Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Materials
2.2. Experiment Settings
2.2.1. Effect of Ultrasonic Treatment on Yeast Fermentation Activity and Non-Viable Cell Count
2.2.2. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Glycolysis Enzymes and the Tricarboxylic Acid Cycle
2.2.3. Combined Treatment with Ultrasound and a Mix of Krebs Cycle Acids: Effect on the Assimilation and Synthesis of Nitrogenous Substances
2.3. Research Methods
3. Results and Discussion
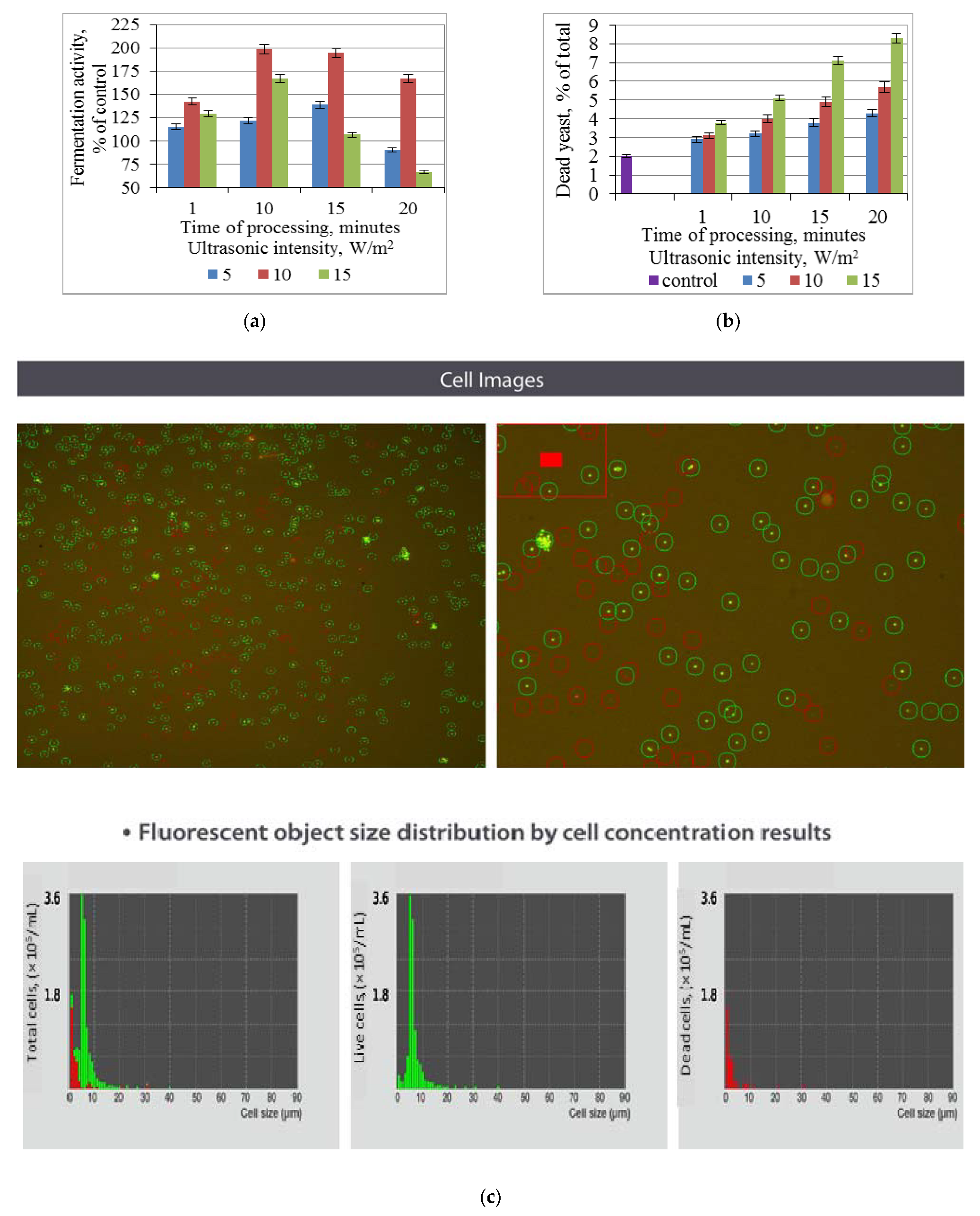
3.1. Effect of Ultrasonic Treatment of Brewer’s Yeast on Fermentation and Non-Viable Cell Count
3.2. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Glycolysis Enzymes and the Tricarboxylic Acid Cycle
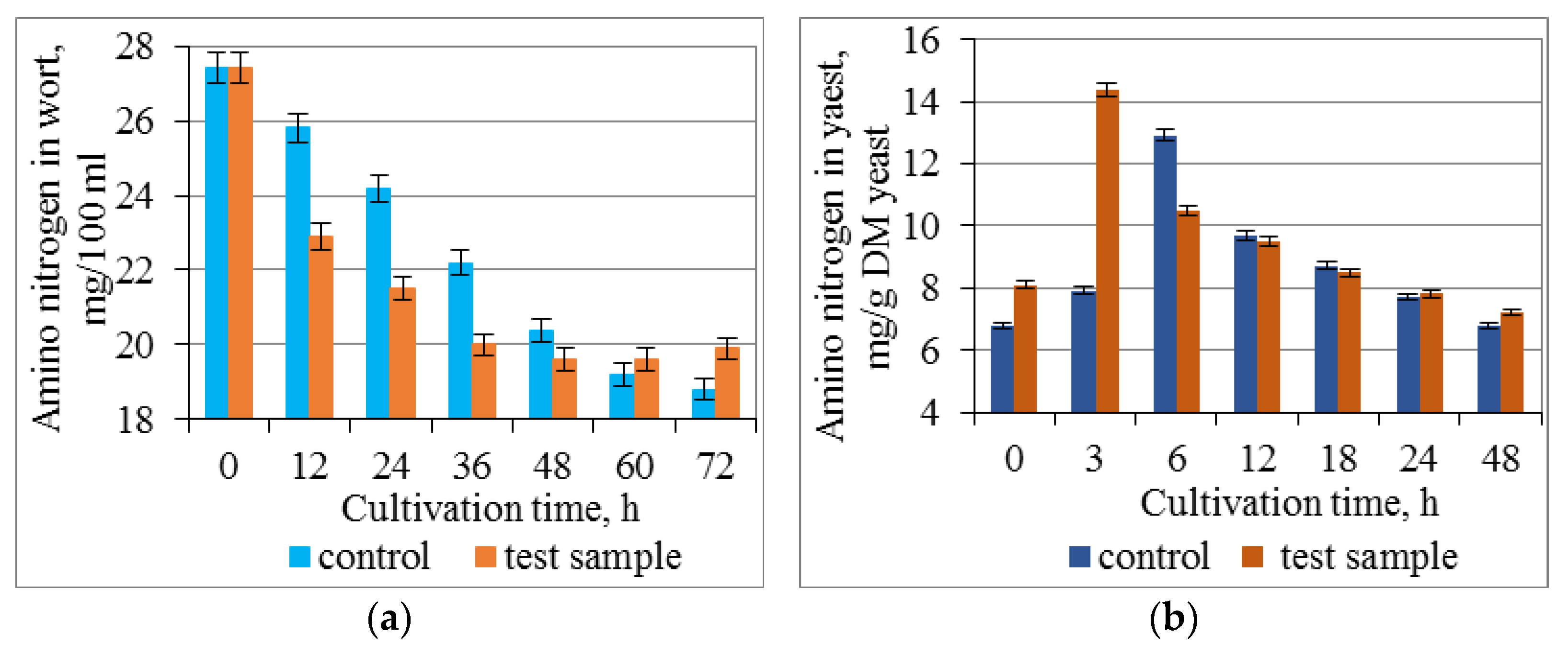
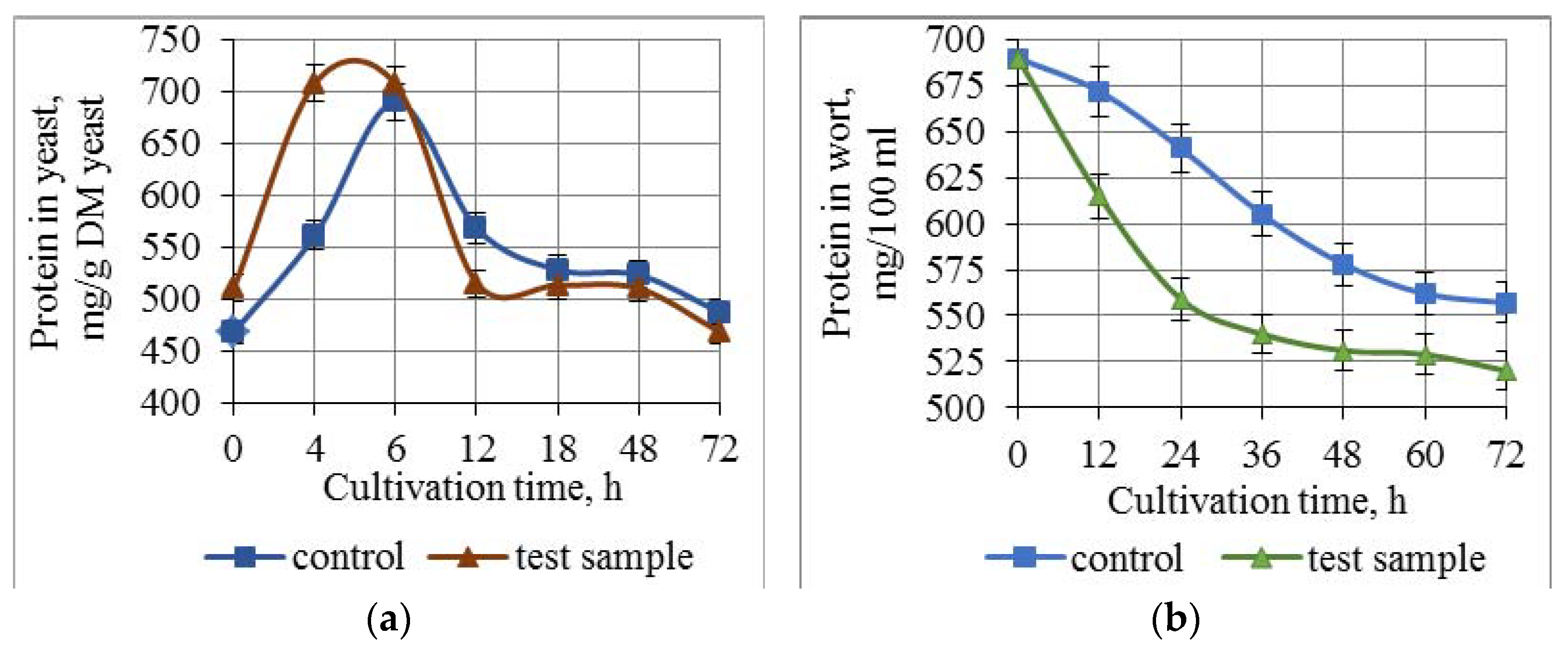
3.3. Effect of Combined Treatment of Yeast with Ultrasound and a Mix of Krebs Cycle Acids on the Assimilation and Synthesis of Nitrogenous Substances
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kurbanova, M.; Voroshilin, R.; Kozlova, O.; Atuchin, V. Effect of Lactobacteria on Bioactive Peptides and Their Sequence Identification in Mature Cheese. Microorganisms 2022, 10, 2068. [Google Scholar] [CrossRef] [PubMed]
- Vesnina, A.; Prosekov, A.; Atuchin, V.; Minina, V.; Ponasenko, A. Tackling Atherosclerosis via Selected Nutrition. Int. J. Mol. Sci. 2022, 23, 8233. [Google Scholar] [CrossRef] [PubMed]
- Kolberg, N.A.; Tikhonova, N.V.; Tikhonov, S.L.; Leontieva, S.A.; Sergeeva, I.Y. Immunotropic Effect of Bursal Peptides on Mice with Experimental Immunodeficiency. Food Process. Tech. Technol. 2022, 52, 296–309. [Google Scholar] [CrossRef]
- Yamada, E.A.; Scarbieri, V.C. Yeast (Saccharomyces cerevisiae) protein concentrate: Preparation, chemical composition, and nutritional and functional properties. J. Agric. Food Chem. 2005, 53, 3931–3936. [Google Scholar] [CrossRef] [PubMed]
- Rimareva, L.V.; Kurbatova, E.I.; Makarova, A.V. Biotechnological aspects of creating food additives with biocorrective action based on microbial biomass. Storage Process. Farm. Prod. 2011, 2, 45–47. [Google Scholar]
- Serba, E.M.; Rachkov, K.V.; Orlova, E.V.; Rimareva, L.V.; Pogorzshelskaya, N.S.; Polyakov, V.A. Fractional composition and fermentolizats functional properties of the yeast biomass. Proc. Samara Sci. Cent. Russ. Acad. Sci. 2013, 15, 1680–1681. [Google Scholar]
