Abstract
Brewers’ spent grain (BSG) constitutes the primary by-product of the brewing industry. The valorization of BSG from a circular economy perspective is of high industrial interest. The objective of this study was the exploitation of BSG for the microbial production of branched-chain fatty acids (BCFAs) and polyunsaturated fatty acids (PUFAs), representing two different classes of high-value fatty acids (FAs). In the present study, this waste material underwent treatment with hot water in an autoclave and the resultant extract was utilized for the preparation of a novel liquid medium (BSG medium) to be employed for microbial fermentation. Screening and subsequent scaling-up experiments confirmed the suitability of the BSG medium to support the microbial production of various high-value FAs. In particular, Streptomyces jeddahensis and Conidiobolus heterosporus could be employed for BCFAs production, Pythium ultimum and Mortierella alpina could be used to provide cis-5,8,11,14,17-eicosapentaenoic acid (EPA) and arachidonic acid (ARA), whereas Mucor circinelloides, when grown in a BSG medium, was able to accumulate γ-linolenic acid (GLA).
1. Introduction
Brewer’s spent grain (BSG) is the main by-product deriving from the brewing process. This lignocellulosic material constitutes the insoluble solid fraction obtained after filtration of the barley wort accounting for 85% (w/w) of the waste materials generated through the overall process of beer production. Currently, the production of 100 L of beer leads to the generation of 20 kg of wet BSG [1]. Beer is one of the most consumed alcoholic beverages and, in 2022, the European production of 34 billion liters of beer [2] generated about 6.8 million tons of BSG.
Until a decade ago, this side product was considered a waste material to be disposed of, with a relevant cost for breweries. Due to its high moisture content, BSG was not stored for an extended period and was immediately utilized, as fertilizer in agriculture or added to ruminants’ feed. However, in recent years, researchers have found new ways for BSG valorization. Indeed, this material is a rather rich matrix containing proteins, cellulose, hemicellulose, lignin, starch, lipids and phenolic derivatives [3]. Additionally, the high level of dietary fibers (β-glucan and arabinoxylan), present in the BSG polysaccharide fraction, makes this material suitable for various applications, including as an ingredient in functional foods for human nutrition. These aspects suggest the potential of BSG as a source of compounds to be used for the preparation of different products of commercial relevance.
Overall, the valorization of BSG from a circular economy perspective contributes to reduce both the carbon footprint and disposal costs associated with brewing processes. Consequently, the number of scientific studies investigating BSG as a low-cost, renewable feedstock for the development of new processes has increased exponentially [3,4,5,6,7,8,9,10,11,12,13].
Recently, we proposed an innovative and efficient method of BSG exploitation, which is based on a multistep fractionation of the biomass [7]. The process involves two sequential applications of different chemical–physical treatments. In the first step, BSG is extracted using water at high temperature to separate soluble proteins and polysaccharides. The solid residue undergoes further fractionation through a deep eutectic solvent-mediated process, allowing the separation of a carbohydrate-rich fraction and a lignin fraction. The first obtained fraction, namely the aqueous extract, constitutes 25–30% (w/w) of the starting dry BSG. This nutrient-rich fraction has been successfully employed for the preparation of a new fermentation medium (referred to as BSG medium) that can be used to support the growth of various microbial strains. Indeed, our previous study demonstrated that some selected fungal and bacterial strains could use BSG medium as a fermentation substrate affording high biomass production. More specifically, some Streptomyces species as well as Phaffia rhodozyma and Rhodococcus opacus, which are microorganisms already used in different industrial processes, exhibited very good results.
According to the latter findings, our research was extended further. The present study focused on the exploitation of BSG medium for the microbial production of high-value fatty acids (FAs). Two different classes of FAs were investigated: branched-chain fatty acids (BCFAs) and polyunsaturated fatty acids (PUFAs) (Figure 1).
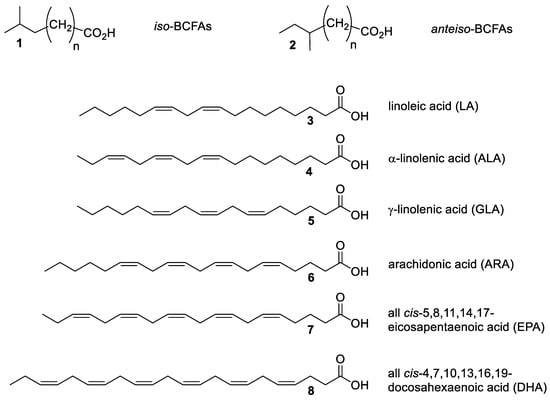
Figure 1.
