The Potential Use of Pseudomonas stutzeri as a Biocatalyst for the Removal of Heavy Metals and the Generation of Bioelectricity
Abstract
1. Introduction
2. Materials and Methods
2.1. Construction of the MFC-SC
2.2. Collection of Samples Used as a Substrate
2.3. Characterization of Contaminated Waters
2.4. Characterization of the Electrochemical Parameters of the MFC-SC
2.5. Bacterial Cultures of the Electrogenic Strains
2.6. Reactivation of the Pseudomonas stutzeri Strain
2.7. Preparation of Bacterial Inoculum
2.8. Commissioning of the Treatment Modules
3. Results and Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. Assess. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Wu, R. Removal of Heavy Metal Ions from Industrial Wastewater Based on Chemical Precipitation Method. Ekoloji Dergisi 2019, 107, 16–25. [Google Scholar]
- Haroon, B.; Ping, A.; Pervez, A.; Faridullah; Irshad, M. Characterization of heavy metal in soils as affected by long-term irrigation with industrial wastewater. J. Water Reuse Desalin. 2019, 9, 47–56. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous removal of organics and heavy metals from industrial wastewater: A review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Karzanova, I.V.; Cordova, S.D.A. Actual Problems of Illegal Mining in Peru: Prospects and Challenges. In Current Problems of the World Economy and International Trade; Emerald Publishing Limited: Lima, Peru, 2022; pp. 83–91. [Google Scholar]
- Holland, L. Illegal gold mining and the struggle to save the Amazon in Peru. Frontiers of Development in the Amazon: Riches, Risks, and Resistances; Rowman & Littlefield: Lanham, MD, USA, 2020; pp. 189–212. [Google Scholar]
- Adimalla, N.; Qian, H.; Wang, H. Assessment of heavy metal (HM) contamination in agricultural soil lands in northern Telangana, India: An approach of spatial distribution and multivariate statistical analysis. Environ. Monit. Assess. 2019, 191, 246. [Google Scholar] [CrossRef] [PubMed]
- Galagarza, O.A.; Ramirez-Hernandez, A.; Oliver, H.F.; Rodríguez, M.V.; Ortiz, M.d.C.V.; Vera, E.P.; Cereceda, Y.; Diaz-Valencia, Y.K.; Deering, A.J. Occurrence of chemical contaminants in Peruvian produce: A food-safety perspective. Foods 2021, 10, 1461. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Flores, S.; Ramirez-Asis, E.; Delgado-Caramutti, J.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Diaz, F.; Delfin-Narcizo, D. An Analysis of Global Trends from 1990 to 2022 of Microbial Fuel Cells: A Bibliometric Analysis. Sustainability 2023, 15, 3651. [Google Scholar] [CrossRef]
- Ghangrekar, M.M.; Shinde, V.B. Performance of membrane-less microbial fuel cell treating wastewater and effect of electrode distance and area on electricity production. Bioresour. Technol. 2007, 98, 2879–2885. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zeng, R.J. Bidirectional extracellular electron transfers of electrode-biofilm: Mechanism and application. Bioresour. Technol. 2019, 271, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhang, J.; Zhang, Y.; Guo, Q.; Hu, T.; Xiao, H.; Lu, W.; Jia, J. Progress on anodic modification materials and future development directions in microbial fuel cells. J. Power Sources 2023, 556, 232486. [Google Scholar] [CrossRef]