- Grishin, D.V.; Podobed, O.V.; Gladilina, Y.A.; Pokrovskaya, M.V.; Aleksandrova, S.S.; Pokrovsky, V.S.; Sokolov, N.N. Bioactive proteins and peptides: Current state and new trends of practical application in the food industry and feed production. Probl. Nutr. 2017, 86, 19–31. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Overchenko, M.B.; Ignatova, N.I.; Shelekhova, N.V.; Pogorzshelskaya, N.S.; Abramova, I.M. Biotechnological aspects of obtaining functional ingredients by the conversion of Saccharomyces cerevisiae 985-T biomass. Biotekhnologiya 2020, 36, 34–41. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Overchenko, M.B.; Ignatova, N.I.; Tadzhibova, P.; Zorin, S.N. Production of peptides and amino acids from microbial biomass in food and feed industries: Biotechnological aspects. Foods Raw Mater. 2020, 8, 268–276. [Google Scholar] [CrossRef]
- Yuraskina, T.V.; Sokolova, E.N.; Fursova, N.A.; Andreeva, S.S.; Serba, E.M. Innovative biotechnological approaches to the production of food ingredients based on enriched microorganisms. Food Ind. 2021, 9, 64–66. [Google Scholar] [CrossRef]
- Pereira, P.R.; Freitas, C.S.; Paschoalin, V.M.F. Saccharomyces cerevisiae biomass as a source of next-generation food preservatives: Evaluating potential proteins as a source of antimicrobial peptides. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4450–4479. [Google Scholar] [CrossRef] [PubMed]
- Jaehrig, S.C.; Rohn, S.; Kroh, L.W.; Wildenauer, F.; Lisdat, F.; Fleischer, L.-G.; Kurz, T. In vitro potential antioxidant activity of (1-3),(1-6)-β-D-glucan and protein fractions from Saccharomyces cerevisiae cell walls. Agric. Food Chem. 2007, 55, 4710–4716. [Google Scholar] [CrossRef]
- Sarmadi, B.H.; Ismail, A. Antioxidative peptides from food proteins: A review. Peptides 2010, 31, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Agyei, D.; Ongkudon, C.M.; Wei, C.Y.; Chan, A.S.; Danquah, M.K. Bioprocess challenges to the isolation and purification of bioactive peptides. Food Bioprod. Process. 2016, 98, 244–256. [Google Scholar] [CrossRef]
- Branco, P.; Francisco, D.; Monteiro, M.; Almeida, M.G.; Caldeira, J.; Arneborg, N.; Prista, C.; Albergaria, H. Antimicrobial properties and death-inducing mechanisms of saccharomycin, a biocide secreted by Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 2017, 101, 159–171. [Google Scholar] [CrossRef]
