Statistical Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment and Enzymatic Hydrolysis for Enhancing Sugar Yields from Corn Cob
Abstract
:1. Introduction
2. Materials and Methods
2.1. Substrate, Chemicals and Enzymes
2.2. Screening for the Optimal Surfactant in Pretreatment
2.3. Optimization of Pretreatment of Corn Cob
2.4. Cellulosic Components Determination
2.5. Optimization of Enzymolysis
2.5.1. Initial Enzymolysis Conditions
2.5.2. Screening of the Optimal Surfactant in Enzymatic Hydrolysis
2.5.3. Enzymatic Hydrolysis Optimization of CC
2.6. Calculations for Conversions of Cellulosic Components
2.7. Data Analysis
3. Results and Discussions
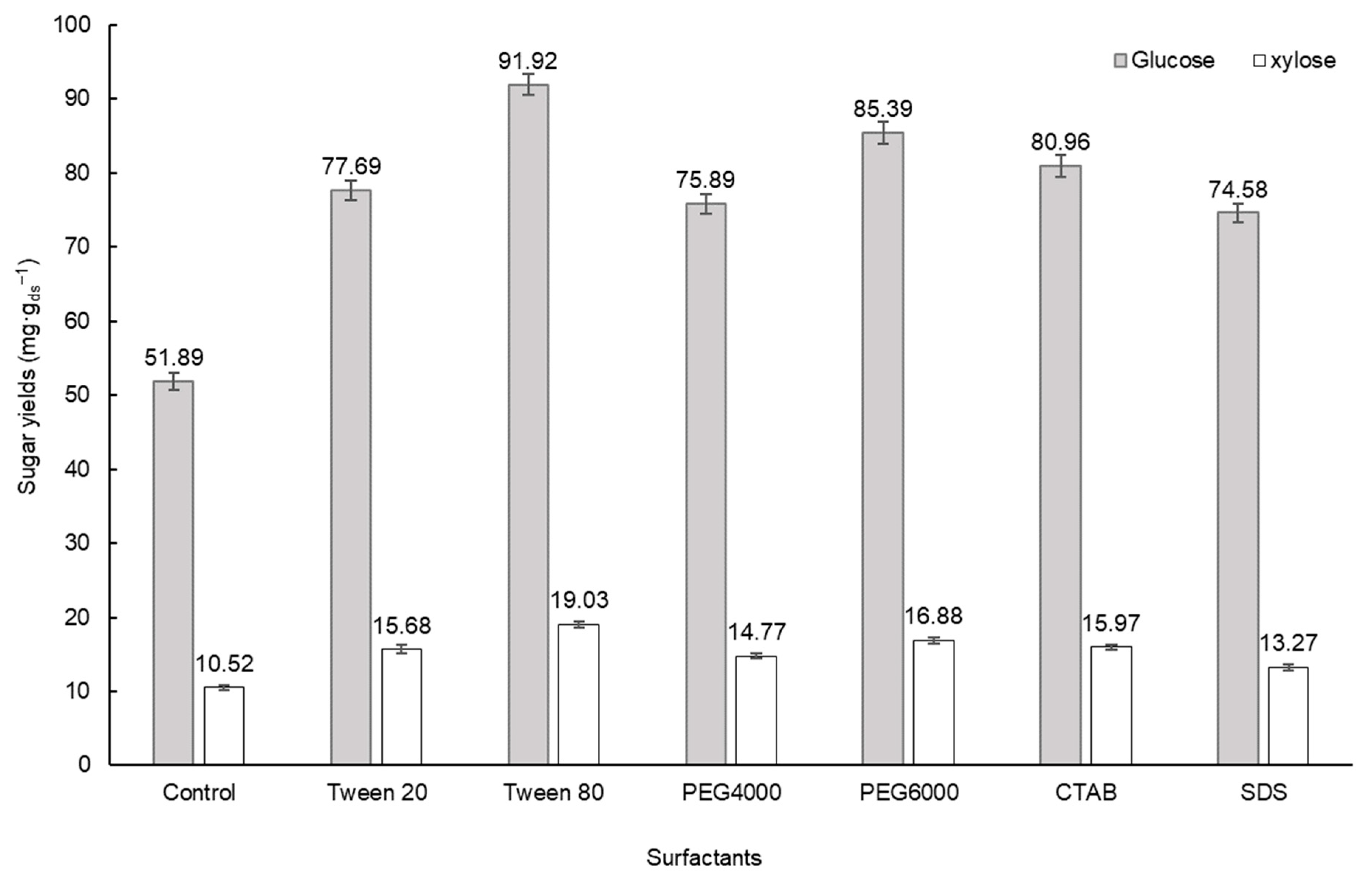
3.1. Screening of the Optimal Surfactant in Pretreatment
3.2. Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment of Corn Cob
3.3. Screening of Surfactant in Enzymatic Hydrolysis
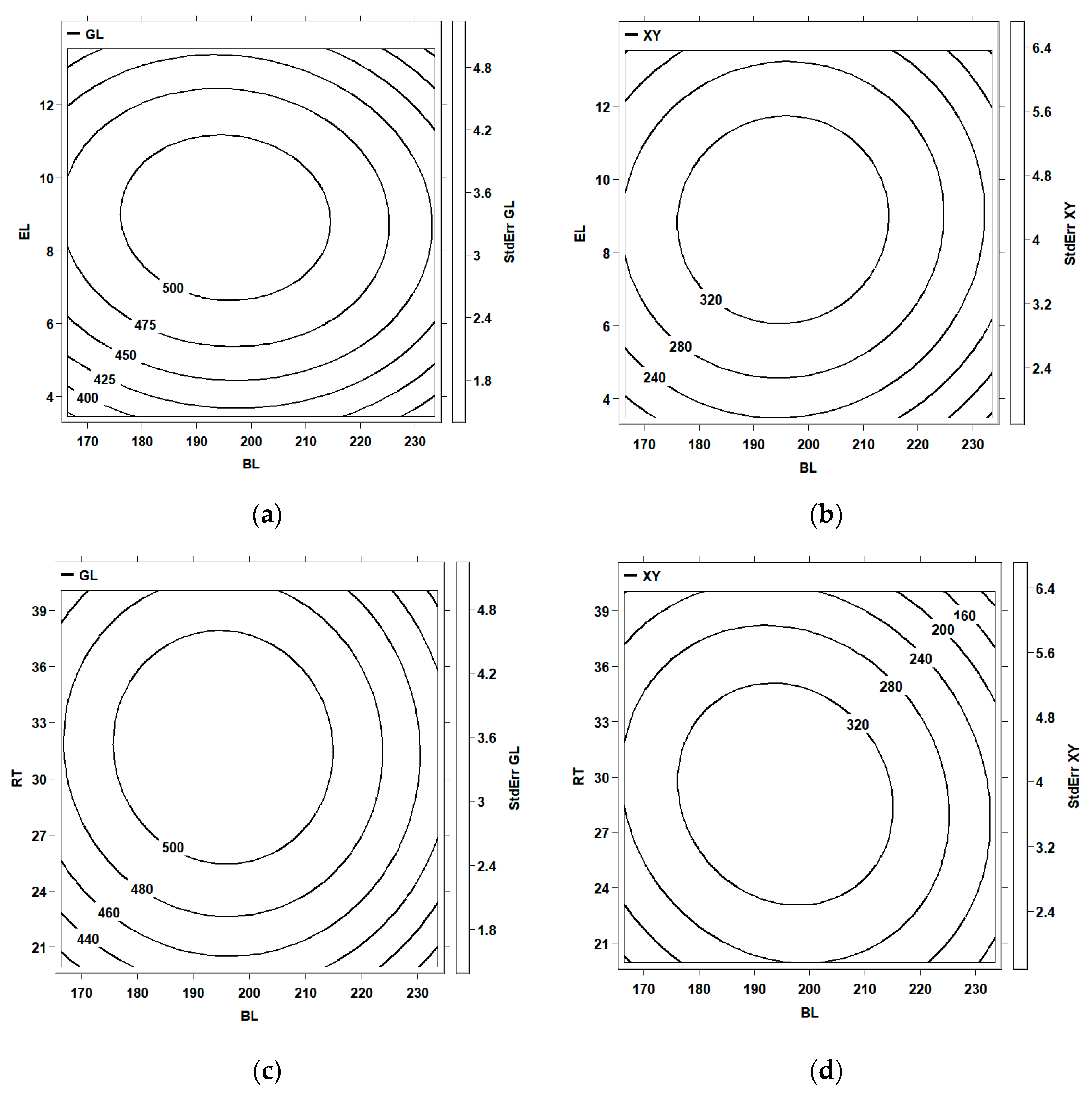
3.4. Optimization of Enzymatic Hydrolysis of Corn Cob
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BBD | Box–Behnken design |
| BL | Biomass loading |
| CBU | Cellubioase activity unit |
| CC | Corn cob |
| CCD | Central composite design |
| CE | Cellulose |
| CEC | Cellulose conversion |
| CER | Cellulose recovery |
| EL | Enzyme loading |
| EU | Endoglucanase activity unit |
| FPU·gds−1 | Filter paper activity units per gram of dry substrate |
| FPU·mL−1 | Filter paper activity units per milliliter |
| GL | Glucose |
| HCE | Hemicellulose |
| HCEC | Hemicellulose conversion |
| HCER | Hemicellulose recovery |
| LGR | Lignin reduction |
| mg·gds−1 | Milligrams per gram of dry substrate |
| PBD | Plackett–Burman design |
| PHC | Potassium hydroxide concentration |
| PTE | Pretreatment temperature |
| PTI | Pretreatment time |
| RSM | Response surface methodology |
| RT | Reaction time |
| SDR | Solid recovery |
| XU·mL−1 | Xylanase activity units per milliliter |
| XY | Xylose |
References
- Bolado-Rodríguez, S.; Toquero, C.; Martín-Juárez, J.; Travaini, R.; García-Encina, P.A. Effect of thermal, acid, alkaline and alkaline-peroxide pretreatments on the biochemical methane potential and kinetics of the anaerobic digestion of wheat straw and sugarcane bagasse. Bioresour. Technol. 2016, 201, 182–190. [Google Scholar] [CrossRef]
- Liu, X.; Zicari, S.M.; Liu, G.; Li, Y.; Zhang, R. Pretreatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour. Technol. 2015, 185, 150–157. [Google Scholar] [CrossRef]
- Korai, R.M.; Li, X. Effect of ultrasonic assisted KOH pretreatment on physiochemical characteristic and anaerobic digestion performance of wheat straw. Chin. J. Chem. Eng. 2020, 28, 2409–2416. [Google Scholar] [CrossRef]