- Rojas-Villacorta, W.; Rojas-Flores, S.; De La Cruz-Noriega, M.; Chinchay Espino, H.; Diaz, F.; Gallozzo Cardenas, M. Microbial Biosensors for Wastewater Monitoring: Mini-Review. Processes 2022, 10, 2002. [Google Scholar] [CrossRef]
- Ahmad, A.; Alshammari, M.B.; Ibrahim MN, M. Impact of self-fabricated graphene–metal oxide composite anodes on metal degradation and energy generation via a microbial fuel cell. Processes 2023, 11, 163. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Serrà, A.; Bhawani, S.A.; Ibrahim, M.N.M.; Khan, A.; Alorfi, H.S.; Asiri, A.M.; Hussein, M.A.; Khan, I.; Umar, K. Utilizing biomass-based graphene oxide–polyaniline–ag electrodes in microbial fuel cells to boost energy generation and heavy metal removal. Polymers 2022, 14, 845. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, M.R.; Ghasemi, S.; Sanaee, Z.; Nejad, Z.G.; Mardanpour, M.M.; Yaghmaei, S.; Ghorbanzadeh, M. Improvement of the microfluidic microbial fuel cell using a nickel nanostructured electrode and microchannel modifications. J. Power Sources 2019, 437, 226891. [Google Scholar] [CrossRef]
- Bustamante-Torres, M.; Torres, O.; Abad-Sojos, S.; Pardo, S.; Bucio, E. Application of Genetically Modified Microorganisms for Bioremediation of Polluted Environments. In Genetically Engineered Organisms in Bioremediation; CRC Press: Boca Raton, FL, USA, 2024; pp. 18–51. [Google Scholar]
- Sharma, R.; Saroop, S. Role of microbes in pesticide bioremediation: Recent advances and biotechnological implications. In Pesticides in a Changing Environment; Elsevier: Amsterdam, The Netherlands, 2024; pp. 223–250. [Google Scholar]
- Palanivel, T.M.; Sivakumar, N.; Al-Ansari, A.; Victor, R. Bioremediation of copper by active cells of Pseudomonas stutzeri LA3 isolated from an abandoned copper mine soil. J. Environ. Manag. 2020, 253, 109706. [Google Scholar] [CrossRef]
- Ridene, S.; Werfelli, N.; Mansouri, A.; Landoulsi, A.; Abbes, C. Bioremediation potential of consortium Pseudomonas Stutzeri LBR and Cupriavidus Metallidurans LBJ in soil polluted by lead. PLoS ONE 2023, 18, e0284120. [Google Scholar] [CrossRef]
- Imron, M.F.; Kurniawan, S.B.; Abdullah, S.R.S. Resistance of bacteria isolated from leachate to heavy metals and the removal of Hg by Pseudomonas aeruginosa strain FZ-2 at different salinity levels in a batch biosorption system. Sustain. Environ. Res. 2021, 31, 1–13. [Google Scholar] [CrossRef]
- Agüero-Quiñones, R.; Ávila-Sánchez, Z.; Rojas-Flores, S.; Cabanillas-Chirinos, L.; Cruz-Noriega, M.D.L.; Cruz-Monzón, J.; Nazario-Naveda, R. Cadmium and COD Removal from Municipal Wastewater Using Chlorella sp. Biomass in Microbial Fuel Cells. Sustainability 2023, 15, 14513. [Google Scholar] [CrossRef]
