Abstract
Trans-2-decenoic acid has a wide range of applications, including those in medicine, food, and health care. Therefore, the industrial production of trans-2-decenoic acid is particularly important. However, few studies have focused on medium-chain unsaturated fatty acids. Therefore, we aimed to optimize the fermentation process of decanoic acid biocatalysis to synthesize trans-2-decenoic acid using an engineered Escherichia coli constructed in the laboratory. Early-stage culture and the effect of the seed liquid culture time, culture temperature, inoculum amount, induction temperature, dissolution effects of the substrate solvent, metal ions, and substrate loading on the titer of trans-2-decenoic acid were evaluated. Based on a single-factor experimental optimization, a Box–Behnken design (BBD) was used for response surface testing using the substrate feeding concentration, inducer concentration, and MnCl2 concentration as response variables and trans-2-decenoic acid production as the response value. The optimal fermentation process was as follows: Seed culture time of 20 h, culture temperature of 37 °C, inoculation amount of 1%, induction temperature of 30 °C, substrate flow of 0.15 g/L, inducer concentration of 5.60 g/L, and MnCl2 concentration of 0.10 mM. Under these conditions, the average production of trans-2-decenoic acid was 1.982 ± 0.110 g/L, which was 1.042 g/L higher than that obtained in the basic LB medium. Compared with that of the previous period, the titer of the trans-2-decenoic acid studied increased by 1.501 ± 0.110 g/L, providing a basis for further research on the fermentation process of the biocatalytic decanoic acid synthesis of trans-2-decenoic acid.
1. Introduction
Trans-2-decenoic acid is a medium-chain fatty acid containing 10 carbon atoms and an unsaturated double bond at the α and β carbon positions. Its molecular formula is C10H18O2, and its relative molecular mass is 170.25. The specific structural formulas are shown in Figure 1. As an essential and uniquely structured medium-chain α, β-unsaturated fatty acid, trans-2-decenoic acid has a wide range of applications; thus, it is important to explore its synthesis method [1]. This α, β-unsaturated fatty acid is typically produced using chemical synthesis methods such as dehydrogenating established carbonyl compounds [2], aldol condensation [3], and Wittig-type [4] reactions. However, the use of various raw materials to synthesize target substances through chemical synthesis has some limitations, such as serious environmental pollution, low reaction controllability, and uncertain safety. Therefore, compared with traditional methods, the biocatalytic synthesis of target substances has attracted wide attention because of its high safety, strong specificity, and low environmental pollution [5].
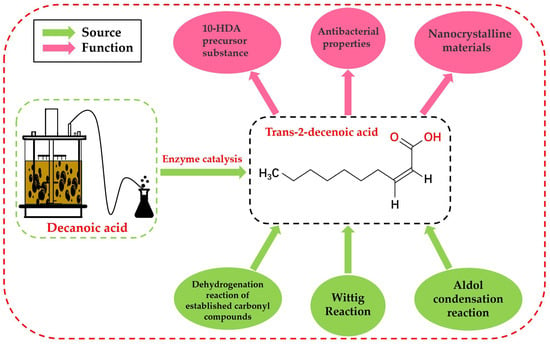
Figure 1.
Source and function of trans-2-decenoic acid.
α, β-Unsaturated fatty acids typically have an aromatic smell and are primarily found in some animal fats and some plant fruits [6]. In the biosynthesis of royal jelly acid (10-hydroxy-2-decenoic, 10-HDA), trans-2-decenoic acid can be used as a precursor for the biosynthesis of 10-HDA [7]. As a key factor in royal jelly, 10-HDA exerts antibacterial [8,9], cancer prevention and treatment, and immunity regulation effects [9]. The biosynthesis of trans-2-decenoic acid provides a basis for the further development of biosynthetic pathways of 10-HDA. Trans-2-decenoic acid can also be used as a ligand to synthesize anisotropic nanocrystalline materials. Lu et al. [10] and others confirmed that the use of organic carboxylic acid ligands strongly influences the size and morphology of synthesized one-dimensional structured nanocrystals. The growth rate is increased only when trans-2-decenoic acid is used as the ligand to produce elongated nanocrystals.
The antibacterial nature of a substance is often closely related to the specific functional groups, chain length, and substituent group position [11,12]. Marques et al. [13] reported that 2-decenoic acid is responsible for inducing a series of Gram-negative and Gram-positive bacteria and yeast, it increases microbial metabolic activity, and significantly enhances the conventional antibacterial bactericidal effect of drugs. Cai et al. [14] reported that trans-2-decenoic acid induces the disintegration of aerobic particles by stripping away the surfaces of aerobic particles, bacteria, and extracellular polymers. Makino et al. [15] found that trans-2-decenoic acid ethyl ester can be used to treat neurological diseases in mice and possesses anti-anxiety effects. These results demonstrate that trans-2-decenoic acid is a promising candidate as a novel anxiolytic with a unique mechanism of action.
