Abstract
Vitamin C, a water-soluble vitamin with strong reducing power, cannot be synthesized by the human body and participates in a variety of important biochemical reactions. Vitamin C is widely used in the pharmaceutical, food, health care, beverage, cosmetics, and feed industries, with a huge market demand. The classical two-step fermentation method is the mainstream technology for vitamin C production. D-sorbitol is transformed into L-sorbose by Gluconobacter oxydans in the first step of fermentation; then, L-sorbose is transformed into 2-keto-L-gulonic acid (2-KGA) by a coculture system composed of Ketogulonicigenium vulgare and associated bacteria; and finally, 2-KGA is transformed into vitamin C through chemical transformation. The conversion of L-sorbose into 2-KGA in the second fermentation step is performed by K. vulgare. However, considering the slow growth and low 2-KGA production of K. vulgare when cultured alone, it is necessary to add an associated bacteria to stimulate K. vulgare growth and 2-KGA production. Although the mechanism by which the associated bacteria promote K. vulgare growth and 2-KGA production has extensively been studied, this remains a hot topic in related fields. Based on the latest achievements and research, this review summarizes the metabolic characteristics of K. vulgare and associated bacteria and elucidates the mechanism by which the associated bacteria promote the growth and 2-KGA production of K. vulgare.
1. Introduction
Vitamin C, a water-soluble vitamin of vital importance to the human body, participates in many important biochemical reactions in organisms and contains an unsaturated enediol structure, thus having a strong reducing ability. In addition to its antioxidant function, vitamin C is involved in collagen synthesis, hormone synthesis, carnitine synthesis, gene transcription, regulation of translation, L-tyrosine catabolism, and iron absorption in humans [1]. As primates, guinea pigs, and fish lack gulonolactone oxidase (GULO), the enzyme required for vitamin C synthesis, they rely on exogenous vitamin C intake [2]. Vitamin C is widely used in the pharmaceutical, food, health, beverage, cosmetic, and feed industries [3] and has a huge market demand.
At present, vitamin C is primarily produced through the classical two-step fermentation process (Figure 1). Specifically, in the first step of fermentation, Gluconobacter oxydans converts D-sorbitol into L-sorbose, which is a fast and efficient process. By adding 300 g/L D-sorbitol in batch feeding, the yield of L-sorbose reaches 279.7 g/L at 16 h [4]. Then, in the second fermentation step, L-sorbose is converted into 2-keto-L-gulonic acid (2-KGA) through a coculture system composed of Ketogulonicigenium vulgare and associated bacteria. Subsequently, 2-KGA is converted to vitamin C by esterification and lactonization via chemical catalysis. Among these reactions, the transformation of L-sorbose to 2-KGA takes a long time, with a low yield and conversion rate (Table 1), so it is the committed step that determines the yield and cost of vitamin C.
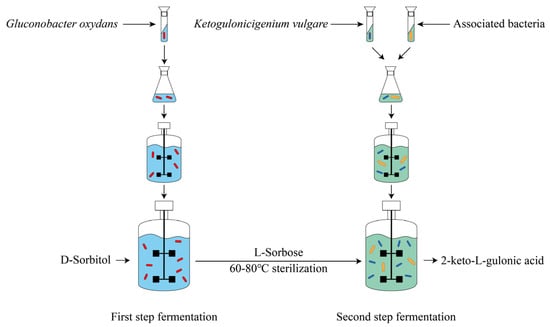
Figure 1.
The process of producing the vitamin C precursor 2-keto-L-gulonic acid by two-step fermentation.

Table 1.
Fermentation yield of the coculture of K. vulgare and different associated bacteria.
Although the two-step fermentation method has advantages such as mature technology and high yield, it suffers from shortcomings such as high energy consumption, large equipment investment, and complex operation brought about by the two sterilization and fermentation steps. Therefore, extensive research has been conducted on one-step fermentation, and some researchers have achieved high 2-KGA yields with such an approach. For example, sorbose dehydrogenase (SDH) and sorbosone dehydrogenase (SNDH) of G. oxydans T100 were coexpressed in G. oxydans G624, which was a chemical mutation to inhibit the L-idonate pathway and the replacement of the original promoter with that of Escherichia coli tufB, resulting in a 2-KGA yield of 130 g/L from 150 g/L of D-sorbitol [14]. Wang et al. [15] deleted the gene involved in the L-sorbose metabolism of G. oxydans and cocultured G. oxydans with K. vulgare, obtaining a 2-KGA yield of 76.6 g/L within 36 h. Zhou et al. used the Gluconobacter oxydans ATCC9937 to direct the production of 2-KGA from D-glucose by balancing intracellular and extracellular D-glucose metabolic flux. The 2-KGA titer reached 30.5 g/L [16]. Despite some progress, the developed one-step fermentation method is not yet suitable for industrial production because of the low yield and low conversion rate.
The coculture process has been widely applied in wastewater treatment, biodegradation of textile azo dyes, treatment of contaminated soil, production of biofuels, and various bulk chemicals and natural products, with high application value [17]. Compared to monoculture, coculture systems can perform more complex tasks and have higher stability and robustness to environmental disturbances [17,18]. In addition, the coculture system can alleviate the growth damage or poor biosynthetic behavior of a single strain caused by excessive cell resource consumption and heavy metabolic burden through division of labor strategies [19]. The coculture system not only makes tasks that cannot be completed by a single strain possible, such as the coculture of Clostridium thermocellum to efficiently hydrolyze cellulose and Thermoanaerobacterium saccharolyticum to produce ethanol from sugars, which produced 38 g/L of ethanol from 92 g/L of avicel [20], but also improves yield and fermentation intensity, such as the coculture of associated bacteria with K. vulgare for 2-KGA production.
In the second step of the two-step fermentation method, K. vulgare, which is responsible for converting L-sorbose into 2-KGA, grows slowly and hardly produces 2-KGA when cultured alone [21,22]. However, when cocultured with associated bacteria that neither metabolize L-sorbose nor produce 2-KGA, K. vulgare can grow smoothly and efficiently to produce 2-KGA. A variety of associated bacteria, such as Xanthomonas maltophilia [5], Bacillus cereus, Bacillus megaterium [6], Bacillus thuringiensis [8], Bacillus endophyticus [9], Bacillus subtilis [10], Bacillus pumilus [12], and Saccharomyces cerevisiae [13], have previously been studied and exploited. Among them, B. megaterium is the most popular one for industrial production.
Stable interactions in microbial consortia generally rely on communication between cells through the coutilization of different substrates in the environment, the sequential conversion and reutilization of substrates, the complement of metabolites, and other ways to meet the normal growth of a single cell in a multicellular system [23]. However, in the second step of the two-step fermentation process for vitamin C production, what mechanism does the associated bacteria use to promote the growth of K. vulgare and the production of 2-KGA? This has always been a research hotspot, and researchers have conducted extensive research and in-depth exploration.
Zou, et al. [22] reviewed the characteristics of K. vulgare and B. megaterium, the research methods of intercellular interaction, and the regulation of artificial microbial ecosystems. Wang et al. [24] expounded on the current classic two-step fermentation process and other potential routes for vitamin C production and summarized the challenges and shortcomings of the one-step fermentation process. Zhang and Lyu [25] reviewed the exchange of substances and intercellular communication between associated bacteria and K. vulgare. Although the previous reviews described the interaction between K. vulgare and associated bacteria in the two-step fermentation process of producing 2-KGA from different perspectives, they have not presented a summary of metabolic defects in different K. vulgare strains or metabolic commonalities among different associated bacteria. The present review summarizes the metabolic defects of K. vulgare and the metabolic characteristics of its associated bacteria and illustrates the mechanism by which associated bacteria can promote K. vulgare growth and 2-KGA production. Not only does this article help provide suggestions and guidance for subsequent research on vitamin C production, but it is also helpful in elucidating the mechanism of mixed microbial symbiosis.
