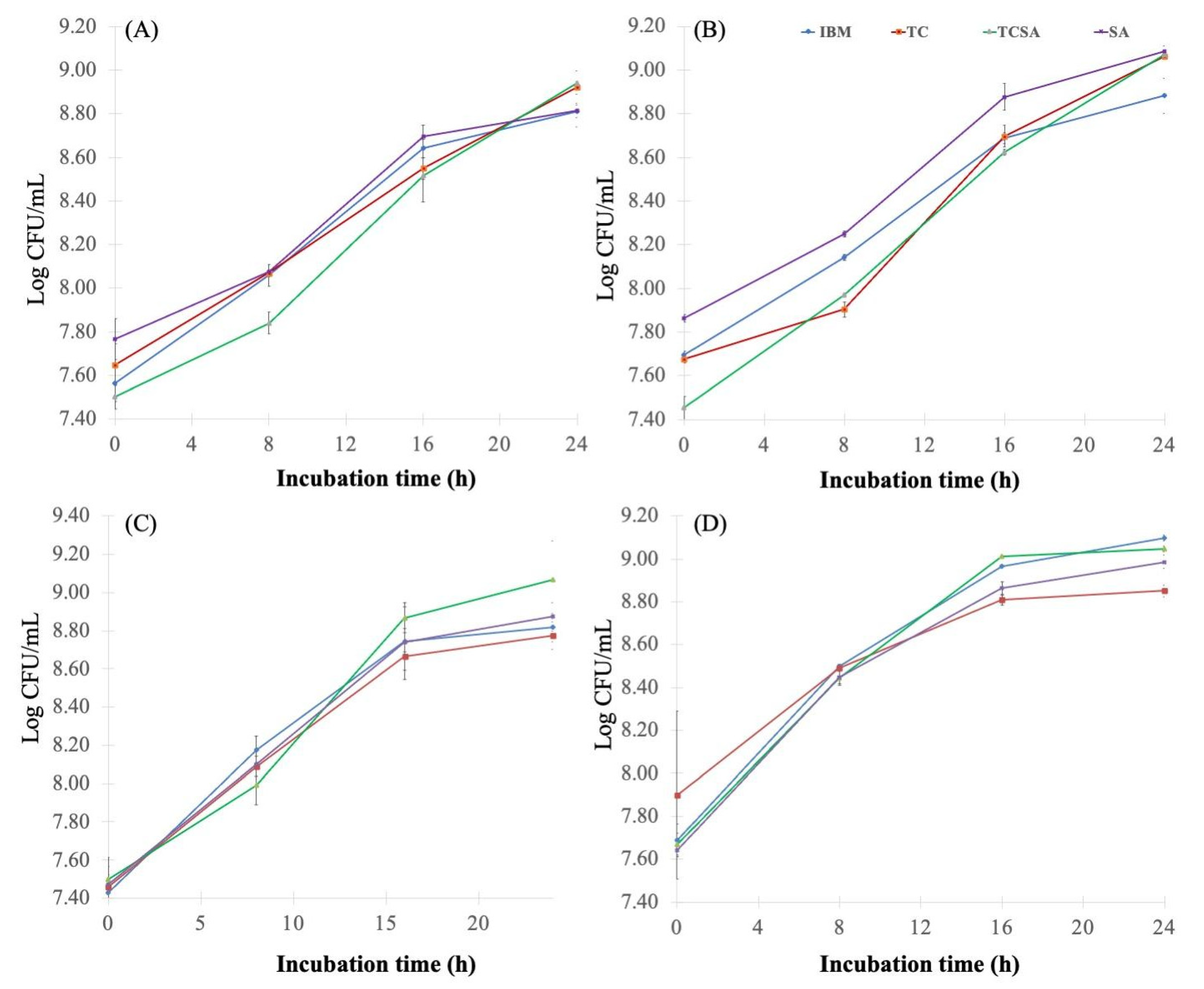
- Sulfuric acid pretreatment (SA): The diluted cane molasses pH was adjusted to 3.0 using concentrated H2SO4 (95–97%) solution and incubated for 1 h in a water bath at 70 °C.