The biosynthesis of fatty acids has gradually become a research hotspot [16]. Liu et al. synthesized crotonic acid through the fatty acid biosynthesis pathway in Escherichia coli strains using a specific thioesterase (bTE) derived from Bacteroides thetaiotaomicron to catalyze the hydrolysis of crotonyl-ACP to synthesize crotonyl-ACP butyric acid [17]. However, the biocatalytic synthesis of trans-2-decenoic acid has not been widely reported. The first report on the biocatalytic synthesis of decenoic acid described a metabolic pathway for the biosynthesis of crotonic acid using a modified β-oxidation pathway; 2-decenoic acid was a by-product of its metabolic pathway, and only trace amounts of 2-decenoic acid were detected in the by-products, with a titer of only 9.45 mg/L. However, the successful synthesis of 2-decenoic acid provided an important foundation for the microbial production of α, β-unsaturated fatty acids [18,19].
The biocatalytic synthesis of trans-2-decenoic acid relies on the β-oxidative decarboxylation of fatty acids [20,21]. As shown in Figure 2, Yan et al. [7] identified key enzymes involved in the β-oxidation process. These enzymes include acyl-CoA synthetase FadD, which activates fatty acids; acyl-CoA dehydrogenase FadE, which forms double bonds through dehydrogenation; and acyl-CoA thioesterase YdiI, which removes CoA from specific carbon chains. Together, these enzymes function in a dual plasmid expression system to overexpress proteins and enhance the metabolism of the β-oxidation pathway; this study laid the foundation for subsequent experiments on the fermentation method to biocatalytically convert decanoic acid into trans-2-decenoic acid. In addition, the genes encoding enoyl-CoA synthase FadB and the protein operon FadR [22] also inhibit a consumption of the intermediate product branch to catalyze the synthesis of trans-2-decenoic acid from decanoic acid [23]. Through this metabolic process, FadD (MaMACS) from Mycobacterium avium activated the substrate decanoic acid, showing the highest activity towards decanoic acid compared with that towards caprylic acid or dodecanoic acid, along with longer chain fatty acids [24]. Therefore, FadD was selected to connect CoA to the saturated fatty acid decanoic acid; the fatty acyl-CoA dehydrogenase (PpFadE) from Pseudomonas putida KT2440 was also screened. PpFadE has a broad substrate specificity; fatty acyl CoA molecules with a chain length of 14 carbon atoms are preferred [25] for catalyzing the dehydrogenation of decanoyl-CoA to generate trans-2-decenoyl-CoA [26]; lipids from Cronobacter turicensis were screened for their acyl-CoA thioesterase (CtYdiI) activity because of their strong catalytic ability for mid-chain trans-2-decenoyl-CoA, achieving a higher efficiency in hydrolyzing CoA in trans-2-decenoyl-CoA to obtain trans-2-decenoic acid [27,28].
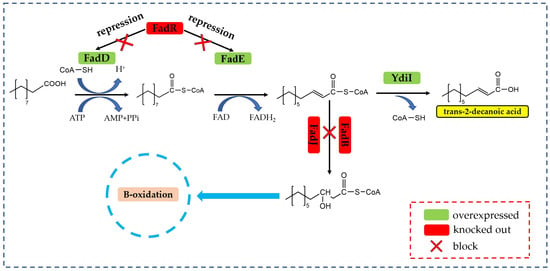
Figure 2.
Synthetic pathway of trans-2-decenoic acid. Note: FadD: acyl-CoA synthetase; FadE: acyl-CoA dehydrogenase; YdiI: acyl-CoA thioesterase; FadR: enoyl-CoA hydratase homologous protein gene; FadJ: enoyl-CoA hydratase homologous protein gene; FadB: enyl-CoA hydratase homologous protein gene.
Owing to the important role of trans-2-decenoic acid in medicine, food, health care, and other applications, the industrial production of trans-2-decenoic acid is necessary. Although microbial fermentation has been widely researched and applied in recent years because of its low cost and ease of operation, few studies have focused on medium-chain unsaturated fatty acids [29]. Therefore, we selected the engineering strain (E. coli BL21(DE3)-△fadB-△fadJ-△fadR/pET28a-sumo-CtydiI/pCDFDuet-1-MaMACS-PpfadE) that has been constructed and saved in the laboratory to design a fermentation method. The culture medium and conditions were optimized to increase the titer and achieve a high industrial production of trans-2-decenoic acid.
The response surface method can be used to determine the optimal parameters of complex systems by establishing mathematical models, regression analysis, and variance analysis [30,31]. This approach can optimize fermentation media and culture conditions [32,33]. The statistical model based on the Box–Behnken design (BBD) is a response surface design method [34]. In this experiment, we used the BBD to explore the effects of different nutrients, such as the substrate dissolution method, substrate flow amount, carbon source, and metal ions, on the synthesis of trans-2-decenoic acid. Through the preliminary screening of single-factor experiments, the key factors affecting fermentation were selected, and a fed-batch fermentation experiment was conducted in a 1 L fermentation tank to efficiently increase the fermentation titer of trans-2-decenoic acid.
2. Materials and Methods
2.1. Strains
We constructed the engineering strain (E. coli BL21(DE3)-△fadB-△fadJ-△fadR/pET28a-sumo-CtydiI/pCDFDuet-1-MaMACS-PpfadE) in a previous study [35].