2. Metabolic Characteristics of K. vulgare
2.1. Enzymes and Electron Transfer Chains for the Production of 2-KGA
The conversion of L-sorbose to 2-KGA by K. vulgare is achieved by two dehydrogenases, sorbose/sorbosone dehydrogenase (SSDH) and SNDH. SSDH exhibits both sorbose dehydrogenase and sorbosone dehydrogenase activities, catalyzing not only the conversion of L-sorbose to L-sorbosone but also the conversion of L-sorbose to 2-KGA [26,27]. SNDH is responsible for converting L-sorbosone into 2-KGA. It is worth noting that SSDH and SNDH usually have multiple isoenzymes in the cell. For example, five SSDHs and two SNDHs were found in K. vulgare WSH-001, and three SNDHs were found in K. vulgare DSM 4025 [27,28]. The dual synthesis pathway of 2-KGA in K. vulgare and the multiple isoenzymes of SSDH and SNDH accelerate the synthesis speed of 2-KGA and increase the environmental adaptability of the enzyme catalytic process, which is an important prerequisite for K. vulgare to become a 2-KGA production strain.
The hydrogen generated during the catalytic process of L-sorbose to 2-KGA by SSDHs and SNDHs requires electron transfer chains to bind with oxygen and generate adenosine triphosphate (Figure 2). With PQQ as the coenzyme, the electrons of SSDHs are transferred to cytochrome c oxidase through cytochrome c551 and cytochrome c552 [29]. SNDHs use PQQ as a coenzyme, and most SNDHs also have a binding site for heme C, transferring electrons from substrate to membrane-bound cytochrome c by PQQ and heme C [28].
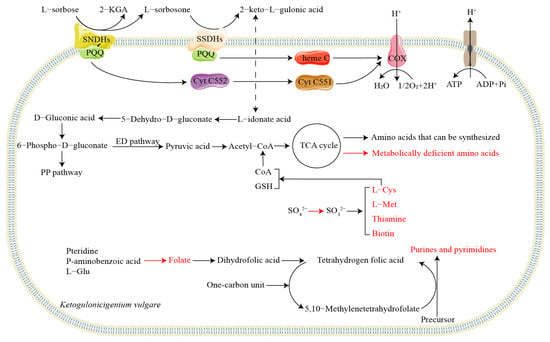
Figure 2.
Metabolic defect and 2-keto-L-gulonic acid synthesis pathway of K. vulgare. Red arrow: defective synthesis steps; Red substance: key nutrients that cannot be synthesized by K. vulgare due to metabolic defects.
2.2. Metabolic Defects of K. vulgare and the Effects of Different Substances on K. vulgare Growth and 2-KGA Production
The bacteria that produce 2-KGA in the second step of the two-step fermentation process have been mistaken for G. oxydans for a long time. However, Ur Ba Nce et al. [21] classified these bacteria as Ketogulonigenium vulgare after phenotype and genotype analysis in 2001. So far, whole-genome sequencing has been completed for K. vulgare Y25 [30], WSH-001 [31], Hbe602 [32], SKV [33], and SPU B805 [34].
Deficiency of carbon metabolism has been reported in K. vulgare. For example, K. vulgare SPU B805 and SKV have complete TCA and PP pathways and invalid EMP and ED pathways, while K. vulgare WSH-001, Y25, and Hbe602 have complete TCA, ED, and PP pathways and a nonfunctional EMP pathway [34]. However, 2-KGA generated by the conversion of L-sorbose can be converted into 6-phospho-D-gluconate through L-iduronic acid, 5-dihydro-D-gluconate, and D-gluonic acid, which can then enter the PP pathway and the ED pathway [35]. Nevertheless, SSDHs in K. vulgare WSH001 can catalyze D-sorbitol, L-sorbitol, glyoxal, methanol, ethanol, glycerol, 1-propanol, 2-propanol, D-mannitol, inositol, D-xylitol, D-glucose, D-galactose, D-mannose, D-xylose, D-rhamnose, D-fructose, D-gluconic acid, D-glucuronic acid, δ-gluconate lactone, and D-lactose, whereas glucose/sorbosone dehydrogenase (GSNDH) and SNDH have higher catalytic activity against glyoxal, D-glucose, D-galactose, D-mannose, D-xylose, and D-rhamnose [27]. Therefore, K. vulgare can utilize various carbon sources for growth. For example, strains 62A-12APP, 266-13BPP, and DSM 4025TP can use carbon sources such as L-arabinose, cellobiose, D-glucose, glycerol, inositol, D-lactose, maltose, D-mannose, D-rhamnose, D-sorbitol, and L-sorbose for growth [21].
Genome-scale metabolic models (GSMMs) of K. vulgare WSH-001 have shown that the insufficient biosynthesis of L-asparagine, L-glycine, L-cysteine, L-methionine, L-tryptophan, adenine, guanine, thymine, thiamine, pantothenic acid, folic acid, and pyridoxal 5-phosphate may be the cause of the poor growth in monoculture. For example, L-glycine, L-cysteine, L-methionine, L-tryptophan, adenine, thymine, thiamine, and pantothenate were separately removed in the fully chemically determined medium (CDM), which resulted in the reduction of biomass formation of K. vulgare to 1, 21, 16, 1, 26, 57, 73, and 24%, respectively [35,36]. K. vulgare Y25 lacks one or more enzymes in the de-novo biosynthesis pathways of L-histidine, L-glycine, L-lysine, L-proline, L-threonine, L-methionine, L-leucine, and L-isoleucine [37]. K. vulgare Hbe602 lacks many amino acid synthesis pathways; only 10 kinds of amino acids are found in the cells, and the metabolic pathways of thiamine, biotin and porphyrin, folic acid, and ubiquinone are incomplete [32]. Wang et al. [34] reconstructed the carbon metabolic pathway of K. vulgare SPU B805 and predicted the presence of synthesis defects in L-histidine, L-alanine, and L-aspartic acid.
Sulfur participates in the formation of important organic compounds such as L-methionine, L-cysteine, L-cystine, biotin, Fe–S cluster proteins, and glutathione (GSH), which play an important role in signal transduction, redox balance, promotion of gene expression, and maintenance of basal metabolic activities [38]. SO42− cannot be converted to SO32− in K. vulgare due to a defect in adenosine monophosphate reductase and adenosine sulfate reductase [35]. Moreover, the insufficient supply of NADPH limits the continued synthesis of SO32− to L-cysteine and L-methionine [39,40]. In addition to being a basic component of proteins, L-cysteine is also an important precursor of GSH and CoA. As an acyl carrier, CoA is involved in a variety of reactions in carbohydrate, fat, and amino acid metabolism, and its deficiency directly limits the growth and metabolism of K. vulgare. The addition of 1 g/L GSH to the culture medium increases cell growth, 2-KGA titer, and intracellular CoA levels by 38.7, 45.5, and 85.3%, respectively, while the addition of 0.4 g/L L-cysteine increases the same indicators by 25.6, 35.8, and 44.7%, respectively [39]. The addition of sulfhydryl compounds such as reduced GSH and dithiothreitol (DTT) increases K. vulgare growth and 2-KGA production approximately twofold and fivefold, respectively [41]. As such, the addition of sulfur-containing compounds not only verifies the sulfur metabolism defect of K. vulgare but also demonstrates that the sulfur metabolism defect is one of the key factors in the slow growth and low 2-KGA production of K. vulgare in monoculture. In addition, the sulfur metabolism defect of K. vulgare can hinder the synthesis of two sulfur-containing vitamins, vitamin B1 and biotin.
