Sensory Assessment of Bi-Enzymatic-Treated Glucose-Galactose Syrup
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Production of GGS
2.2.1. Hydrolysis of Permeates
2.2.2. Isomerisation
2.3. Lactose, Glucose, Fructose and Galactose Analyses
2.4. Galacto-Oligosaccharides (GOS) Analyses
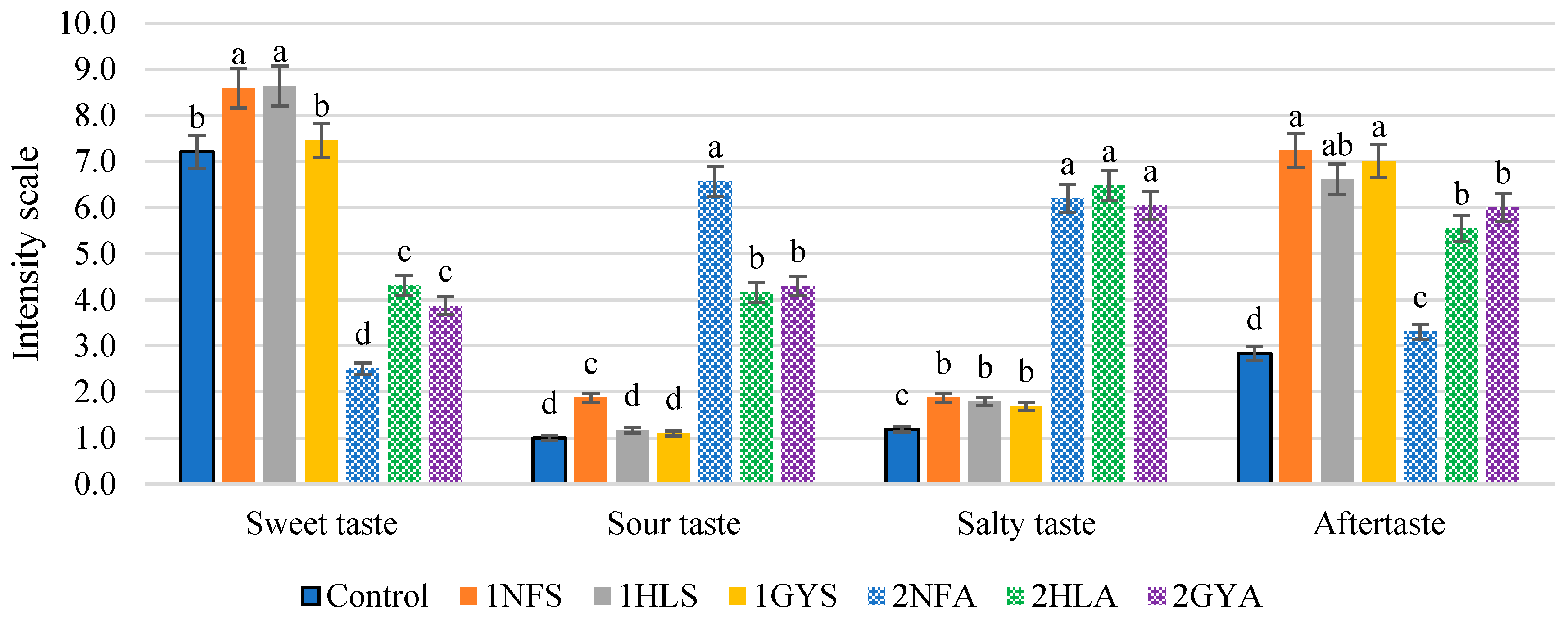
2.5. Sensory Evaluation
2.6. Statistical Analysis
3. Results
3.1. First-Stage Fermentation with β-Galactosidase
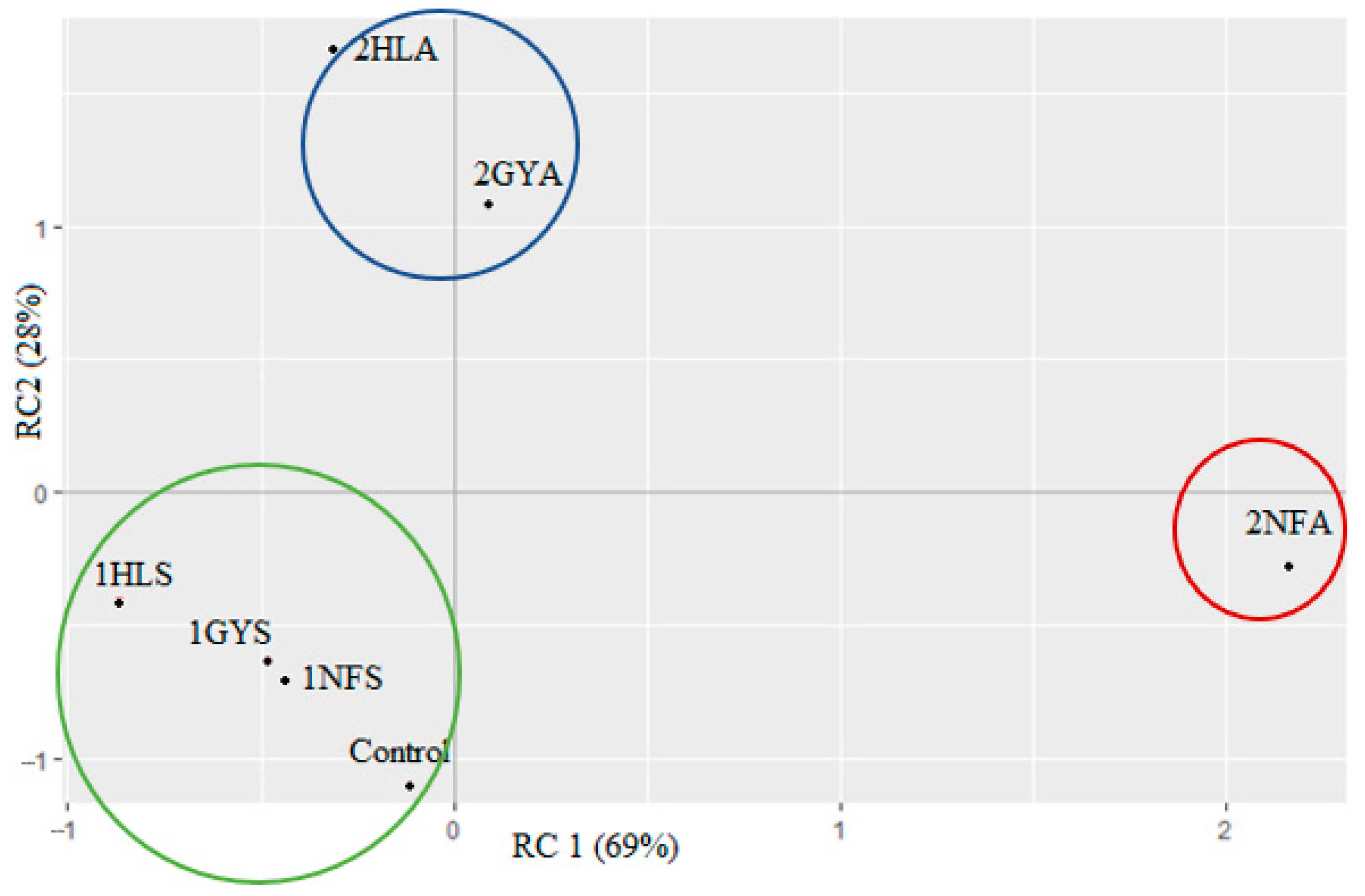
The Results of Sensory Test 1
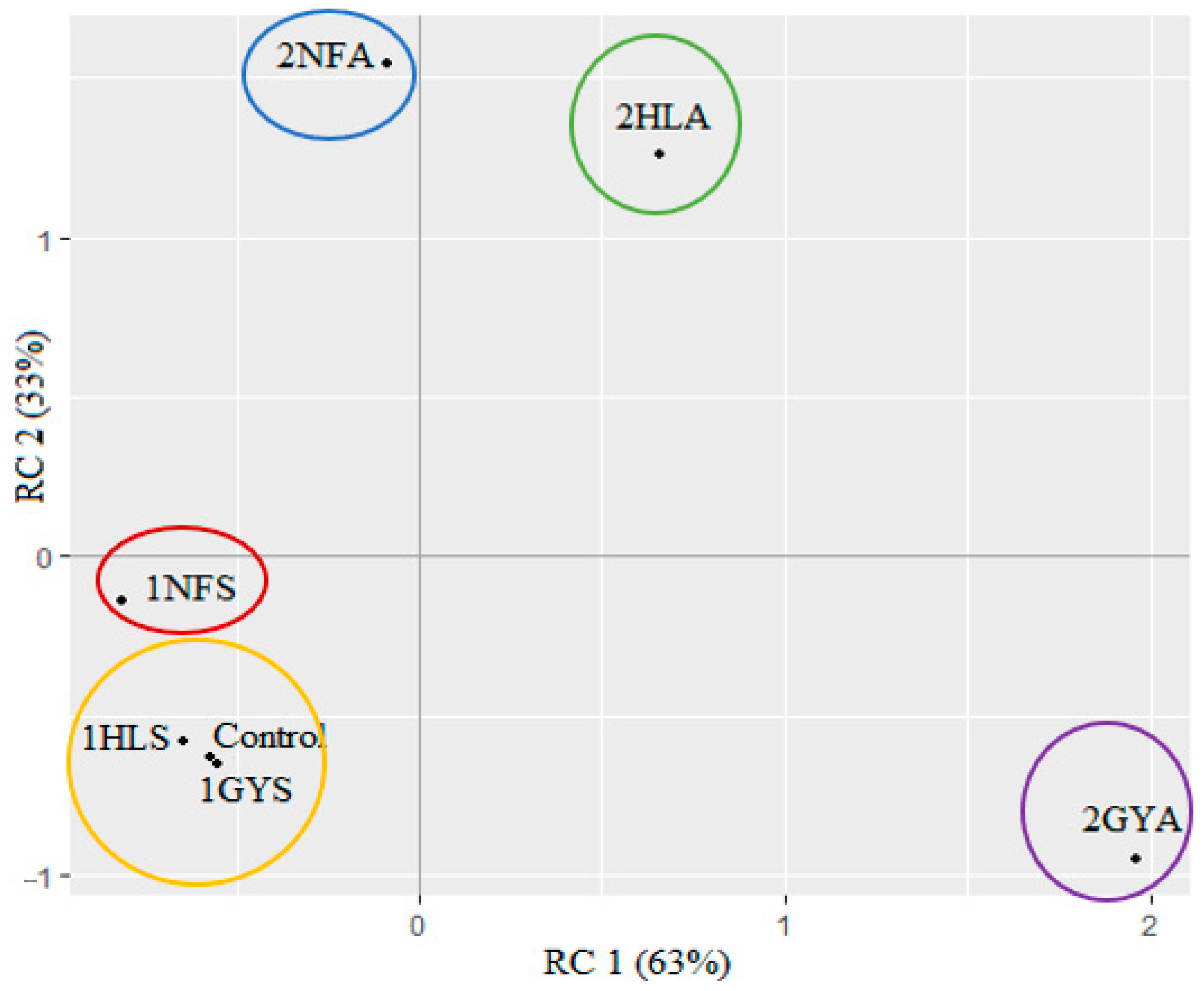
3.2. Second-Stage Fermentation with Glucose Isomerase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ryan, M.P.; Walsh, G. The biotechnological potential of whey. Rev. Environ. Sci. Biotechnol. 2016, 15, 479–498. [Google Scholar] [CrossRef]
- Simović, M.; Milivojević, A.; Ćorović, M.; Banjanac, K.; Bezbradica, D. Whey valorization using transgalactosylation activity of immobilized β-galactosidase. Int. J. Food Sci. 2019, 54, 3074–3082. [Google Scholar] [CrossRef]
- Anand, S.; Som Nath, K.; Chenchaiah, M. Whey and whey products. In Milk and Dairy Products in Human Nutrition: Production, Composition and Health; Park, Y.W., Haenlein, G.F.W., Eds.; John Wiley and Sons, Ltd.: Chichester, UK, 2013; pp. 477–497. [Google Scholar] [CrossRef]
- Carpin, M.; Bertelsen, H.; Bech, J.K.; Jeantet, R.; Risbo, J.; Schuck, P. Caking of lactose: A critical review. Trends Food Sci. Technol. 2016, 53, 1–12. [Google Scholar] [CrossRef]