- Xie, X.; Feng, X.; Chi, S.; Zhang, Y.; Yu, G.; Liu, C.; Li, Z.; Li, B.; Peng, H. A sustainable and effective potassium hydroxide pretreatment of wheat straw for the production of fermentable sugars. Bioresour. Technol. Rep. 2018, 3, 169–176. [Google Scholar] [CrossRef]
- Chi, X.; Liu, C.; Bi, Y.H.; Yu, G.; Zhang, Y.; Wang, Z.; Li, B.; Cui, Q. A clean and effective potassium hydroxide pretreatment of corncob residue for the enhancement of enzymatic hydrolysis at high solids loading. RSC Adv. 2019, 9, 11558–11566. [Google Scholar] [CrossRef]
- Zahoor; Wang, W.; Tan, X.; Guo, Y.; Zhang, B.; Chen, X.; Yu, Q.; Zhuang, X.; Yuan, Z. Mild urea/KOH pretreatment to enhance enzymatic hydrolysis of corn stover with liquid waste recovery for plant growth. J. Clean. Prod. 2021, 284, 125392. [Google Scholar] [CrossRef]
- Zahoor; Wang, W.; Tan, X.; Imtiaz, M.; Wang, Q.; Miao, C.; Yuan, Z.; Zhuang, X. Rice straw pretreatment with KOH/urea for enhancing sugar yield and ethanol production at low temperature. Ind. Crops Prod. 2021, 170, 113776. [Google Scholar] [CrossRef]
- Popy, R.S.; Ni, Y.; Salam, A.; Jahan, M.S. Mild potassium hydroxide-based alkaline integrated biorefinery process of Kash (Saccharum spontaneum). Ind. Crops Prod. 2020, 154, 112738. [Google Scholar] [CrossRef]
- Pandey, A.K.; Negi, S. Impact of surfactant assisted acid and alkali pretreatment on lignocellulosic structure of pine foliage and optimization of its saccharification parameters using response surface methodology. Bioresour. Technol. 2015, 192, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nasirpour, N.; Mousavi, S.M.; Shojaosadati, S.A. A novel surfactant-assisted ionic liquid pretreatment of sugarcane bagasse for enhanced enzymatic hydrolysis. Bioresour. Technol. 2014, 169, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Chen, X.; Wana, Y. Pretreatment of wheat straw by nonionic surfactantassisted dilute acid for enhancing enzymatic hydrolysis and ethanol production. Bioresour. Technol. 2010, 101, 4875–4883. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Impact of surfactants on pretreatment of corn stover. Bioresour. Technol. 2010, 101, 5941–5951. [Google Scholar] [CrossRef] [PubMed]
- Saini, J.K.; Anurag, R.K.; Arya, A.; Kumbhar, B.K.; Tewari, L. Optimization of saccharification of sweet sorghum bagasse using response surface methodology. Ind. Crop. Prod. 2013, 44, 211–219. [Google Scholar] [CrossRef]
- Akimkulova, A.; Zhou, Y.; Zhao, X.; Liu, D. Improving the enzymatic hydrolysis of dilute acid pretreated wheat straw by metal ion blocking of non-productive cellulase adsorption on lignin. Bioresour. Technol. 2016, 208, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hou, S.; Shen, B.; Zhang, D.; Li, R.; Xu, X.; Wang, K.; Lai, C.; Yong, Q. Understanding of promoting enzymatic hydrolysis of combined hydrothermal and deep eutectic solvent pretreated poplars by Tween 80. Bioresour. Technol. 