In addition to being the basic unit of peptides and proteins, some amino acids are involved in a variety of important microbial metabolisms, such as energy metabolism, sulfur metabolism, and purine and pyrimidine synthesis; thus, metabolic defects of amino acids directly limit the growth and metabolic activities of microorganisms. Some amino acids can also play a crucial role in microbial resistance to adverse external environmental factors. L-proline not only prevents the osmotic pressure generated by high concentrations of 2-KGA from damaging K. vulgare cells by stabilizing proteins, subcellular structures, and cell membranes, but also provides antioxidant stress protection for cells by maintaining intracellular redox homeostasis and increasing catalase activity [42,43].
Four key enzymes in the folate synthesis pathway of K. vulgare WSH001 are defective. Thus, K. vulgare WSH001 cannot synthesize folate [36]. Folate acts as a cofactor in the cell, providing one-carbon compounds in many reactions and participating in the de-novo biosynthetic pathways of amino acids, purines, and pyrimidines [44]. Therefore, the supply of folate and synthetic precursors can promote the growth of K. vulgare and its production of 2-KGA. Folate derivatives, especially dihydrofolic acid, significantly increase the biomass of K. vulgare LMP P-20356 and its production of 2-KGA [45]. In addition, by overexpressing the folate synthesis gene cluster in K. vulgare, the biomass and yield of the recombinant bacteria in the fermentor can be increased by 25 and 36%, respectively [44]. Adenine, guanine, and thymidine all significantly promote the growth of K. vulgare, and since the purine nucleotide and deoxythymidine biosynthesis pathways are folate-dependent, this may be due to the requirement for folate compounds [45].
In addition to the effects on growth and metabolism, some nutrients can also affect the cell size, morphology, and membrane structure of K. vulgare. Intracytoplasmic membranes are formed by the invagination of the plasma membrane due to nutrient restriction or stimulation by some nutrients, which is accompanied by a significant increase in membrane-bound dehydrogenase activity [46]. Addition of 1 g/L GSH to the medium can lead to 4–6-fold elongation of K. vulgare cells (from (0.6 ± 0.2) × (0.5 ± 0.1) μm to (2.4 ± 0.7) × (0.6 ± 0.1) μm), a 3.6-fold increase in biomass, a 5.6-fold increase in 2-KGA production, and the formation of intracytoplasmic membranes [40]. Similarly, reconstruction of the L-threonine biosynthesis pathway in K. vulgare enables the cell size to change from (0.7 ± 0.2) × (0.6 ± 0.1) μm to (1.5 ± 0.2) × (0.6 ± 1) μm, and the gene transcription levels of SNDH and idonate-dehydrogenase (IDH) increase by 3.78 and 1.54 times, respectively [47].
Genome sequencing and the reconstruction of GSMMs systematically have predicted the metabolic defects of K. vulgare. The addition of nutrients has further verified these metabolic defects and revealed the effect of the metabolic defects on the growth and 2-KGA production of K. vulgare. Notably, the study of the K. vulgare metabolic defects provides a theoretical basis for the mechanism by which associated bacteria promote K. vulgare growth and 2-KGA production, in addition to guiding endeavors to further improve the yield under the coculture system.
3. Species and Metabolic Characteristics of Associated Bacteria
3.1. Species of Associated Bacteria
In the 1960s, Yin et al. invented a mixed culture system for 2-KGA production from L-sorbose, which consisted of K. vulgare and an associated strain (Pseudomonas sp. or Bacillus sp.) [24]. Although the associated bacteria can neither utilize L-sorbose nor transform L-sorbose into 2-KGA [48], the addition of these bacteria to the mixed fermentation system can significantly enhance the growth of K. vulgare and its production of 2-KGA. Subsequently, a variety of associated bacteria have been developed and applied to promote 2-KGA production by K. vulgare (Table 1), and the strains of the Bacillus genus have shown strong potential for application. Despite differences in fermentation containers, cultivation conditions, and L-sorbose concentrations, these associated bacteria significantly increased the yield and conversion rate, shortening the fermentation cycle. It is worth noting that the coculture of B. megaterium and B. cereus as associated bacteria in mixed fermentation with K. vulgare could achieve a higher 2-KGA yield than the addition of a single associated bacteria [6]. Moreover, the mixed fermentation system composed of K. vulgare and G. oxydans achieves a one-step fermentation with a high conversion rate from D-sorbitol to 2-KGA. In that system, G. oxydans is not only responsible for converting D-sorbitol to L-sorbose but also plays a role similar to associated bacteria [15].
3.2. Metabolic Characteristics of Associated Bacteria
According to the records of Bergey’s Manual of Systematic Bacteriology [49], B. subtilis, B. licheniformis, B. megaterium, B. thuringiensis, and most Pseudomonas species can grow with ammonium salt or nitrate as the only nitrogen source. B. macerans can grow in an ammonium medium supplemented with vitamin H and vitamin B1. The growth of B. cereus requires the addition of one or more amino acids to the culture medium, and some strains of B. pumilus also require amino acids in addition to vitamin H. The growth requirements of Xanthomonas are complex, and the culture medium usually needs to contain L-methionine, L-glutamate, and niacin. The fermentation medium usually contains corn steep liquor as an organic nitrogen source, which can fully meet the nutritional requirements of all types of associated bacteria. Furthermore, the metabolic defects of the associated bacteria do not conflict with the metabolic defects of K. vulgare; the medium can provide nutrients that neither the associated bacteria nor K. vulgare can synthesize. In addition, these associated bacteria with metabolic defects can meet the needs of K. vulgare in the synthesis of other nutrients. Thus, although B. macerans, B. cereus, B. pumilus, and X. maltophilia lack the ability to synthesize certain amino acids or vitamins, they can still serve as excellent associated bacteria.
Various nutrients that are easily destroyed by high-temperature sterilization, such as vitamins and sulfur-containing amino acids—which can promote the growth and 2-KGA production of K. vulgare but cannot be synthesized due to K. vulgare’s metabolic defects—are primarily synthesized by the associated bacteria. Therefore, accompanying bacteria must have strong biosynthetic abilities. The reported related bacteria can grow in inorganic nitrogen sources or inorganic nitrogen source media with only a few amino acids and vitamins added, demonstrating their potential to synthesize all or most of the nutrients required for their growth and reproduction. For example, in the GSMM of B. megaterium WSH002, the reactions of amino acid metabolism, nucleic acid metabolism, and cofactor and vitamin metabolism account for 23.1, 12.9, and 14.0%, respectively [50], ensuring its strong ability to synthesize nutrients.
Rapid growth, vigorous metabolism, and abundant enzyme systems are also common characteristics and important prerequisites of associated bacteria. First, in the early stage of fermentation, associated bacteria secrete various digestive enzymes to degrade the macromolecular nutrients, especially nitrogen sources, such as converting corn steep liquor powder into small peptides or amino acids that can be easily absorbed and utilized by K. vulgare. Second, the vigorous metabolism of associated bacteria ensures their rapid utilization of various nutrients synthesized in the culture medium, except for L-sorbose, for growth and conversion into their sugars, lipids, amino acids, proteins, and vitamins. In addition, Bacillus species can produce spores and show greater potential than other associated bacteria, possibly due to the release of intracellular nutrients into the extracellular space by the formation and release of the spores at the appropriate time.
