1. Introduction
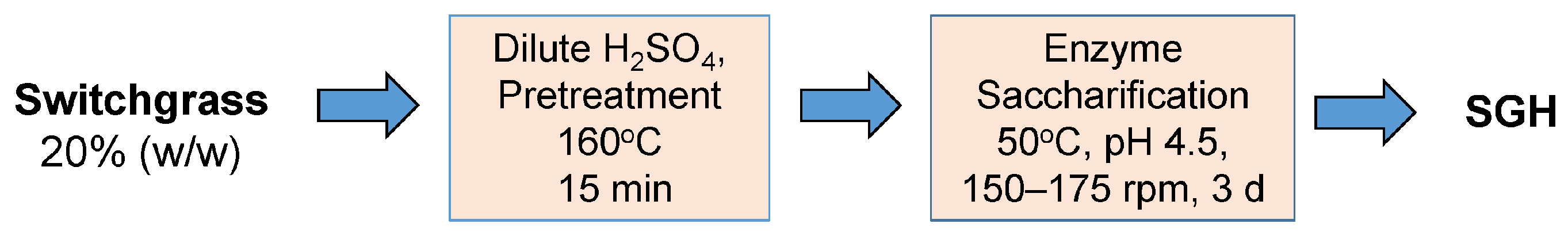
Switchgrass is a perennial grass that is being developed as a feedstock for production of renewable fuels and bioproducts. The grass is harvested post-frost and milled, then it is processed to yield fermentable sugars by chemical and biological catalysis (
Scheme 1), which involves pretreating, and hydrolyzing with cellulases and associated enzymes. The most common pretreatment consists of reacting the biomass with heated dilute sulfuric acid, which directly hydrolyzes xylan and activates the cellulose for enzymatic cleavage, as recently reviewed [
1]. Dilute sulfuric acid pretreatment offers low costs and versatility with a wide range of severities for the highly efficient pretreatment of a variety of biomass types [
2]. The favorable economics of dilute acid pretreatment followed by enzyme saccharification are attributed to high conversion rates and efficiencies and low costs of feedstock, acid catalysts, and enzymes which balance the moderate costs of capital investment and environmental management [
3,
4,
5].
One characteristic of post-frost harvested switchgrass is a low protein content [
6], and therefore, hydrolysates of switchgrass need to be supplemented with amino acids prior to fermentation [
7].
Scheffersomyces stipitis and other pentose-fermenting yeasts [
8], as well as traditional and engineered
Saccharomyces sp. yeasts [
9], need a supply of amino acids to support optimal growth and ethanol production, particularly on xylose, and especially when furan aldehyde inhibitors are present as an additional tax on metabolism [
10,
11]. Soy flour is a low-cost and readily available source of protein often used to supplement microbial processes [
12]. For example, Li [
13] found soy flour addition greatly beneficial to
S. cerevisiae growth and the fermentation of sugar beets and pointed out that both protein-based amino acids and fatty acids could be potential contributors. However, when used unhydrolyzed, soy flour provides only a low concentration of soluble free amino acids, a fraction of what would be readily available if the protein was fully hydrolyzed. Seredynski et al. [
14] showed that the proteolytic activity of
S. cerevisiae is substrate inducible, but complex, requiring several days to develop. Thus, amino acids from hydrolyzed protein are preferred to provide a ready supply of amino acids for timely utilization by yeast during growth and fermentation.
In prior studies to select hydrolysate-tolerant evolved strains of
Scheffersomyces stipitis, amino acids were supplied to screening fermentations by adding defatted soy flour to the dilute acid pretreatment of the switchgrass [
7]. During pretreatment, amino acids were released from the flour along with sugars (mainly xylose) from the hemicellulose fraction of the switchgrass. Enzyme saccharification was then applied to release glucose from the pretreated cellulosic fiber and finish xylose release from any remaining xylan (
Scheme 2).
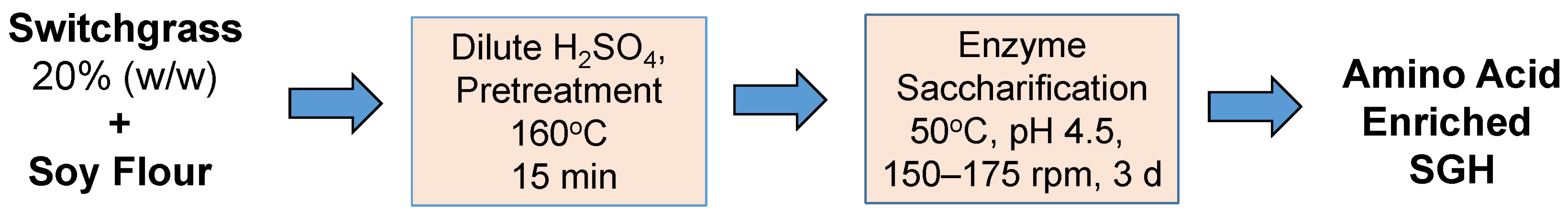
The current study aims to elucidate the efficiency of amino acid release from soy flour when hydrolyzed with dilute sulfuric acid. Two basic flour treatment strategies were primarily explored: either the soy flour and switchgrass were co-pretreated with dilute sulfuric acid to improve hydrolysate fermentability (
Scheme 2), or the flour was treated alone to maximize amino acid yield (
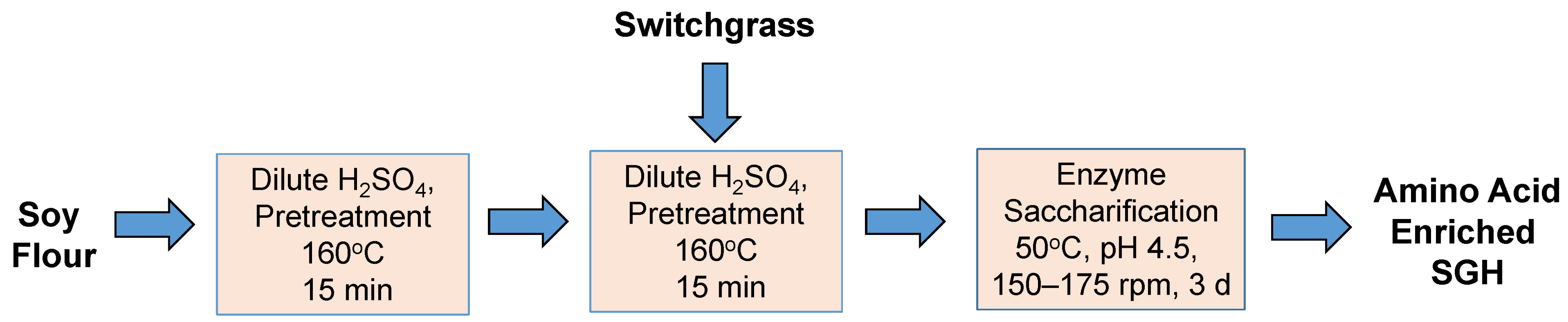
Scheme 3). In the course of the investigation, a hybrid of
Scheme 2 and
Scheme 3 was also considered as indicated in
Scheme 4, in which the soy flour was first hydrolyzed and then added to switchgrass pretreatment. Processing conditions (including concentrations of flour and acid, reaction time and temperature) were explored for various reaction schemes to elucidate their impact on hydrolysate composition relative to fermentability and the best route to maximize amino acid yields. The carbon source, key metabolic inhibitors (acetic acid, furfural and 5-hydroxymethylfurfural), and primary amino acid content were analyzed as indicators of fermentability. The results from these novel studies are expected to be directly pertinent to the postharvest protein fortification of switchgrass and other protein-deficient lignocellulosic substrates and to support microbial growth and sugar conversion to biofuels and bioproducts. No prior literature to date has addressed methods of applying soy flour during the pretreatment of lignocellulosic biomass to solve a problem with amino acid deficiency in fermentation feedstocks or to otherwise improve lignocellulosic hydrolysate fermentability.
Additionally, the application of dilute sulfuric acid under conditions typical of biomass pretreatment has not been thoroughly studied for soy flour hydrolysis, particularly in the 0.09–1.8 M acid concentration range at 160–180 °C for less than 60 min. It is this operating space that will be pursued in the current research because it is consistent with the common pretreatment equipment and materials that would be used in a biorefinery fed by switchgrass and other herbaceous biomass. The collection of amino acid yield and kinetic data with soy flour as a model will add to the literature supporting the development of a biorefinery process for producing primary amino acids from protein. It also aids our understanding and interpretation of the scenario in which we combine soy flour with the switchgrass pretreatment process.