- Wong, S.Y.; Hartel, R.W. Crystallization in lactose refining: Review. J. Food Sci. 2014, 79, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Batista, K.A.; Silva, C.N.S.; Fernandes, P.M.; Campos, I.T.N.; Fernandes, K.F. Development of a new bioaffinity stationary phase for lactose removal using a lactose-binding lectin immobilized onto polyaniline. Sep. Purif. Technol. 2017, 185, 54–60. [Google Scholar] [CrossRef]
- Panesar, P.; Kumari, S.; Panesar, R. Potential applications of immobilized β-galactosidase in food processing industries. Enzyme Res. 2010, 2010, 473137. [Google Scholar] [CrossRef]
- Dutra Rosolen, M.; Gennari, A.; Volpato, G.; De Souza, C.F.V. Lactose hydrolysis in milk and dairy whey using microbial β-galactosidases. Enzyme Res. 2015, 2015, 806240. [Google Scholar] [CrossRef]
- Saqib, S.; Akram, A.; Halim, S.A.; Tassaduq, R. Sources of β-galactosidase and its applications in food industry. 3 Biotech 2017, 7, 79. [Google Scholar] [CrossRef]
- Adalberto, P.R.R.; Massabni, A.C.C.; Carmona, E.C.C.; Goulart, A.J.J.; Marques, D.P.P.; Monti, R. Effect of divalent metal ions on the activity and stability of β-galactosidase isolated from Kluyveromyces lactis. Rev. Ciênc. Farm. Básica E Apl. 2010, 31, 143–150. [Google Scholar]
- Shen, J.; Chen, J.; Jensen, P.R.; Solem, C. Sweet as sugar—Efficient conversion of lactose into sweet sugars using a novel whole—Cell catalyst. J. Agric. Food Chem. 2019, 67, 6257–6262. [Google Scholar] [CrossRef]
- Joesten, M.D.; Wood, J.L.; Castellion, M.E. The world of chemistry: Essentials, 3rd ed.; Brooks/Cole: Belmont, CA, USA, 2007; p. 231. [Google Scholar]
- Rocha, J.M.; Guerra, M.A. On the valorisation of lactose and its derivatives from cheese whey as a dairy industry by-product: An overview. Eur. Food Res. Technol. 2020, 246, 2161–2174. [Google Scholar] [CrossRef]