2022, 362, 127825. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fan, M.; Li, X.; Zhang, A.; Xie, J. Enhancing enzymatic hydrolysis of sugarcane bagasse by ferric chloride catalyzed organosolv pretreatment and Tween 80. Bioresour. Technol. 2018, 258, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sang, Q.; Zhang, W. Statistical optimization of cellulases production by Aspergillus niger HQ-1 in solid-state fermentation and partial enzymatic characterization of cellulases on hydrolyzing chitosan. Ann. Microbiol. 2012, 62, 629–645. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J. Statistical optimization of sodium hydroxide pretreatment and enzymatic hydrolysis of corn stover powder for enhancing sugar production using response surface methodology. Biomass Convers. Bior. 2023, 13, 7111–7125. [Google Scholar] [CrossRef]
- Fang, H.; Zhao, C.; Song, X.Y. Optimization of enzymatic hydrolysis of steam-exploded corn stover by two approaches: Response surface methodology or using cellulase from mixed cultures of Trichoderma reesei RUT-C30 and Aspergillus niger NL02. Bioresour. Technol. 2010, 101, 4111–4119. [Google Scholar] [CrossRef] [PubMed]
- Verma, U.; Naik, J.B.; Patil, J.S.; Yadava, S.K. Screening of process variables to enhance the solubility of famotidine with 2-HydroxyPropyl-β-Cyclodextrin & PVP K-30 by using Plackett-Burman design approach. Mat. Sci. Eng. C 2017, 77, 282–292. [Google Scholar] [CrossRef]
- Venkataraghavan, R.; Thiruchelvi, R.; Sharmila, D. Statistical optimization of textile dye effluent adsorption by Gracilaria edulis using Plackett-Burman design and response surface methodology. Heliyon 2020, 6, e05219. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, W.; Du, D.; Shang, X. Plackett-Burman experimental design of modified wood ceramics for ammonia nitrogen removal from water and response optimization of the modified wood ceramic’s comprehensive performance. Ceram. Int. 2023, 49, 11612–11622. [Google Scholar] [CrossRef]
- Fang, M.; Yu, Z.; Zhang, W.; Cao, J.; Liu, W. Friction coefficient calibration of corn stalk particle mixtures using Plackett-Burman design and response surface methodology. Powder Technol. 2022, 396, 731–742. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Laboratory Analytical Procedure (LAP): Determination of Structural Carbohydrates and Lignin in Biomass. In Technical Report: NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Zhang, H.; Chen, W.; Han, X.; Zeng, Y.; Zhang, J.; Gao, Z.; Xie, J. Intensification of sugar production by using Tween 80 to enhance metal-salt catalyzed pretreatment and enzymatic hydrolysis of sugarcane bagasse. Bioresour. Technol. 2021, 339, 125522. [Google Scholar] [CrossRef] [PubMed]