As further background, a review of the literature shows that prior proteolysis studies have focused on applications of 6–10 N HCl and 6–12 N H
2SO
4 at 50 to 110 °C for 24–48 h to a variety of substrates, including casein, hemoglobin, and coconut and soybean meals [
15,
16,
17,
18]. The end products targeted by all of these studies were mainly soluble proteins as mixtures of peptides (~10 kDa), although low yields of primary amino acids were also reported. Focusing on amino acids rather than peptides as the goal of proteolysis, Scott et al. [
19] reviewed the literature to describe how biorefineries can add value to protein-containing coproducts such as DDGS (dried distiller grains with solubles) by producing amino acids with versatile functional groups (-NH
2 and –COOH) to support conversion to many other valuable chemicals. Weighing the pros and cons of available proteolysis methodologies suggests that more development is needed in this area in order to realize this scenario. The use of 6 N HCl requires prolonged high temperatures (24 h, 110 °C) to produce primary amino acids [
20], but these conditions lead to yield loss through sugar-amino Maillard and Amadori reactions [
21], and high equipment costs due to corrosion are a disadvantage [
19]. A few studies in the last decade have considered sulfuric acid as a potentially more economical alternative to HCl to catalyze proteolysis to produce primary amino acids. Most recently, Warnakulasuriya et al. [
22] compared 6 N HCl with 6 N H
2SO
4 at 110 °C and 24 h to hydrolyze canola cakes (post oil extraction) to, respectively, yield primary amino acids at 279 mg/g and 700 mg/g cake protein, showing an impressive yield advantage for sulfuric acid over hydrochloric acid, but acid concentrations are still high. Proteases are another alternative, but they are costly and have led to incomplete hydrolysis to amino acids, resulting in dilute solutions of amino acid-peptide mixtures [
23,
24]. Recently, however, improved thermophilic enzyme cocktails have been reported that synergistically produce ~10 mg/mL amino acids in 3 h at 60 °C [
25]. Supercritical water extraction has also been studied, but it requires acid/base catalysis for good yields and has prohibitively high energy and capital costs [
19].
Considering this background, low-cost dilute sulfuric acid proteolysis seems the most promising for economical amino acid production in a biorefinery, and it would also be consistent with lignocellulosic biomass pretreatment. Given a plant-based protein substrate, such as soy flour, which contains both carbohydrates and protein [
26], prolonged heating under acidic conditions will be conducive to the Maillard–Amadori reactions of the free sugars and amino acids, and these have potential to reduce the amino acid yield but increase sugar degradation to furfural and hydroxymethylfurfural [
21]. In this situation, optimization of acid concentration, reaction time, and temperature is pertinent to maximizing amino acids and sugars while avoiding furanaldehyde development and will be applied during the current investigation. For example, Lujan-Rhenals et al. [
27] treated soybean meal with 0.9–1.9% (
w/
w) H
2SO
4 1–4 h at 80 °C to produce a protein-enriched cake and a liquid fraction of soluble sugars with significant furanaldehyde levels, but in later studies [
28] optimal cake protein enrichment with lower furanaldehyde levels in the soluble sugar stream was found using higher temperatures (120 °C) and shorter times (<30 min) with 1.5% (
w/
v) H
2SO
4.
To summarize, protein hydrolysis methods are of interest in the development of profitable biorefineries not only to supply amino acids for microbial processes, but also to provide amino acids as building blocks for the development of other higher value specialty products. The current research is aimed at optimizing the dilute sulfuric acid hydrolysis of soy flour to amino acids in the presence and absence of the biomass substrate, switchgrass, which is protein poor. Although soy flour is the protein source studied in the present research article, the isolation and hydrolysis of proteins from lignocellulosic biomass or even yeast products could be incorporated into a biorefinery, as well, by applying the optimization principles discovered in the current research. Furthermore, those biorefineries that incorporate dilute sulfuric acid pretreatment of lignocellulosic biomass for biofuels and products may benefit by extending the same processing technology to broaden their portfolio to include amino acid production since the demand for amino acids is growing, especially for those derived from non-animal sources [
29].
2. Materials and Methods
2.1. Standard Switchgrass Hydrolysis in Serum Bottles—Dilute Acid Pretreatment and Enzyme Saccharification
Switchgrass dilute-acid hydrolysis was adopted from the prior method [
7] by reducing the amount of switchgrass used from 20.0 g to 2.0 g (dry).
Scheme 2 shows the flow diagram of this process. The source of switchgrass was field grown post-frost Kanlow N1, dried, and baled in Mead, NE, USA. The post-frost Kanlow N1 composition (g/Kg, dry basis) has been previously described as follows: glucan, 385.0 ± 7.3; xylan, 245.6 ± 0.6; arabinan, 29.7 ± 0.2; acetate, 49.1 ± 0.3; total Klasson lignin, 165.0 ± 24.5 [
30]; and 59.4 mg/g average crude protein [
31]. The switchgrass was ground in a knife mill to pass through a 2 mm screen. Switchgrass (SG) was pretreated at 20% solids by mixing 2 g dry weight of biomass with 8 mL of 0.936% (
v/
v) sulfuric acid (H
2SO
4) solution and 30 mg Pluronic F68 Biochemica surfactant (AppliChem GmbH, Darmstadt, Germany) in 50-mL serum bottles (Wheaton Industries Inc., Millville, NJ, USA). The standard acid to switchgrass mixing ratio H
2SO
4:SG was 0.0689 (g/g dry basis). The bottles were crimp sealed and loaded into steel vessels (stacked 2 per vessel) along with 10 mL of water, which promotes heat transfer. The cannisters were loaded into a Mathis Labomat IR Dyer Oven Model BFA12 (Werner Mathis AG, Oberhasli, Switzerland) where they were rotated at 50 rpm (1 min right then 1 min left) and heated to 160 °C for 15 min before being water cooled to 40 °C. In experiments requiring fewer than 24 treatment bottles, serum bottles filled with 10 mL water were used as placeholders.
After pretreatment, the product in each bottle was adjusted to pH 4.5 by adding 30% Ca(OH)2 solution and 1 M citric acid buffer (50 mM, pH = 4.8) directly into each serum bottle and then tumbling 15 min in the Labomat. Pretreatment hydrolysates were loaded with 0.05 mL of Ctec and 0.27 mL of Htec enzymes (Novozyme, Bagsvaerd, Denmark), and tightly capped to incubate approximately 72 h at 50 °C and 150–170 rpm (1”orbit, Innova, New Brunswick Scientific, NJ, USA). Resulting hydrolysates were directly clarified and sterilized by passing through 0.2 μm Nalgene filter units and stored at either 4 or −20 °C. Samples collected were analyzed for sugars, furans, acetic acid, individual basic amino acids, primary amino acids, urea, and ammonia concentrations. Processing conditions were varied from above as described in the experimental designs.
2.2. Hydrolysis of Soy Flour during Switchgrass Pretreatment
The source of soy flour for this study was Toasted Nutrisoy Flour [Product code 063160, Archer Daniels Midland Co. (ADM), Chicago, IL, USA], with 6.15% moisture (or 93.85% dry mass). Unless stated otherwise, soy flour was added to the switchgrass in pretreatment bottles at a rate of 0.24 g dry weight per 8 mL of dilute acid, or 30 g/L (3% w/v).
2.3. Dilute Sulfuric Acid Hydrolysis of Soy Flour in the Absence of Switchgrass
Soy flour was mixed with 8 mL sulfuric acid solution in 50 mL borosilicate glass serum bottles with crimp-sealed septa which were heated and mixed as described above for switchgrass pretreatment using the Mathis Labomat Oven. The soy flour content, concentrations of sulfuric acid, and the reaction temperature and time were as specified in experiment designs and each reaction was run in triplicate. The septa on bottles allowed for syringe withdrawal of 400 µL samples between heating cycles when additional cycles were run.
2.4. HPLC Quantitation of Individual Amino Acids
To characterize individual amino acids within hydrolysates, both the Waters AccQ-Tag method and AccQ-Fluor Reagent Kit (WAT052880-2B, Waters Corporation, Milford, MA, USA) were used to assay 17 basic amino acids (AccQTag Amino Acid Standard Kit, WAT088122) via derivatization and HPLC analysis with fluorescence detection. Hydrolysate samples, adjusted to pH ~6.2, were serially diluted in 20 mM HCl in order to find the optimal dilution in the linear range of fluorometry detection for each amino acid component in the sample.
Derivatizations were done, in a heating block preheated to 55 °C. Briefly, an aliquot of 70 µL of AccQ-Fluor borate buffer was added to 10 µL of each properly diluted standard (or 80 µL buffer to each 20 µL of appropriately diluted hydrolysate sample). After mixing a few seconds, 20 µL of AccQ-Fluor Reagent was added to each assay tube followed with immediate mixing. Then, the tubes were allowed to stand 1 min at room temperature.