- Evdokimov, I.A.; Kulikova, I.K.; Volkova, E.I.; Oleshkevich, O.I.; Shramko, M.I.; Voblikova, T.V. Modification of the carbohydrate component of ice cream and frozen desserts using glucose-galactose syrup. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Veliky Novgorod, Russia, 22 October 2020. [Google Scholar]
- Lindsay, M.J.; Walker, T.W.; Dumesic, J.A.; Rankin, S.A.; Huber, G.W. Production of monosaccharides and whey protein from acid whey waste streams in the dairy industry. Green Chem. 2018, 20, 1824–1834. [Google Scholar] [CrossRef]
- Suárez, S.; Guerrero, C.; Vera, C.; Illanes, A. Effect of particle size and enzyme load on the simultaneous reactions of lactose hydrolysis and transgalactosylation with glyoxyl-agarose immobilized β-galactosidase from Aspergillus oryzae. Process Biochem. 2018, 73, 56–64. [Google Scholar] [CrossRef]
- Belitz, H.D.; Grosch, W.; Schieberle, P. Amino Acids, Peptides, Proteins. In Food Chemistry, 4th ed.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 27–56. [Google Scholar]
- Majore, K.; Ciprovica, I. Bioconversion of lactose into glucose–galactose syrup by two-stage enzymatic hydrolysis. Foods 2022, 11, 400. [Google Scholar] [CrossRef]
- Žolnere, K.; Ciproviča, I.; Ķirse, A.; Cinkmanis, I. A study of commercial β-galactosidase stability under simulated in vitro gastric conditions. Agron. Res. 2018, 16, 1555–1562. [Google Scholar] [CrossRef]
- Srivastava, A.; Tripathi, R.; Verma, S.; Srivastava, N.; Rawat, A.K.S.; Deepak, D. A novel method for quantification of lactose in mammalian milk through HPTLC and determination by a mass spectrometric technique. Anal. Methods 2014, 6, 7268–7276. [Google Scholar] [CrossRef]