The high-value fatty acids examined in this study.
BCFAs are saturated fatty acids possessing one methyl branch on the carbon chain located at the ultimate (iso, general structure 1) or penultimate (anteiso, general structure 2) carbon. BCFAs possess distinctive physical, chemical and biological properties. The presence of the methyl branch greatly decreases the melting point of the BCFAs as well as of their esters that have been selected as the biofuels of choice for use in cold environments [14]. In addition, BCFAs have shown potential health effects in relation to various human diseases [15]. In particular, recent studies have proven that the latter class of FAs reduces the incidence of neonatal necrotizing enterocolitis (NEC) [16]. For these reasons, BCFAs have been employed as ingredients in infant food formulas as human milk fat substitutes [17].
PUFAs exhibit protective activity against cardiovascular diseases and are of fundamental relevance in brain, nerve and eye functioning [18]. PUFAs are essential fatty acids because humans are not able to synthesize them de novo. Therefore, these compounds have become of great commercial value, being widely employed as food supplements. Among the PUFAs present on the market (compounds 3–8), only linoleic acid (LA, 3) is easily available by extraction from vegetable oils. The natural occurrence of the other two C18-linolenic acid isomers, namely α-linolenic (ALA, 4) and γ-linolenic (GLA, 5) acids, is restricted to a limited number of seed oils such as linseed oil and borage seed oil, respectively. Finally, the most health-beneficial (and most sought-after) PUFAs are arachidonic acid (ARA, 6) [19], cis-5,8,11,14,17-eicosapentaenoic acid (EPA, 7) and cis-4,7,10,13,16,19-docosahexaenoic acid (DHA, 8) [20]. These FAs are usually supplied to the human body from dietary animal sources, where they occur in very minute amounts. A relevant exception concerns EPA and DHA, whose main dietary sources are marine fish (salmon, sardines, mackerel and shrimps) that accumulate the above-mentioned FAs as a result of their marine phytoplankton consumption. Until recent years, fish oils have been largely used to prepare food supplements containing PUFAs. Despite this fact, extreme overfishing and climate change have affected fish fat composition, which has shown a remarkable reduction in PUFAs. In addition, the quality of the fish oils can also be reduced by the possible contamination with organo-mercury derivatives. Overall, these drawbacks created a relevant gap between demand and supply, fostering the search for alternative sources of PUFAs [21]. In this context, different microbial fermentation processes turned out to be the best methods for PUFA production and, currently, the industrial production of ARA and DHA is performed using specific strains of the fungi Mortierella alpina and of the protists Crypthecodinium cohnii, respectively. Different agro-food industrial waste materials have been used as substrates for microbial growth [13,21], but the aqueous extract of BSG has not yet been exploited.
According to the scope of our study, the potential use of a BSG medium for the microbial production of BCFAs and PUFAs was investigated. Overall, 28 strains were tested and the most promising microorganisms were employed in scaling-up experiments.
2. Materials and Methods
2.1. BSG Aqueous Extraction and Preparation of BSG Media
BSG waste was obtained as a wet residue directly from the brewery within 12 h after a batch of beer production. The BSG used in this study is derived from the production of pale ale beer. This material was brought to dryness in a ventilated oven (60 °C, 24 h) and was finely minced by using an electric blender. The obtained dried BSG, designated as BSGD, contained 4.3% of residual humidity and was stored, sealed and refrigerated (4 °C). BSGD was used for the preparation of BSG medium and marine BSG medium (MBSG medium).
BSG medium: BSGD (40 g) was suspended in deionized water (700 mL) and was heated in an autoclave (121 °C, 30 min). The solid was filtrated, washed three times with deionized water (3 × 250 mL) and dried in a ventilated oven (60 °C, 24 h), leading to 28.9 g of exhausted BSG (4.1% of residual humidity). The combined liquid phases were used for the preparation of the BSG microbial growth medium. Accordingly, ammonium sulfate (4.5 g), yeast extract (1.5 g) and trace elements solution (15 mL) were added to the extract. Hence, the final volume of the liquid was adjusted to 1.5 L by the addition of further deionized water and the obtained medium was sterilized by autoclaving (121 °C, 15 min).
MBSG medium: The extraction of BSGD (40 g) was performed as described above, for the preparation of the BSG medium. Then, the combined liquid phases were used for the preparation of the MBSG medium. Accordingly, unrefined sea salt (30 g), MgCl2∙6H2O (7 g), MgSO4∙7H2O (3 g), CaCl2 (1.5 g), KCl (0.75 g), SrCl2∙6H2O (0.75 g), NaBr (0.75 g), NH4Cl (4.5 g), soil extract (80 mL), NaOAc (1.5 g), yeast extract (1.5 g) and trace elements solution (15 mL) were added to the extract. Hence, the final volume of the liquid was adjusted to 1.5 L by the addition of further deionized water and the obtained medium was sterilized by autoclaving (121 °C, 15 min).