- Lopez, F.; Pitarch, E.; Botero-Coy, A.; Fabregat-Safont, D.; Ibáñez, M.; Marin, J.; Peruga, A.; Ontañón, N.; Martínez-Morcillo, S.; Olalla, A.; et al. Removal efficiency for emerging contaminants in a WWTP from Madrid (Spain) after secondary and tertiary treatment and environmental impact on the Manzanares River. Sci. Total Environ. 2022, 812, 152567. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Benites, S.M.; Nazario-Naveda, R.; Delfín-Narciso, D.; Gallozzo-Cardemas, M.; Díaz, F.; Murga-Torres, E.; Rojas-Villacorta, W. Use of Kiwi Waste as Fuel in MFC and Its Potential for Use as Renewable Energy. Fermentation 2023, 9, 446. [Google Scholar] [CrossRef]
- Rojas-Villacorta, W.; Rojas-Flores, S.; Benites, S.M.; Delfín-Narciso, D.; Cruz-Noriega, M.D.L.; Cabanillas-Chirinos, L.; Rodríguez-Serin, H.; Rebaza-Araujo, S. Potential use of pepper waste and microalgae Spirulina sp. for bioelectricity generation. Energy Rep. 2023, 9, 253–261. [Google Scholar] [CrossRef]
- Flores, S.R.; Nazario-Naveda, R.; Delfín-Narciso, D.; Cardenas, M.G.; Diaz, N.D.; Ravelo, K.V. Generation of bioelectricity from organic fruit waste. Environ. Res. Eng. Manag. 2021, 77, 6–14. [Google Scholar] [CrossRef]
- Agüero-Quiñones, R.; Ávila-Sánchez, Z.; Rojas-Flores, S.; Cabanillas-Chirinos, L.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Rojas-Villacorta, W. Activated Carbon Electrodes for Bioenergy Production in Microbial Fuel Cells Using Synthetic Wastewater as Substrate. Sustainability 2023, 15, 13767. [Google Scholar] [CrossRef]
- Jibaja, S.; Oyola, V.; Berastain, A.; Ramos, D.; Roncal, E.; Medina, J.C.; Untiveros, G.; Sheen, P.; Hurtado, J. Producción de electricidad empleando Acidithiobacillus ferrooxidans a partir de iones tiosulfato y férrico. Rev. De La Soc. Química Del Perú 2019, 85, 3–13. [Google Scholar] [CrossRef]
- Yan, H.; Catania, C.; Bazan, G.C. Membrane-Intercalating Conjugated Oligoelectrolytes: Impact on Bioelectrochemical Systems. Adv. Mater. 2015, 27, 2958–2973. [Google Scholar] [CrossRef]
- Ramalingam, G.; Priya, A.K.; Gnanasekaran, L.; Rajendran, S.; Hoang, T.K. Biomass and waste derived silica, activated carbon and ammonia-based materials for energy-related applications–A review. Fuel 2024, 355, 129490. [Google Scholar] [CrossRef]
- Rasmussen, M.; Minteer, S.D. Long-term arsenic monitoring with an Enterobacter cloacae microbial fuel cell. Bioelectrochemistry 2015, 106, 207–212. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.-F.; Sekar, R.; Chang, H.-C.; Zhang, J.; Wells, M.; Ren, Y.-X.; Chen, Z. Arsenic mitigation in paddy soils by using microbial fuel cells. Environ. Pollut. 2018, 238, 647–655. [Google Scholar] [CrossRef]
- Gustave, W.; Yuan, Z.F.; Sekar, R.; Ren, Y.X.; Liu, J.Y.; Zhang, J.; Chen, Z. Soil organic matter amount determines the behavior of iron and arsenic in paddy soil with microbial fuel cells. Chemosphere 2019, 237, 124459. [Google Scholar] [CrossRef] [PubMed]
- Bose, D.; Santra, M.; Sanka RV, S.P.; Krishnakumar, B. Bioremediation analysis of sediment-microbial fuel cells for energy recovery from microbial activity in soil. Int. J. Energy Res. 2021, 45, 6436–6445. [Google Scholar] [CrossRef]
