Scale-Up of Pigment Production by the Marine-Derived Filamentous Fungus, Talaromyces albobiverticillius 30548, from Shake Flask to Stirred Bioreactor
Abstract
1. Introduction
2. Materials and Methods
2.1. Organism and Maintenance
2.2. Inoculum Preparation
2.3. Fermentation in Shake Flasks
2.4. Fermentation in Bioreactor
2.5. Biomass Estimation
2.6. Estimation of Pigments Absorbance
2.7. Color Characteristics
3. Results
3.1. Importance and Regulation of pH for Pigment Production
3.2. Changes in Fungal Morphology and Rheology
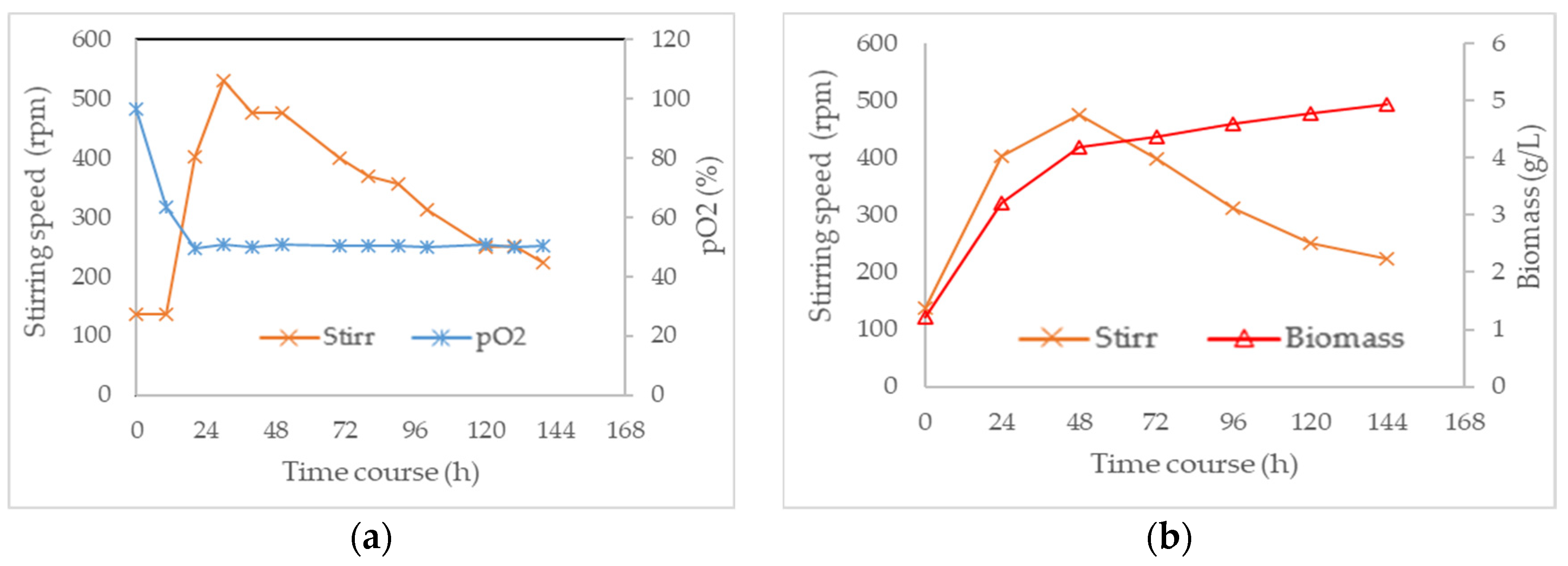
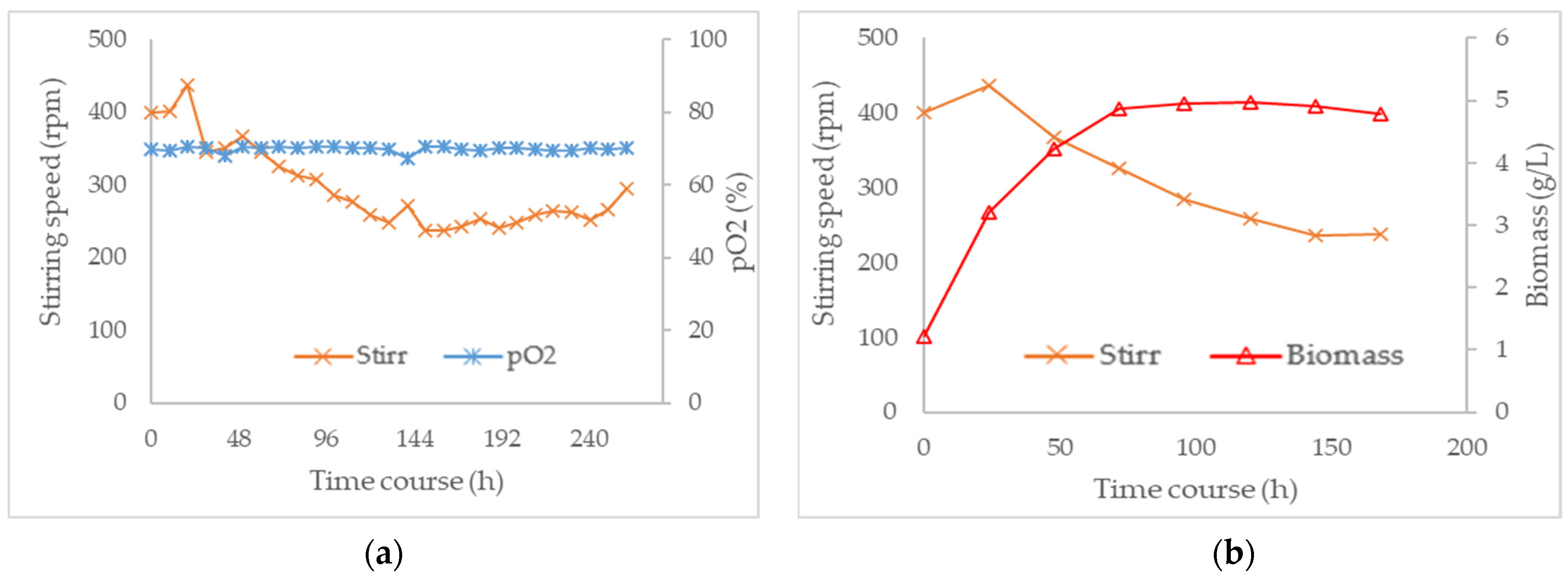
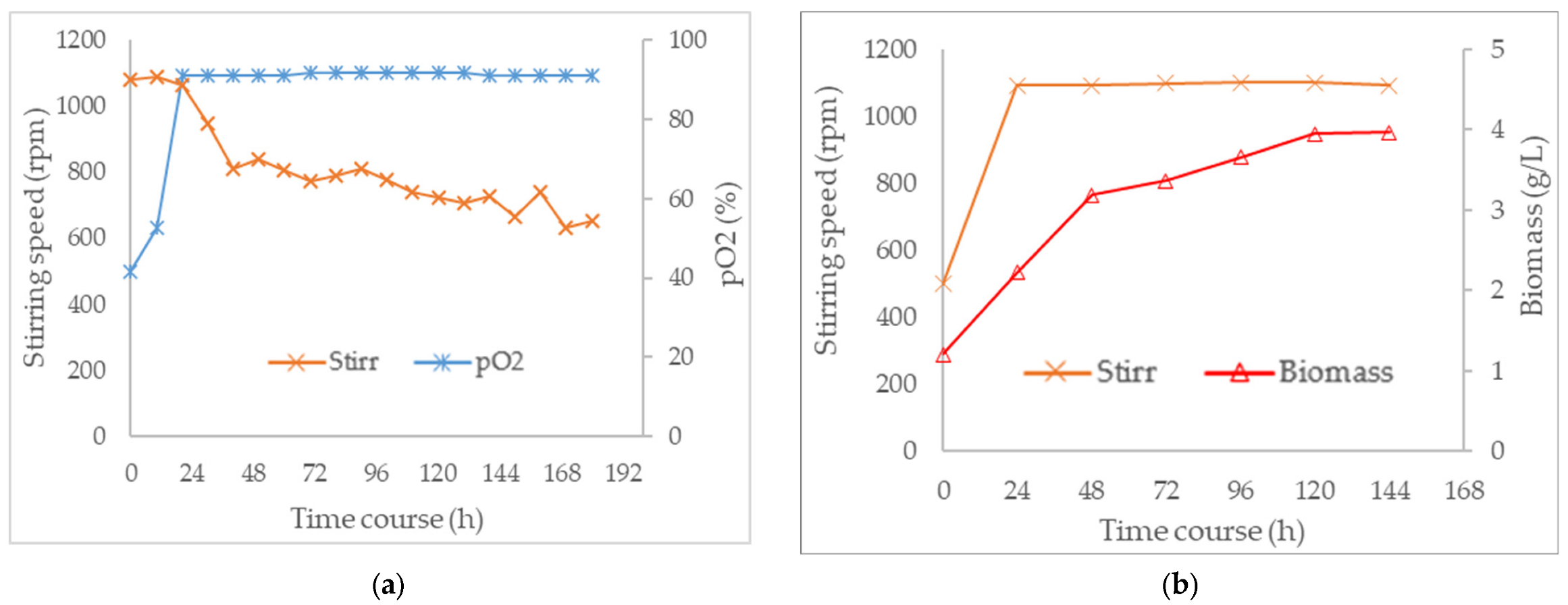
3.3. Combined Effect of Agitation Speed and Dissolved Oxygen on Biomass Growth
3.4. Effect of Agitation Speed and Dissolved Oxygen on Pigment Production
3.5. Colorimetric Characterization of Extracellular Pigments
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Velmurugan, P.; Lee, Y.H.; Venil, C.K.; Lakshmanaperumalsamy, P.; Chae, J.C.; Oh, B.T. Effect of light on growth, intracellular and extracellular pigment production by five pigment-producing filamentous fungi in synthetic medium. J. Biosci. Bioeng. 2010, 109, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, B.; Humphrey, A.; Taguchi, H. Dynamic measurement of the volumetric oxygen transfer coefficient in fermentation systems. Biotechnol. Bioeng. 1967, 9, 533–544. [Google Scholar] [CrossRef]
- Kalra, R.; Conlan, X.A.; Goel, M. Fungi as a potential source of pigments: Harnessing filamentous fungi. Front. Chem. 2020, 8, 369. [Google Scholar] [CrossRef]
- Dufosse, L.; Fouillaud, M.; Caro, Y.; Mapari, S.A.; Sutthiwong, N. Filamentous fungi are large-scale producers of pigments and colorants for the food industry. Curr. Opin. Biotechnol. 2014, 26, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Gmoser, R.; Ferreira, J.A.; Lennartsson, P.R.; Taherzadeh, M.J. Filamentous ascomycetes fungi as a source of natural pigments. Fungal Biol. Biotechnol. 2017, 4, 1–25. [Google Scholar] [CrossRef]