- Lee, C.H.; Kim, H.T.; Yun, E.J.; Lee, A.R.; Kim, S.R.; Kim, J.H.; Choi, I.G.; Kim, K.H. A novel agarolytic β-galactosidase acts on agaro-oligosaccharides for complete hydrolysis of agarose into monomers. Appl. Environ. Microbiol. 2014, 80, 5965–5973. [Google Scholar] [CrossRef] [PubMed]
- ISO 4121:2003; Sensory Analysis—Guidelines for the Use of Quantitative Response Scales. International Organization for Standardization: Geneva, Switzerland, 2003.
- Rodrigues, J.F.; Siman, I.B.; de Oliveira, L.E.A.; de Fátima Barcelos, A.; Oliveira, R.A.A.; Silva, R.; da Cruz, A.G. Use of diaries as a research strategy on sensory perception and consumer behavior of Canastra cheese. J. Sens. Stud. 2021, 36, e12627. [Google Scholar] [CrossRef]
- Fischer, C.; Kleinschmidt, T. Synthesis of galacto-oligosaccharides using sweet and acid whey as a substrate. Int. Dairy J. 2015, 48, 15–22. [Google Scholar] [CrossRef]
- Juajun, O.; Nguyen, T.-H.H.; Maischberger, T.; Iqbal, S.; Haltrich, D.; Yamabhai, M. Cloning, purification, and characterization of β-galactosidase from Bacillus licheniformis DSM 13. Appl. Microbiol. Biotechnol. 2011, 89, 645–654. [Google Scholar] [CrossRef]
- Xavier, J.R.; Ramana, K.V.; Sharma, R.K. β-Galactosidase: Biotechnological applications in food processing. J. Food Biochem. 2018, 42, e12564. [Google Scholar] [CrossRef]
- Demirhan, E.; Apar, D.K.; Ozbek, B. Product inhibition of whey lactose hydrolysis. Chem. Eng. Commun. 2007, 195, 293–304. [Google Scholar] [CrossRef]