- Kleingesinds, E.K.; José, Á.H.M.; Brumano, L.P.; Silva-Fernandes, T.; Rodrigues, D., Jr.; Rodrigues, R.C. Intensification of bioethanol production by using Tween 80 to enhance dilute acid pretreatment and enzymatic saccharification of corncob. Ind. Crops Prod. 2018, 124, 166–176. [Google Scholar] [CrossRef]
- Ma, Q.; Zhou, W.; Du, X.; Huang, H.; Gong, Z. Combined dilute sulfuric acid and Tween 80 pretreatment of corn stover significantly improves the enzyme digestibility: Synergistic removal of hemicellulose and lignin. Bioresour. Technol. 2023, 382, 129218. [Google Scholar] [CrossRef]
- Song, G.; Madadi, M.; Sun, C.; Shao, L.; Tu, M.; Abdulkhani, A.; Zhou, Q.; Lu, X.; Hu, J.; Sun, F. Surfactants facilitated glycerol organosolv pretreatment of lignocellulosic biomass by structural modification for co-production of fermentable sugars and highly reactive lignin. Bioresour. Technol. 2023, 383, 129178. [Google Scholar] [CrossRef]
- Nasirpour, N.; Mousavi, S.M. RSM based optimization of PEG assisted ionic liquid pretreatment of sugarcane bagasse for enhanced bioethanol production: Effect of process parameters. Biomass Bioenerg. 2018, 116, 89–98. [Google Scholar] [CrossRef]
- Goshadroua, A.; Lefsrud, M. Synergistic surfactant-assisted [EMIM]OAc pretreatment of lignocellulosic waste for enhanced cellulose accessibility to cellulase. Carbohyd. Polym. 2017, 166, 104–113. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, N.; Tong, Q.; Jin, Z.; Xu, X. Intensification of sodium hydroxide pretreatment of corn stalk using magnetic field in a fluidic system. Bioresour. Technol. 2016, 220, 1–7. [Google Scholar] [CrossRef]
- Garai, D.; Kuma, V. A Box-Behnken design approach for the production of xylanase by Aspergillus candidus under solid state fermentation and its application in saccharification of agro residues and Parthenium hysterophorus L. Ind. Crops Prod. 2013, 44, 352–363. [Google Scholar] [CrossRef]
- Oruganti, R.K.; Sunar, S.L.; Panda, T.K.; Shee, D.; Bhattacharyy, D. Kraft lignin recovery from de-oiled Jatropha curcas seed by potassium hydroxide pretreatment and optimization using response surface methodology. Bioresour. Technol. Rep. 2023, 23, 101572. [Google Scholar] [CrossRef]
- Sharma, R.; Palled, V.; Sharma-Shivappa, R.R.; Osborne, J. Potential of potassium hydroxide pretreatment of switchgrass for fermentable sugar production. Appl. Biochem. Biotechnol. 2013, 169, 761–772. [Google Scholar] [CrossRef]
- Xu, Q.Q.; Zhao, M.J.; Yu, Z.Z.; Yin, J.Z.; Li, G.M.; Zhen, M.Y.; Zhang, Q.Z. Enhancing enzymatic hydrolysis of corn cob, corn stover and sorghum stalk by dilute aqueous ammonia combined with ultrasonic pretreatment. Ind. Crops Prod. 2017, 109, 220–226. [Google Scholar] [CrossRef]