- Lagashetti, A.C.; Dufossé, L.; Singh, S.K.; Singh, P.N. Fungal pigments and their prospects in different industries. Microorganisms 2019, 7, 604. [Google Scholar] [CrossRef] [PubMed]
- Fouillaud, M.; Venkatachalam, M.; Llorente, M.; Magalon, H.; Cuet, P.; Dufossé, L. Biodiversity of pigmented fungi isolated from marine environment in la réunion island, indian ocean: New resources for colored metabolites. J. Fungi 2017, 3, 36. [Google Scholar] [CrossRef]
- Frisvad, J.C.; Yilmaz, N.; Thrane, U.; Rasmussen, K.B.; Houbraken, J.; Samson, R.A. Talaromyces atroroseus, a new species efficiently producing industrially relevant red pigments. PloS ONE 2013, 8, e84102. [Google Scholar] [CrossRef]
- Pimenta, L.P.; Gomes, D.C.; Cardoso, P.G.; Takahashi, J.A. Recent findings in azaphilone pigments. J. Fungi 2021, 7, 541. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Zelena, M.; Cacciola, F.; Ceslova, L.; Girard-Valenciennes, E.; Clerc, P.; Dugo, P.; Mondello, L.; Fouillaud, M.; Rotondo, A. Partial characterization of the pigments produced by the marine-derived fungus talaromyces albobiverticillius 30548. Towards a new fungal red colorant for the food industry. J. Food Compos. Anal. 2018, 67, 38–47. [Google Scholar] [CrossRef]
- Caro, Y.; Venkatachalam, M.; Lebeau, J.; Fouillaud, M.; Dufossé, L. Pigments and colorants from filamentous fungi. In Fungal Metabolites; Merillon, J.-M., Ramawat, G.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 499–568. [Google Scholar]
- Santos-Ebinuma, V.C.; Teixeira, M.F.S.; Pessoa Jr, A. Submerged culture conditions for the production of alternative natural colorants by a new isolated penicillium purpurogenum dpua 1275. J. Microbiol. Biotechnol. 2013, 23, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Mapari, S.A.; Hansen, M.E.; Meyer, A.S.; Thrane, U. Computerized screening for novel producers of monascus-like food pigments in penicillium species. J. Agric. Food Chem. 2008, 56, 9981–9989. [Google Scholar] [CrossRef] [PubMed]