- Serba, E.M.; Rimareva, L.V.; Kurbatova, E.I.; Volkova, G.S.; Polyakov, V.A.; Varlamov, V.P. The study of the process of enzymatic hydrolysis of yeast biomass to generate food ingredients with the specified fractional composition of protein substances. Probl. Nutr. 2017, 86, 76–83. [Google Scholar] [CrossRef]
- Fakruddin, M.; Hossain, M.N.; Ahmed, M.M. Antimicrobial and antioxidant activities of Saccharomyces cerevisiaeIFST062013, a potential probiotic. BMC Complement. Altern. Med. 2017, 17, 64. [Google Scholar] [CrossRef]
- Agarkova, E.Y.; Kruchinin, A.G. Enzymatic conversion as a method of producing biologically active peptides. Bull. MSTU 2018, 21, 412–419. [Google Scholar] [CrossRef]
- Rizk, Z.; El Rayess, Y.; Ghanem, C.; Mathieu, F.; Taillandier, P.; Nehme, N. Identification of multiple-derived peptides produced by Saccharomyces cerevisiae involved in malolactic fermentation inhibition. FEMS Yeast Res. 2018, 18, foy080. [Google Scholar] [CrossRef]
- Al-Sahlany, S.T.G.; Altemim, A.B.; Al-Manhel, A.J.A.; Niamah, A.K.; Lakhssassi, N.; Ibrahim, S.A. Purification of bioactive peptide with antimicrobial properties produced by Saccharomyces cerevisiae. Foods 2020, 9, 324. [Google Scholar] [CrossRef]
- Tikhonov, S.L.; Tikhonova, N.V.; Kolberg, N.A.; Kudryashov, L.S. Systematization of scientific knowledge about the production technology and mechanism of action of certain biologically active peptides. Agro-Ind. Complex Russ. 2022, 29, 254–261. [Google Scholar] [CrossRef]
- Huang, G.; Chen, S.; Tang, Y.; Dai, C.; Sun, L.; Ma, H.; He, R. Stimulation of low intensity ultrasound on fermentation of skim milk medium for yield of yoghurt peptides by Lactobacillus paracasei. Ultrason. Sonochem. 2019, 51, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Mofa, N.N.; Zhapekova, A.O.; Bakkara, A.E. Ultrasonic processing is an effective method of directed synthesis of nanostructured systems (review). Combust. Plasma Chem. 2021, 19, 67–77. [Google Scholar] [CrossRef]
- Konovalov, S.A. Biochemistry of Yeast; Food Industry: Moscow, Russia, 1980. [Google Scholar]
- Annemuller, G.; Manger, H.-J.; Lietz, P. The Yeast in the Brewery; VLB Berlin: Berlin, Germany, 2011. [Google Scholar]