2.2. Fermentation with a BSG Microbial Growth Medium
The BSG medium, prepared as described above, was used for the growth of all the investigated strains, except for Crypthecodinium cohnii, Shewanella hanedai and Moritella marina, which were grown in the MBSG medium. The experiments were carried out in triplicate and the presented results are the media of three experimental samples.
Preparation of microbial inoculum: The microbial strain was grown in its suitable medium (40 or 100 mL) contained in 100 mL or 250 mL Erlenmeyer flasks, respectively. The pre-growth was stirred at 130 rpm for 4–7 days (depending on the strain). Then, the microbial biomass was separated by centrifugation, was suspended in a small volume of BSG or MBSG medium and was used to inoculate the flask or the bioreactor.
Screening experiments: Each fermentation trial was performed using a 1 L Erlenmeyer flask, sealed with a cotton plug and containing 250 mL of either BSG or MBSG medium. After the inoculum of the microbial strain, the flask was stirred at 130 rpm until the complete growth of the same. All the strains tested were grown at 24 °C, except Shewanella hanedai and Moritella marina, which were grown at 15 °C. Hence, the biomass was collected by centrifugation and was treated, under a static atmosphere of nitrogen, with hydroquinone (10 mg), aqueous NaOH (5% w/v, 16 mL per gram of biomass) and methanol (4 mL per gram of biomass). The mixture was vigorously stirred and was heated at reflux for 6 h. Hence, the reaction was cooled (0 °C), acidified by the dropwise addition of concentrated HCl aqueous (37% w/v) and was extracted twice with ethyl acetate. The combined organic phases were washed in turn with water and brine, then were dried (Na2SO4) and concentrated under reduced pressure. The residue was treated at 0 °C with an excess of an ethereal solution of freshly prepared diazomethane and was submitted to GC/MS analysis.
Bioreactor fermentation: Each fermentation trial was performed using a 5 L bioreactor (Biostat A BB-8822000, Sartorius-Stedim, Göttingen, Germany) loaded with 1.5 L of the BSG medium. Stirring and aeration were set at 200 rpm and 0.6 L/L/min, respectively, whereas the temperature, the pH and the fermentation duration were set depending on the strain used. As soon as the fermentation was stopped, the biomass was collected by centrifugation and was freeze-dried in high vacuum (0.05 mmHg) until the sample reached a constant weight. The obtained dry biomass was treated, under a static atmosphere of nitrogen, with hydroquinone (10 mg), aqueous NaOH (10% w/v, 16 mL per gram of biomass) and methanol (4 mL per gram of biomass). The mixture was vigorously stirred and was heated at reflux for 6 h. Hence, the reaction was cooled (0 °C), acidified by the dropwise addition of concentrated HCl aqueous (37% w/v) and was extracted twice with ethyl acetate. The combined organic phases were washed in turn with water and brine, then were dried (Na2SO4) and concentrated under reduced pressure. The residue consisted of a mixture of free fatty acids. The presence of other metabolites was negligible, as indicated by the chromatographic purification, affording the fatty acids mixture with a weight loss of only 10%. A sample of this mixture was treated at 0 °C with an excess of an ethereal solution of freshly prepared diazomethane. The obtained methyl esters mixture was submitted to GC-MS analysis.
3. Results and Discussion
3.1. BSG Medium Preparation and Characterization
As described before [7], the BSG medium was prepared by extraction with water of ground BSGD, heating in an autoclave for a rather short time (121 °C, 15 min). In these conditions, about 25% (w/w) of the starting BSGD was extracted. According to additional experiments, the efficiency of the extraction procedure can be improved by prolonging the autoclave cycle. A contact time of 30 min at 121 °C allowed the extraction of 28% (w/w) BSGD whereas the highest amount of extracted material (30% w/w) was obtained from very long thermic treatment (121 °C, 2 h). The process cannot improve further. Hence, the extraction protocol using a contact time of 30 min at 121 °C, represented the best compromise between extraction efficiency and process sustainability.
Considering that cellulose and lignin are not soluble in neutral water, even at high temperatures, the obtained solution must consist of soluble polysaccharides, proteins and a very minor amount of fats, phenolic compounds and inorganic salts. The protein content was measured according to the Bradford assay [22], indicating a value of 0.43 g/L. This result proved that the nitrogen content of the extract was rather modest, supporting our previous protocol that included an ammonium salt in the BSG medium. Overall, the main nutrients of BSG extract are polysaccharides, most likely consisting of soluble starch, β-glucans and hemicellulose.