- Espinoza-Hernández, T.C.; Rodríguez-Herrera, R.; Aguilar-González, C.N.; Lara-Victoriano, F.; Reyes-Valdés, M.H.; Castillo-Reyes, F. Characterization of three novel pigment-producing penicillium strains isolated from the mexican semi-desert. Afr. J. Biotechnol. 2013, 12, 3405–3413. [Google Scholar]
- Méndez, A.; Pérez, C.; Montañéz, J.C.; Martínez, G.; Aguilar, C.N. Red pigment production by penicillium purpurogenum gh2 is influenced by ph and temperature. J. Zhejiang Univ. Sci. B 2011, 12, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Lyu, Y.; Yu, H.; Chen, W.; Ye, L.; Yang, R. Biotechnological advances for improving natural pigment production: A state-of-the-art review. Bioresour. Bioprocess. 2022, 9, 1–38. [Google Scholar] [CrossRef]
- Crater, J.S.; Lievense, J.C. Scale-up of industrial microbial processes. FEMS Microbiol. Lett. 2018, 365, fny138. [Google Scholar] [CrossRef]
- Potumarthi, R.; Ch, S.; Jetty, A. Alkaline protease production by submerged fermentation in stirred tank reactor using bacillus licheniformis ncim-2042: Effect of aeration and agitation regimes. Biochem. Eng. J. 2007, 34, 185–192. [Google Scholar] [CrossRef]
- Cui, Y.Q.; van der Lans, R.G.; Luyben, K.C. Effects of dissolved oxygen tension and mechanical forces on fungal morphology in submerged fermentation. Biotechnol. Bioeng. 1998, 57, 409–419. [Google Scholar] [CrossRef]
- Jüsten, P.; Paul, G.; Nienow, A.; Thomas, C. Dependence of mycelial morphology on impeller type and agitation intensity. Biotechnol. Bioeng. 1996, 52, 672–684. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Oh, H.J.; Shin, C.S. Morphology control of monascus cells and scale-up of pigment fermentation. Process Biochem. 2002, 38, 649–655. [Google Scholar] [CrossRef]