- Popescu, L.; Bulgaru, V.; Siminiuc, R. Effect of temperature, Ph and amount of enzyme used in the lactose hydrolysis of milk. Food Sci. Nutr. 2021, 12, 1243–1254. [Google Scholar] [CrossRef]
- Botvynko, A.; Bednářová, A.; Henke, S.; Shakhno, N.; Čurda, L. Production of galacto-oligosaccharides using various combinations of the commercial β-galactosidases. Biochem. Biophys. Res. Commun. 2019, 517, 762–766. [Google Scholar] [CrossRef]
- Osmak, T.; Mleko, S.; Bass, O.; Mykhalevych, A.; Kuzmyk, U. Enzymatic hydrolysis of lactose in concentrates of reconstituted demineralized whey, intended for ice cream production. Ukr. Food J. 2021, 10, 277–288. [Google Scholar] [CrossRef]
- Vidya, B.; Palaniswamy, M.; Gopalakrishnan, V.K. Screening and optimization of β-galactosidase from fungal strains by using agro residues. World J. Pharm. Sci. 2014, 3, 1809–1821. [Google Scholar]
- Bozanic, R.; Barukcic, I.; Lisak, K.; Jakopovic, T.L. Possibilities of whey utilisation. Austin J. Nutr. Food Sci. 2014, 2, 1036–1042. [Google Scholar]
- Kumar, A.; Kathuria, D.; Sharma, S.K.; Kumar, S. Optimizing process to prepare lactose hydrolysed milk by enzymatic treatment. J. Hill Agric. 2018, 3, 361–369. [Google Scholar] [CrossRef]
- Pawlos, M.; Znamirowska, A.; Kluz, M.; Szajnar, K.; Kowalczyk, M. Low-lactose fermented goat milks with Bifidobacterium animalis ssp. lactis Bb-12. J. Microbiol. Biotechnol. Food Sci. 2021, 9, 751–755. [Google Scholar] [CrossRef]
- Li, X.E.; Lopetcharat, K.; Qiu, Y.; Drake, M.A. Sugar reduction of skim chocolate milk and viability of alternative sweetening through lactose hydrolysis. J. Dairy Sci. 2015, 98, 1455–1466. [Google Scholar] [CrossRef]