- Zhang, J.; Li, K.; Liu, S.; Huang, S.; Xu, C. Alkaline hydrogen peroxide pretreatment combined with bio-additives to boost high-solids enzymatic hydrolysis of sugarcane bagasse for succinicacid processing. Bioresour. Technol. 2022, 345, 126550. [Google Scholar] [CrossRef]
- Asghar, U.; Irfan, M.; Nadeem, M.; Nelofer, R.; Syed, Q. Effect of KOH pretreatment on lignocellulosic waste to be used as substrate for ethanol production. Iran J. Sci. Technol. Trans. Sci. 2017, 41, 659–663. [Google Scholar] [CrossRef]
- Ma, L.; Cui, Y.; Cai, R.; Liu, X.; Zhang, C.; Xiao, D. Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour. Technol. 2015, 180, 1–6. [Google Scholar] [CrossRef]
- Li, K.; Wan, J.; Wang, X.; Wang, J.; Zhang, J. Comparison of dilute acid and alkali pretreatments in production of fermentable sugars from bamboo: Effect of Tween 80. Ind. Crops Prod. 2016, 83, 414–422. [Google Scholar] [CrossRef]
- Buyukkileci, A.O.; Temelli, N. Organosolv pretreatment of corncob for enzymatic hydrolysis of xylan. Biomass Convers. Bior. 2023, 13, 6385–6394. [Google Scholar] [CrossRef]
- Costa Filho, J.D.B.; Araújo Padilha, C.E.; Bivar Matias, S.C.; Ribeiro, V.T.; Santos, E.S.; Santana Souza, D.F. Combining glycerol pretreatment with persulfate oxidation to obtain cellulosic ethanol and dye adsorbent from maize cob. Biomass Convers. Bior. 2023. [Google Scholar] [CrossRef]
- Qiao, H.; Ouyang, S.; Shi, J.; Zheng, Z.; Ouyang, J. Mild and efficient two-step pretreatment of lignocellulose using formic acid solvent followed by alkaline salt. Cellulose 2021, 28, 1283–1293. [Google Scholar] [CrossRef]
- Su, Y.; Du, R.; Guo, H.; Cao, M.; Wu, Q.; Su, R.; Qi, W.; He, Z. Fractional pretreatment of lignocellulose by alkaline hydrogen peroxide: Characterization of its major components. Food Bioprod. Process. 2015, 94, 322–330. [Google Scholar] [CrossRef]
- Bu, L.; Xing, Y.; Yu, H.; Gao, Y.; Jiang, J. Comparative study of sulfite pretreatments for robust enzymatic saccharification of corn cob residue. Biotechnol. Biofuels 2012, 5, 87. [Google Scholar] [CrossRef]
- Ling, R.; Wu, W.; Yuan, Y.; Wei, W.; Jin, Y. Investigation of choline chloride-formic acid pretreatment and Tween 80 to enhance sugarcane bagasse enzymatic hydrolysis. Bioresour. Technol. 2021, 326, 124748. [Google Scholar] [CrossRef] [PubMed]
- Parnthong, J.; Kungsanant, S.; Chavadej, S. The Influence of nonionic surfactant adsorption on enzymatic hydrolysis of oil palm fruit bunch. Appl. Biochem. Biotechnol. 2018, 186, 895–908. [Google Scholar] [CrossRef]
- Ribeiro, V.T.; Costa Filho, J.D.B.; Araújo Padilha, C.E.; Santos, E.S. Using Tween 80 in pretreatment, enzymatic hydrolysis, and fermentation processes for enhancing ethanol production from green coconut fiber. Biomass Convers. Bior. 2023. [Google Scholar] [CrossRef]