Since many microorganisms are not able to metabolize hemicellulose or their monosaccharide components, the BSG extract was analyzed to characterize its hemicellulose content [23]. BSG contains mainly arabinoxylanes; namely, the hemicellulose consisting of arabinose and xylose copolymer. Taking into account that starch and β-glucans are glucose polymers, a BSG extract sample was hydrolyzed with mineral acid (1 M H2SO4 at reflux). This treatment completely broke down the polymer molecules that were converted into their monosaccharide components. Their reduction to alditol derivatives and the following acetylation afforded the corresponding alditol acetate, which was analyzed by GC-MS. The analysis indicated the presence of only four components; namely, glucose (84.2%), arabinose (8.5%), xylose (5.8%) and galactose (1.5%). Hence, we can conclude that about 16% of the organic content of BSG extract corresponds to hemicellulose whereas the remaining 84% is due to glucose polymers, most likely starch and β-glucans.
These results point to the potential employment of BSG extract as a medium for microorganisms able to metabolize these compounds, such as fungi and bacteria that in nature grow on cereals or decaying vegetation.
It is worth noting that different marine microorganisms are EPA or DHA producers. Since the production of these high-value PUFAs by fermentation of the BSG extract was one of the objectives of our research, two different BSG media, one for the most common microorganisms and a second one for marine microorganisms, were prepared. The first medium (reported as BSG medium) consisted of BSG extract (7.5 g/L), yeast extract (1 g/L) and ammonium sulfate (3 g/L). As previously reported [7], the addition of yeast extract was essential because it supplied vitamins and microelements necessary for microbial growth, whereas the choice of ammonium sulfate as a nitrogen source was due to the fact that this salt is very cheap and easily available. The second medium (reported as marine BSG medium) was made up of the above-described BSG medium with the addition of the salts necessary for the growth of marine microorganisms. Therefore, marine BSG medium contained a high amount of unrefined sea salt (20 g/L) as well as magnesium and calcium salts and other minor inorganic components.
3.2. Microbial Growth on the BSG Medium: Strains Screening
The above-described media were tested for the fermentation of several selected microorganisms. Since both BCFAs and PUFAs are potentially employed in human nutrition, the present study makes use of strains belonging to the BL-1, with a high preference for those generally recognized as safe for human health (GRAS). All the strains were screened, evaluating the fatty acids composition of the obtained biomass. More specifically, each strain was incubated in the BSG medium at a suitable temperature until complete growth. Hence, the biomass was collected, treated with sodium hydroxide to hydrolyze lipids and phospholipids and then the free fatty acids were transformed in methyl esters using a diazomethane addition. The obtained mixture was analyzed by GC-MS. The resulting complex fatty acid profile did not provide information about the overall strain productivity, but it clearly indicated its capability in the transformation of the BSG medium nutrients into a given fatty acid.
Accordingly, all the data were collected in Table 1, where the FAs composition was expressed as a percentage of the sum of the compounds detected by GC-MS analysis. The identified FAs were divided into four groups; namely, branched-chain FAs (BCFAs), linear saturated FAs (SFAs), monounsaturated FAs (MUFAs) and polyunsaturated FAs (PUFAs). For each class, the identity and the abundance of the most relevant FA components were given in brackets.

Table 1.
Microbial growth on a BSG medium: fatty acids composition of the biomass.
At first, a group of strains connected to the prospective production of BCFAs was investigated. According to our previous work [7], Streptomyces species properly grow on a BSG medium, accumulating BCFAs. Hence, Streptomyces jeddahensis (DSM 101878) [24], a new oleaginous [25] bacterium isolated from desert soil, was tested. Its FAs profile (entry 2) was compared with those of Streptomyces cavourensis (DSM 112466), which is the best BCFAs producer, according to our previous study (entry 1). The two bacterial strains showed very similar results, producing 63 and 66% of BCFAs, respectively.
Usually, fungi do not produce BCFAs, which are typically present in bacterial cell membranes. A very interesting exception is represented by some fungal strains belonging to the Entomophthora and Conidiobolus [26,27] genera, which are able to produce both BCFAs and PUFAs. More specifically, Entomophthora exitialis and Conidiobolus heterosporus have been described as oleaginous fungi, suitable for the production of arachidonic acid [28] and of the BCFA iso-C14 [29]. Accordingly, Entomophthora exitialis (CBS 180.60) and Conidiobolus heterosporus (CBS 543.63) used BSG media as fermentation substrate, as shown in entries 3 and 4, respectively. The fatty acid mixtures extracted from the two biomasses showed the concurrent presence of BCFAs and PUFAs in both samples. E. exitialis produced 21% of BCFAs and 24% of ARA whereas C. heterosporus produced 56% of BCFAs, 10% of ARA and 1.5% of EPA.