The contents of sample tubes were transferred to autosampler limited volume inserts (LVI) (0.125 µL, 8 mm × 32 mm, clear crimp cap vials, Product Number (P/N) 10800100, Thermo Fisher Scientific, Waltham, MA, USA) with 8 mm crimp caps having Teflon/silicon septa (Thermo-Fisher P/N C4008-4A). LVIs were placed in 1-mL HPLC vials and heated in the preheated heating block for 10 min at 55 °C. The amino acid derivatives thus prepared were cooled and kept tightly sealed at ambient temperature for up to a week prior to analysis.
The basic amino acids were eluted at 1 mL/min through an AccQ tag column (3.9 × 150 mm) at 25 °C eluted at a flow rate of 1 mL/min using a gradient mobile phase consisting of (A) AccQTag Eluent A (Waters WAT052890), (B) water, and (C) acetonitrile. Before running samples, the column was brought to 37 °C and conditioned using the following program: 5 min of 40:60 B:C, then 10 min of 97:3 A:C. After injection of 10 µL sample (Waters 717 Plus Autosampler at 25 °C), the column was eluted 0.5 min 97:3 Eluant A:C; 17.5 min 98.5:1.5 A:C; 1 min 93.1:6.9 A:C; 10.5 min 89.2:10.8 A:C; 2.5 min 81.3:18.7 A:C; 3 min of 39.2:60.8 B:C; and finally, 3 min 97:3 Eluant A:C. Sample peaks were quantitated via fluorescence detection (Waters 2475 Multi-wavelength Fluorescence Detector) during a total run time per sample of 45 min.
2.5. HPLC Quantitation of Sugars, Furanaldehydes and Acetic Acid
Furfural, 5-hydrolxymethyl furfural (HMF), and acetic acid in culture samples were analyzed using a Waters HPLC system equipped with a Biorad HPX-87H Aminex ion exclusion column (Bio-Rad, Hercules, CA, USA) fitted with a Micro-guard Cation H Micro-Guard Cartridge (125–0129) precolumn. Samples (10 μL) injected to the precolumn were eluted at 60 °C with acidified water (15 mM HNO3) at 0.6 mL/min to achieve component separations which were monitored by both refractive index and ultraviolet absorbance (215 nm) detectors. For hydrolysate compositional analysis, Biorad Aminex HPX-87P carbohydrate analysis column (125–0098) with deashing cartridge (125–0118) and Carbo-P Micro-Guard Cartridge (125–0119)) was used at 80 °C with water as the mobile phase.
2.6. Enzymatic Assay of PAN, Ammonia, Urea
Enzyme-based test kits were used to assay primary amino nitrogen (PAN), ammonia, and urea (Megazyme K-PANOPA and K-URAMR, Megazyme International Ireland Ltd., Wicklow, Ireland).
2.7. LECO Analysis of Total N
The mass N content was measured from complete combustion gases of soy flour samples using a LECO analysis instrument equipped with a thermal conductivity detector, CHN 628, Laboratory Equipment Corporation (LECO), Saint Joseph, MI. A LECO certified EDTA standard was used to develop a four-point linear calibration at 25 mg, 50 mg, 100 mg, and 150 mg resulting in ±0.03% of the certified 9.57% nitrogen in the EDTA standard. Every five samples were interrupted to assure accuracy or drift relative to the standard. The nitrogen results were converted to protein content using a conversion factor of 6.25 for soy flour. The instrument uses the Dumas combustion method.
2.8. Statistics
Analysis of variance (ANOVA) and Student–Newman–Keuls (SNK) pairwise comparison analyses were performed at significance criterion p ≤ 0.05 using Sigmaplot 15 (Systat Software, Inc., San Jose, CA, USA).
3. Results and Discussion
3.1. Total Nitrogen Content of Nutrisoy
The defatted soy flour (Nutrisoy) selected for this study contained 8.64 ± 0.0271% N on a mass basis. This equates to a protein content of 54.0%, assuming that protein averages 16% N on a mass basis. If 30 g/L soy flour were hydrolyzed, the maximum theoretical yield would be 2.592 g/L of N as amino acids, or 0.0864 g N/g soy flour. However, some of the nitrogen can be in the form of ammonia and urea. Total amino acids, urea, and ammonia were all monitored via enzymatic assays. Basic amino acids were monitored using HPLC for selected experiments. In addition, total N was followed throughout selected processes using LECO combustion-based analysis.
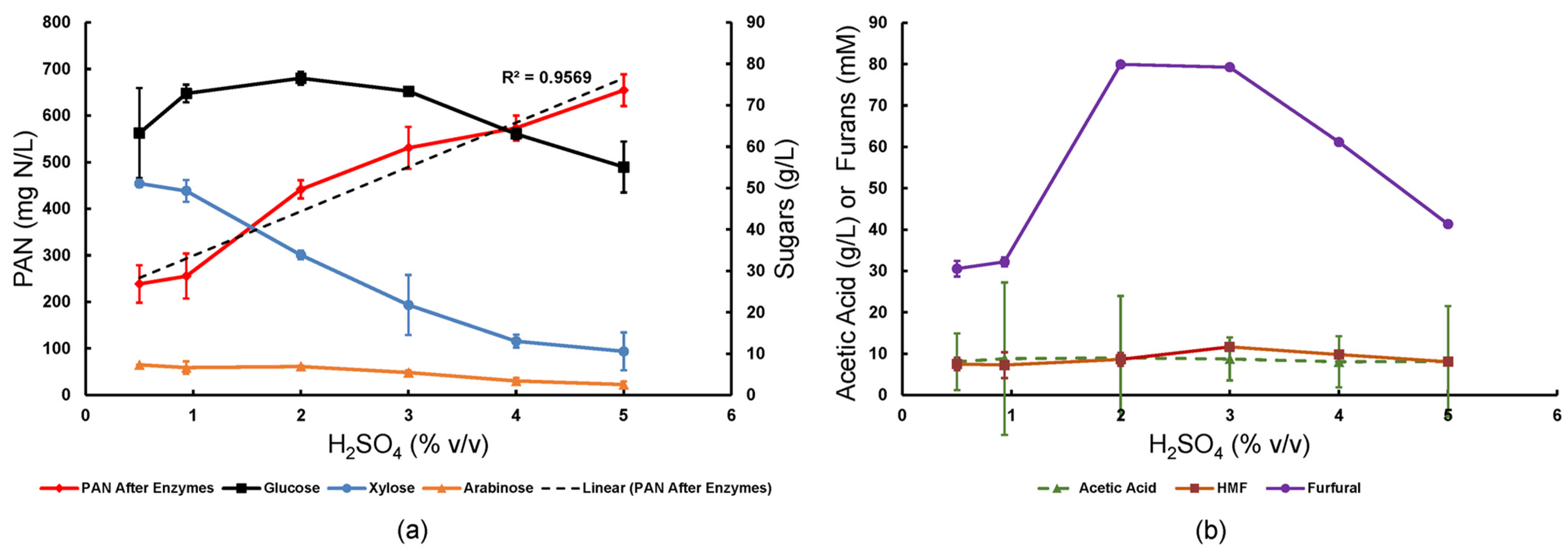
3.2. Impact of Sulfuric Acid Concentration and Switchgrass on Amino Acid Release from 30 g/L Soy Flour
The effect of pretreatment acid loading on yields of amino acids, glucose, and xylose was studied when switchgrass and soy flour (30 g/L) were co-pretreated and then enzyme saccharified (
Scheme 2). The yield of primary amino acid nitrogen (PAN) increased almost linearly with sulfuric acid concentration (
Figure 1a). The maximum PAN titer was 654 mg N/L, a yield of 0.022 g N/g soy flour (25% of theoretical). However, the yield of glucose was optimized using 0.936 to 3% (
v/
v) dilute acid, while xylose and arabinose were maximized at lower acid concentrations of 0.5–0.936% (
v/
v) (
Figure 1b). This result coincided with inhibitory furfural concentrations peaking at 80 mM at 2–3% acid, which was consistent with xylose degradation via Maillard reactions [
21]. The HMF arising similarly from glucose degradation remained below 10 mM, except for 11.7 mM at the 3% acid level, and acetic acid arising from hemicellulose hydrolysis remained at 8–8.7 g/L, and both were statistically insensitive to sulfuric acid concentration within the tested range.
For comparison, the impact of sulfuric acid on the hydrolysis of soy flour alone was also investigated (
Scheme 3). PAN recovery improved in the absence of switchgrass (
Figure 2 versus
Figure 1). The effect of subjecting the soy flour–sulfuric acid treatments to two heating cycles was an increase in amino acid production by 10 to 17% (
Figure 2). For both heating cycles, the steepest gain in PAN accumulation occurred at <2% (
v/
v) H
2SO
4 and plateaued at ~4%. The PAN maximum titers were 1046 and 1165 mg N/L for cycles 1 and 2, respectively, while yields were 0.035 g/g (40.5% theoretical) and 0.039 g/g (45% theoretical), respectively.