- Kudryasheva, A.A.; Tikhomirov, A.A. Features of protein biosynthesis by a unicellular organisms wand ways of its regulation. Food Ind. 2016, 7, 40–43. [Google Scholar]
- Starovojtova, O.V.; Sadrieva, A.A.; Mingaleeva, Z.S.; Reshetnik, O.A. Activation of yeast Saccharomyces cerevisiae in the technology of bread preparation. Bull. Kazan Technol. Univ. 2014, 1, 235–237. [Google Scholar]
- Krikunova, L.N.; Rjabova, S.M.; Peschanskaja, V.A.; Urusova, L.M. Influence of succinic acid on the metabolism of yeast Saccharomyces cerevisiae. Beer Drink. 2015, 1, 36–38. [Google Scholar]
- Miller, Y.Y.; Kiseleva, T.F.; Arysheva, I.V. Forming soy malt quality with organic growth promoters. Food Process. Tech. Technol. 2021, 51, 248–259. [Google Scholar] [CrossRef]
- Vereschagin, A.L.; Eremina, V.V.; Zakhareva, Y.I.; Khmeleva, A.N.; Kunets, L.L. Biological activity of ultra-small concentrations of some natural organic acids—Krebs cycle intermediates. Proc. Universities. Appl. Chem. Biotechnol. 2012, 2, 72–75. [Google Scholar]
- Vereshhagin, A.L.; Kunec, L.L. Influence of ultra-low concentrations of intermediates of the Krebs cycle on the growth and development of pure culture Staphylococus aureus. Proc. Universities. Appl. Chem. Biotechnol. 2012, 2, 143–144. [Google Scholar]
- Vereshchagin, A.L.; Nurminsky, V.N.; Eremina, V.V.; Zakharyeva, Y.I.; Ozolina, N.V.; Salyaev, R.K. Influence of a number of dicarboxylic acids in ultra-low concentrations on the barrier function of the membrane of an isolated vacuole. Bull. Irkutsk. State Univ. Ser. Biology. Ecol. 2013, 6, 3–7. [Google Scholar]
- Vereshchagin, A.L.; Tatarnikova, T.V.; Borina, L.L. Effect of Krebs cycle intermediates on the bactericidal activity of ampicillin and chloramphenicol against Staphylococcus aureus and Salmonella typhimurium. Polzunovskiy Vestn. 2015, 2, 128–130. [Google Scholar]
- Borina, L.L.; Vereshchagin, A.L. An effect of Krebs cycle intermediates on the growth and development of Pseudomonas aeruginosa. Proc. Universitets. Appl. Chem. Biotechnol. 2018, 8, 85–91. [Google Scholar] [CrossRef]
- Permyakova, L.V.; Pomozova, V.A.; Vereshchagin, A.L. Use of mix of acids of Krebs cycle in ultralow concentration for activation of culture of beer yeast. Beer Beverages 2018, 1, 20–24. [Google Scholar]