Conversely, Pythium ultimum (CBS 805.95), a eukaryotic microorganism belonging to the Oomycetes class [30,31], produced both EPA (5.4%) and ARA (3%), without the formation of BCFAs (entry 5).
The production of ARA and GLA, which are the biosynthetic precursors of ARA, have been also observed in fungi belonging to the Mucoromycota division, such as Mucor, Cunninghamella, Rhizopus and Mortierella species [21,25]. Our experiments, performed on Mucor circinelloides (DSM 1191), Mucor plumbeus (DSM 62759), Cunninghamella echinulata (DSM 1905) and Rhizopus stolonifer (DSM 855) showed that all these species, when grown in BSG media, produced GLA with a percentage that ranged from 20.6 to 23.3% of the total fatty acids content (entries 6–9). Other Mucoromycota species that are potentially GLA producers were also tested. Rhizopus oryzae (CBS 112.07), Syncephalastrum racemosus (MUT 2770) and Absidia coerulea (AM93) afforded mainly saturated and monounsaturated FAs whereas the GLA percentage ranged from 7.1 to 12.7% of the total fatty acids content (entries 10–12), thus indicating that these strains are not a suitable producer of linolenic acid. This behavior was even more evident for the thermophilus strain Rhizomucor pusillus (CBS 354.68), which afforded GLA in only 1.6% (entry 13). This result can be explained by taking into account that an increase in growing temperature usually ends up with a decrease in the PUFA content.
Currently, Mortierella alpina CBS 754.68 is the most used strain for the industrial production of ARA [21], and BSG has been already employed as a substrate for the growth of different Mortierella species [32]. According to the literature, the use of the BSG medium also exhibited very good results (entry 14) as the ARA percentage of the total fatty acids content was superior to 40%. On the contrary, another Mortierella species, namely M. isabellina (CBS 208.32), did not produce ARA with the concomitant increase in oleic acid and GLA formation (entry 15).
Since patent literature [33] indicates the possibility that strains belonging to Aspergillum, Fusarium, Cladosporium and Trichoderma genera could be able to produce lipids with a high PUFA content, the screening was extended to a large number of these species (entry 16–24). However, Trichoderma viride (DSM 63065), Chaetomium globosum (DSM 1962), Aspergillus niger (CBS 626.66), Aspergillus kanagawaensis (CBS 424.68), Cladosporium cucumerinum (CBS 158.51), Sodiomyces alcalophilus (CBS 114.92), Nigrospora oryzae (MUT 5844), Fusarium culmorum (MUT 5855) and Beauveria bassiana (AM278) did not afford high-value PUFAs, as only LA was generated by the fungal metabolism. Slightly different results were obtained by fermenting BSG medium with Neurospora crassa (DSM 894) or Alternaria alternata (DSM 1102). Besides producing LA, both fungal strains produced a significant amount of ALA corresponding to 21.1 and 13.9%, respectively, of the total fatty acids content (entries 25 and 26).
Finally, the marine BSG medium was tested as a possible medium for PUFAs-producing marine microorganisms. Three different strains were singled out. The bacteria Shewanella hanedai (DSM 6066) and Moritella marina (DSM 104096) are producers of EPA or DHA [34], respectively, whereas Crypthecodinium cohnii (CCMP 316) is a marine heterotrophic dinoflagellate that can accumulate high amounts of DHA and has been already employed in the transformation of recycled biomasses into high-value FAs [35]. The results of the three experiments were disappointing. None of the investigated strains was able to grow in the MBSG medium, as confirmed by the very small amount of the formed biomass. Likely, the nutrients contained in the BSG medium are not metabolized by the evaluated marine microorganisms, making their employment unsuitable.
3.3. Microbial Growth on the BSG Medium: Scaling-Up and Evaluating the PUFAs Productivity
Overall, the above-described results suggest that the BSG medium can support the microbial production of BCFAs and different high-value PUFAs. According to our screening experiments, we singled out the strains that have shown the best results. More specifically, Streptomyces jeddahensis was selected as a potential BCFAs producer while Conidiobolus heterosporus and Entomophthora exitialis were further studied because they simultaneously synthesized both BCFAs and ARA. Pythium ultimum and Mortierella alpina were selected for their capability to accumulate EPA and ARA, respectively. Finally, Rhizopus stolonifer and Mucor circinelloides were chosen as prospective GLA producers.