- Tricalcium phosphate pretreatment (TC): The diluted cane molasses was pretreated with 2% (w/v) tricalcium phosphate and autoclaved at 105 °C for 5 min.
- Tricalcium phosphate and sulfuric acid pretreatment (TCSA): The tricalcium phosphate-pretreated sample (TC) was adjusted to pH 3.0 using concentrated H2SO4 and incubated at 70 °C for 1 h.
2.3. Effect of Molasses-Based Medium Formulation on the Growth of Engineered Agrobacterium tumefaciens
2.4. Effect of Minerals and Heavy Metals on Growth of Engineered Agrobacterium tumefaciens
2.5. Effects of Antibiotics on Cell Growth and Genetic Instability
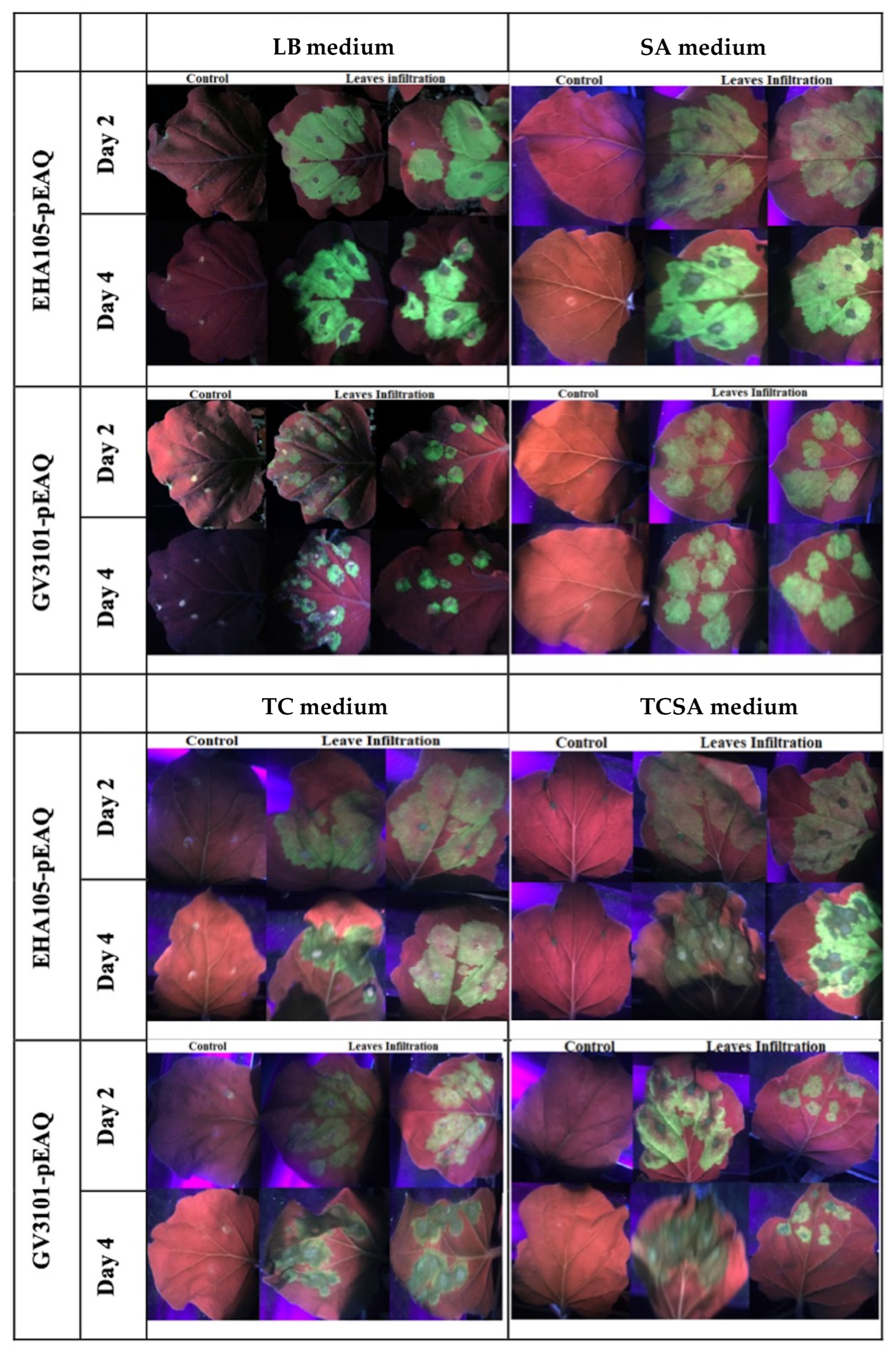
2.6. Plant Preparation, Agrobacterium Infiltration and Expression of Gfp in Nicotiana benthamiana
3. Statistical Analysis
4. Results and Discussion
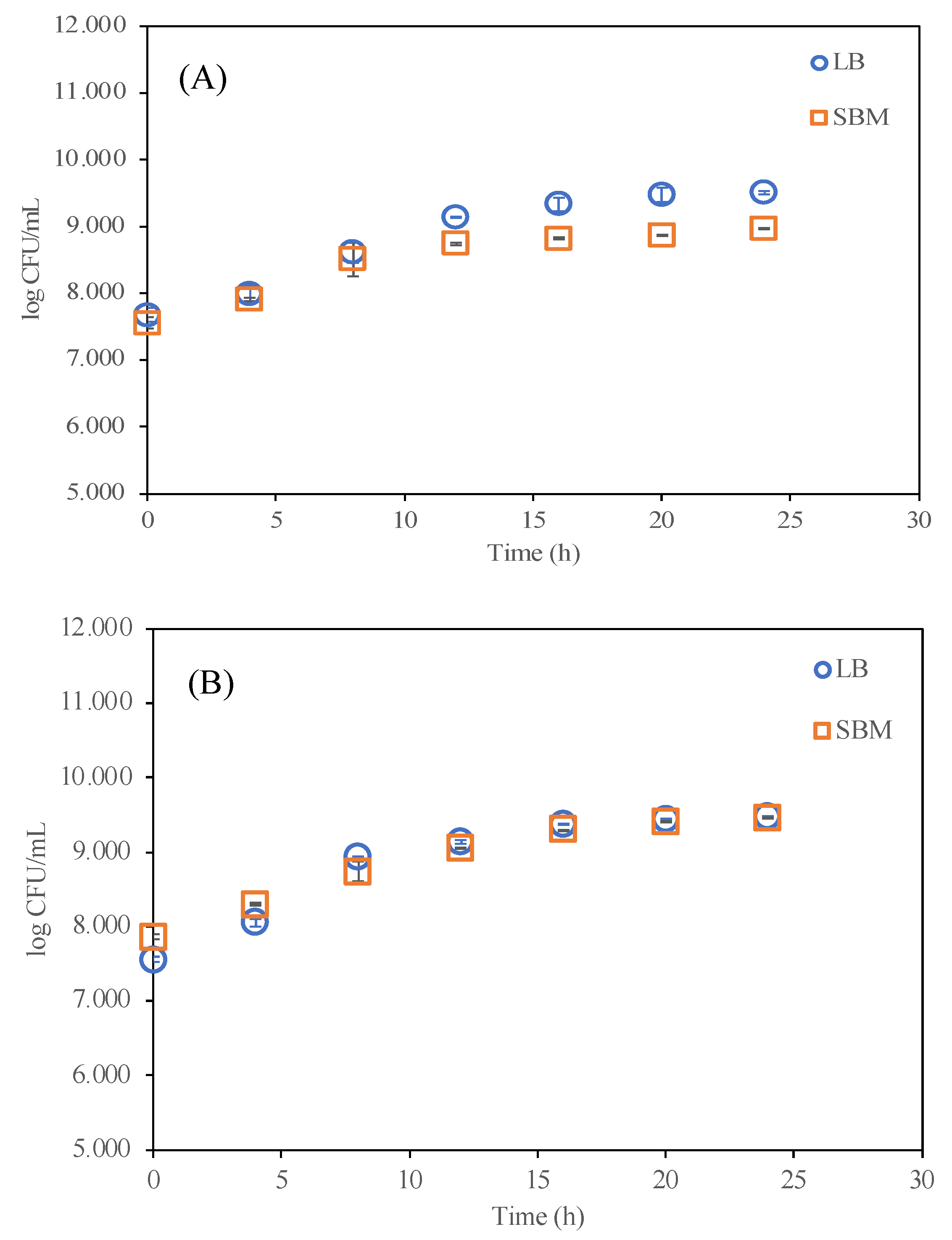
4.1. Effects of Sucrose on Engineered A. tumefaciens Growth
4.2. Effect of Pretreatment Methods on Molasses and Growth of Agrobacterium
4.3. Effect of Minerals and Heavy Metals in Molasses on Growth of Agrobacterium
4.4. Effects of Antibiotics on Cell Growth and Genetic Instability
4.5. Cost Evaluations of Molasses Culture Media
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, J.F.; Park, E.; von Arnim, A.G.; Nebenführ, A. The FAST technique: A simplified Agrobacterium-based transformation method for transient gene expression analysis in seedlings of Arabidopsis and other plant species. Plant Methods 2009, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, P.B.; Venkateshwaran, M.; Wu, L.; Ané, J.M.; Jiang, J. Agrobacterium-mediated transient gene expression and silencing: A rapid tool for functional gene assay in potato. PLoS ONE 2009, 4, e5812. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, K.; Qi, Y.; Nguyen le, V.; Bethke, G.; Tsuda, Y.; Glazebrook, J.; Katagiri, F. An efficient Agrobacterium-mediated transient transformation of Arabidopsis. Plant J. Cell Mol. Biol. 2012, 69, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Leth, I.K.; McDonald, K.A. Media development for large scale Agrobacterium tumefaciens culture. Biotechnol. Prog. 2017, 33, 1218–1225. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Hyun, E.K.; Kim, Y.S.; Lee, Y.J.; Oh, D.K. Characterization of an Agrobacterium tumefaciens D-psicose 3-epimerase that converts D-fructose to D-psicose. Appl. Environ. Microbiol. 2006, 72, 981–985. [Google Scholar] [CrossRef]
- Clarke, M.A. SYRUPS. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003. [Google Scholar] [CrossRef]
- Abdel-Rahman, G.N.; Nassar, N.R.; Heikal, Y.A.; Abou-Donia, M.A.; Naguib, M.M.; Fadel, M. Effect of Different Treatments on Heavy Metal Concentration in Sugar Cane Molasses. Int. Sch. Sci. Res. Innov. 2016, 10, 6. [Google Scholar]
- Goksungur, Y.; Ucan, A.; Guvenc, U. Production of pullulan from beet molasses and synthetic medium by Aureobasidium pullulans. Turk. J. Biol. 2004, 28, 23–30. [Google Scholar]
- Küçükaşik, F.; Kazak, H.; Güney, D.; Finore, I.; Poli, A.; Yenigün, O.; Nicolaus, B.; Oner, E.T. Molasses as fermentation substrate for levan production by Halomonas sp. Appl. Microbiol. Biotechnol. 2011, 89, 1729–1740. [Google Scholar] [CrossRef]
- Chudobova, D.; Dostalova, S.; Ruttkay-Nedecky, B.; Guran, R.; Rodrigo, M.A.; Tmejova, K.; Krizkova, S.; Zitka, O.; Adam, V.; Kizek, R. The effect of metal ions on Staphylococcus aureus revealed by biochemical and mass spectrometric analyses. Microbiol. Res. 2015, 170, 147–156. [Google Scholar] [CrossRef]
- Tokdar, P.; Ranadive, P.; Deshmukh, S.; Khora, S. Optimization of Fermentation Process Conditions for the Production of CoQ10 using Paracoccus denitrificans ATCC 19367 Fusant Strain PF-P1. Int. J. Eng. Res. 2017, 6, 135–143. [Google Scholar] [CrossRef]
- Acosta-Piantini, E.; Rodríguez-Díez, E.; Chavarri, M.; López-de-Armentia, I.; Villaran, M.C.; Lombraña, J.I. Preparation of Hydrolyzed Sugarcane Molasses as a Low-Cost Medium for the Mass Production of Probiotic Lactobacillus paracasei ssp. paracasei F19. Separations 2023, 10, 33. [Google Scholar] [CrossRef]