Although various excellent associated bacteria have been developed, screening new types of associated bacteria, modification of the already known associated bacteria, and evolution of bacteria under human intervention may still be effective means to achieve higher production yields. For example, L-sorbose in the culture medium can inhibit the growth of associated bacteria; however, the strain of L-sorbose–tolerant associated bacteria, B. cereus 21, obtained through experimental evolution, can achieve higher conversion rates at higher concentrations of L-sorbose when cocultured with K. vulgare [11].
4. Effect of Associated Bacteria on the Growth and 2-KGA Production of K. vulgare
4.1. Fermentation Process
In the second step of the two-step fermentation method, K. vulgare and associated bacteria are usually prepared into mixed bacterial seeds through cocultivation and then inoculated into the fermentation medium [51]. It has been shown that the centrifugation supernatant and cytoplasmic matrix of B. megaterium can promote the growth of K. vulgare, and the presence of B. megaterium significantly shortens the lag time of K. vulgare [52]. At this stage, the associated bacteria grow rapidly, and the biomass of K. vulgare increases slowly, producing only a small amount of 2-KGA [48,51].
The coculture fermentation medium usually includes L-sorbose, corn steep liquor, urea, potassium dihydrogen phosphate, magnesium sulfate, and calcium carbonate [10,53]. Given that the associated bacteria cannot use L-sorbose, corn steep liquor is the only energy source for the associated bacteria, besides providing carbon sources, nitrogen sources, and growth factors. With the progress of fermentation, the nutrient components in the medium are rapidly consumed, leading to a lack of nutrition and especially energy. Poor nutritional conditions lead to the formation of spores by Bacillus [48,54]. Subsequently, Bacillus releases spores, which are accompanied by cell lysis, and the associated bacteria that cannot form spores undergo autolysis. As a result, peptides, proteins, purines, pyrimidines, and small molecules from the cytoplasm are released, which provides new nutrients for K. vulgare. From this moment on, K. vulgare enters the stage of rapid growth and rapid transformation of L-sorbose to 2-KGA [51].
The associated bacteria quickly absorb nutrients from the culture medium, except for L-sorbose, and synthesize the nutrients required for their own growth. Subsequently, the associated bacteria release new components and ratios of nutrients through secretion, release of spores, or cell lysis, which are utilized by K. vulgare. This phenomenon of sequential conversion and reutilization of substrates is common in cocultivation systems, such as the process of cellulose-to-ethanol transformation in the cocultivation of cellulose-decomposing microorganisms and ethanol-producing microorganisms [17]. Nutrients with new compositions and proportions are released by associated bacteria, stimulating the growth of K. vulgare and the production of 2-KGA.
4.2. Supplementation of Key Substance
Although K. vulgare has defects in amino acid, vitamin, coenzyme, and sulfur metabolism, it has a strong resource utilization and transportation system. In the GSMM of K. vulgare WSH-001, 103 genes were found responsible for the transport of exogenous polypeptides, and about 58 genes encoded aminopeptidases or peptidases that could hydrolyze polypeptides into amino acids [35]. Maximizing the use of nutrient elements in the environment is the way for many auxotrophic strains to survive in nature, and this is why associated bacteria can promote the growth and 2-KGA production of K. vulgare.
On the soft agar medium plate, the increase in amino acid concentration in the medium around K. vulgare colonies attracted B. megaterium to move and aggregate to K. vulgare; erythrose, erythritol, avian purines, and inositol were depleted by K. vulgare; and 2-KGA content in agar increased dramatically [54]. The same movement phenomenon was found when K. vulgare was cocultured with B. thuringiensis. Erythrose, erythritol, guanine, and inositol accumulated around B. thuringiensis were consumed by K. vulgare, and the production of 2-KGA increased sharply [55]. Additionally, B. megaterium WSH002 can secrete pantothenic acid and L-cysteine, which may be two potential growth promoters of K. vulgare because they are precursors of CoA [50]. Other studies have also shown that the adenine, guanine, and hypoxanthine required for K. vulgare in the middle stage of fermentation probably come from the decomposition of the associated bacteria [56].
In addition to the small-molecule metabolites, the associated bacteria also secrete proteins that can promote the growth and 2-KGA production of K. vulgare. B. megaterium secretes two different proteins, with molecular weights of 30–50 kDa and > 100 kDa, which can promote the growth of K. vulgare and increase the production of 2-KGA [37]. However, the specific protein and the mechanism by which it promotes K. vulgare growth and 2-KGA production have not yet been isolated and studied.
Reasonable utilization of material exchange between microorganisms can improve the biomass and product yield of coculture systems. For example, in the nitrogen- and fatty acid-limiting environment of water kefir, the presence of Saccharomyces cerevisiae improves the growth of Lactobacillus hordei by providing gluconate, fructose, amino acids, fatty acids, etc. [57]. Yeast utilizes oxygen respiration to produce carbon dioxide, while microalgae convert carbon dioxide into lipids and oxygen through photosynthesis [58]. Coculture of Rhodotorula glutinis and Scenedesmus obliquus in a photobioreactor increases the fermentation biomass by 40–50% and total lipids by 60–70%. [59]. However, in the continuous transformation and reutilization process of substrates, microorganisms that first utilize these substances will consume a portion for growth and metabolism; reducing these losses can improve the conversion efficiency of substrates. Similarly, in the coculture process of Bacillus and K. vulgare, a considerable part of the limited nutrients in the medium is locked in the spores. It has been shown that using lysozyme to lyse B. megaterium releases more intracellular substances than spore formation; the growth rate of K. vulgare increases by 27.4%, and the productivity of 2-KGA increases by 28.2%. [52]. Second, in the fermentation stage after the formation of spores, most of the nutrients for K. vulgare growth are provided by the lysate produced during the process of spore release by Bacillus, which leads to the problem that the substances released after spore decomposition may not completely meet the needs of K. vulgare. For example, in the coculture system with B. megaterium as associated bacteria, B. megaterium forms spores within 7–22 h, but the extracellular contents of L-proline, L-cysteine, L-valine, L-phenylalanine, and L-arginine remain 0 during 10–70 h of fermentation [60]. This indicates that these nutrients are leaked at low concentrations or that they are quickly absorbed and utilized by K. vulgare during spore release, and nutrients such as L-cysteine and L-proline, which cannot be synthesized by K. vulgare itself, may be factors limiting K. vulgare’s growth and 2-KGA production at this time.
4.3. Alleviation of Oxidative Pressure in Fermentation Systems
Autoxidation of flavin dehydrogenase [61,62] and non-respiratory flavoproteins in some metabolic pathways [63,64,65] can generate reactive oxygen species (ROS), including superoxide anion, hydrogen peroxide, hydroxyl radicals, and nitric oxide. ROS are natural products of oxygen metabolism in aerobic organisms. Excessive levels of ROS in cells cause oxidative stress, which results in DNA damage, cell membrane peroxidation, inactivation of enzymes and cofactors, and eventually cell death [66]. The presence of cytochrome c in K. vulgare couples the production of 2-KGA with the respiratory chain [27], and ROS inhibits the growth of K. vulgare and the production of 2-KGA [67]. Except for the conversion process of L-sorbose to 2-KGA and other metabolic activities that produce a lot of ROS, 2-KGA as the target product also causes oxidative stress in K. vulgare [68,69].