In order to quantify the FAs productivity of each strain, in the BSG medium, a new set of experiments (Table 2) was devised. Accordingly, each fermentation trial was performed in a bioreactor, using 1.5 L of the BSG medium and while controlling for pH and aeration. After complete growth, the biomass was collected and freeze-dried to establish biomass productivity. Hence, sodium hydroxide treatment followed by acidification and solvent extraction allowed for isolation of the free FAs, which were then converted into their methyl ester derivatives. The latter esters mixture was weighed and analyzed by GC-MS to measure the fatty acids yields and the PUFAs content, respectively.

Table 2.
Biomass and fatty acid productivity of selected microbial strains growth in the BSG medium.
The results of these experiments deserve some relevant considerations. Streptomyces jeddahensis (entry 2) showed similar biomass yield and FAs composition than Streptomyces cavourensis (entry 1) but with higher FAs yield (313 mg/L vs. 220 mg/L).
Surprisingly, Conidiobolus heterosporus (entry 3) and Pythium ultimum (entry 5) greatly increased their ARA and EPA content when switching the fermentation from the flask to bioreactor experiment. Accordingly, ARA and EPA percentages increased from 10.4 and 1.5% to 19.2 and 3.7%, for Conidiobolus heterosporus, whereas they increased from 3.0 and 5.4% to 9.3 and 8.3%, for Pythium ultimum. For the latter two microorganisms, the measured FA productivities corresponded to 460 and 400 mg/L, respectively.
Conversely, Entomophthora exitialis exhibited disappointing results (entry 4), as both biomass and FA productivity were rather low.
The best results were recorded for Mortierella alpina (entry 6) and Mucor circinelloides (entry 8), which showed very high productivity, both in terms of biomass and FAs content, which reached 7 and 6.5 g/L, respectively, and 900 and 600 mg/L, respectively. In particular, Mortierella alpina was confirmed to be the strain of choice for ARA production, as about 41% of the produced FAs correspond to the latter PUFA. Similarly, Mucor circinelloides compared favorably over the last investigated GLA producer strain, namely Rhizopus stolonifer (entry 7), which showed greatly inferior FA productivity (250 mg/L).
4. Conclusions
Overall, the present study established that the formulated BSG medium, prepared according to the present protocol, is suitable to support the microbial production of high-value BCFAs and PUFAs. In particular, the fermentation of the BSG medium with Streptomyces jeddahensis and Conidiobolus heterosporus can be employed for BCFAs production whereas Pythium ultimum can be used to provide a FAs mixture enriched in EPA and ARA. Moreover, Mortierella alpina and Mucor circinelloides, when grown in the BSG medium, produce very high amounts of ARA and GLA, respectively. On the contrary, the production of DHA through the employment of some selected marine microorganisms seems not possible when the BSG medium is employed.
The described findings are noteworthy as the proposed process efficiently exploits a waste material (BSG), generating high-value FAs. Taking into account that the preparation of the BSG medium recycles up to 30% (w/w) of the initial BSGD, the process is of considerable significance from a circular economy perspective. To improve sustainability, it is worth noting that different steps could be avoided in a prospective industrial application. For example, BSG can be ground and extracted without a drying process. In addition, the extraction and sterilization steps could be merged to only one step, with considerable energy savings. In this context, further studies will be conducted in order to scale up the process.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9121008/s1, S1: Materials and General Methods; S2: Microorganisms and Growth Media; S3: General Analytical Methods and Analysis of the BSG medium.
Author Contributions
S.S. conceived this study; S.S., P.D., L.A.M.R. and E.R. designed and performed the experiments and analyzed the data; S.S. and P.D. wrote, reviewed and edited the paper and provided resources and funding. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the European Union—Next Generation EU through MUR (Ministero dell’Università e della Ricerca), PRIN 2022 under the project ‘Biotechnological synthesis of valuable Lipids and fatty acid derivatives from Agro-food industrial Residues’ (BioLAR), grant number 20223E9C8S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article or Supplementary Materials.
Acknowledgments
L.A.M.R. acknowledges MUR for a Ph.D. grant (XXXVI Research Doctorate Cycle); S.S acknowledges Filip Boratyński for providing us with Absidia coerulea (AM93) and Beauveria bassiana (AM278) strains.
Conflicts of Interest
The authors declare no conflict of interest.