As described in the protocol, bottles were stacked in twos for each steel reaction canister inserted into the Mathis IR oven. PAN production was conducted in quadruplicate reaction bottles with 30 g/L soy flour across the range of 0.5 to 5% (v/v) H2SO4 concentrations and was analyzed as a function of bottle position. There was no significant difference between top and bottom bottles.
Several interpretations are important to convey here. The results are consistent with prior findings that high acid concentration during switchgrass pretreatment leads to an excessive loss of pentoses to furfural production [
32,
33,
34]. Relative to the pentose loss, degradation of glucose to HMF was much less pronounced, and its derivatives, levulinic and formic acids (not measured), were presumed low [
34]. Furfural production is unwanted because it reduces sugar yield and severely inhibits microbial growth and metabolism [
35]. Based on the data analysis, sulfuric acid should be limited to no more than 1% (
v/
v) for co-pretreatment of switchgrass and soy flour. However, we still do not know if the soy flour impacts sugar and inhibitor yields. Also, given that the presence of switchgrass reduced PAN production, it was of interest to determine if amino acid yield losses occurred during the pretreatment or subsequent cellulase hydrolysis of
Scheme 2.
3.3. Effect of Soy Flour Concentration and Switchgrass Content on Amino Acid Release
PAN production by either hydrolyzing soy flour with or without switchgrass (
Scheme 2 and
Scheme 3) was compared in duplicate experiments (
Figure 3). Following the acid hydrolysis step, the PAN concentration increased linearly with the concentration of soy flour when it was included during the pretreatment of switchgrass (
Scheme 2). However, when soy flour alone (no switchgrass) was treated under the same conditions (
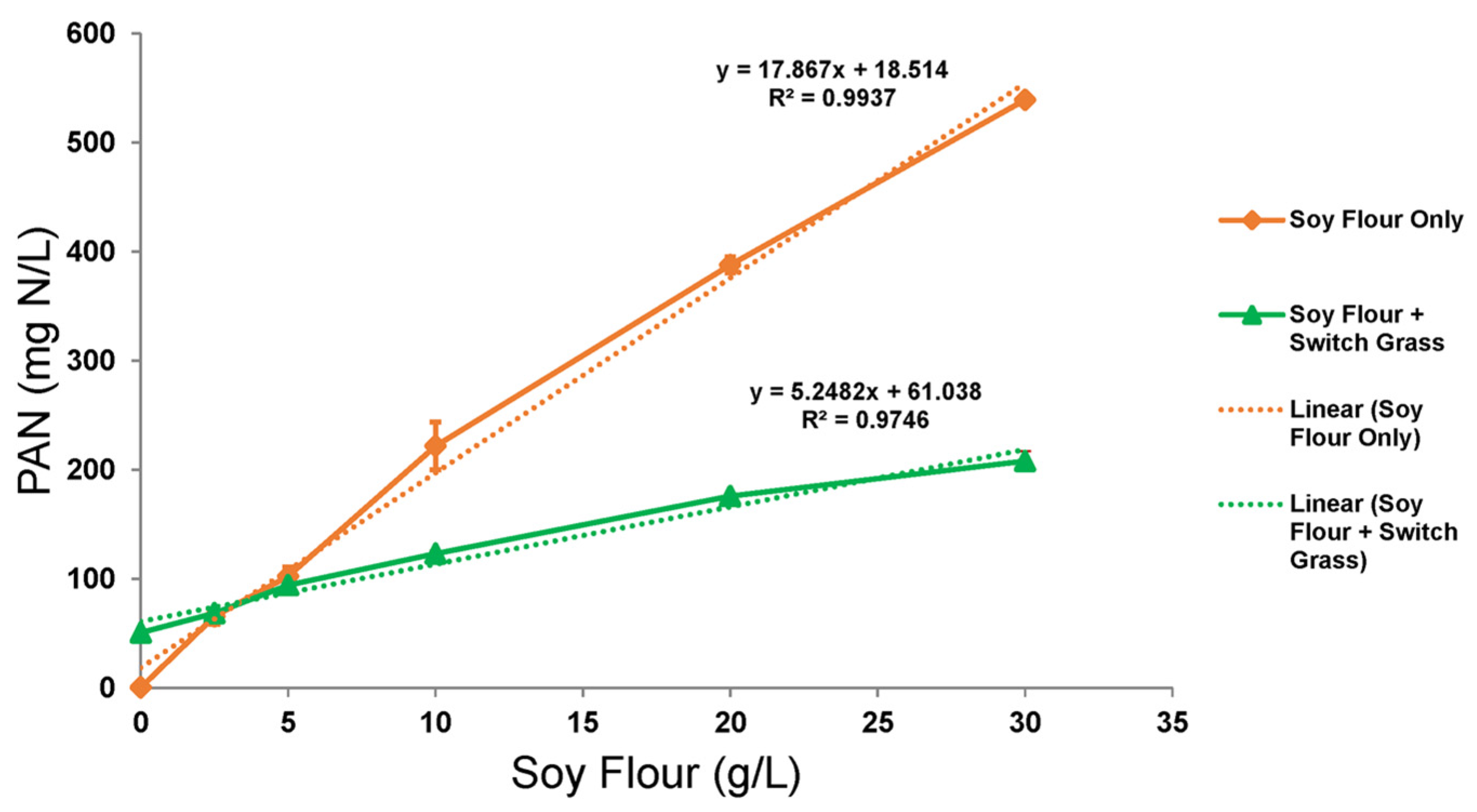
Scheme 3), the linear rate of PAN release per g/L soy flour was much higher, resulting in 539 mg N/L released from 30 g/L soy flour compared to only 208 mg N/L in the presence of switchgrass (
Figure 3). Note that this 208 mg N/L includes 51 mg/L derived from switchgrass itself, as determined by treating switchgrass without soy flour. Thus, the amino acid released from the hydrolysis of 30 g/L soy flour by itself was ~3.4 times (539/157) that from the hydrolysis of soy flour in combination with switchgrass. Still, when switchgrass pretreatment was supplemented with 30 g/L soy flour, the level of PAN in the hydrolysate was boosted by 4 times that available from switchgrass when pretreated alone. The amino acid yields from soy flour in the two processes were 0.018 g/g and 0.0052 g/g, or 21 and 6% of theoretical yield, respectively. Additional experiments were conducted to address the low amino acid yield found for both process schemes.
3.4. Pre-Hydrolysis of Soy Flour and Inclusion in Switchgrass Pretreatment and Enzyme Saccharification
Co-pretreatment of soy flour and switchgrass (
Scheme 2) was modified to allow for pre-hydrolyzing soy flour (
Scheme 4) in attempt to improve amino acid yield. In
Scheme 2, soy flour was mixed directly with the switchgrass and hydrolyzed (0.936% (
v/
v), 15 min, 160 °C), but in
Scheme 4 the soy flour was pre-hydrolyzed by itself (0.936%
v/v sulfuric acid, 15 min, 160 °C) prior to mixing with switchgrass followed by pretreatment with dilute acid (0.936% (
v/
v) acid, 15 min, 160 °C). In each case, the progress of PAN release was monitored at 0, 24, 48 and 72 h during enzyme saccharification (
Figure 4). A three-way ANOVA was applied to determine PAN dependencies on soy flour concentration (0–30 g/L), soy flour pre-hydrolysis (+/−) prior to switchgrass addition, and enzyme saccharification time (0–72 h). All three factors significantly impacted the yield of PAN. Increasing the amount of soy flour used increased the final PAN concentration. The pre-hydrolysis of soy flour only increased the PAN concentration by 14% (131 mg N/L compared to 115 mg N/L based on overall means). The net PAN accumulation was less responsive to the 20 and 30 g/L soy flour supply levels if pre-hydrolyzed (
Scheme 4) versus if not pre-hydrolyzed (
Scheme 2). Additionally, significant amounts of amino acids generated from pre-hydrolysis were lost during pretreatment and enzymic hydrolysis with switchgrass. This loss was ~34, 42 and 53%, for the 10, 20 and 30 g/L soy flour levels, which is seen when we compare the soy flour only hydrolysis plot in
Figure 3 with the 0 h enzyme plot in
Figure 4a. Regardless of whether soy flour was pre-hydrolyzed or not, the enzymatic hydrolysis of cellulose led to a loss in PAN since the overall mean for PAN trended downward from 130 to 112 mg N/L as enzyme reaction time progressed from 0 h to 72 h (averaged across all soy flour concentrations and both pre-hydrolysis conditions). In summary, the pre-hydrolysis of the soy flour prior to dilute acid pretreatment with switchgrass, while releasing more PAN into the system, had only a small net benefit after the apparent loss of amino acids during the enzyme hydrolysis process.