To combat the ROS-induced damage to cells, biological organisms have developed a series of enzymes that can eliminate ROS, including superoxide dismutase, catalase, and peroxidase. The associated bacteria can induce the upregulation of these ROS-scavenging enzymes. For example, S. cerevisiae induces upregulation of superoxide dismutase, catalase, and oxidative stress–related genes (sod, cat, and gpd), thereby leading to increased 2-KGA production by K. vulgare [13]. Proteomic studies have shown that the enzymes in the microbial consortium that maintain the metabolic reduction environment of cells, including superoxide dismutase, glutathione S-transferase, NADPH: quinone oxidoreductase, and glucose-6-phosphate dehydrogenase, reach higher levels of expression during 18–23 h of fermentation [56].
Some small-molecular substances released by the associated bacteria may also help K. vulgare resist ROS. Proline can directly eliminate ROS and may also protect the stability of a variety of antioxidant enzymes [70]. L-cysteine can be used to synthesize GSH—the most important antioxidant in cells. Pyridoxine can quench ROS [71]. Vitamin C can alleviate oxidative stress in the environment and promote the growth and 2-KGA production of K. vulgare. Hence, using Saccharomyces cerevicae VTC2, which is genetically engineered to produce vitamin C from D-glucose, as an associated bacteria could increase 2-KGA production by 25% compared to the original strain [13]. Adenine, guanine, xanthine, and hypoxanthine produced by the associated bacteria may contribute to K. vulgare’s resistance to ROS [56]. Furthermore, the effect of B. megaterium–engineered bacteria that cannot form spores in the coculture system is weakened, which may be due to the absence or reduction of antioxidant substances called sporulenes, produced during the sporulation stage of B. megaterium [72,73].
Siderophores are secreted by microorganisms such as bacteria and fungi to obtain iron from the environment, and they can efficiently combine with low-molecular-weight substances of iron in the surrounding environment [74,75]. Most aerobic and facultative anaerobic microorganisms can synthesize at least one siderophore, and microorganisms growing under aerobic conditions require iron for a variety of functions, including the reduction of oxygen for ATP synthesis, the reduction of nucleoside precursors of DNA, heme formation, and other basic purposes [76]. It has been shown that K. vulgare cannot synthesize siderophores but has a siderophore-absorption system, and the addition of 500 µg/L Bacillus pumilus SY-A9 siderophores increases the titer of 2-KGA by 71.45% [12]. Further research has shown that siderophores cause overexpression of iron absorption system–related genes, electron transfer chain–related genes, ATP synthase–related genes, antioxidant enzyme–related genes, and 2-KGA production enzyme–related factors in K. vulgare 25B-1, thereby reducing oxidative stress and ensuring energy metabolism [12].
Despite the fact that the associated bacteria play a significant role in helping K. vulgare resist oxidation, the addition of antioxidant substances can further alleviate the oxidative stress of the fermentation system and improve the yield of 2-KGA. The addition of glutathione and oxidized glutathione with a concentration ratio of 50:1 in the fermentation system of B. endophyticus ST-1 as the associated bacteria increases the activities of total antioxidant capacity (t-AOC), total superoxide dismutase (T-SOD), and catalase (CAT) in the fermentation system and upregulates the expression of genes related to superoxide dismutase, such as sod, gst, gr, zwf, and gp, thereby eliminating oxidative stress, improving 2-KGA production, and shortening fermentation time [67]. Moreover, during the period when K. vulgare produces ROS due to the rapid dehydrogenation of L-sorbose to 2-KGA, the associated bacteria usually form spores or cell lysates. The antioxidant substances provided by associated bacteria lack a sustained supply during the high-speed production period of 2-KGA, so manual intervention to alleviate the oxidative pressure of K. vulgare in the later stage of fermentation may further improve the fermentation intensity.
5. Conclusions
The second fermentation process in the production of vitamin C by the two-step fermentation method is an artificial microbial community system with a clear division of labor composed of the strain K. vulgare (responsible for converting L-sorbose into 2-KGA) and associated bacteria (responsible for promoting K. vulgare growth and its production of 2-KGA). Due to deficiencies in the synthesis of amino acids, purines, pyrimidines, and vitamins, as well as carbon metabolism and sulfur metabolism pathways, K. vulgare grows slowly and produces low 2-KGA when cultured alone. However, associated bacteria possess extremely strong biosynthetic abilities and act as nutrient synthesis factories in coculture systems to synthesize a variety of nutrients for K. vulgare. Moreover, the nutrient availability in the culture medium is relatively scarce, which leads to the formation of spores by the associated bacteria, such as Bacillus, thereby releasing a large amount of intracellular nutrients during cell lysis and spore release after rapidly consuming nutrients. In the coculture system, nutrients, especially nitrogen sources, are transformed by associated bacteria and subsequently utilized by K. vulgare, supplementing K. vulgare’s metabolic defects and meeting K. vulgare’s growth and 2-KGA production. In addition to producing and providing necessary nutrients for K. vulgare, the associated bacteria can also regulate the redox environment of the fermentation system, slow down the oxidative stress of K. vulgare, and ensure a good external environment for K. vulgare’s 2-KGA production.
Previous studies have sequenced the genomes of several K. vulgare strains, reconstructed the metabolic models, and predicted the metabolic defects of K. vulgare. However, cultivating K. vulgare alone to achieve a high yield of 2-KGA has not yet been realized. More accurate exploration of enzymatic properties and gene annotation, research on the promoting effects of more types of nutrients on the growth and 2-KGA production of K. vulgare, and the development of a more complex high-yield 2-KGA fermentation medium will further deepen the understanding of K. vulgare metabolic defects and nutrient exchange with associated strains in mixed fermentation.
The sequential conversion and reutilization process of substrates in coculture systems usually results in substrate loss; take carbon sources, for instance, which are used for respiratory metabolism and cell components. For Bacillus, some nutrients are locked in the spore and cannot be utilized by K. vulgare. Further promoting the release of nutrients from Bacillus spores may further promote K. vulgare’s growth and 2-KGA production. Moreover, in the later stage of cocultivation, K. vulgare produces a large amount of ROS due to the production of 2-KGA, and the associated bacteria are in a spore or cell lysis state. During this period, using chemical reducing agents to stabilize the redox potential of the fermentation environment will be beneficial for the fermentation process. Furthermore, the nutrients released by the associated bacteria cannot perfectly meet the needs of K. vulgare in terms of composition and proportion. It is necessary to study the addition of nutrients to meet the growth needs of K. vulgare after the associated bacteria form spores or lysis. Additionally, enhancing the synthesis and release of key nutrients in associated bacteria through genetic engineering is an ideal means to improve the industrial production of 2-KGA. Moreover, adding different forms of associated bacteria and their secreted nutrients in the later stage of fermentation, such as active or inactivated cell-containing cultures, cultures that, after spore release or bacterial autolysis, or the supernatant of these cultures, may further shorten the fermentation cycle and increase 2-KGA production.
Author Contributions
Conceptualization, H.Y. and T.W.; methodology, W.C.; software, Q.L.; validation, H.L. and D.H.; formal analysis, Y.J.; investigation, T.W. and H.Y.; resources, T.W.; data curation, M.L.; writing—original draft preparation, W.C.; writing—review and editing, Q.L.; visualization, M.L.; supervision, D.H.; project administration, T.W. and H.L.; funding acquisition, T.W., W.C. and Q.L. contributed equally to this work. All authors have read and agreed to the published version of this manuscript.