- Merkel, A.; Voropaeva, D.; Ondrušek, M. The impact of integrated nanofiltration and electrodialytic processes on the chemical composition of sweet and acid whey streams. J. Food Eng. 2021, 298, 110500. [Google Scholar] [CrossRef]
- Wen, X.; Chen, A.; Wu, Y.; Yang, Y.; Xu, Y.; Xia, W.; Chen, S. Comparative evaluation of proximate compositions and taste attributes of three Asian hard clams (Meretrix meretrix) with different shell colors. Int. J. Food Prop. 2020, 23, 400–411. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hong, H.; Seo, H.; Pan, J.G.; Kim, E.J.; Maeng, P.J.; Yang, T.H.; Kim, K.J. High galacto-oligosaccharide production and a structural model for transgalactosylation of β-galactosidase ii from Bacillus circulans. J. Agric. Food Chem. 2020, 68, 13806–13814. [Google Scholar] [CrossRef] [PubMed]
- Van De Voorde, I.; Goiris, K.; Syryn, E.; Van den Bussche, C.; Aerts, G. Evaluation of the cold-active Pseudoalteromonas haloplanktis β-galactosidase enzyme for lactose hydrolysis in whey permeate as primary step of d-tagatose production. Process Biochem. 2014, 49, 2134–2140. [Google Scholar] [CrossRef]
- Cieśliński, H.; Wanarska, M.; Pawlak-Szukalska, A.; Krajewska, E.; Wicka, M.; Kur, J. Cold-active β-galactosidases: Sources, biochemical properties and their biotechnological potential. In Biotechnology of Extremophiles, 1st ed.; Rampelotto, P.H., Ed.; Springer: Cham, Switzerland, 2016; Volume 1, pp. 445–469. [Google Scholar] [CrossRef]
- Frankowski, K.M.; Miracle, R.E.; Drake, M.A. The role of sodium in the salty taste of permeate. J. Dairy Sci. 2014, 97, 5356–5370. [Google Scholar] [CrossRef]