- Mandal, S.K.; Das, N. Application of microbial fuel cells for bioremediation of environmental pollutants: An overview. J.Microbiol. Biotechnol. Food Sci. 2018, 7, 437. [Google Scholar]
- Arun, S.; Sinharoy, A.; Pakshirajan, K.; Lens, P.N. Algae based microbial fuel cells for wastewater treatment and recovery of value-added products. Renew. Sustain. Energy Rev. 2020, 132, 110041. [Google Scholar] [CrossRef]
- Moradian, J.M.; Fang, Z.; Yong, Y.C. Recent advances on biomass-fueled microbial fuel cell. Bioresour. Bioprocess. 2021, 8, 14. [Google Scholar] [CrossRef]
- Ahmadi, H.; Gheshlaghi, R.; Mahdavi, M.A.; Abazarian, E. Comparison of sediment microbial fuel cell performance with and without sediment Autoclaving. In Proceedings of the 17th National Chemical Engineering Congress & Exhibition (IChEC 2021), Mashhad, Iran, 9 November 2021. [Google Scholar]
- Rahdar, M.; Gheshlaghi, R.; Mahdavi, M.A.; Abazarian, E.; Elkamel, A. Probing the effect of distance between sediment microbial fuel cells and sediment storage on electricity generation and organic matter removal in open channels. Fuel 2024, 357, 129932. [Google Scholar] [CrossRef]
- Idris, M.O.; Kim, H.C.; Yaqoob, A.A.; Ibrahim MN, M. Exploring the effectiveness of microbial fuel cell for the degradation of organic pollutants coupled with bio-energy generation. Sustain. Energy Technol. Assess. 2022, 52, 102183. [Google Scholar] [CrossRef]
- Khan, N.; Anwer, A.H.; Ahmad, A.; Sabir, S.; Khan, M.Z. Investigating microbial fuel cell aided bio-remediation of mixed phenolic contaminants under oxic and anoxic environments. Biochem. Eng. J. 2020, 155, 107485. [Google Scholar] [CrossRef]
- Daud, N.N.M.; Al-Zaqri, N.; Yaakop, A.S.; Ibrahim, M.N.M.; Guerrero-Barajas, C. Stimulating bioelectric generation and recovery of toxic metals through benthic microbial fuel cell driven by local sago (Cycas revoluta) waste. Environ. Sci. Pollut. Res. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhao, Y.G.; Liu, X.; Fu, Y. Anode modification of sediment microbial fuel cells (SMFC) towards bioremediating mariculture wastewater. Mar. Pollut. Bull. 2022, 182, 114013. [Google Scholar] [CrossRef] [PubMed]
- Sevak, P.; Pushkar, B. Arsenic pollution cycle, toxicity and sustainable remediation technologies: A comprehensive review and bibliometric analysis. J. Environ. Manag. 2024, 349, 119504. [Google Scholar] [CrossRef] [PubMed]
- Lepikash, R.; Lavrova, D.; Stom, D.; Meshalkin, V.; Ponamoreva, O.; Alferov, S. State of the Art and Environmental Aspects of the Plant Microbial Fuel Cells Application. Energies 2024, 17, 752. [Google Scholar] [CrossRef]
- Do, M.H.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Pandey, A.; Sharma, P.; Varjani, S.; Nguyen, T.A.H.; Hoang, N.B. A dual chamber microbial fuel cell based biosensor for monitoring copper and arsenic in municipal wastewater. Sci. Total Environ. 2022, 811, 152261. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Cheng, J.; Wang, J.; Hu, S. Simultaneous removal of trivalent arsenic and nitrate using microbial fuel cells. Processes 2021, 9, 673. [Google Scholar] [CrossRef]