Funding
This research was funded by the Key Research and Development Program of Shandong Province (2022CXGC010506), the National Natural Science Foundation of China (22308180, 32001632), the Natural Science Foundation of Shandong Province (ZR2020QB041, ZR2021QB074, ZR2021QB113), the Qilu University of Technology (Shandong Academy of Sciences) of talent research projects (2023RCKY221, 2023RCKY224, 2023RCKY225), the Qilu University of Technology of Cultivating Subject for Biology and Biochemistry (No. 202003, No. 202018, No. 202007), and the Key Research and Development Program of Zibo (2021XCYF0085).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.K.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C-Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Valdés, F. Vitamin C. Actas Dermo-Sifiliogr. 2006, 97, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Xu, H. Industrial Fermentation of Vitamin C. In Industrial Biotechnology of Vitamins, Biopigments, and Antioxidants; Vandamme, E., Revuelta, J.L., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 161–192. [Google Scholar]
- Giridhar, R.N.; Srivastava, A.K. Productivity improvement in L-sorbose biosynthesis by fedbatch cultivation of Gluconobacter oxydans. J. Biosci. Bioeng. 2002, 94, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Takagi, Y.; Sugisawa, T.; Hoshino, T. Continuous 2-Keto-l-gulonic acid fermentation by mixed culture of Ketogulonicigenium vulgare DSM 4025 and Bacillus megaterium or Xanthomonas maltophilia. Appl. Microbiol. Biotechnol. 2010, 86, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Mandlaa; Yang, W.C.; Han, L.T.; Wang, Z.Y.; Xu, H. Two-helper-strain co-culture system: A novel method for enhancement of 2-keto-L-gulonic acid production. Biotechnol. Lett. 2013, 35, 1853–1857. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, M.; Lv, Y.; Wang, Y.; Song, H.; Yuan, Y.J. Enhancement of 2-keto-gulonic acid yield by serial subcultivation of co-cultures of Bacillus cereus and Ketogulonicigenium vulgare. Bioresour. Technol. 2013, 132, 370–373. [Google Scholar] [CrossRef]
- Yang, W.; Han, L.; Mandlaa, M.; Chen, H.; Jiang, M.; Zhang, Z.; Xu, H. Spaceflight-induced enhancement of 2-keto-L-gulonic acid production by a mixed culture of Ketogulonigenium vulgare and Bacillus thuringiensis. Lett. Appl. Microbiol. 2013, 57, 54–62. [Google Scholar] [CrossRef]
- Jia, N.; Du, J.; Ding, M.Z.; Gao, F.; Yuan, Y.J. Genome Sequence of Bacillus endophyticus and Analysis of Its Companion Mechanism in the Ketogulonigenium vulgare-Bacillus Strain Consortium. PLoS ONE 2015, 10, 17. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, M.; Yu, X.D.; Zhang, Y.H.; Lyu, S.X. Optimization of medium composition for two-step fermentation of vitamin C based on artificial neural network-genetic algorithm techniques. Biotechnol. Biotechnol. Equip. 2015, 29, 1128–1134. [Google Scholar] [CrossRef]
- Mandlaa; Sun, Z.Y.; Wang, R.G.; Han, X.D.; Xu, H.; Yang, W.C. Enhanced 2-keto-L-gulonic acid production by applying L-sorbose-tolerant helper strain in the co-culture system. AMB Express 2018, 8, 7. [Google Scholar] [CrossRef]
- Zhang, Q.; Lin, Y.; Shen, G.; Zhang, H.; Lyu, S. Siderophores of Bacillus pumilus promote 2-keto-L-gulonic acid production in a vitamin C microbial fermentation system. J. Basic Microb. 2022, 62, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.C.; Liu, Y.; Zhou, M.Y.; Ding, M.Z.; Yuan, Y.J. Construction of synthetic microbial consortia for 2-keto-L-gulonic acid biosynthesis. Synth. Syst. Biotechnol. 2022, 7, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Saito, Y.; Ishii, Y.; Hayashi, H.; Imao, Y.; Akashi, T.; Yoshikawa, K.; Noguchi, Y.; Soeda, S.; Yoshida, M.; Niwa, M.; et al. Cloning of genes coding for L-sorbose and L-sorbosone dehydrogenases from Gluconobacter oxydans and microbial production of 2-keto-L-gulonate, a precursor of L-ascorbic acid, in a recombinant G. oxydans strain. Appl. Environ. Microbiol. 1997, 63, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.X.; Ding, M.Z.; Ma, Q.; Dong, X.T.; Yuan, Y.J. Reorganization of a synthetic microbial consortium for one-step vitamin C fermentation. Microb. Cell. Fact. 2016, 15, 11. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Li, D.; Zeng, W.; Qin, Z.; Chen, J.; Zhou, J. Efficient production of 2-keto-L-gulonic acid from D-glucose in Gluconobacter oxydans ATCC9937 by mining key enzyme and transporter. Bioresour. Technol. 2023, 384, 129316. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wu, R.; Zhou, J.; He, A.; Xu, J.; Xin, F.; Zhang, W.; Ma, J.; Jiang, M.; Dong, W. Recent advances of biofuels and biochemicals production from sustainable resources using co-cultivation systems. Biotechnol. Biofuels. 2019, 12, 155. [Google Scholar] [CrossRef]
- Brenner, K.; You, L.; Arnold, F.H. Engineering microbial consortia: A new frontier in synthetic biology. Trends Biotechnol. 2008, 26, 483–489. [Google Scholar] [CrossRef]
- Agapakis, C.M.; Boyle, P.M.; Silver, P.A. Natural strategies for the spatial optimization of metabolism in synthetic biology. Nat. Chem. Biol. 2012, 8, 527–535. [Google Scholar] [CrossRef]
- Argyros, D.A.; Tripathi, S.A.; Barrett, T.F.; Rogers, S.R.; Feinberg, L.F.; Olson, D.G.; Foden, J.M.; Miller, B.B.; Lynd, L.R.; Hogsett, D.A.; et al. High ethanol titers from cellulose by using metabolically engineered thermophilic, anaerobic microbes. Appl. Environ. Microbiol. 2011, 77, 8288–8294. [Google Scholar] [CrossRef]
- Urbance, J.W.; Bratina, B.J.; Stoddard, S.F.; Schmidt, T.M.; Microbiology, E. Taxonomic characterization of Ketogulonigenium vulgare gen. nov., sp. nov. and Ketogulonigenium robustum sp. nov., which oxidize L-sorbose to 2-keto-L-gulonic acid. Int. J. Syst. Evol. Microbiol. 2001, 51, 1059–1070. [Google Scholar] [CrossRef][Green Version]
- Zou, W.; Liu, L.M.; Chen, J. Structure, mechanism and regulation of an artificial microbial ecosystem for vitamin C production. Crit. Rev. Microbiol. 2013, 39, 247–255. [Google Scholar] [CrossRef]