- Steward Nababan, M.Y.; Fatriasari, W.; Wistara, N.J. Response surface methodology for enzymatic hydrolysis optimization of jabon alkaline pulp with Tween 80 surfactant addition. Biomass Convers. Bior. 2022, 12, 2165–2174. [Google Scholar] [CrossRef]
- Alhammad, A.; Adewale, P.; Kuttiraja, M.; Christopher, L.P. Enhancing enzyme-aided production of fermentable sugars from poplar pulp in the presence of non-ionic surfactants. Bioproc. Biosyst. Eng. 2018, 41, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, N.; Chandraraj, K. Improved high solids loading enzymatic hydrolysis and fermentation of cotton microdust by surfactant addition and optimization of pretreatment. Process Biochem. 2021, 106, 60–69. [Google Scholar] [CrossRef]
- Gupta, P.; Parkhey, P. A two-step process for efficient enzymatic saccharification of rice straw. Bioresour. Technol. 2014, 173, 207–215. [Google Scholar] [CrossRef]
- Alrumman, S.A. Enzymatic saccharification and fermentation of cellulosic date palm wastes to glucose and lactic acid. Braz. J. Microbiol. 2016, 47, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Su, X.; Yang, W.; Wang, Y.; Kuang, M.; Ma, L.; Fang, D.; Zhou, D. Enzymatic saccharification of high pressure assist-alkali pretreated cotton stalk and structural characterization. Carbohyd. Polym. 2016, 140, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Grubišić, M.; Perečinec, M.G.; Peremin, I.; Mihajlovski, K.; Beluhan, S.; Šantek, B.; Šantek, M.I. Optimization of pretreatment conditions and enzymatic hydrolysis of corn cobs for production of microbial lipids by Trichosporon oleaginosus. Energies 2022, 15, 3208. [Google Scholar] [CrossRef]
- David, A.N.; Sewsynker-Sukai, Y.; Sithole, B.; Gueguim Kana, E.B. Development of a green liquor dregs pretreatment for enhanced glucose recovery from corn cobs and kinetic assessment on various bioethanol fermentation types. Fuel 2020, 274, 117797. [Google Scholar] [CrossRef]
- Gao, W.; Li, Z.; Liu, T.; Wang, Y. Production of high-concentration fermentable sugars from lignocellulosic biomass by using high solids fed-batch enzymatic hydrolysis. Biochem. Eng. J. 2021, 176, 108186. [Google Scholar] [CrossRef]
- Kuntapa, M.; Sudaprasert, K.; Tachaapaikoon, C. Pretreatment of corn cobs using 1-butyl-3-methylimidazolium tetrafluoroborate and dimethyl sulfoxide to enhance enzymatic saccharification. Biomass Convers. Bior. 2023, 13, 6941–6952. [Google Scholar] [CrossRef]
- Jain, L.; Kurmi, A.K.; Kumar, A.; Narani, A.; Bhaskar, T.; Agrawal, D. Exploring the flexibility of cellulase cocktail obtained from mutant UV-8 of Talaromyces verruculosus IIPC 324 in depolymerising multiple agro-industrial lignocellulosic feedstocks. Int. J. Biol. Macromol. 2020, 154, 538–544. [Google Scholar] [CrossRef]