- Shin, C.S.; Kim, H.J.; Kim, M.J.; Ju, J.Y. Morphological change and enhanced pigment production of monascus when cocultured with saccharomyces cerevisiae or aspergillus oryzae. Biotechnol. Bioeng. 1998, 59, 576–581. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Shum-Chéong-Sing, A.; Caro, Y.; Dufossé, L.; Fouillaud, M. Ovat analysis and response surface methodology based on nutrient sources for optimization of pigment production in the marine-derived fungus talaromyces albobiverticillius 30548 submerged fermentation. Mar. Drugs 2021, 19, 248. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Magalon, H.; Dufossé, L.; Fouillaud, M. Production of pigments from the tropical marine-derived fungi talaromyces albobiverticillius: New resources for natural red-colored metabolites. J. Food Compos. Anal. 2018, 70, 35–48. [Google Scholar] [CrossRef]
- Venkatachalam, M.; Gérard, L.; Milhau, C.; Vinale, F.; Dufossé, L.; Fouillaud, M. Salinity and temperature influence growth and pigment production in the marine-derived fungal strain talaromyces albobiverticillius 30548. Microorganisms 2019, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.G.; Ramesh, K.P.M.; Puttananjaiah, M.H.; Dhale, M.A. Cicer arietinum (bengal gram) husk as alternative for talaromyces purpureogenus cfrm02 pigment production: Bioactivities and identification. Lwt 2019, 116, 108499. [Google Scholar] [CrossRef]
- Afshari, M.; Shahidi, F.; Mortazavi, S.A.; Tabatabai, F.; Es’ haghi, Z. Investigating the influence of ph, temperature and agitation speed on yellow pigment production by penicillium aculeatum atcc 10409. Nat. Prod. Res. 2015, 29, 1300–1306. [Google Scholar] [CrossRef]
- Blechert, O.; Zheng, H.; Zang, X.; Wang, Q.; Liu, W. Influence of the cultivation medium and ph on the pigmentation of trichophyton rubrum. PloS ONE 2019, 14, e0222333. [Google Scholar] [CrossRef]
- Joshi, V.; Attri, D.; Bala, A.; Bhushan, S. Microbial pigments. Indian J. Biotechnol. 2003, 2, 362–369. [Google Scholar]