Notations
| ALA | α-linolenic acid |
| ARA | Arachidonic acid |
| BCFA | Branched-chain fatty acid |
| BSG | Brewers’ spent grain |
| DHA | cis-4,7,10,13,16,19-docosahexaenoic acid |
| EPA | cis-5,8,11,14,17-eicosapentaenoic acid |
| FA | Fatty acid |
| GLA | γ-linolenic acid |
| LA | Linolenic acid |
| OA | Oleic acid |
| PAL | Palmitic acid |
| PUFA | Polyunsaturated fatty acid |
References
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Eurostat. Available online: https://ec.europa.eu/eurostat/en/web/products-eurostat-news/w/ddn-20230803-1 (accessed on 8 October 2023).
- Agrawal, D.; Gopaliya, D.; Willoughby, N.; Khare, S.K.; Kumar, V. Recycling potential of brewer’s spent grains for circular biorefineries. Curr. Opin. Green Sustain. Chem. 2023, 40, 100748. [Google Scholar] [CrossRef]
- Schmidt, A.R.; Dresch, A.P.; Alves Junior, S.L.; Bender, J.P.; Treichel, H. Applications of brewer’s spent grain hemicelluloses in biorefineries: Extraction and value-added product obtention. Catalysts 2023, 13, 755. [Google Scholar] [CrossRef]
- Chetrariu, A.; Dabija, A. Spent grain: A functional ingredient for food applications. Foods 2023, 12, 1533. [Google Scholar] [CrossRef]
- Mitri, S.; Salameh, S.-J.; Khelfa, A.; Leonard, E.; Maroun, R.G.; Louka, N.; Koubaa, M. Valorization of brewers’ spent grains: Pretreatments and fermentation, a review. Fermentation 2022, 8, 50. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Griffini, G.; Marzorati, S.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Towards a complete exploitation of brewers’ spent grain from a circular economy perspective. Fermentation 2022, 8, 151. [Google Scholar] [CrossRef]
- Llimós, J.; Martínez-Avila, O.; Marti, E.; Corchado-Lopo, C.; Llenas, L.; Gea, T.; Ponsá, S. Brewer’s spent grain biotransformation to produce lignocellulolytic enzymes and polyhydroxyalkanoates in a two-stage valorization scheme. Biomass Convers. Biorefinery 2022, 12, 3921–3932. [Google Scholar] [CrossRef]
- De Crane d’Heysselaer, S.; Bockstal, L.; Jacquet, N.; Schmetz, Q.; Richel, A. Potential for the valorisation of brewer’s spent grains: A case study for the sequential extraction of saccharides and lignin. Waste Manag. Res. 2021, 40, 1007. [Google Scholar] [CrossRef] [PubMed]
- Chetrariu, A.; Dabija, A. Brewer’s spent grains: Possibilities of valorization, a review. Appl. Sci. 2020, 10, 5619. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Duan, Y.; Zhang, H.; Ma, H. A mini-review on brewer’s spent grain protein: Isolation, physicochemical properties, application of protein, and functional properties of hydrolysates. J. Food Sci. 2019, 84, 3330–3340. [Google Scholar] [CrossRef] [PubMed]
- Outeiriño, D.; Costa-Trigo, I.; Pinheiro de Souza Oliveira, R.; Pérez Guerra, N.; Domínguez, J.M. A novel approach to the biorefinery of brewery spent grain. Process Biochem. 2019, 85, 135–142. [Google Scholar] [CrossRef]
- Patel, A.; Mikes, F.; Bühler, S.; Matsakas, L. Valorization of brewers’ spent grain for the production of lipids by oleaginous yeast. Molecules 2018, 23, 3052. [Google Scholar] [CrossRef]
- Yi, J.S.; Yoo, H.-W.; Kim, E.-J.; Yang, Y.-H.; Kim, B.-G. Engineering Streptomyces coelicolor for production of monomethyl branched chain fatty acids. J. Biotechnol. 2020, 307, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gozdzik, P.; Magkos, F.; Sledzinski, T.; Mika, A. Monomethyl branched-chain fatty acids: Health effects and biological mechanisms. Prog. Lipid Res. 2023, 90, 101226. [Google Scholar] [CrossRef] [PubMed]