- Kravtsov, V.; Kulikova, I.; Mikhaylin, S.; Bazinet, L. Alkalinization of acid whey by means of electrodialysis with bipolar membranes and analysis of induced membrane fouling. J. Food Eng. 2020, 277, 109891. [Google Scholar] [CrossRef]
- Onwulata, C.I.; Isobe, S.; Tomasula, P.M.; Cooke, P.H. Properties of whey protein isolates extruded under acidic and alkaline conditions. J. Dairy Sci. 2006, 89, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Luzzi, G.; Steffens, M.; Clawin-Rädecker, I.; Hoffmann, W.; Franz, C.M.; Fritsche, J.; Lorenzen, P.C. Enhancing the sweetening power of lactose by enzymatic modification in the reformulation of dairy products. Int. J. Dairy Tech. 2020, 73, 502–512. [Google Scholar] [CrossRef]
- Majore, K.; Ciproviča, I. Optimisation of lactose hydrolysis by combining solids and ß-galactosidase concentrations in whey permeates. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2020, 74, 263–269. [Google Scholar] [CrossRef]
- Lorenzen, P.C.; Breiter, J.; Clawin-Rädecker, I.; Dau, A. A novel bi-enzymatic system for lactose conversion. Int. J. Food Sci. 2013, 48, 1396–1403. [Google Scholar] [CrossRef]
- Araya, E.; Urrutia, P.; Romero, O.; Illanes, A.; Wilson, L. Design of combined crosslinked enzyme aggregates (combi-CLEAs) of β-galactosidase and glucose isomerase for the one-pot production of fructose syrup from lactose. Food Chem. 2019, 288, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, F.V.; Neifar, S.; Merdzo, Z.; Viña-Gonzalez, J.; Fernandez-Arrojo, L.; Ballesteros, A.O.; Fernandez-Lobato, M.; Bejar, S.; Plou, F.J. A three-step process for the bioconversion of whey permeate into a glucose D-free tagatose syrup. Catalysts 2020, 10, 647. [Google Scholar] [CrossRef]