Ammonia and urea were also assayed enzymatically during this study. Ammonia production was minor and urea negligible (
Figure 4). Of the factors tested (pre-hydrolysis, soy flour concentration, and saccharification time) only the enzyme saccharification time significantly impacted the levels of urea (
p < 0.05), and none of these variables significantly impacted the ammonia concentration (
Table 1). However, it is possible that some ammonia was released as a gas, which was not accounted for here. While our goal is to maximize the conversion of proteins to amino acids and minimize their degradation to ammonia and carbon dioxide, ammonia is still a useful source of N for yeast, and therefore the relative yields of N in both forms will be reported for selected studies. However, amino acids are much more valuable than either ammonia or urea:
$0.73/lb for ammonia < ~
$2/lb for soy flour < ~
$100/lb for amino acids (pending scale and quality) [
36]. Furthermore, the global amino acids market was USD 25.21 billion in 2022 and is expected to grow at a compound annual growth rate (CAGR) of 8.4% from 2023 to 2030 [
19,
29,
36].
The effect of the tested variables on the production of sugars, furan, and acetic acid are also of interest because the sugars will be fermented, and furan and acetic acid are microbial inhibitors. The impacts of soy flour concentration and dilute acid pre-hydrolysis on sugar, furan, and acetic acid components of the final hydrolysate after 72 h enzyme saccharification are summarized in
Figure 5. The results are reported as a comparison of final hydrolysates from
Scheme 4 (pre-hydrolyzed soy flour) and
Scheme 2 (soy flour not pre-hydrolyzed). The means were calculated from two repeated experiments. Soy flour had a significant impact (
p < 0.001) on the final furfural concentration regardless of whether soy flour was pre-hydrolyzed or not (
Scheme 4 versus
Scheme 2). Adding just 2.5 g/L soy flour to the switchgrass hydrolysate reduced the final furfural concentration by ~25%. HMF accumulation in hydrolysates increased gradually with increasing soy flour supplementation (
p < 0.001) and was further increased by including soy flour that had not been pre-hydrolyzed (
p < 0.001). Acetic acid accumulation remained ~6.3–6.9 regardless of soy flour concentration and pre-hydrolysis. Net glucose production gradually declined with increasing soy flour concentration, with the decline becoming significant as soy flour reached 20–30 g/L; and pre-hydrolysis resulted in lower glucose accumulation, reaching significant differences at 30 g/L soy flour. Consistent with the furfural findings, xylose accumulation significantly increased when at least 2.5 g/L soy flour was added for both
Scheme 2 and
Scheme 4. The final arabinose concentrations were low and were not significantly impacted by soy flour concentration or the pre-hydrolysis condition. In summary, supplementation with a low level of ~2.5 to 10 g/L soy flour benefited sugar production to 112 g/L total neutral sugars and reduced production of the potent microbial inhibitors furfural and HMF. Most notably, supplementation with soy flour during pretreatment reduced furfural production by 25%.
3.5. LECO Tracking of N from Reactants to Products through Hydrolysis Process Steps
The complete N mass balance was monitored throughout the process (
Scheme 2 and
Scheme 3). The steps monitored included: after mixing soy flour with dilute sulfuric acid, after pretreatment, and after carrying out enzyme saccharification. The mass of N entering the process in reactants and the mass of N leaving the process in products was balanced within about 1–6% for all of the process steps except the heating of soy flour alone with dilute 0.936% (
v/
v) H
2SO
4 (
Scheme 3). In the latter situation approximately 13% of the N put into the system was not recovered in the liquid and solid product fractions, as surmised from the ratio of product N:reactant N (
Table 2). This suggests that thermal decomposition may have led to N exiting as ammonia gas for the soy flour only dilute acid hydrolysis system (
Scheme 3) [
37]. The apparent better retention of ammonia in the dilute acid pretreatments of both switchgrass and soy flour, as compared with the treatment of soy flour alone, could be explained by the binding of the soluble ammonia with both lignin and cellulose in the processes charged with switchgrass, effectively entrapping the ammonia and preventing its escape in gas form [
38,
39]. It is also a possibility that amino acids become attached to cellulases and cellulose fiber, thus causing assays to miss large quantities of amino acids that have been released by acid hydrolysis and causing low calculations of the amino acid yield in the processes containing switchgrass. There is evidence of this phenomenon in the literature where aromatic amino acids (tyrosine, tryptophan, histidine, phenylalanine), glutamine, and asparagine attach to the cellulose binding domain of cellulase based upon structural modelling studies [
40,
41,
42]. Additionally, Maillard reactions of amino acids with sugars likely divert them to other side products [
21].
Although low levels of soy flour added during switchgrass hydrolysis were beneficial to minimizing furfural and improving neutral sugar accumulations, the co-pretreatment of switchgrass and soy flour did not appear to lead to optimal amino acid recovery because some of the amino acids released appeared to be lost. The optimal acid concentration for amino acid release from soy flour is much higher than that for the pretreatment of the switchgrass, and free amino acids disappeared during switchgrass pretreatment and saccharification. The mechanisms of the latter are hypothesized to involve degradation to ammonia [
37], Maillard reactions [
21], and possibly the binding of amino acids or ammonia to lignin, cellulose, and/or cellulases [
38,
39,
40,
41,
42]. As a consequence, additional experiments were conducted to further optimize PAN recovery from dilute sulfuric acid treatment of soy flour alone via
Scheme 3. High titers of PAN could be harvested from this route and utilized to enrich final hydrolysates post enzyme saccharification and prior to microbial cultivations, or as a source of amino acids for other valued products.
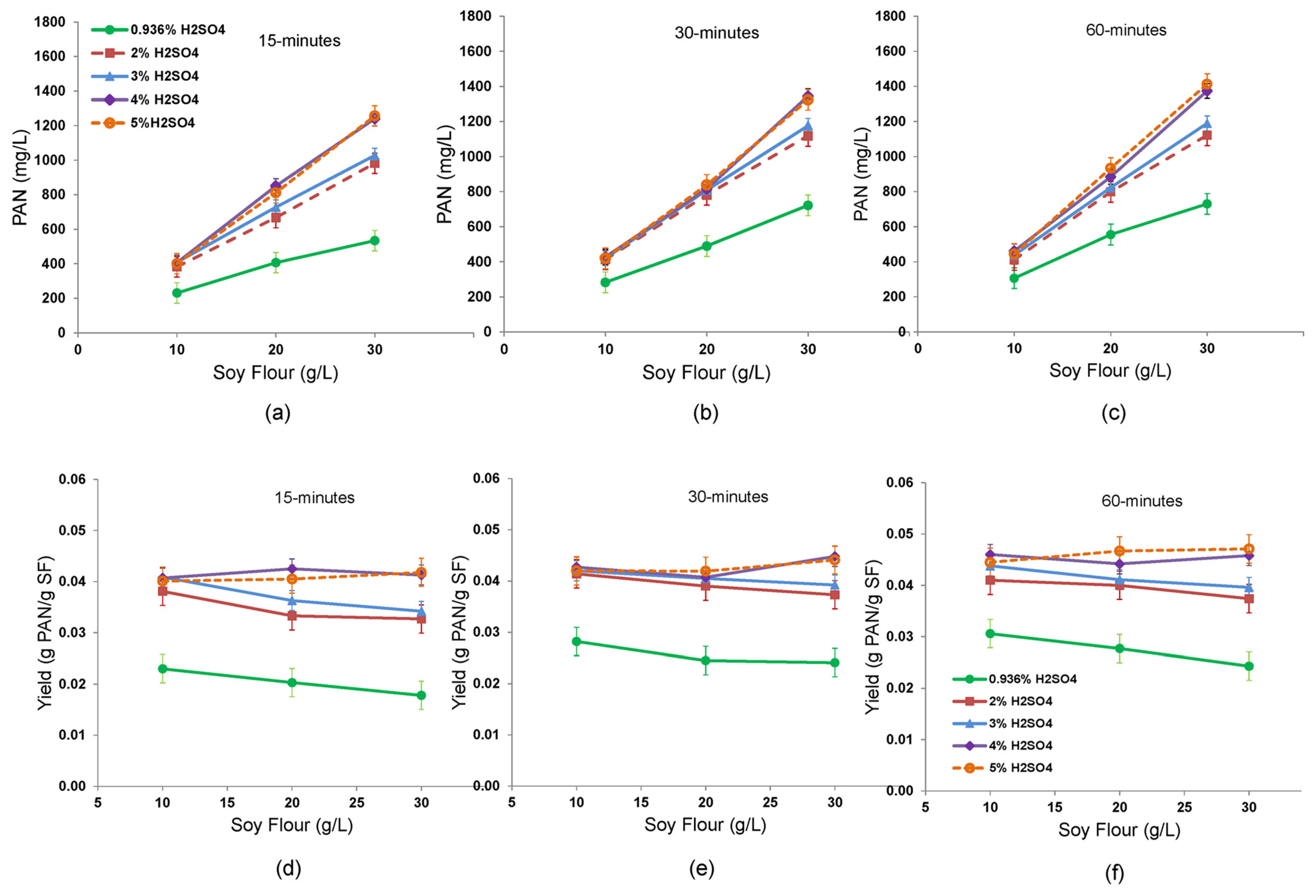
3.6. Optimization of PAN Titer and Yield by Varying Reaction Time and Concentrations of Sulfuric Acid and Soy Flour
The reaction time, sulfuric acid concentration, and soy flour concentration all significantly impacted the final PAN concentration (
p < 0.001;
Figure 6a–c). The statistical analysis was based on duplicate treatments and repeated experiments. Primary amino acid titers, increased linearly with the initial soy flour concentration (10–30 g/L) (
p < 0.001) for all sulfuric acid concentrations tested (0.936–5%). When comparing the PAN product concentrations occurring across time, a 15 min reaction time led to incomplete hydrolysis for the higher soy flour levels (20 and 30 g/L) at the tested sulfuric acid concentrations, but the reaction rate supported by the 0.936% H
2SO
4 was significantly slower than the rates afforded by higher acid concentrations (
p < 0.001). Even after 60 min at 160 °C, the PAN accumulation in the 0.936% acid treatments still lagged far behind the more concentrated acid treatments. Amino acid production was lower for 15 min reactions (regardless of acid concentration) than for longer reaction times (
p < 0.05), but there was not a significant difference in the extent of the reaction between the 30 and 60 min time intervals. In the 60 min window for 30 g/L soy flour, the 0.936% acid treatment slowed in PAN accumulation and began to lose linearity with soy flour concentration. This result signals that amino acids were being degraded faster than they were produced, and that the net yield of PAN per g of protein consumed was dropping. In contrast, a reaction rate advantage of the 4 and 5% (
v/
v) acid treatments is indicated over all lower acid concentrations for the 30 g/L soy flour treatments across all time intervals (
p < 0.001). The reason for this advantage may lie in the relative rates of amino acid formation becoming higher than the thermal degradation of amino acids to ammonia or diversion into Maillard reactions as the acid concentration was increased. Traditional kinetic plots of PAN with time as a function of sulfuric acid within each soy flour level further confirm the critical need for sulfuric acid to be high enough to ensure a high amino acid production rate that outpaces losses in order to rapidly convert concentrated soy flour to high amino acid titers (
Supplemental Figure S1).