- Ninchan, B.; Sirisatesuwon, C.; Rattanaporn, K.; Sriroth, K. Understanding and Efficiently Manipulating Environmental Stress Caused by Metal Ions to Improve Ethanol Fermentation. Appl. Sci. Eng. Prog. 2022, 15, 4717. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, K. Molasses fermentation to produce low-cost carbon source for denitrification. Biotechnol. Biotechnol. Equip. 2022, 36, 878–890. [Google Scholar] [CrossRef]
- Ojkic, N.; Serbanescu, D.; Banerjee, S. Antibiotic Resistance via Bacterial Cell Shape-Shifting. Mbio 2022, 13, e0065922. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Khani, M.; Bahrami, A.; Chegeni, A.; Ghafari, M.D.; Zadeh, A.M. Optimization of carbon and nitrogen sources for extracellular polymeric substances production by Chryseobacterium indologenes MUT.2. Iran J. Biotechnol. 2016, 14, 13. [Google Scholar] [CrossRef]
- Srinivasan, R.; Gothandam, K.M. Synergistic Action of D-Glucose and Acetosyringone on Agrobacterium Strains for Efficient Dunaliella Transformation. PLoS ONE 2016, 11, e0158322. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Boanini, E.; Gazzano, M.; Nervi, C.; Chierotti, M.R.; Rubini, K.; Gobetto, R.; Bigi, A. Strontium and Zinc Substitution in β-Tricalcium Phosphate: An X-ray Diffraction, Solid State NMR and ATR-FTIR Study. J. Funct. Biomater. 2019, 10, 20. [Google Scholar] [CrossRef]
- Yin, X.; Calderin, L.; Stott, M.J.; Sayer, M. Density functional study of structural, electronic and vibrational properties of Mg- and Zn-doped tricalcium phosphate biomaterials. Biomaterials 2002, 23, 4155–4163. [Google Scholar] [CrossRef]
- Wein, T.; Hülter, N.; Mizrahi, I.; Dagan, T. Emergence of plasmid stability under non-selective conditions maintains antibiotic resistance. Nat. Commun. 2019, 10, 2595. [Google Scholar] [CrossRef] [PubMed]