- Vereshchagin, A.L.; Kropotkina, V.V.; Khmeleva, A.N. On the mechanism of growth-stimulating action of ultra-low doses of natural organic acids. Bull. Altai State Agrar. Univ. 2010, 63, 46–48. [Google Scholar]
- Rogov, I.A.; Danilchuk, T.N. The mechanism of biological effects of extremely low doses of oscillatory and wave effects in the field of sound frequencies. Part 1. Biological effects of low-intensity physical impacts in food technologies. Elektron. Obrab. Mater. 2017, 53, 63–69. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Gernet, M.V.; Krjukova, E.V.; Gribkova, I.N.; Nurmukhanbetova, D.E.; Assembayeva, E.K. Acoustic vibration effect on genus Saccaromyces yeast population development. Bull. NAS RK 2019, 4, 103–112. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Karaycheva, A.I. Influence of polyfrequency acoustic impacts on the development of yeast populations. Colloq. J. Chem. Sci. Colloq. J. 2020, 8, 49–51. [Google Scholar] [CrossRef]
- Bodrova, O.J.; Krechetnikova, A.N. Activating and disintegrating effects of ultrasonic treatment of microorganisms. Hist. Sci. Technol. 2006, 4, 51–54. [Google Scholar]
- Chemat, F.; Zill-e-Huma; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–883. [Google Scholar] [CrossRef]
- Vereshchagin, A.L.; Khmeleva, A.N. Effect of Ultrasonic Irradiation and Growth Regulators on the Rhizogene Activity of Plant Objects; AltSTU Publishing House: Biysk, Russia, 2010. [Google Scholar]
- Yaldagard, M.; Mortazavi, S.A.; Tabatabaie, F. Application of ultrasonic waves as a priming technique for accelerating and enhancing the germination of barley seed: Optimization of method by the taguchi approach. J. Inst. Brew. 2008, 114, 14–21. [Google Scholar] [CrossRef]
- Ponomareva, E.I.; Alekhina, N.N.; Skvortsova, O.B. Changing the nutritional value of buckwheat grains when germination with using ultrasound-treated water. J. Izv. Vuzov. Food Technol. 2020, 1, 30–32. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Escott, C.; Loira, I.; Cuerda, R.; Suárez-Lepe, J.A. Sonication of yeast biomasses to improve the ageing on lees technique in red wines. Molecules 2019, 24, 635. [Google Scholar] [CrossRef] [PubMed]
- Gavahian, M.; Manyatsi, T.S.; Morata, A.; Tiwari, B.K. Ultrasound-assisted production of alcoholic beverages: From fermentation and sterilization to extraction and aging. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5243–5271. [Google Scholar] [CrossRef] [PubMed]