- Chen, G.; Xu, R.; Liu, L.; Shi, H.; Wang, G.; Wang, G. Limited carbon source retards inorganic arsenic release during roxarsone degradation in Shewanella oneidensis microbial fuel cells. Appl. Microbiol. Biotechnol. 2018, 102, 8093–8106. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, Q.; Huang, G.; Zhang, L.; Liu, Y. Effect of dissolved oxygen concentration on nitrogen removal and electricity generation in self pH-buffer microbial fuel cell. Int. J. Hydrogen Energy 2020, 45, 34099–34109. [Google Scholar] [CrossRef]
- Ren, Y.; Lv, Y.; Wang, Y.; Li, X. Effect of heterotrophic anodic denitrification on anolyte pH control and bioelectricity generation enhancement of bufferless microbial fuel cells. Chemosphere 2020, 257, 127251. [Google Scholar] [CrossRef] [PubMed]
- Mohyudin, S.; Farooq, R.; Jubeen, F.; Rasheed, T.; Fatima, M.; Sher, F. Microbial fuel cells a state-of-the-art technology for wastewater treatment and bioelectricity generation. Environ. Res. 2022, 204, 112387. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.; Solorza-Feria, O.; López-Díaz, J. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. Int. J. Hydrogen Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, X.; Wang, H.; Long, X.; Li, X. Simultaneous enhancement of heavy metal removal and electricity generation in soil microbial fuel cell. Ecotoxicol. Environ. Saf. 2020, 192, 110314. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Khatoon, A.; Mohd Setapar, S.H.; Umar, K.; Parveen, T.; Mohamad Ibrahim, M.N.; Ahmad, A.; Rafatullah, M. Outlook on the role of microbial fuel cells in remediation of environmental pollutants with electricity generation. Catalysts 2020, 10, 819. [Google Scholar] [CrossRef]
- De La Cruz-Noriega, M.; Benites, S.M.; Rojas-Flores, S.; Otiniano, N.M.; Vargas, A.M.S.; Alfaro, R.; Cabanillas-Chirinos, L.; Rojas-Villacorta, W.; Nazario-Naveda, R.; Delfín-Narciso, D. Use of Wastewater and Electrogenic Bacteria to Generate Eco-Friendly Electricity through Microbial Fuel Cells. Sustainability 2023, 15, 10640. [Google Scholar] [CrossRef]
- Silva-Palacios, F.; Salvador-Salinas, A.; Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Gallozzo-Cardenas, M.; Delfin-Narciso, D.; Díaz, F. Use of Mine Tailings as a Substrate in Microbial Fuel Cells for Electric Energy Generation. In International Conference on Clean Energy and Electrical Systems; Springer Nature: Singapore, 2023; pp. 333–342. [Google Scholar]
- Mkilima, T.; Zharkenov, Y.; Utepbergenova, L.; Smagulova, E.; Fazylov, K.; Zhumadilov, I.; Kirgizbayeva, K.; Baketova, A.; Abdukalikova, G. Carwash wastewater treatment through the synergistic efficiency of microbial fuel cells and metal-organic frameworks with graphene oxide integration. Case Stud. Chem. Environ. Eng. 2024, 9, 100582. [Google Scholar] [CrossRef]
- Rojas-Flores, S.; De La Cruz-Noriega, M.; Nazario-Naveda, R.; Benites, S.M.; Delfín-Narciso, D.; Angelats-Silva, L.; Murga-Torres, E. Use of Banana Waste as a Source for Bioelectricity Generation. Processes 2022, 10, 942. [Google Scholar] [CrossRef]