- Orozco, S.F.B.; Kilikian, B.V. Effect of ph on citrinin and red pigments production by monascus purpureus cct3802. World J. Microbiol. Biotechnol. 2008, 24, 263–268. [Google Scholar] [CrossRef]
- Chen, M.-H.; Johns, M.R. Effect of ph and nitrogen source on pigment production by monascus purpureus. Appl. Microbiol. Biotechnol. 1993, 40, 132–138. [Google Scholar] [CrossRef]
- Garcia-Ochoa, F.; Gomez, E. Bioreactor scale-up and oxygen transfer rate in microbial processes: An overview. Biotechnol. Adv. 2009, 27, 153–176. [Google Scholar] [CrossRef] [PubMed]
- Limón, M.C.; Rodríguez-Ortiz, R.; Avalos, J. Bikaverin production and applications. Appl. Microbiol. Biotechnol. 2010, 87, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.-H.; Shin, C.S. Analysis of the morphologic changes of monascus sp. J101 cells cocultured with saccharomyces cerevisiae. FEMS Microbiol. Lett. 2000, 193, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gmoser, R.; Ferreira, J.A.; Lundin, M.; Taherzadeh, M.J.; Lennartsson, P.R. Pigment production by the edible filamentous fungus neurospora intermedia. Fermentation 2018, 4, 11. [Google Scholar] [CrossRef]
- Fujita, M.; Iwahori, K.; Tatsuta, S.; Yamakawa, K. Analysis of pellet formation of aspergillus niger based on shear stress. J. Ferment. Bioeng. 1994, 78, 368–373. [Google Scholar] [CrossRef]
- Liu, Y.; Liao, W.; Chen, S. Study of pellet formation of filamentous fungi rhizopus oryzae using a multiple logistic regression model. Biotechnol. Bioeng. 2008, 99, 117–128. [Google Scholar] [CrossRef]
- Saraswathy, A.; Hallberg, R. Mycelial pellet formation by penicillium ochrochloron species due to exposure to pyrene. Microbiol. Res. 2005, 160, 375–383. [Google Scholar] [CrossRef]
- Zhou, Y.; Du, J.; Tsao, G.T. Mycelial pellet formation by rhizopus oryzae atcc 20344. Appl. Biochem. Biotechnol. 2000, 84, 779–789. [Google Scholar] [CrossRef]
- Nair, R.B.; Lennartsson, P.R.; Taherzadeh, M.J. Mycelial pellet formation by edible ascomycete filamentous fungi, neurospora intermedia. AMB Express 2016, 6, 1. [Google Scholar] [CrossRef]