The corresponding yield plots for these reaction conditions are shown in
Figure 6d–f, where PAN yield was based on g PAN produced per g of soy flour supplied. Based on three-way ANOVA, the PAN yield was more sensitive to heat time and acid concentration (
p < 0.001) than to initial soy flour concentration (
p = 0.04). There were no significant interactions of these variables impacting yield. Treatments with 4 and 5% (
v/
v) acid displayed a slightly increasing yield across the range of soy flour concentrations provided, regardless of the heating time, but treatments with lower acid concentrations had yields that declined linearly with increasing soy flour. The downward shift of yield lines with decreasing acid concentration in
Figure 6d–f for all heating times indicated that lower acid concentrations in the 1–3% (
v/
v) range were insufficient to allow amino acid production rate to exceed detrimental side reactions. The upward shifting of all yield lines with heating time suggested the benefit to longer heating times to allow more complete soy flour utilization, especially in the slower reactions. However, treatments with slower reaction rates not only lose amino acid yield due to residual unreacted protein, but also potentially due to amino acids entering Maillard reactions with sugars or thermal degradation to form ammonia as discussed above (
Section 3.5).
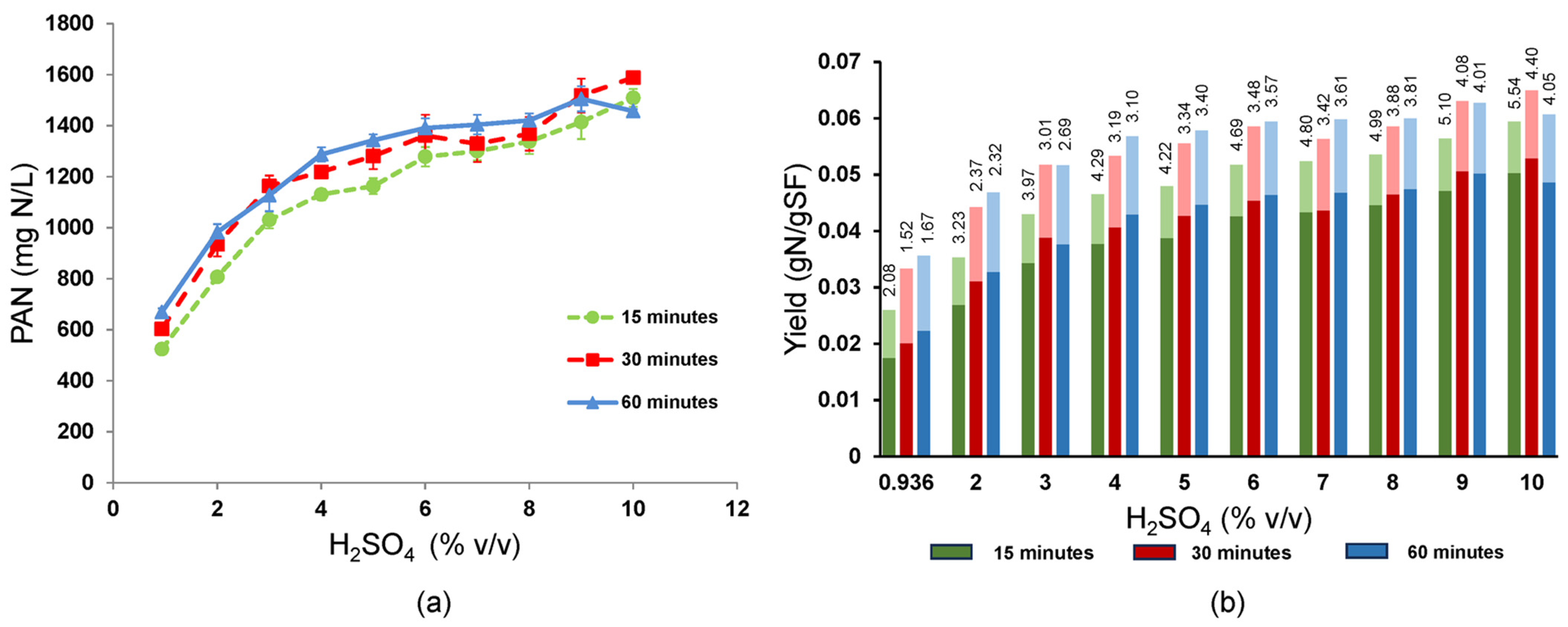
3.7. Advantage of Increasing the Sulfuric Acid Concentration
Given its critical role in determining conversion efficiency at a soy flour concentration of 30 g/L, sulfuric acid concentration was tested over a range of 0.936% to 10% (
v/
v) (
Figure 7). Two-way ANOVA indicated significant effects (
p < 0.001) of the sulfuric acid concentration and heating time at 160 °C. PAN titers were generally higher when hydrolyzed for 30 and 60 min versus 15 min. Significant increases in PAN with sulfuric acid continued until it reached ~6% (
v/
v), when PAN curves began to flatten and neighboring PAN values were sometimes not significantly different from one another, especially at 30 and 60 min times. The highest PAN titers of 1414 to 1587 mg PAN/L were achieved using 9–10% (
v/
v) H
2SO
4 even when hydrolyzed for only 15 min, although the highest PAN value occurred at 10% and 30 min, where the standard error was ±24.96 mg PAN/L. The PAN yield showed a trend similar to the PAN titer, being highest with 9–10% (
v/
v) acid (0.0486 to 0.0529 ± 0.00083 gN/g soy flour, i.e., 56.3 to 61.2% of theoretical, respectively) and reaching a maximum occurring at 10% (
v/
v) acid and 30 min reaction time. The total N yields, including PAN and ammonia N, were highest at 0.0564 to 0.0650, where the peak at 0.065 ± 0.0009 g N/g soy flour was 75% of theoretical and occurred at 10% (
v/
v) acid and 30 min reaction time. Interestingly, the overall average PAN selectivity was highest at the shortest reaction time (15 min) and the highest acid concentration (10%
v/
v). This parameter peaked at 5.539 +/− 0.0971 after only 15 min at 10% (
v/
v) acid, indicating that less degradation of amino acids to ammonia significantly benefited the short time PAN yields although highest yields were obtained in the 30 min reactions at 10% (
v/
v) H
2SO
4. Thus, rapid hydrolysis to amino acids afforded by the higher sulfuric acid concentrations were key to improving yield by outpacing amino acid degradation to ammonia and/or loss to Maillard reactions.