- Ida, T.K.; Mandal, B. Microbial fuel cell design, application and performance: A review. Mater. Today Proc. 2023, 76, 88–94. [Google Scholar]
- Madondo, N.I.; Rathilal, S.; Bakare, B.F.; Tetteh, E.K. Application of Magnetite-Nanoparticles and Microbial Fuel Cell on Anaerobic Digestion: Influence of External Resistance. Microorganisms 2023, 11, 643. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Al-Zaqri, N.; Alamzeb, M.; Hussain, F.; Oh, S.E.; Umar, K. Bioenergy generation and phenol degradation through microbial fuel cells energized by domestic organic waste. Molecules 2023, 28, 4349. [Google Scholar] [CrossRef] [PubMed]
- Raychaudhuri, A.; Sahoo, R.N.; Behera, M. Sequential anaerobic–aerobic treatment of rice mill wastewater and simultaneous power generation in microbial fuel cell. Environ. Technol. 2023, 44, 3176–3182. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Ahmed, S.; Hossain, N.; Rana, M.; Aoyon, H.; Ali, R.; Islam, S.R.; Hossain, J.; Chowdhury, D. Enhancement of microbial fuel cell performance by introducing dosing materials in waste water to increase microorganism growth. Renew. Energy 2023, 219, 119497. [Google Scholar] [CrossRef]
- Liu, S.H.; Lai, C.Y.; Chang, P.H.; Lin, C.W.; Chen, Y.H. Enhancing copper recovery and electricity generation from wastewater using low-cost membrane-less microbial fuel cell with a carbonized clay cup as cathode. J. Clean. Prod. 2020, 247, 119118. [Google Scholar] [CrossRef]
- Choudhury, P.; Ray, R.N.; Bandyopadhyay, T.K.; Basak, B.; Muthuraj, M.; Bhunia, B. Process engineering for stable power recovery from dairy wastewater using microbial fuel cell. Int. J. Hydrogen Energy 2021, 46, 3171–3182. [Google Scholar] [CrossRef]
- Omenesa Idris, M.; Al-Zaqri, N.; Warad, I.; Hossain AM, A.; Masud, N.; Ali, M. Impact of Commercial Sugar as a Substrate in Single-Chamber Microbial Fuel Cells to Improve the Energy Production with Bioremediation of Metals. Int. J. Chem. Eng. 2023, 2023. [Google Scholar] [CrossRef]
- Alshammari, A.S.; Aleid, G.M.; Ahmad AR, D.; Alomari, A.D.; Abdullahi SS, A.; Mohammad RE, A. Oil Palm Biomass Sap-Rotten Rice as a Source to Remove Metal Ions and Generate Electricity as By-Products through Microbial Fuel Cell Technology. J. Chem. 2024, 2024. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Bassi, A. Investigation of heavy metal removal from salty wastewater and voltage production using Shewanella oneidensis MR-1 nanowires in a dual-chamber microbial fuel cell. Environ. Prog. Sustain. Energy 2024, 43, e14237. [Google Scholar] [CrossRef]
- Wang, H.; Li, Y.; Mi, Y.; Wang, D.; Wang, Z.; Meng, H.; Jiang, C.; Dong, W.; Li, J.; Li, H. Cu (II) and Cr (VI) Removal in Tandem with Electricity Generation via Dual-Chamber Microbial Fuel Cells. Sustainability 2023, 15, 2388. [Google Scholar] [CrossRef]