- Tough, A.; Prosser, J. Experimental verification of a mathematical model for pelleted growth of streptomyces coelicolor a3 (2) in submerged batch culture. Microbiology 1996, 142, 639–648. [Google Scholar] [CrossRef]
- Cui, Y.; Van der Lans, R.; Luyben, K. Effect of agitation intensities on fungal morphology of submerged fermentation. Biotechnol. Bioeng. 1997, 55, 715–726. [Google Scholar] [CrossRef]
- de Oliveira, F.; Ferreira, L.C.; Neto, Á.B.; Teixeira, M.F.S.; Ebinuma, V.d.C.S. Biosynthesis of natural colorant by talaromyces amestolkiae: Mycelium accumulation and colorant formation in incubator shaker and in bioreactor. Biochem. Eng. J. 2020, 161, 107694. [Google Scholar] [CrossRef]
- Cho, Y.; Park, J.; Hwang, H.; Kim, S.; Choi, J.; Yun, J. Production of red pigment by submerged culture of paecilomyces sinclairii. Lett. Appl. Microbiol. 2002, 35, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Kurono, M.N.K.; Shindo, K.; Tada, M. Biosyntheses of monascorubrin and monascoflavin. Chem. Pharm. Bull. 1963, 11, 359–362. [Google Scholar] [CrossRef]
- de Oliveira, F.; Lima, C.d.A.; Lopes, A.M.; Marques, D.d.A.V.; Druzian, J.I.; Pessoa Júnior, A.; Santos-Ebinuma, V.C. Microbial colorants production in stirred-tank bioreactor and their incorporation in an alternative food packaging biomaterial. J. Fungi 2020, 6, 264. [Google Scholar] [CrossRef]
- Lee, B.-K.; Park, N.-H.; Piao, H.Y.; Chung, W.-J. Production of red pigments bymonascus purpureus in submerged culture. Biotechnol. Bioprocess Eng. 2001, 6, 341–346. [Google Scholar] [CrossRef]
- Pisareva, E.; Savov, V.; Kujumdzieva, A. Pigments and citrinin biosynthesis by fungi belonging to genus monascus. Z. Für Nat. C 2005, 60, 116–120. [Google Scholar] [CrossRef]
- de Oliveira, F.; Pedrolli, D.B.; Teixeira, M.F.S.; de Carvalho Santos-Ebinuma, V. Water-soluble fluorescent red colorant production by talaromyces amestolkiae. Appl. Microbiol. Biotechnol. 2019, 103, 6529–6541. [Google Scholar] [CrossRef]