- Rogov, I.A.; Danilchuk, T.N. The mechanism of biological effects of extremely low doses of oscillatory and wave effects in the field of sound frequencies. Part II. Physicochemical model of the influence of low-intensity physical factors on the activity of hydrolytic enzymes. Elektron. Obrab. Mater. 2017, 53, 70–77. [Google Scholar] [CrossRef]
- Karpenko, D.V.; Kravchenko, V.S.; Shalaginov, K.V. Activation of an amylolytic enzyme preparation by wave action. Beer Drink. 2017, 5, 16–19. [Google Scholar]
- Kaluzhina, O.Y.; Yakovleva, K.S.; Kashapova, R.A.; Chernenkov, E.N.; Chernenkova, A.A.; Bodrov, A.Y. The effect of ultrasound on brewing yeast. Proc. VSUET 2020, 82, 103–109. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Salameh, D.; Maroun, R.G.; Louka, N.; Vorobiev, E. Ultrasound-assisted fermentation for cider production from Lebanese apples. Ultrason. Sonochem. 2020, 63, 104952. [Google Scholar] [CrossRef]
- Rakowska, R.; Sadowska, A.; Dybkowska, E.; Świderski, F. Spent yeast as natural source of functional food additives. Rocz Panstw. Zakl. Hig. 2017, 68, 115–121. [Google Scholar]
- Jaeger, A.; Arendt, E.K.; Zannini, E.; Sahin, A.W. Brewer’s spent yeast (BSY), an underutilized brewing by-product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Pomozova, V.A.; Permyakova, L.V.; Safonova, E.A.; Artemasov, V.V. Activation of brewer’s yeast. Beer Drink. 2002, 2, 26–27. [Google Scholar]
- Shershenkov, B.S.; Suchkova, E.P. Ultrasonic Modulation of Propionibacterium freudenreichii subsp. shermanii Metabolic Activity in Production of Enriched by B12 Vitamin Food Products. Available online: https://cyberleninka.ru/article/n/ultrazvukovaya-modulyatsiya-metabolicheskoy-aktivnosti-propionibacterium-freudenreichii-subsp-shermanii-pri-poluchenii-pischevyh (accessed on 25 October 2022).
- Vereshchagin, A.L.; Glushkova, Y.I. Influence of Krebs Cycle Intermediates and Physical Factors on Phytotoxicity of Solid Herbicides; AltSTU Publishing House: Biysk, Russia, 2017. [Google Scholar]
- Evstafev, S.N.; Khoang, K.K. Ultrasonic treatment of wheat straw in 1-butyl-3-methylimidazolium chloride. Chem. Plant Raw Mater. 2018, 1, 5–12. [Google Scholar] [CrossRef]