- Ran-Ressler, R.R.; Khailova, L.; Arganbright, K.M.; Adkins-Rieck, C.K.; Jouni, Z.E.; Koren, O.; Ley, R.E.; Brenna, J.T.; Dvorak, B. Branched chain fatty acids reduce the incidence of necrotizing enterocolitis and alter gastrointestinal microbial ecology in a neonatal rat model. PLoS ONE 2011, 6, e29032. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xiaohan, W.; Chen, Y.; Jin, W.; Jin, Q.; Wang, X. Enrichment of branched chain fatty acids from lanolin via urea complexation for infant formula use. LWT 2020, 117, 108627. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 polyunsaturated fatty acids and their health benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Tallima, H.; El Ridi, R. Arachidonic acid: Physiological roles and potential health benefits—A review. J. Adv. Res. 2018, 11, 33–41. [Google Scholar] [CrossRef]
- Jesionowska, M.; Ovadia, J.; Hockemeyer, K.; Clews, A.C.; Xu, Y. EPA and DHA in microalgae: Health benefits, biosynthesis, and metabolic engineering advances. J. Am. Oil Chem. Soc. 2023, 100, 831–842. [Google Scholar] [CrossRef]
- Jovanovic, S.; Dietrich, D.; Becker, J.; Kohlstedt, M.; Wittmann, C. Microbial production of polyunsaturated fatty acids—High-value ingredients for aquafeed, superfoods, and pharmaceuticals. Curr. Opin. Biotechnol. 2021, 69, 199–211. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Allegretti, C.; Bellinetto, E.; D’Arrigo, P.; Ferro, M.; Griffini, G.; Rossato, L.A.M.; Ruffini, E.; Schiavi, L.; Serra, S.; Strini, A.; et al. Fractionation of raw and parboiled rice husks with deep eutectic solvents and characterization of the extracted lignins towards a circular economy perspective. Molecules 2022, 27, 8879. [Google Scholar] [CrossRef]
- Röttig, A.; Atasayar, E.; Meier-Kolthoff, J.P.; Spröer, C.; Schumann, P.; Schauer, J.; Steinbüchel, A. Streptomyces jeddahensis sp. Nov., an oleaginous bacterium isolated from desert soil. Int. J. Syst. Evol. Microbiol. 2017, 67, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Ghazani, S.M.; Marangoni, A.G. Microbial lipids for foods. Trends Food Sci. Technol. 2022, 119, 593–607. [Google Scholar] [CrossRef]
- Tyrrell, D. The fatty acid composition of some Entomophthoraceae. III. Can. J. Microbiol. 1971, 17, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Nagai, T.; Ishiyama, I. Fatty acid and lipid compositions of Conidiobolus. J. Appl. Bacteriol. 1983, 54, 85–90. [Google Scholar] [CrossRef]
- Kendrick, A.; Ratledge, C. Lipid formation in the oleaginous mold Entomophthora exitalis grown in continuous culture: Effects of growth rate, temperature and dissolved oxygen tension on polyunsaturated fatty acids. Appl. Microbiol. Biotechnol. 1992, 37, 18–22. [Google Scholar] [CrossRef]
- Fraatz, M.A.; Goldmann, M.; Geissler, T.; Gross, E.; Backes, M.; Hilmer, J.-M.; Ley, J.; Rost, J.; Francke, A.; Zorn, H. Biotechnological production of methyl-branched aldehydes. J. Agric. Food Chem. 2018, 66, 2387–2392. [Google Scholar] [CrossRef]
- Gandhi, S.R.; Weete, J.D. Production of the polyunsaturated fatty acids arachidonic acid and eicosapentaenoic acid by the fungus Pythium ultimum. Microbiology 1991, 137, 1825–1830. [Google Scholar] [CrossRef]
- Stredansky, M.; Conti, E.; Salaris, A. Production of polyunsaturated fatty acids by Pythium ultimum in solid-state cultivation. Enzyme Microb. Technol. 2000, 26, 304–307. [Google Scholar] [CrossRef]
- Jacobs, A.; Botha, A.; Van Zyl, W.H. The production of eicosapentaenoic acid by representatives of the genus Mortierella grown on brewers’ spent grain. Biologia 2009, 64, 871–876. [Google Scholar] [CrossRef]
- Hideki, F. Process for Secretive Fermentation of Lipids by Fungi or Algae. European Patent Application EP207475, 7 January 1987. [Google Scholar]
- Moi, I.M.; Leow, A.T.C.; Ali, M.S.M.; Rahman, R.N.Z.R.A.; Salleh, A.B.; Sabri, S. Polyunsaturated fatty acids in marine bacteria and strategies to enhance their production. Appl. Microbiol. Biotechnol. 2018, 102, 5811–5826. [Google Scholar] [CrossRef] [PubMed]
- Didrihsone, E.; Dubencovs, K.; Grube, M.; Shvirksts, K.; Suleiko, A.; Suleiko, A.; Vanags, J. Crypthecodinium cohnii growth and omega fatty acid production in mediums supplemented with extract from recycled biomass. Mar. Drugs 2022, 20, 68. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).