- Wein, T.; Wang, Y.; Hülter, N.F.; Hammerschmidt, K.; Dagan, T. Antibiotics Interfere with the Evolution of Plasmid Stability. Curr. Biol. 2020, 30, 3841–3847. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; Thuenemann, E.C.; Sainsbury, F.; Lomonossoff, G.P. Virus-Derived Vectors for the Expression of Multiple Proteins in Plants. Methods Mol. Biol. 2016, 1385, 39–54. [Google Scholar] [CrossRef]

| Culture Medium | Compositions | pH | References | |
|---|---|---|---|---|
| Main Carbon Source | Medium Composition | |||
| Lysogeny Broth (LB) | Casein tryptone type I (10 g/L) | Yeast extract (5 g/L) NaCl (10 g/L) | 7.0 | [12] |
| Initial basal medium (IBM) | Sucrose (20 g/L) | Yeast extract (5 g/L), K2HPO4 (6 g/L), NaH2PO4 (7 g/L), NH4Cl (0.7 g/L), MgSO4 (0.5 g/L) | 7.0 | [17] |
| Sucrose-based defined media (SBM) | Sucrose (4 g/L) | (NH4)2SO4 (2.5 g/L), MgSO4·7H2O (0.6 g/L), CaCl2·2H2O (0.066 g/L), FeSO4·7H2O (10 mg/L), MnSO4·H2O (0.6 mg/L), ZnSO4·7H2O (0.6 mg/L), Phosphate buffer 0.05 M | 7.0 | [4] |
| Molasses-based media (MBM) | Molasses (X g/L) * | (NH4)2SO4 (2.5 g/L) MgSO4·7H2O (~0.25 g/L) ** CaCl2·2H2O (~0.031 g/L) ** Phosphate buffer 0.05 M | 7.0 | This study |
| Minerals | Units | Molasses | SA | TC | TCSA | Reference Methods |
|---|---|---|---|---|---|---|
| Total sugar | g/100 g | 57.5 | 45.2 | 53.4 | 50.8 | Estimated as total carbohydrates by phenol-sulfuric acid method [19] |
| Ash | g/100 g | 7.7 | 3.8 | 4.3 | 3.8 | AOAC (2016) 920.153 |
| Sulfate ash | g/100 g | 9.0 | 4.9 | 5.9 | 4.1 | AOAC (2016) 900.02 |
| Calcium (Ca) | mg/kg | 7173.5 | 589.5 | 3029.8 | 589.4 | In-house method based on AOAC (2012) 984.27 |
| Magnesium (Mg) | mg/kg | 2949.2 | 2121.8 | 2163.6 | 2087.6 | In-house method based on AOAC (2012) 984.27 |
| Potassium (K) | mg/kg | 9530.5 | 9449.4 | 9692.3 | 9665.5 | In-house method based on AOAC (2012) 984.27 |
| Nitrogen (N) | g/kg | 0.085 | 0.016 | 0.011 | 0.036 | Determination of Kjeldahl Nitrogen Horizontal, 2003 |
| Composition | Size | Bulk Cost (USD) | Source | Amount Used (g/L) | Price (USD/L) | |
|---|---|---|---|---|---|---|