- Davydenko, S.G.; Dedegkayev, A.T.; Meledina, T.V. Development of a new express method of beer physiological influence assessment. Beer Drink. 2012, 5, 20–23. [Google Scholar]
- Klenova, N.A.; Makurina, O.N.; Pisareva, E.V.; Yazykova, M.Y. Special Workshop on the Biochemistry of Animals, Plants and Microorganisms; Educational allowance; Publishing House “S-Print”: Samara, Russia, 2013. [Google Scholar]
- Polygalina, G.V.; Cherednichenko, V.S.; Rimareva, L.V. Determination of Enzyme Activity: A Reference Book for Universities; DeLi Print: Moscow, Russia, 2003. [Google Scholar]
- Wordona, B.A.; Mortimerb, B.; McMaster, L.D. Comparative real-time analysis of Saccharomyces cerevisiae cell viability, injury and death induced by ultrasound (20 kHz) and heat for the application of hurdle technology. Food Res. Int. 2012, 47, 134–139. [Google Scholar] [CrossRef]
- Soro, A.B.; Oliveira, M.; O’Donnell, C.P.; Tiwari, B.K. Ultrasound assisted modulation of yeast growth and inactivation kinetics. Ultrason. Sonochem. 2021, 80, 105819. [Google Scholar] [CrossRef] [PubMed]
- Salogub, E.V.; Bogolyubova, J.S. The study of the influence of ultrasonic treatment on the biosorption properties of yeast. Probl. Mod. Sci. Educ. 2017, 22, 15–17. [Google Scholar]
- Soh, E.Y.; Lim, S.S.; Chew, K.W.; Phuang, X.W.; Ho, V.M.; Chu, K.Y.; Wong, R.; Lee, L.Y.; Tiong, T.J. Valorization of spent brewery yeast biosorbent with sonication-assisted adsorption for dye removal in wastewater treatment. Environ. Res. 2021, 204, 112385. [Google Scholar] [CrossRef]
- Iida, Y.; Tuziuti, T.; Yasui, K.; Kozuka, T.; Towata, A. Protein release from yeast cells as an evaluation method of physical effects in ultrasonic field. Ultrason. Sonochem. 2008, 15, 995–1000. [Google Scholar] [CrossRef]


| Variant | Treatment | Concentration of Krebs Cycle Acids, mol/L | Dose of Krebs Cycle Acids, % to Suspension Volume | Ultrasound Treatment Volume, W/m2 | Treatment Time, min |
|---|---|---|---|---|---|
| Control 1 (C1) | No treatment | - | - | - | - |
| Control 2 (C2) | Krebs cycle acids | 1 × 10−10 | 1 | - | 60 |
| Control 3 (C3) | Ultrasound | - | - | 10 | 10 |
| Test sample 1 (TS1) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 10 |
| Test sample 2 (TS2) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 5 |
| Test sample 3 (TS3) | Krebs cycle acids + ultrasound | 1 × 10−10 | 1 | 10 | 3 |