| Terms | Glucose Yield | Xylose Yield |
|---|---|---|
| Constant | 118.101 | 29.9542 |
| Potassium hydroxide concentration | 8.228 ## | 3.4408 ## |
| Solid-to-liquid ratio | 0.088 | 0.0008 |
| Pretreatment temperature | 10.411 ## | 4.2392 ## |
| Pretreatment time | 7.329 ## | 3.2708 ## |
| Tween 80 | 0.179 | 0.0608 |
| R2 | 99.45% | 99.29% |
| Adj-R2 | 99.03% | 98.75% |
| Lack of fit | 0.135 | 0.134 |
| Terms | Glucose Yield (Y1) | Xylose Yield (Y2) |
|---|---|---|
| Constant | 229.500 | 123.143 |
| Potassium hydroxide concentration (x1) | −6.749 ## | −8.164 ## |
| Pretreatment temperature (x2) | −6.710 ## | −7.548 ## |
| Pretreatment time (x3) | 4.554 ## | −7.276 ## |
| Potassium hydroxide concentration × potassium hydroxide concentration (x1 × x1) | −25.358 ## | −20.058 ## |
| Pretreatment temperature × pretreatment temperature (x2 × x2) | −19.090 ## | −14.330 ## |
| Pretreatment time × pretreatment time (x3 × x3) | −15.797 ## | −12.278 ## |
| Potassium hydroxide concentration × pretreatment temperature (x1 × x2) | 0.177 | 1.750 |
| Potassium hydroxide concentration × pretreatment time (x1 × x3) | −0.185 | −3.973 ## |
| Pretreatment temperature × pretreatment time (x2 × x3) | −0.682 | −0.410 |
| R2 | 99.6% | 99.6% |
| Adj-R2 | 98.8% | 98.8% |
| Lack of fit | 0.118 | 0.120 |
| Substance | Conditions | CER | HCER | LGR | Refs. |
|---|---|---|---|---|---|
| CC | Potassium permanganate 20 g·L−1, solid dose 100 g·L−1, 50 °C, 360 min. | 94.56% | 81.47% | 46.79% | [38] |
| CC | Ethanol solution 70% (v/v), solid dose 100 g·L−1, 170 °C, 60 min. | 85.1% | 81.1% | 51.1% | [40] |
| CC | Mixture of glycerol and water and H2SO4 (80:19:1, w/w), solid dose 61 g·L−1, 121 °C, 60 min. | 89.9% | 47.77% | 54.12% | [41] |
| CC | Formic acid 880 g·L−1, solid dose 100 g·L−1, 80 °C, 180 min. | 87.2% | 9.9% | 87.1% | [42] |
| CC | Hydrogen peroxide solution 2.0% (w/w) with pH 11.5, solid dose 50 g·L−1, 50 °C, 360 min. | 81.3% | 61.3% | 75.4% | [43] |
| CC | Sodium hydroxide solution 5.0 g·L−1, solid dose 100 g·L−1, 80 °C, 180.0 min. | 84.15% | - | 34.98% | [44] |
| CC | Potassium hydroxide solution 46 g·L−1, solid dose 200 g·L−1, Tween 80 3.0 g·L−1, 78 °C, 50 min. | 97.8% | 68.0% | 89.7% | This work |
| Terms | Glucose Yield | Xylose Yield |
|---|---|---|
| Constant | 220.229 | 128.361 |
| Biomass loading | 14.563 ## | 8.176 ## |
| Enzyme loading | 15.498 ## | 10.573 ## |
| Reaction temperature | 0.384 | 0.794 |
| Reaction pH | 0.983 | 0.346 |
| Reaction time | 19.208 ## | 10.054 ## |
| Tween 80 concentration | 0.968 | 0.456 |
| R2 | 99.43% | 99.40% |
| Adj-R2 | 98.86% | 98.80% |
| Lack of fit | 0.108 | 0.104 |
| Terms | Glucose Yield | Xylose Yield |
|---|---|---|
| Constant | 515.824 | 347.833 |
| Biomass loading (X1) | −8.651 ## | −15.640 ## |