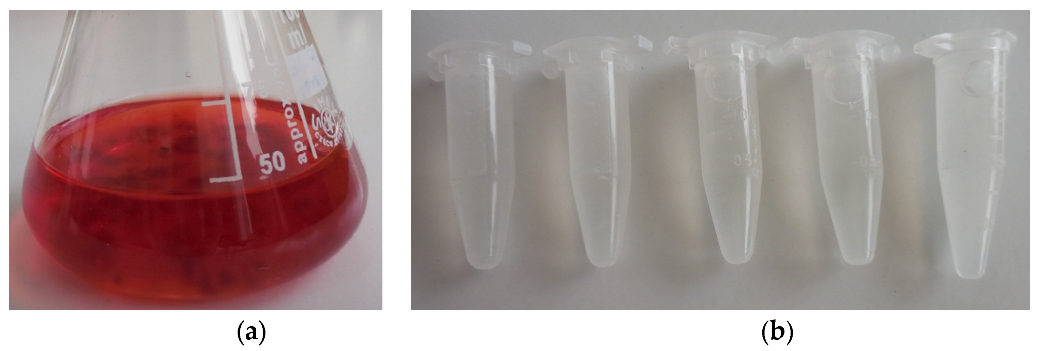
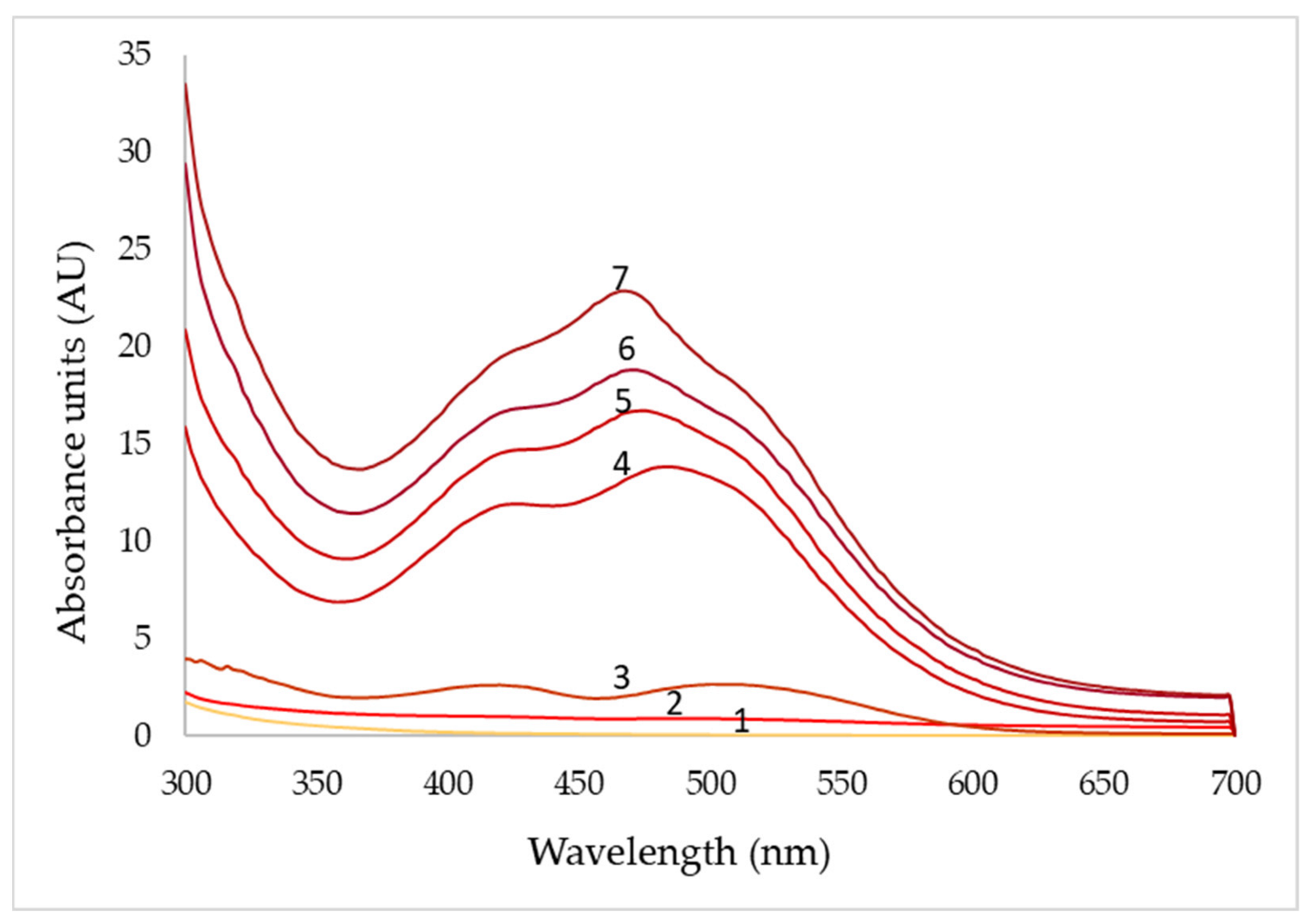
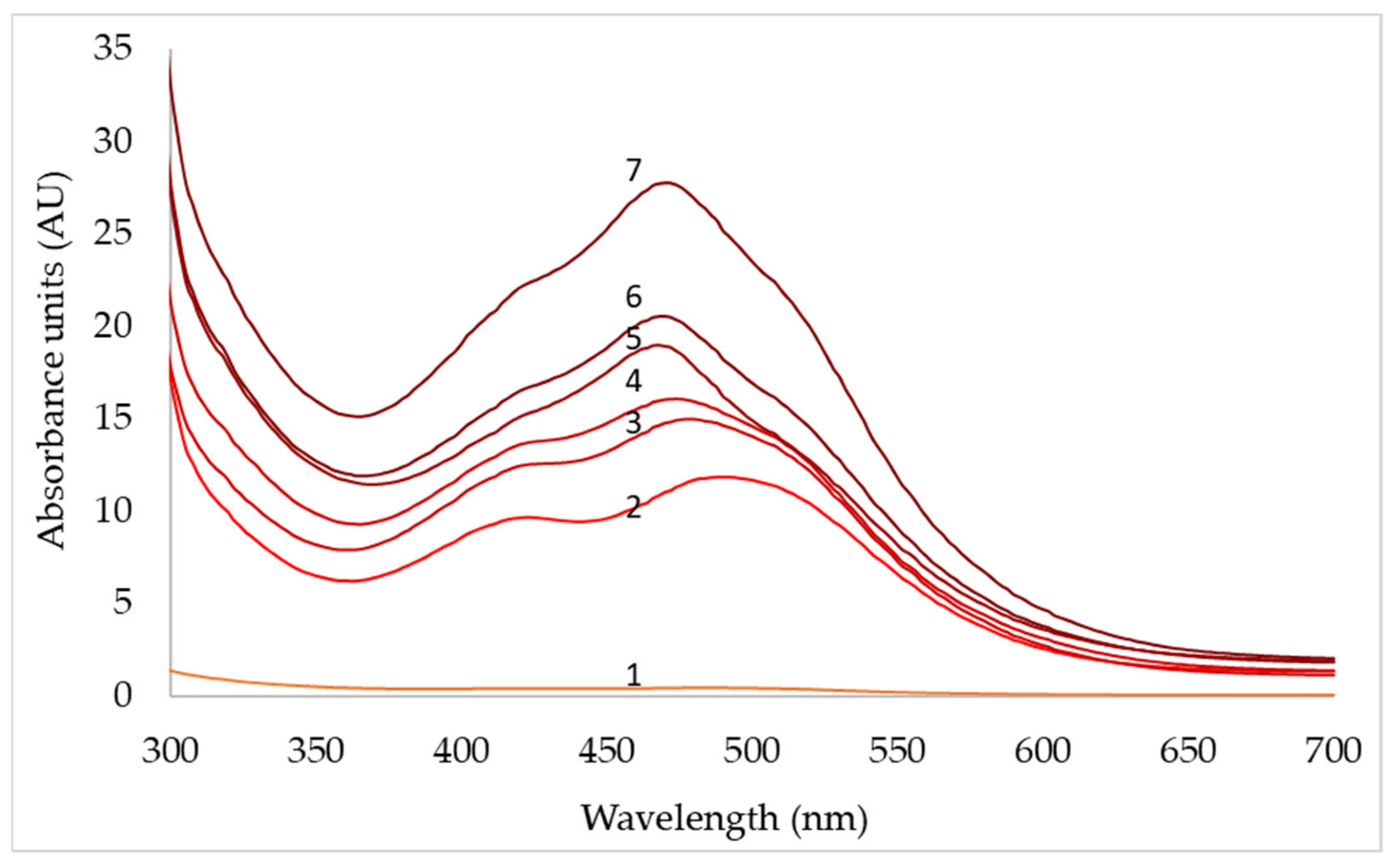
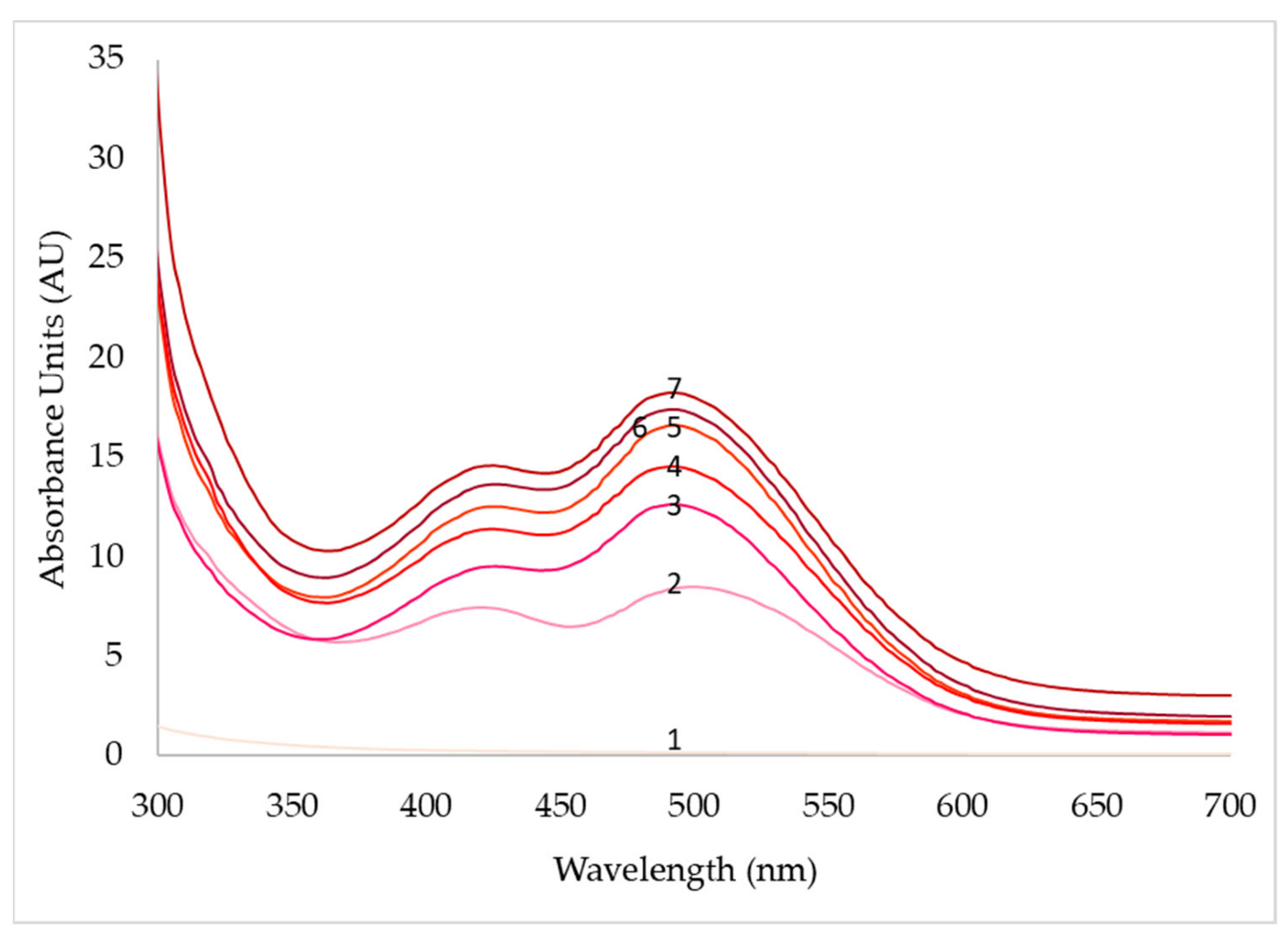


| pH | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|
| Day of maximum absorbance | 9 | 7 | 8 | 9 | 8 | 9 |
| Maximum absorbance (AU) ± Standard error | 6.74 ± 0.84 | 14.91 ± 1.03 | 22.39 ± 2.59 | 21.70 ± 1.66 | 10.14 ± 0.91 | 7.22 ± 0.33 |
| pH | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|
| Day of maximum absorbance | 9 | 7 | 8 | 8 | 8 | 9 |
| Maximum absorbance (AU) ± Standard error | 4.04 ± 0.36 | 13.29 ± 0.42 | 14.84 ± 1.93 | 13.42 ± 0.83 | 9.94 ± 0.35 | 7.41 ± 0.26 |
| Days | pO2 (50%) | pO2 (70%) | pO2 (90%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 430 nm | 470 nm | 500 nm | 430 nm | 470 nm | 500 nm | 430 nm | 470 nm | 500 nm | |
| 1 | 0.1 | 0.1 | 0.1 | 0.4 | 0.2 | 0.2 | 0.3 | 0.2 | 0.2 |
| 2 | 0.9 | 1.0 | 1.2 | 9.5 | 11.3 | 11.6 | 7.4 | 7.1 | 8.4 |
| 3 | 2.3 | 2.2 | 2.8 | 12.5 | 15.1 | 14.4 | 9.2 | 11.1 | 12.3 |
| 4 | 12.2 | 13.5 | 134 | 14.6 | 16.0 | 14.6 | 11.4 | 13.2 | 14.8 |
| 5 | 14.7 | 16.8 | 15.4 | 16.4 | 19.2 | 15.0 | 12.7 | 15.4 | 16.6 |
| 6 | 16.9 | 19.6 | 17.4 | 18.2 | 20.7 | 16.6 | 13.6 | 15.6 | 17.4 |
| 7 | 20.2 | 24.6 | 19.2 | 22.8 | 27.2 | 23.5 | 14.5 | 16.4 | 18.0 |
| pO2 at 50% | |||||
|---|---|---|---|---|---|
| Days | L* (D65) | a* (D65) | b* (D65) | C* (D65) | h (D65) |
| 1 | 96.36 | 1.56 | 0.87 | 3.13 | 152.43 |
| 2 | 94.91 | 3.75 | 1.85 | 4.66 | 151.57 |
| 3 | 68.26 | 41.39 | 27.11 | 49.49 | 33.33 |
| 4 | 39.44 | 60.13 | 66.77 | 89.87 | 48.02 |
| 5 | 31.76 | 58.21 | 54.93 | 80.04 | 43.35 |
| 6 | 32.07 | 57.81 | 55.01 | 79.81 | 43.59 |
| 7 | 31.24 | 57.54 | 55.30 | 79.81 | 43.86 |
| pO2 at 70% | |||||
|---|---|---|---|---|---|
| Days | L* (D65) | a* (D65) | b* (D65) | C* (D65) | h (D65) |