- Cheng, S.; Hummel, M.; Dahal, B.; Gu, Z.; Kharel, P.; Martínez-Monteagudo, S.I. A two-step process for the synthesis of sweetening syrup from aqueous lactose. LWT 2020, 117, 108659. [Google Scholar] [CrossRef]
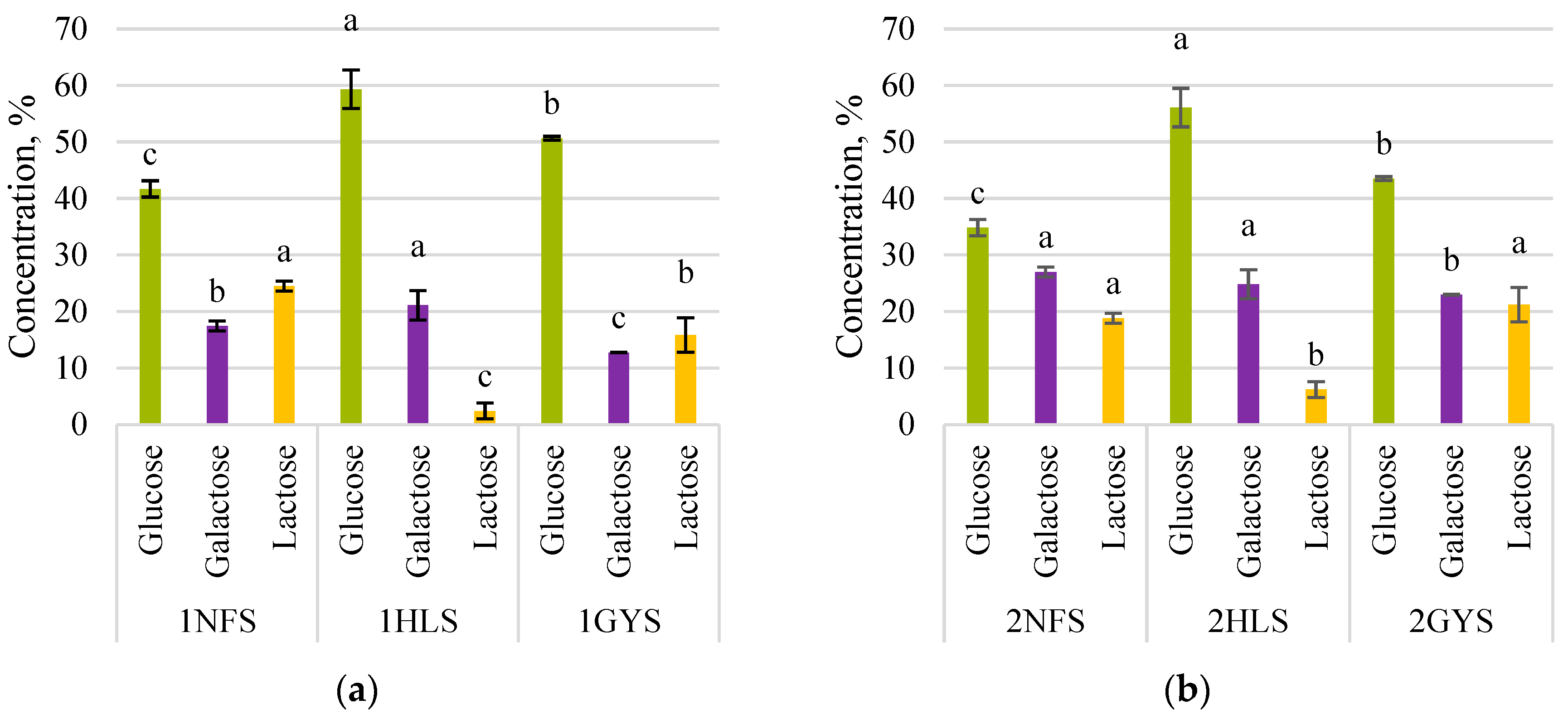


| Fructose | Galactose | Glucose | Lactose | GOS | |
|---|---|---|---|---|---|
| Control | - | 34 ± 2 c | 42 ± 2 a | 9 ± 1 b | 15 ± 1 b |
| 1NFS | 23 ± 2 ab | 37 ± 1 b | 22 ± 1 c | 6 ± 1 c | 12 ± 2 b |
| 1HLS | 25 ± 1 a | 30 ± 2 c | 16 ± 2 de | 5 ± 1 c | 22 ± 1 a |
| 1GYS | 20 ± 4 abc | 21 ± 3 d | 12 ± 4 e | 13 ± 1 a | 34 ± 7 a |
| 2NFA | 15 ± 6 bc | 49 ± 7 a | 29 ± 5 b | 5 ± 2 c | 2 ± 1 d |
| 2HLA | 16 ± 1 bc | 44 ± 1 a | 28 ± 5 bc | 4 ± 1 c | 8 ± 1 c |
| 2GYA | 14 ± 3 c | 38 ± 1 b | 23 ± 2 c | 10 ± 2 ab | 14 ± 4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majore, K.; Ciprovica, I. Sensory Assessment of Bi-Enzymatic-Treated Glucose-Galactose Syrup. Fermentation 2023, 9, 136. https://doi.org/10.3390/fermentation9020136
Majore K, Ciprovica I. Sensory Assessment of Bi-Enzymatic-Treated Glucose-Galactose Syrup. Fermentation. 2023; 9(2):136. https://doi.org/10.3390/fermentation9020136
Chicago/Turabian StyleMajore, Kristine, and Inga Ciprovica. 2023. "Sensory Assessment of Bi-Enzymatic-Treated Glucose-Galactose Syrup" Fermentation 9, no. 2: 136. https://doi.org/10.3390/fermentation9020136
APA StyleMajore, K., & Ciprovica, I. (2023). Sensory Assessment of Bi-Enzymatic-Treated Glucose-Galactose Syrup. Fermentation, 9(2), 136. https://doi.org/10.3390/fermentation9020136