- Aleid, G.M.; Alshammari, A.S.; Alomari, A.D.; AAlmukhlifi, H.; Ahmad, A.; Yaqoob, A.A. Dual role of sugarcane waste in benthic microbial fuel to produce energy with degradation of metals and chemical oxygen demand. Processes 2023, 11, 1060. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, C.Y.; Lin, C.W.; Zhu, T.J. Promoting removal of copper from sediment and production of bioelectricity by sediment microbial fuel cells using tea extracts. J. Water Process Eng. 2023, 51, 103454. [Google Scholar] [CrossRef]
- Fan, M.; Zhuang, X.; Gao, Z.; Lv, Z.; Dong, W.; Xin, F.; Chen, Y.; Jia, H.; Wu, X. Electroactive microorganisms synthesizing iron sulfide nanoparticles for enhanced hexavalent chromium removal in microbial fuel cells. Sci. Total Environ. 2023, 889, 164311. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.-S.; Zheng, T.; Yong, X.-Y.; Zhai, D.-D.; Si, R.-W.; Li, B.; Yu, Y.-Y.; Yong, Y.-C. Trace heavy metal ions promoted extracellular electron transfer and power generation by Shewanella in microbial fuel cells. Bioresour. Technol. 2016, 211, 542–547. [Google Scholar] [CrossRef]
- Wareen, G.; Saeed, M.; Ilyas, N.; Asif, S.; Umair, M.; Sayyed, R.; Alfarraj, S.; Alrasheed, W.A.; Awan, T.H. Comparison of pennywort and hyacinth in the development of membraned sediment plant microbial fuel cell for waste treatment. Chemosphere 2023, 313, 137422. [Google Scholar] [CrossRef]
- Aleid, G.M.; Alshammari, A.S.; Alomari, A.D.; Sa’ad Abdullahi, S.; Mohammad RE, A.; Abdulrahman RM, I. Degradation of Metal Ions with Electricity Generation by Using Fruit Waste as an Organic Substrate in the Microbial Fuel Cell. Int. J. Chem. Eng. 2023, 2023. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Ibrahim MN, M.; Al-Zaqri, N. A pilot trial in the remediation of pollutants simultaneously with bioenergy generation through microbial fuel cell. J. Environ. Chem. Eng. 2023, 11, 110643. [Google Scholar] [CrossRef]
- Lin, C.W.; Jhan, Y.C.; Zhu, T.J.; Liu, S.H. Enhancement of chromium (VI) removal and power generation by adding biochar to a single-medium sediment-based microbial fuel cell. J. Water Process Eng. 2023, 53, 103612. [Google Scholar] [CrossRef]
- Zhang, H.L.; Cai, H.; Xia, Y.; Zhang, P.; Xiong, S.W.; Gai, J.G. An l-cystine/l-cysteine impregnated nanofiltration membrane with the superior performance of an anchoring heavy metal in wastewater. RSC Adv. 2020, 10, 3438–3449. [Google Scholar] [CrossRef] [PubMed]



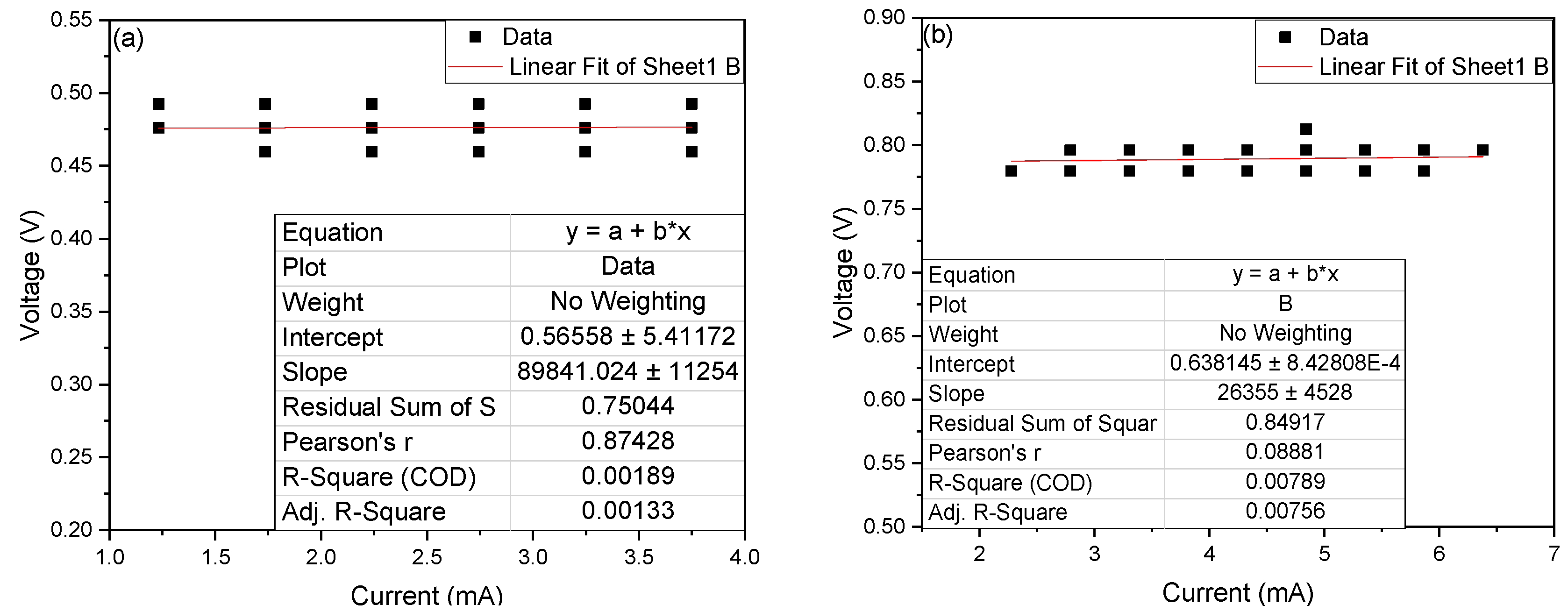
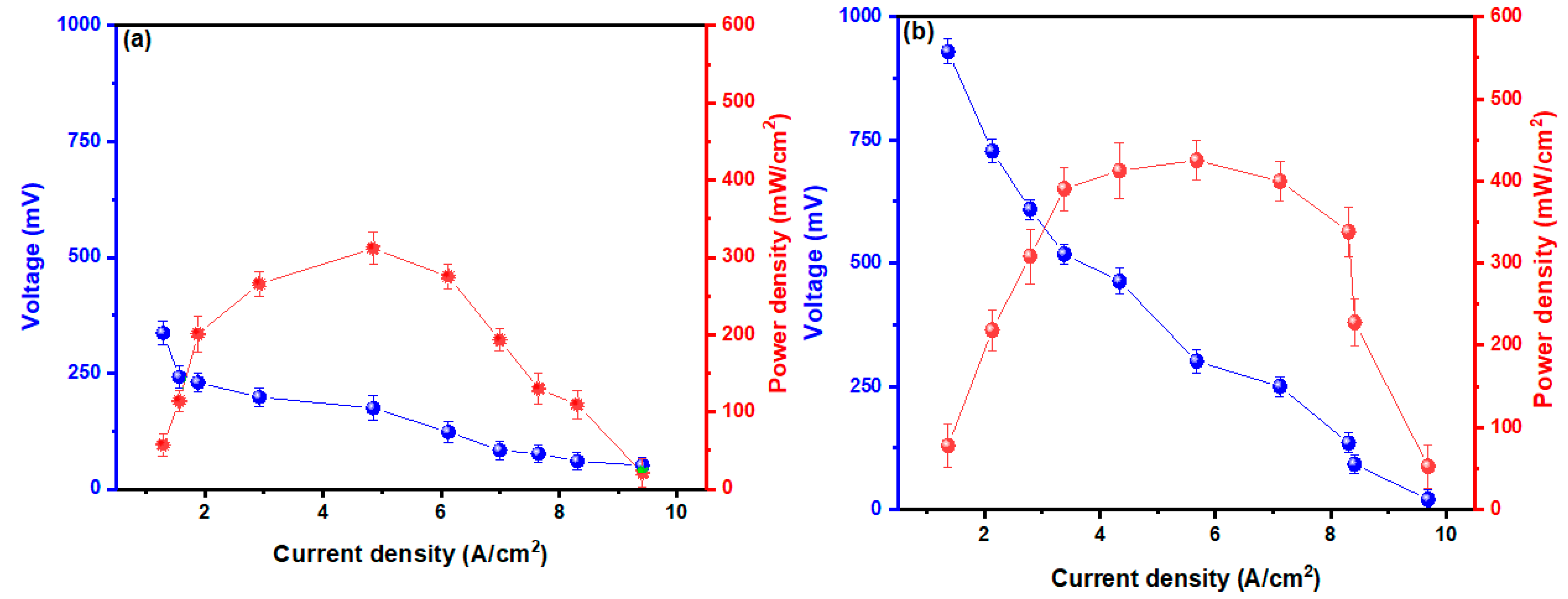
| Coding | Identified Species | Category | pb | % Identity | Accession Number |
|---|---|---|---|---|---|
| BAC4 | Pseudomonas stutzeri | Bacteria | 1439 | 100.00 | MT027239.1 |
| Initial | Final | ||
|---|---|---|---|
| Parameters | Target | Pseudomonas stutzeri | |
| Nitrogen (mg/L) | 1.68 | 0.56 | 0.82 |
| Total organic carbon (mg/L) | 383.1 | 76.9 | 110.8 |
| Total inorganic carbon (mg/L) | 988.0 | 346.2 | 393.8 |
| Carbon lost on ignition (mg/L) | 114.9 | 23.1 | 43.2 |
| Dissolved organic carbon—DOC (mg/L) | 245.2 | 49.2 | 84.9 |
| Chemical oxygen demand, COD (mgO2/L) | 416.1 | 56.3 | 96.4 |
| Initial | Target | P. stutzeri | |||