| 1 | 93.85 | 4.36 | 3.71 | 5.75 | 36.98 |
| 2 | 51.88 | 60.31 | 71.75 | 93.88 | 49.65 |
| 3 | 45.21 | 61.84 | 77.24 | 99.08 | 51.15 |
| 4 | 37.53 | 59.60 | 64.48 | 87.92 | 47.13 |
| 5 | 34.25 | 60.08 | 58.73 | 84.01 | 44.35 |
| 6 | 34.03 | 58.96 | 58.40 | 83.00 | 44.70 |
| 7 | 36.94 | 58.82 | 63.42 | 86.52 | 47.15 |
| pO2 at 90% | |||||
|---|---|---|---|---|---|
| Days | L* (D65) | a* (D65) | b* (D65) | C* (D65) | h (D65) |
| 1 | 98.05 | 0.24 | 0.3 | 0.38 | 52.08 |
| 2 | 93.71 | 5.48 | 3.74 | 6.63 | 34.3 |
| 3 | 76.16 | 30.1 | 24.6 | 38.88 | 39.26 |
| 4 | 56.08 | 55.97 | 62.1 | 83.6 | 47.98 |
| 5 | 53.24 | 58.43 | 65.08 | 87.46 | 48.08 |
| 6 | 52.59 | 56.16 | 61.51 | 83.29 | 47.61 |
| 7 | 56.87 | 52.86 | 54.61 | 76.01 | 45.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatachalam, M.; Mares, G.; Dufossé, L.; Fouillaud, M. Scale-Up of Pigment Production by the Marine-Derived Filamentous Fungus, Talaromyces albobiverticillius 30548, from Shake Flask to Stirred Bioreactor. Fermentation 2023, 9, 77. https://doi.org/10.3390/fermentation9010077
Venkatachalam M, Mares G, Dufossé L, Fouillaud M. Scale-Up of Pigment Production by the Marine-Derived Filamentous Fungus, Talaromyces albobiverticillius 30548, from Shake Flask to Stirred Bioreactor. Fermentation. 2023; 9(1):77. https://doi.org/10.3390/fermentation9010077
Chicago/Turabian StyleVenkatachalam, Mekala, Gary Mares, Laurent Dufossé, and Mireille Fouillaud. 2023. "Scale-Up of Pigment Production by the Marine-Derived Filamentous Fungus, Talaromyces albobiverticillius 30548, from Shake Flask to Stirred Bioreactor" Fermentation 9, no. 1: 77. https://doi.org/10.3390/fermentation9010077
APA StyleVenkatachalam, M., Mares, G., Dufossé, L., & Fouillaud, M. (2023). Scale-Up of Pigment Production by the Marine-Derived Filamentous Fungus, Talaromyces albobiverticillius 30548, from Shake Flask to Stirred Bioreactor. Fermentation, 9(1), 77. https://doi.org/10.3390/fermentation9010077