| Enzyme | Activity | |||||
|---|---|---|---|---|---|---|
| C1 | C2 | C3 | TS1 | TS2 | TS3 | |
| Zymase complex, µmol glucose/g DM·min | 85.4 ± 2.3 | 135.7 ± 3.4 | 105.1 ± 2.9 | 118.1 ± 2.6 | 146.3 ± 2.1 | 169.8 ± 3.2 |
| α-glucosidase, µmol maltose/g DM·min | 15.8 ± 0.4 | 36.5 ± 1.3 | 21.0 ± 0.8 | 29.8 ± 0.9 | 43.1 ± 1.5 | 52.0 ± 1.6 |
| β-fructofuranosidase, µmolsucrose/g DM·min | 32.5 ± 0.8 | 47.2 ± 1.6 | 39.2 ± 1.4 | 46.7 ± 1.3 | 62.5 ± 1.6 | 75.4 ± 1.5 |
| Proteolytic activity | Sample | Cultivation time, h | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 12 | 24 | 36 | 48 | 60 | 72 | ||
| extracellular protease, units/10 cm3 × 10−3 | Control | - | 0.63 ± 0.02 | 1.21 ± 0.03 | 1.36 ± 0.03 | 1.45 ± 0.03 | 0.75 ± 0.02 | 0.55 ± 0.02 |
| Test | - | 0.80 ± 0.02 | 1.34 ± 0.03 | 1.61 ± 0.03 | 0.83 ± 0.02 | 0.66 ± 0.02 | 0.60 ± 0.02 | |
| intracellular protease, units/g protein × 10−5 | Control | 9.10 ± 0.35 | 7.83 ± 0.30 | 6.62 ± 0.30 | 8.30 ± 0.35 | 13.23 ± 0.50 | 17.36 ± 0.50 | 11.90 ± 0.44 |
| Test | 12.21 ± 0.40 | 11.50 ± 0.40 | 11.94 ± 0.40 | 13.62 ± 0.50 | 20.55 ± 0.60 | 17.60 ± 0.50 | 12.41 ± 0.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Permyakova, L.; Sergeeva, I.; Dolgolyuk, I.; Starovoitova, K.; Atuchin, V.; Vereshchagin, A.; Romanenko, V.; Lashitsky, S. Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation 2023, 9, 132. https://doi.org/10.3390/fermentation9020132
Permyakova L, Sergeeva I, Dolgolyuk I, Starovoitova K, Atuchin V, Vereshchagin A, Romanenko V, Lashitsky S. Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation. 2023; 9(2):132. https://doi.org/10.3390/fermentation9020132
Chicago/Turabian StylePermyakova, Larisa, Irina Sergeeva, Irina Dolgolyuk, Kseniya Starovoitova, Victor Atuchin, Alexander Vereshchagin, Vasiliy Romanenko, and Sergey Lashitsky. 2023. "Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae" Fermentation 9, no. 2: 132. https://doi.org/10.3390/fermentation9020132
APA StylePermyakova, L., Sergeeva, I., Dolgolyuk, I., Starovoitova, K., Atuchin, V., Vereshchagin, A., Romanenko, V., & Lashitsky, S. (2023). Combined Effect of Ultrasound Treatment and a Mix of Krebs Cycle Acids on the Metabolic Processes in Saccharomyces cerevisiae. Fermentation, 9(2), 132. https://doi.org/10.3390/fermentation9020132