| LB medium | ||||||
| 1 | Yeast Extract | 500 g/bot | 35.18 | Hi-media | 5 | 0.3518 |
| 2 | Tryptone type I | 500 g/bot | 55.06 | Hi-media | 10 | 1.1012 |
| 3 | NaCl | 1 kg | 6.42 | Kemaus | 10 | 0.0642 |
| Total | 1.52 | |||||
| Molasses-based medium | ||||||
| 1 | (NH4)2SO4 | 500 g/bot | 11.32 | Kemaus | 2.4 | 0.0543 |
| 2 | MgSO4·7H2O | 500 g/bot | 11.32 | Kemaus | 0.25 | 0.0056 |
| 3 | Na2HPO4 | 500 g/bot | 14.07 | Kemaus | 4.543 | 0.1278 |
| 4 | KH2PO4 | 500 g/bot | 11.93 | Kemaus | 2.448 | 0.0584 |
| 5 | Molasses | 1 kg/bot | 0.81 | 8.7 | 0.0070 | |
| Total | 0.25 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watthanasakphuban, N.; Nguyen, L.V.; Cheng, Y.-S.; Show, P.-L.; Sriariyanun, M.; Koffas, M.; Rattanaporn, K. Development of a Molasses-Based Medium for Agrobacterium tumefaciens Fermentation for Application in Plant-Based Recombinant Protein Production. Fermentation 2023, 9, 149. https://doi.org/10.3390/fermentation9020149
Watthanasakphuban N, Nguyen LV, Cheng Y-S, Show P-L, Sriariyanun M, Koffas M, Rattanaporn K. Development of a Molasses-Based Medium for Agrobacterium tumefaciens Fermentation for Application in Plant-Based Recombinant Protein Production. Fermentation. 2023; 9(2):149. https://doi.org/10.3390/fermentation9020149
Chicago/Turabian StyleWatthanasakphuban, Nisit, Luan Van Nguyen, Yu-Shen Cheng, Pau-Loke Show, Malinee Sriariyanun, Mattheos Koffas, and Kittipong Rattanaporn. 2023. "Development of a Molasses-Based Medium for Agrobacterium tumefaciens Fermentation for Application in Plant-Based Recombinant Protein Production" Fermentation 9, no. 2: 149. https://doi.org/10.3390/fermentation9020149
APA StyleWatthanasakphuban, N., Nguyen, L. V., Cheng, Y.-S., Show, P.-L., Sriariyanun, M., Koffas, M., & Rattanaporn, K. (2023). Development of a Molasses-Based Medium for Agrobacterium tumefaciens Fermentation for Application in Plant-Based Recombinant Protein Production. Fermentation, 9(2), 149. https://doi.org/10.3390/fermentation9020149