|---|---|---|---|---|---|
| 0 h | 24 h | 72 h | 24 h | 72 h | |
| As (mg/Kg) | 0.360 | 0.210 | <0.0033 | 0.195 | <0.0033 |
| Cu (mg/Kg) | 2.12 | 2.011 | 1.812 | 1.924 | 1.582 |
| Fe (mg/Kg) | 15.09 | 6.374 | 2.32 | 4.671 | 1.55 |
| Types of MFC | Metal Ions | Recovery (%) | Electrodes | Time (Days) | Ref. |
|---|---|---|---|---|---|
| Single Chamber | Pb2+, Cd2+ and Hg2+ | 89, 76.45 and 89.45 | graphite rods | 12 | [66] |
| One Chamber | Pb2+, Cd2+, Cr3+ and Ni2+ | 83.67, 84.10, 84.55 and 95.99 | graphite rods | 25 | [67] |
| Dual Chamber | Cu (II), Mg (II), Mn (II), Zn (II), and Na | 98, 49, 57, 59, and 36 | Carbon felt | 4 | [68] |
| Dual Chamber | Cu (II) and Cr(VI) | 67.09 and 37.06 | Carbon felt and stainless steel | 18 | [69] |
| Single Chamber | Pb (II), Cd (II), and Cr (III | more than 90% | graphite | 70 | [70] |
| Single Chamber | Cu | 75 | carbon cloth | 60 | [71] |
| Dual Chamber | Cr6+ | 99.18 ± 0.1 | carbon felts | 10 | [72] |
| Microbes | Metal Ions | Recovery (%) | Voltage (mV) | Power Density (mW/m2) | Ref. |
|---|---|---|---|---|---|
| Shewanella oneidensis | Cu2+ and Cd2+ | 21.8 and 18.2 | 175 ± 8 | 92.43 | [73] |
| Leptothrix discophora | Zn, Ni and Cr | 99.8, 98.4 and 94.3 | 1120 | 135.14 | [74] |
| Klebsiella pneumoniae | Cr3+, Co2+ and Ni2+ | 69.24, 72 and 70.11 | 102 | 99.84 | [75] |
| Bacillus and Pseudomonas | Pb2+ | 88 | 1390 | 111.731 | [76] |
| Proteobacteria | Cr6+ | 97.7 | 1020 | 55.1 | [77] |
| Shewanella oneidensis | Cu | 93 | 516.6 | 56.2 | [78] |
| Ochrobactrum sp. | Pb2+ | 98 | 9.3 | 225.1 | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Segundo, R.-F.; De La Cruz-Noriega, M.; Cabanillas-Chirinos, L.; Otiniano, N.M.; Soto-Deza, N.; Rojas-Villacorta, W.; De La Cruz-Cerquin, M. The Potential Use of Pseudomonas stutzeri as a Biocatalyst for the Removal of Heavy Metals and the Generation of Bioelectricity. Fermentation 2024, 10, 113. https://doi.org/10.3390/fermentation10020113
Segundo R-F, De La Cruz-Noriega M, Cabanillas-Chirinos L, Otiniano NM, Soto-Deza N, Rojas-Villacorta W, De La Cruz-Cerquin M. The Potential Use of Pseudomonas stutzeri as a Biocatalyst for the Removal of Heavy Metals and the Generation of Bioelectricity. Fermentation. 2024; 10(2):113. https://doi.org/10.3390/fermentation10020113
Chicago/Turabian StyleSegundo, Rojas-Flores, Magaly De La Cruz-Noriega, Luis Cabanillas-Chirinos, Nélida Milly Otiniano, Nancy Soto-Deza, Walter Rojas-Villacorta, and Mayra De La Cruz-Cerquin. 2024. "The Potential Use of Pseudomonas stutzeri as a Biocatalyst for the Removal of Heavy Metals and the Generation of Bioelectricity" Fermentation 10, no. 2: 113. https://doi.org/10.3390/fermentation10020113
APA StyleSegundo, R.-F., De La Cruz-Noriega, M., Cabanillas-Chirinos, L., Otiniano, N. M., Soto-Deza, N., Rojas-Villacorta, W., & De La Cruz-Cerquin, M. (2024). The Potential Use of Pseudomonas stutzeri as a Biocatalyst for the Removal of Heavy Metals and the Generation of Bioelectricity. Fermentation, 10(2), 113. https://doi.org/10.3390/fermentation10020113