| Enzyme loading (X2) | 7.871 ## | 9.549 ## |
| Reaction time (X3) | 9.232 ## | −11.097 ## |
| Biomass loading × biomass loading (X1 × X1) | −18.894 ## | −32.468 ## |
| Enzyme loading × enzyme loading (X2 × X2) | −30.526 ## | −33.709 ## |
| Reaction time × reaction time (X3 × X3) | −16.813 ## | −30.603 ## |
| Biomass loading × enzyme loading (X1 × X2) | −2.469 | 2.004 |
| Biomass loading × reaction time (X1 × X3) | −1.111 | −7.526 ## |
| Enzyme loading × reaction time (X2 × X3) | 0.566 | −1.466 |
| R2 | 99.4% | 99.5% |
| Adj-R2 | 98.9% | 99.1% |
| Lack of fit | 0.105 | 0.103 |
| Substance | Cellulase | Enzymolysis Conditions | GL (mg·gds−1) | XY (mg·gds−1) | CEC | HCEC | Refs. |
|---|---|---|---|---|---|---|---|
| CC | Cellulase from Sigma-Aldrich, St. Louis, MO, USA | EL 10.0 FPU·gds−1, BL 50 g·L−1, 48 h. | 433 | - | 60.9% | [41] | |
| CC | Celluclast 1.5 L and Novozyme 188 from Sigma Co., St. Louis, MO, USA | EL 61.62 FPU·gds−1 and 27 CBU·gds−1, BL 25 g·L−1, 96 h. | 500.8 | 81.2% | [44] | ||
| CC | Cellulases from Novozymes | EL 75.15 FPU·gds−1, BL 200 g·L−1, 72 h. | 408.2 | 282.1 | 92.0% | 72.7% | [54] |
| CC | Cellulases from Novozymes | EL 10.0 FPU·gds−1, BL 100 g·L−1, 72 h. | 420 | [55] | |||
| CC | Commercial cellulase from Qingdao Vland Biological Co., Ltd., Qingdao, China | EL 31.1 FPU·gds−1, BL 50 g·L−1, 24 h. | 385.02 | 221.25 | 88.6% | 70.8% | [56] |
| CC | Cellulase from Novozymes | EL 31.1 FPU·gds−1, BL 10 g·L−1, 72 h. | 332.04 | - | 92.98% | - | [57] |
| CC | Talaromyces verruculosus IIPC 324 | EL 2155.4 EU·gds−1, BL 75 g·L−1, 72 h. | 515.3 | 19.5 | 83.9% | 35.8% | [58] |
| CC | A. niger HQ-1 | EL 8.9 FPU·gds−1, BL 195 g·L−1, Tween 80 3.0 g·L−1, 30.4 h. | 518.48 | 351.14 | 97.2% | 82.9% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Wu, J. Statistical Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment and Enzymatic Hydrolysis for Enhancing Sugar Yields from Corn Cob. Fermentation 2023, 9, 1009. https://doi.org/10.3390/fermentation9121009
Zhang H, Wu J. Statistical Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment and Enzymatic Hydrolysis for Enhancing Sugar Yields from Corn Cob. Fermentation. 2023; 9(12):1009. https://doi.org/10.3390/fermentation9121009
Chicago/Turabian StyleZhang, Hui, and Junhui Wu. 2023. "Statistical Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment and Enzymatic Hydrolysis for Enhancing Sugar Yields from Corn Cob" Fermentation 9, no. 12: 1009. https://doi.org/10.3390/fermentation9121009
APA StyleZhang, H., & Wu, J. (2023). Statistical Optimization of Tween-80-Assisted Potassium Hydroxide Pretreatment and Enzymatic Hydrolysis for Enhancing Sugar Yields from Corn Cob. Fermentation, 9(12), 1009. https://doi.org/10.3390/fermentation9121009