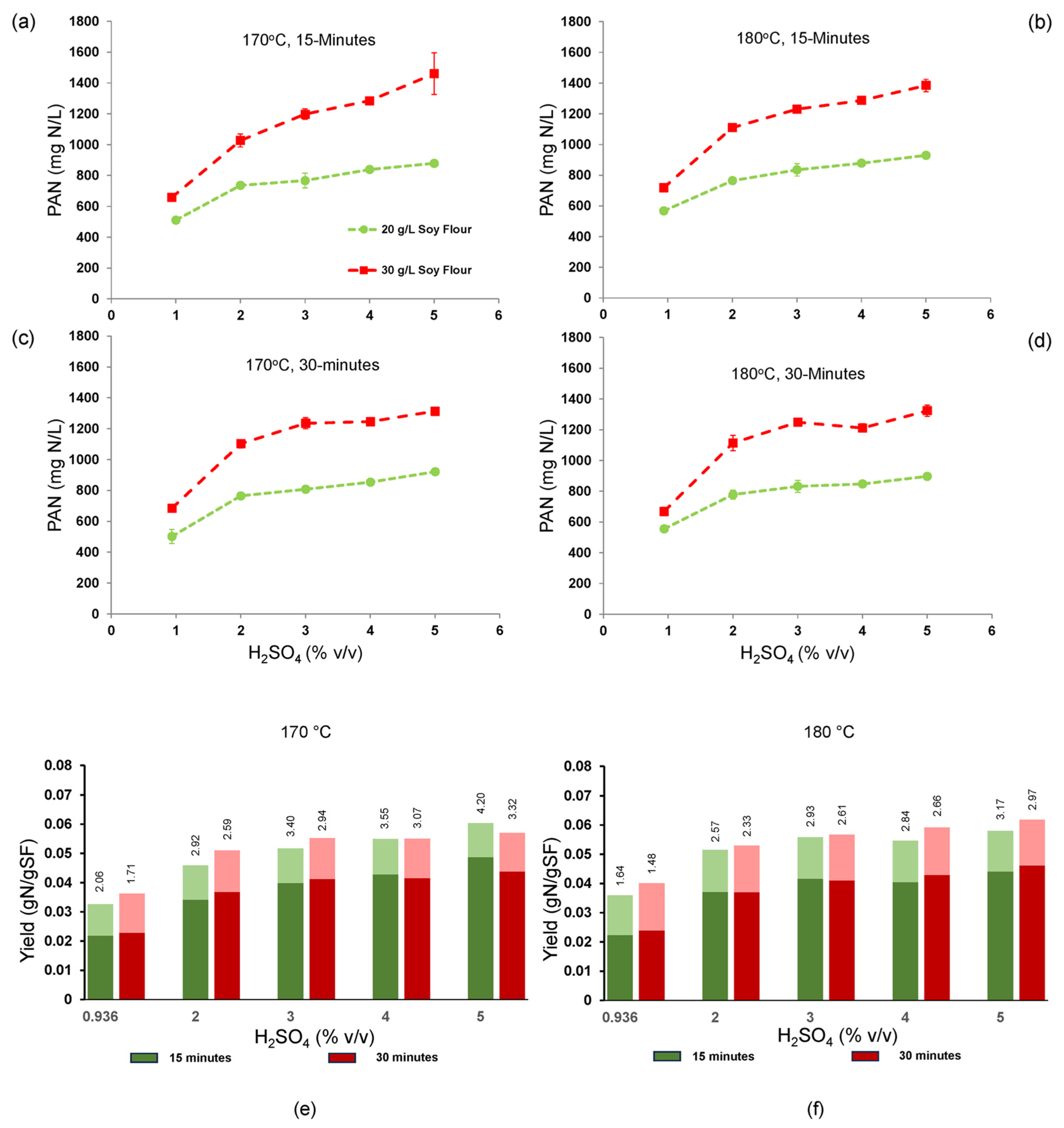
3.8. Impact of Increasing Temperatures on PAN Accumulation and Yield
Hydrolysis temperature (170 and 180 °C), time (15 and 30 min), sulfuric acid concentration (0.936–5%
v/
v), and soy flour concentration (20 and 30 g/L) were also tested to check if PAN titer and yield could be further improved at the lower acid levels. Acid concentration had the strongest impact and both PAN titer and yield increased similarly as acid concentration increased (
Figure 8). Significantly higher titers were seen with the higher soy flour loading, but yield was not very sensitive to soy flour level, indicating conversion efficiency was constant. PAN was sensitive to all three reaction factors, and the highest PAN titer and yield obtained were 1460 mg N/L and 0.0487 g N/g soy flour (56% of theoretical), respectively (170 °C, 15 min, 5% (
v/
v) H
2SO
4, 30 g/L soy flour). The highest total N yield (ammonia and PAN) observed was 0.0603–0.0617 g/g (70–71% of theoretical) (170 °C,15 min and 180 °C, 30 min, respectively, at 5% (
v/
v) H
2SO
4). Thus, comparing these results to those presented in
Figure 7a suggests that operating at 170 °C could lead to PAN titers and yields at 5% (
v/
v) H
2SO
4 that are comparable to those obtained with 8–9% (
v/
v) H
2SO
4 at 160 °C. Economic analysis will determine the merits of each optimum. Raising the temperature to 180 °C did not appear to be advantageous. Based on statistical analysis of the main effects, the hydrolysis conditions 170 °C, 15 min reaction time, and 5% (
v/
v) H
2SO
4 was a second set of conditions maximizing PAN titer, yield, but also selectivity, the latter being the ratio of PAN to ammonia N. In this temperature study, the highest selectivity of PAN over ammonia N was 4.197 (170 °C, 15 min reaction time and 5% H
2SO
4) which was lower than that observed in the study at 160 °C (
Figure 7b). It is notable in
Figure 7b and in these data (
Figure 8e,f) that the product selectivity was always higher at the lower reaction times, indicating the advantage of rapid reaction rates and short reaction times to reduce the amount of N going to ammonia.
3.9. Traditional Hydrolysis of Soy Flour with 6 N HCl as Compared to Dilute H2SO4
A side-by-side comparison of dilute sulfuric acid hydrolysis of soy flour with a traditional hydrochloric acid hydrolysis was carried out. The “sealed flask method” developed by Cavins et al. [
20] served as a control to directly connect our results with a previous method aimed at the quantitation of amino acids in soybean meal. Briefly, 30 g/L soy flour was treated with 6 N HCL in sealed vials with an N
2 atmosphere during heating in an oven at 110 °C for 22 h. At selected times, duplicate vials were sacrificed for analysis of amino acids. Under these conditions, the hydrolysis reaction was complete by 16 h with a peak PAN concentration of 628 mg PAN/L and a yield of 0.021 g PAN/g soy flour (24.3% of theoretical yield). A slight decline in PAN was beginning to be apparent by 24 h (
Figure 9). In comparison, the optimal dilute sulfuric acid process, which required 10% (
v/
v) H
2SO
4 (1.8 M) for hydrolysis of 30 g/L soy flour 30 min at 160 °C, gave a PAN titer (1588 mg PAN/L) and yield (0.0529 g PAN/g flour) that were 2.5 times those seen for the 6 N HCl hydrolysis process and in just 3% of the time required. The distributions of the amino acids produced from the Nutrisoy soy flour submitted to various reaction conditions are compared in
Table 3. A two-way ANOVA of amino acid percentage as a function of reaction condition x amino acid type indicated that the product amino acid profiles did not vary significantly with the reaction condition applied to the soy flour (
p = 0.075). However, aspartic acid, glutamic acid and glycine were significantly predominant over other primary amino acid types formed (
p < 0.001). Methionine and cystine were the most sparce components, and tryptophan (not shown) was not detectable using this method. It is typically destroyed by acid hydrolysis [
20].
In addition to the hydrolysis efficiency advantages of the optimized 1.8 M H2SO4 process at 160 °C, there are significant cost differences between H2SO4 and HCl catalysts to consider. Concentrated H2SO4 is 18 M, i.e., 36 N, and its pKa is −3. Concentrated HCl is 12 M, i.e., 12 N, but pKa is −6.3. Thus, HCl is a stronger acid, and it is much more corrosive than sulfuric acid to stainless steel, so capital costs will be higher when using it. Concentrated H2SO4 costs ~$900 per 55 gallons of 93% technical grade, while concentrated HCl costs ~$1000 per 55 gallons of 37% technical grade (Alliance Chemical). Based on our findings, optimal soy flour hydrolysis required 10% (v/v) of concentrated sulfuric acid, or 1.8 M (3.6 N), and a 55-gallon barrel would supply a 550-gallon reaction at 1.8 M for ~$900. For the same cost, only a 99-gallon 6 N HCl hydrolysis reaction would be supported. Thus, considering just the catalyst cost, a 6 N HCl process would cost ~5.6 times that of an equal volume 1.8 M H2SO4 process.