- Ding, M.Z.; Song, H.; Wang, E.X.; Liu, Y.; Yuan, Y.J. Design and construction of synthetic microbial consortia in China. Synth. Syst. Biotechnol. 2016, 1, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.P.; Zeng, W.Z.; Xu, S.; Du, G.C.; Zhou, J.W.; Chen, J. Current challenges facing one-step production of L-ascorbic acid. Biotechnol. Adv. 2018, 36, 1882–1899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lyu, S. Microbial Interactions in a Vitamin C Industrial Fermentation System: Novel Insights and Perspectives. Appl. Environ. Microbiol. 2022, 88, e0121222. [Google Scholar] [CrossRef] [PubMed]
- Asakura, A.; Hoshino, T. Isolation and Characterization of a New Quinoprotein Dehydrogenase, L-Sorbose/L-Sorbosone Dehydrogenase. Biosci. Biotechnol. Biochem. 1999, 63, 46–53. [Google Scholar] [CrossRef]
- Wang, P.P.; Zeng, W.Z.; Du, G.C.; Zhou, J.W.; Chen, J. Systematic characterization of sorbose/sorbosone dehydrogenases and sorbosone dehydrogenases from Ketogulonicigenium vulgare WSH-001. J. Biotechnol. 2019, 301, 24–34. [Google Scholar] [CrossRef]
- Miyazaki, T.; Sugisawa, T.; Hoshino, T. Pyrroloquinoline quinone-dependent dehydrogenases from Ketogulonicigenium vulgare catalyze the direct conversion of L-sorbosone to L-ascorbic acid. Appl. Environ. Microbiol. 2006, 72, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Han, X.D.; Xiong, X.G.; Jiang, D.Q.; Chen, S.H.; Huang, E.Y.; Zhang, W.C.; Liu, X.Q. Crystal structure of L-sorbose dehydrogenase, a pyrroloquinoline quinone-dependent enzyme with homodimeric assembly, from Ketogulonicigenium vulgare. Biotechnol. Lett. 2014, 36, 1001–1008. [Google Scholar] [CrossRef]
- Xiong, X.H.; Han, S.A.; Wang, J.H.; Jiang, Z.H.; Chen, W.; Jia, N.; Wei, H.L.; Cheng, H.; Yang, Y.X.; Zhu, B.; et al. Complete Genome Sequence of the Bacterium Ketogulonicigenium vulgare Y25. J. Bacteriol. 2011, 193, 315–316. [Google Scholar] [CrossRef]
- Liu, L.M.; Li, Y.; Zhang, J.; Zhou, Z.M.; Liu, J.; Li, X.M.; Zhou, J.W.; Du, G.C.; Wang, L.; Chen, J. Complete Genome Sequence of the Industrial Strain Ketogulonicigenium vulgare WSH-001. J. Bacteriol. 2011, 193, 6108–6109. [Google Scholar] [CrossRef]
- Jia, N.; Ding, M.Z.; Du, J.; Pan, C.H.; Tian, G.; Lang, J.D.; Fang, J.H.; Gao, F.; Yuan, Y.J. Insights into mutualism mechanism and versatile metabolism of Ketogulonicigenium vulgare Hbe602 based on comparative genomics and metabolomics studies. Sci. Rep. 2016, 6, 23068. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Ding, M.Z.; Du, Y.Z.; Feng, S.; Gao, F.; Yuan, Y.J. Complete Genome Sequence of the Industrial Bacterium Ketogulonicigenium vulgare SKV. Genome Announc. 2016, 4, e01426-16. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Li, Y.; Gao, Z.W.; Liu, L.C.; Wu, Y.C.; Zhang, M.Y.; Zhang, T.Y.; Zhang, Y.X. Reconstruction and analysis of carbon metabolic pathway of Ketogulonicigenium vulgare SPU B805 by genome and transcriptome. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.; Liu, L.M.; Zhang, J.; Yang, H.R.; Zhou, M.D.; Hua, Q.; Chen, J. Reconstruction and analysis of a genome-scale metabolic model of the vitamin C producing industrial strain Ketogulonicigenium vulgare WSH-001. J. Biotechnol. 2012, 161, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.C.; Zhang, Z.Y.; Zou, W.; Huang, Z.; Liu, J.; Liu, L.M. Development of a minimal chemically defined medium for Ketogulonicigenium vulgare WSH001 based on its genome-scale metabolic model. J. Biotechnol. 2014, 169, 15–22. [Google Scholar] [CrossRef]
- Liu, L.M.; Chen, K.J.; Zhang, J.; Liu, J.; Chen, J. Gelatin enhances 2-keto-L-gulonic acid production based on Ketogulonigenium vulgare genome annotation. J. Biotechnol. 2011, 156, 182–187. [Google Scholar] [CrossRef]
- Wang, R. Physiological implications of hydrogen sulfide: A whiff exploration that blossomed. Physiol. Rev. 2012, 92, 791–896. [Google Scholar] [CrossRef]
- Huang, Z.; Zou, W.; Liu, J.; Liu, L.M. Glutathione enhances 2-keto-L-gulonic acid production based on Ketogulonicigenium vulgare model iWZ663. J. Biotechnol. 2013, 164, 454–460. [Google Scholar] [CrossRef]
- Ma, Q.; Zhang, W.; Zhang, L.; Qiao, B.; Pan, C.; Yi, H.; Wang, L.; Yuan, Y.J. Proteomic analysis of Ketogulonicigenium vulgare under glutathione reveals high demand for thiamin transport and antioxidant protection. PLoS ONE 2012, 7, e32156. [Google Scholar] [CrossRef]
- Zhou, J.; Yi, H.; Wang, L.L.; Zhang, W.W.; Yuan, Y.J. Metabolomic Analysis of the Positive Effects on Ketogulonigenium vulgare Growth and 2-Keto-L-Gulonic Acid Production by Reduced Glutathione. Omics 2012, 16, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, J.W.; Liu, J.; Chen, K.J.; Liu, L.M.; Chen, J.A. Development of chemically defined media supporting high cell density growth of Ketogulonicigenium vulgare and Bacillus megaterium. Bioresour. Technol. 2011, 102, 4807–4814. [Google Scholar] [CrossRef] [PubMed]
- Goswami, G.; Hazarika, D.J.; Chowdhury, N.; Bora, S.S.; Sarmah, U.; Naorem, R.S.; Boro, R.C.; Barooah, M. Proline confers acid stress tolerance to Bacillus megaterium G18. Sci. Rep. 2022, 12, 8875. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Yuan, M.Q.; Li, Z.J.; Chen, J.C.; Chen, G.Q. Genetic engineering of Ketogulonigenium vulgare for enhanced production of 2-keto-L-gulonic acid. J. Biotechnol. 2012, 157, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Leduc, S.; Troostembergh, J.; Lebeault, J.-M. Folate requirements of the 2-keto-L-gulonic acid-producing strain Ketogulonigenium vulgare LMP P-20356 in L-sorbose/CSL medium. Appl. Microbiol. Biotechnol. 2004, 65, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Claus, G.W.; Batzing, B.L.; Baker, C.A.; Goebel, E.M. Intracytoplasmic membrane formation and increased oxidation of glycerol growth of Gluconobacter oxydans. J. Bacteriol. 1975, 123, 1169–1183. [Google Scholar] [CrossRef]
- Pan, C.H.; Wang, E.X.; Jia, N.; Dong, X.T.; Liu, Y.; Ding, M.Z.; Yuan, Y.J. Reconstruction of amino acid biosynthetic pathways increases the productivity of 2-keto-l-gulonic acid in Ketogulonicigenium vulgare-Bacillus endophyticus consortium via genes screening. J. Ind. Microbiol. Biotechnol. 2017, 44, 1031–1040. [Google Scholar] [CrossRef]
- Wei, D.; Yuan, W.; Yin, G.; Yuan, Z.; Chen, M. Studies on kinetic model of vitamin C two-step fermentation process. Chin. J. Biotechnol. 1992, 8, 195–201. [Google Scholar]
- Bergey, D.H.; Buchanan, R.E.; Gibbons, N.E. Bergey’s Manual of Determinative Bacteriology, 8th ed.; Science Press: Beijing, China, 1974; pp. 729–759. [Google Scholar]
- Zou, W.; Zhou, M.D.; Liu, L.M.; Chen, J. Reconstruction and analysis of the industrial strain Bacillus megaterium WSH002 genome-scale in silico metabolic model. J. Biotechnol. 2013, 164, 503–509. [Google Scholar] [CrossRef]
- Yang, W.C.; Sun, H.; Dong, D.; Ma, S.; Mandlaa; Wang, Z.X.; Xu, H. Enhanced 2-keto-L-gulonic acid production by a mixed culture of Ketogulonicigenium vulgare and Bacillus megaterium using three-stage temperature control strategy. Braz. J. Microbiol. 2021, 52, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, J.; Shi, Z.P.; Liu, L.M.; Chen, J. Manipulation of B-megaterium growth for efficient 2-KLG production by K-vulgare. Process. Biochem. 2010, 45, 602–606. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Zhu, X.J.; Xie, P.; Sun, J.W.; Yuan, J.Q. Macrokinetic model for Gluconobacter oxydans in 2-keto-L-gulonic acid mixed culture. Biotechnol. Bioprocess Eng. 2012, 17, 1008–1017. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, Q.; Yi, H.; Wang, L.; Song, H.; Yuan, Y.J. Metabolome profiling reveals metabolic cooperation between Bacillus megaterium and Ketogulonicigenium vulgare during induced swarm motility. Appl. Environ. Microbiol. 2011, 77, 7023–7030. [Google Scholar] [CrossRef]
- Jia, N.; Ding, M.Z.; Gao, F.; Yuan, Y.J. Comparative genomics analysis of the companion mechanisms of Bacillus thuringiensis Bc601 and Bacillus endophyticus Hbe603 in bacterial consortium. Sci. Rep. 2016, 6, 28794. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Zhou, J.; Zhang, W.; Meng, X.; Sun, J.; Yuan, Y.J. Integrated proteomic and metabolomic analysis of an artificial microbial community for two-step production of vitamin C. PLoS ONE 2011, 6, e26108. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Behr, J.; Geißler, A.J.; Bechtner, J.; Ludwig, C.; Vogel, R.F. Label-free quantitative proteomic analysis reveals the lifestyle of Lactobacillus hordei in the presence of Sacchromyces cerevisiae. Int. J. Food Microbiol. 2019, 294, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Szotkowski, M.; Holub, J.; Šimanský, S.; Hubačová, K.; Sikorová, P.; Mariničová, V.; Němcová, A.; Márová, I. Bioreactor Co-Cultivation of High Lipid and Carotenoid Producing Yeast Rhodotorula kratochvilovae and Several Microalgae under Stress. Microorganisms 2021, 9, 1160. [Google Scholar] [CrossRef]
- Yen, H.W.; Chen, P.W.; Chen, L.J. The synergistic effects for the co-cultivation of oleaginous yeast-Rhodotorula glutinis and microalgae-Scenedesmus obliquus on the biomass and total lipids accumulation. Bioresour. Technol. 2015, 184, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Diao, J.; Xiang, B.T.; Cao, Z. Studies on metabolism of nitrogen source in fermentation of 2-keto-gulonic acid. Acta Microbiol. Sinica. 1996, 36, 19–24. [Google Scholar]
- Messner, K.R.; Imlay, J.A. The identification of primary sites of superoxide and hydrogen peroxide formation in the aerobic respiratory chain and sulfite reductase complex of Escherichia coli. J. Biol. Chem. 1999, 274, 10119–10128. [Google Scholar] [CrossRef]
- Kussmaul, L.; Hirst, J. The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc. Natl. Acad. Sci. USA 2006, 103, 7607–7612. [Google Scholar] [CrossRef]
- Massey, V.; Strickland, S.; Mayhew, S.G.; Howell, L.G.; Engel, P.C.; Matthews, R.G.; Schuman, M.; Sullivan, P.A. The production of superoxide anion radicals in the reaction of reduced flavins and flavoproteins with molecular oxygen. Biochem. Biophys. Res. Commun. 1969, 36, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Grinblat, L.; Sreider, C.M.; Stoppani, A.O. Superoxide anion production by lipoamide dehydrogenase redox-cycling: Effect of enzyme modifiers. Biochem. Int. 1991, 23, 83–92. [Google Scholar] [PubMed]
- Geary, L.E.; Meister, A. On the mechanism of glutamine-dependent reductive amination of alpha-ketoglutarate catalyzed by glutamate synthase. J. Biol. Chem. 1977, 252, 3501–3508. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Kogoma, T. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 1991, 55, 561–585. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.H.; Yao, S.; Ma, D.; Yu, X.D.; Zhang, Q.; Lyu, S.X. Antioxidant effect of glutathione on promoting 2-keto-l-gulonic acid production in vitamin C fermentation system. J. Appl. Microbiol. 2018, 125, 1383–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lyu, S. 2-Keto-L-gulonic acid inhibits the growth of Bacillus pumilus and Ketogulonicigenium vulgare. World J. Microbiol. Biotechnol. 2023, 39, 257. [Google Scholar] [CrossRef]
- Fang, J.; Wan, H.; Zeng, W.; Li, J.; Chen, J.; Zhou, J. Transcriptome Analysis of Gluconobacter oxydans WSH-003 Exposed to Elevated 2-Keto-L-Gulonic Acid Reveals the Responses to Osmotic and Oxidative Stress. Appl. Biochem. Biotechnol. 2021, 193, 128–141. [Google Scholar] [CrossRef]
- Kaur, G.; Asthir, B.J.B.P. Proline: A key player in plant abiotic stress tolerance. Biol. Plant. 2015, 59, 609–619. [Google Scholar] [CrossRef]
- Dalto, D.B.; Matte, J.J. Pyridoxine (Vitamin B6) and the Glutathione Peroxidase System; a Link between One-Carbon Metabolism and Antioxidation. Nutrients 2017, 9, 189. [Google Scholar] [CrossRef]
- Zhu, Y.B.; Liu, J.; Du, G.C.; Zhou, J.W.; Chen, J. Sporulation and spore stability of Bacillus megaterium enhance Ketogulonigenium vulgare propagation and 2-keto-L-gulonic acid biosynthesis. Bioresour. Technol. 2012, 107, 399–404. [Google Scholar] [CrossRef]
- Bosak, T.; Losick, R.M.; Pearson, A. A polycyclic terpenoid that alleviates oxidative stress. Proc. Natl. Acad. Sci. USA 2008, 105, 6725–6729. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, D.; Monllor-Satoca, D.; Kim, K.; Lee, W.; Choi, W. Homogeneous photocatalytic Fe3+/Fe2+ redox cycle for simultaneous Cr(VI) reduction and organic pollutant oxidation: Roles of hydroxyl radical and degradation intermediates. J. Hazard. Mater. 2019, 372, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Sloderbach, A.; Marszałł, M.P. Siderophore-drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Neilands, J.B. Siderophores: Structure and function of microbial iron transport compounds. J. Biol. Chem. 1995, 270, 26723–26726. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).