3.10. Comparison of Optimal Dilute H2SO4 Hydrolysis Results with the Literature
In recent studies of canola cakes after oil extraction, Warnakulasuriya et al. [
22] compared the efficiencies of 6 N HCl with 6 N H
2SO
4 during the hydrolysis of 2.5 mg canola protein/mL at 110 °C for 24 h. The respective process yields of primary amino acids were 279 mg/g and 700 mg/g of canola cake protein, showing that the sulfuric acid process yielded 2.5 times more primary amino acids than the hydrochloric acid process did. A similar PAN yield advantage for H
2SO
4 over HCL hydrolysis of defatted soy flour was seen in the current study. Using the same yield basis as Warnakulasuriya et al. [
22], the optimized amino acid yield on soy flour in the current study was comparable at 612 mg/g protein (given 54% protein at 16% N in the defatted soy flour) at the conditions 10% (
w/
v) H
2SO
4 (3.6 N), 160 °C, 30 min, and 30 g/L soy flour. It is notable that operation at 160 °C allowed a relatively efficient amino acid yield from soy flour protein despite an acid loading per g protein that was an order of magnitude lower and reaction times that were just 2% of those applied in the canola protein study. However, it is important to remember that differences in the substrate types studied are likely to contribute to proteolytic efficiency differences.
Proteases are growing in importance for biorefinery use in proteolysis, but cost is a major barrier [
19]. Recently, improved thermophilic enzyme cocktails have been reported that synergistically produce ~10 g/L amino acids in 3 h at 60 °C from soybean pulp (i.e., okara), which is a low-value byproduct from soybean processing [
25]. This is a significant improvement over past protease technologies that mainly yielded dilute solutions of peptide-amino acid mixtures [
23,
24]. For perspective, optimized PAN titers from dilute acid hydrolyzed defatted soy flour in the current study were comparable at 1588 mg PAN/L, which corresponds to 9.9 g/L amino acids.
3.11. Summary of Results, Significance, and Applications in Biorefining
Primary amino acids were recognized as potential coproducts of biorefineries fed lignocellulosic biomass. They have relevance in biorefining both as nutrients for microbial bioconversion processes, such as fermentation of nitrogen-poor switchgrass, but also as functionalized coproducts that could be valorized through specialty chemical manufacturing. A review of the literature identified the lack of economical processes for efficient proteolysis of plant-based protein sources to primary amino acids as a key barrier to commercialization [
19]. In an effort to fill this gap, the current research was aimed at optimizing proteolysis conditions compatible with rapid, high temperature dilute acid hydrolysis, the most commonly used pretreatment for lignocellulose. Dilute acid hydrolysis is controllable and versatile enough to handle a variety of feedstocks, and it is currently the best compromise to allow efficient biomass hydrolysis at low costs with moderate waste stream management for environmental sustainability. However, literature reports on process data extending rapid, high temperature dilute acid hydrolysis to proteolysis are lacking.
In this research, soy flour hydrolysis was studied as a representative low-cost plant protein source requiring proteolysis to free primary amino acids for timely application. Two rapid, high-temperature dilute sulfuric acid process strategies to hydrolyze the soy flour were optimized. Either the soy flour was co-processed with nitrogen poor switchgrass biomass using a dilute-acid pretreatment, or it was hydrolyzed alone with dilute acid. In the first case, soy flour hydrolysis was aimed at enriching the switchgrass hydrolysate with nitrogen to support microbial cultivations, but in the second case, the hydrolysis was optimized to produce amino acids for any purpose.
Significant improvement to hydrolysate fermentability was accomplished by adding 2.5–10 g/L soy flour to switchgrass (20%
w/
v) pretreatment with dilute sulfuric acid (0.936%
v/
v) for 15 min at 160 °C. This practice optimized the accumulation of neutral sugars and resulted in a 25% reduction in the microbial inhibitor furfural while boosting xylose by 7% and doubling primary amino nitrogen (PAN) from 51 to 110 mg/L when 10 g/L soy flour, as compared to no soy flour, wass added to switchgrass pretreatment. When the sulfuric acid concentration was increased above 1% (
v/
v) or the soy flour was increased above 10 g/L in the co-hydrolysis of switchgrass and soy flour, higher PAN release occurred, but unfortunately sugar degradation and furanaldehyde inhibitor production also increased somewhat. The inhibitor-reducing capacity of low-level soy flour addition to dilute acid pretreatment was an unexpected finding that has not previously been shown in the literature. However, the utility of supplementing switchgrass with 30 g/L soy flour has been demonstrated previously for the production of ethanol by native and evolved strains of the pentose-fermenting yeast
S. stipitis [
7] and also for the production of lipids by several genera of yeast [
43], including
Lipomyces,
Rhodotorula (
Rhodosporidium), and
Saitoella. The latter two genera also produce carotenoids, with
Saitoella being rare among the collected strains [
44]. The vision of the production of lipids for biofuel has been gaining interest among researchers [
45,
46,
47], and valuable co-products, such as carotenoids and amino acids, may help the economics [
48], as will advancements in proteolysis in the context of bioconversion and biorefining [
19].
Despite adding amino acids and reducing inhibitors to improve fermentability, the co-hydrolysis of soy flour and switchgrass led to low amino acid yields at only ~6% of theoretical from 30 g/L soy flour. Consequently, other hydrolysis schemes were explored to improve yield and diversify amino acid applications. One scenario examined the pre-hydrolysis of the soy flour before dilute acid pretreatment with switchgrass (
Scheme 4). Although more PAN could be released into the system, this practice had only a small net benefit due to an apparent loss of amino acids during the cellulolytic enzyme hydrolysis.
LECO tracking of N from reactants to products indicated that around 14% of N may be lost as ammonia gas during the hydrolysis of soy flour alone (
Scheme 3), while very little loss was detected from the switchgrass hydrolysis and enzyme saccharification processes (
Scheme 2 and
Scheme 4). Prior literature reports suggest Maillard reactions, thermal amino acid degradation to ammonia, lignin and/or cellulosic fiber binding of soluble ammonia, and amino acid involvement in cellulose–enzyme interactions [
20,
21,
37,
38,
39,
40,
41,
42] as key potential mechanisms consistent with the LECO results that could explain the apparent losses of ammonia and soluble amino acids observed during the co-hydrolysis of soy flour and switchgrass.
Research then shifted to the second strategy of hydrolyzing soy flour alone (
Scheme 3), which allowed for a rapid, high temperature reaction with the flexibility to operate at dilute acid conditions above 1% (
v/
v). As a result, PAN titers were greatly improved to 1588 mg/L, and yield, to 0.0529 g PAN/g, i.e., 61% of theoretical, by increasing the sulfuric acid to 10% (
v/
v) (1.8 M) during a 30 min hydrolysis at 160 °C. Under these conditions the total N in amino acids plus ammonia peaked at 0.065 ± 0.0009 g N/g soy flour, or 75% of the theoretically available N. Operating at 170 °C for 15 min could lead to titers and yields at 5% (
v/
v) H
2SO
4 that were comparable to those seen at 8–10% H
2SO
4 at 160 °C, so the option exists to use even lower sulfuric acid concentrations and higher temperatures to accomplish similar amino acid titers and yields, pending process economics. It is notable that as a general rule, the PAN selectivity was higher at the lower reaction times indicating the advantage of rapid reaction rates and short reaction times to lowering N losses to Maillard reactions or thermal degradation to ammonia.
In comparison to the conventional hydrolysis of protein by the reaction of 30 g/L soy flour with 6 N HCl 24 h at 110 °C, optimal 1.8 M dilute sulfuric acid hydrolysis provided 2.5 times the amino acid yields in just 3% of the reaction time. Preliminary cost comparisons indicated that an optimized sulfuric acid processes would likely be less expensive to operate than 6 N HCl processes, not only due to greater proteolysis efficiency, but also due to lower operating and capital costs associated with the less concentrated, less corrosive catalyst. Favorable comparisons of the process titer and yield of the current research with other processes reported in the literature were also noted for new enzymatic proteolysis of okara, a soy bean waste [
25], and dilute 6 N H
2SO
4 hydrolysis of canola cake protein at 110 °C [
22]. Relative to these reports, the extremely short reaction times (15–30 min) and very low catalyst loading per gram of substrate protein used in the current study (0.056 to 0.111 moles H
2SO
4/g protein) were especially noteworthy, potentially leading to large cost advantages over other reported systems.