2.2. Activation and Culture of Engineered Escherichia coli
2.2.1. Strain Activation
Solid LB seed culture medium was prepared and sterilized at 121 °C for 20 min. The preserved engineering strain described in Section 2.1 was streaked onto a solid LB plate and cultured at a 37 °C for 16 h.
2.2.2. Strain Culture and Preservation
A single colony strain grown on LB solid medium was added to and cultured in a 250 mL triangular flask containing 50 mL LB medium, to which, 40 μL (0.08%) streptomycin and 50 μL (0.1%) kanamycin were added. The flask was incubated in a constant-temperature shaking incubator at 37 °C (200 rpm) for 12 h. The bacterial solution was removed, aliquoted, and mixed with 50% glycerin and stored at −80 °C.
2.2.3. Type of Inducer and Timing of Addition
We chose α-lactose hydrate (≥99.5%) as the inducer. After inoculation, the bacterial cells were grown in the fermentation tank at 37 °C. When the bacterial cells OD600 = 0.8–1.2, α-lactose hydrate (≥99.5%) and the substrate decanoic acid were added to the system.
2.3. Determination of Substrates and Target Products Using Gas Chromatography
2.3.1. Sample Pretreatment
The removed sample (1 mL) was dispensed into a 2 mL tube and mixed with 1 mL of 4 M hydrochloric acid solution. The mixture was then subjected to various treatments. Firstly, it was placed in a shaking incubator (200 rpm) at 30 °C for 1 h. After that, it was reacted in a constant temperature water bath for 10 min. Subsequently, the sample was transferred to a −80 °C ultra-low temperature refrigerator for 15 min. Upon thawing, the frozen sample was divided into two parts. Each part was mixed with 750 μL of ethyl acetate in separate 2 mL tubes, which were then vigorously vortexed for 20 s to facilitate extraction. The resulting mixture was centrifuged at 8000 rpm for 10 min to separate the aqueous layer from the organic layer. This centrifugation process was repeated twice, and the supernatant from each extraction was collected. The collected samples were dried using a nitrogen blower (37 °C) and then mixed with 500 μL ethyl acetate, 500 μL n-hexane, and 100 μL DTFA-TMCS (99:1). The mixture was allowed to stand at room temperature for 5 min, followed by incubation at 70 °C for 50 min. After the final silanization step, the resulting liquid was filtered using a 0.22 μm organic filter membrane and stored in a gas chromatography vial for subsequent use.
2.3.2. Gas Chromatography Detection
Six standard samples with different concentration gradients were prepared as the substrates and products. The concentrations were 0.0625, 0.125, 0.25, 0.5, 1, and 2 g/L. A Shimadzu GC-2030 gas chromatograph (Kyoto, Japan) was used for analysis of an HP-5 chromatographic capillary column and the instrument carrier gas. The inlet conditions were set to a constant flow mode, the injection volume was 1 μL, the split injection mode was selected, the injection split ratio was maintained at 1:50, the injection temperature was 250 °C, and the constant flow rate was set to 1 mL/min. Based on the experimental procedure and product characteristics, the temperature was maintained at 50 °C for 1 min, raised to 250 °C at 15 °C/min, and held for 10 min.
2.4. Single-Factor Analysis
Based on our preliminary laboratory work, the seed culture temperature, seed liquid culture time, inoculation amount, induction temperature, substrate dissolution method, inducer concentration, substrate flow concentration, and reaction of different metal compounds were determined in single-factor experiments to determine their effects on the trans-2-decenoic acid titer and conversion rate. The seed culture temperatures were 25 °C, 28 °C, 30 °C, 33 °C, and 37 °C; the seed liquid culture times were 8, 10, 12, 16, and 20 h; the inoculum amounts were 1%, 3%, 5%, 8%, and 10%; the induction temperatures were 20 °C, 25 °C, 28 °C, 30 °C, and 37 °C; absolute ethanol, Tween-80, decane, N, N-dimethylformamide, and dimethyl sulfoxide were used as organic solvents to dissolve decanoic acid [36,37,38]; the substrate flow concentrations were 0, 0.1, 0.2, 0.3, and 0.4 g/L; and the metal compounds [38,39] were 0.1 mM MnCl2, CaCl2, MgCl2·6H2O, ZnCl2, and CuCl2.
2.5. Box–Behnken Design (BBD)
We used a BBD to design and optimize fermentation conditions and adopted a three-factor and three-level experimental method. Based on the results of previous single-factor experiments, three factors that significantly impacted the production of trans-2-decenoic acid (substrate flow concentration, inducer concentration, and MnCl2) were selected, as shown in Table 1. The response surface method was used to evaluate the fermentation conditions. Optimization was performed to determine the optimal fermentation conditions.

Table 1.
Coded and actual values of factors in Box–Behnken design.
2.6. Comparison of Expression of E. coli Transporter Proteins before and after Optimization
Through the complex metabolic pathways in E. coli, the substrate decanoic acid not only participates in the biosynthetic pathway but also partially participates in the metabolic cycle. We removed fatty acids of specific chain lengths from CoA by enhancing YdiI, FadD, and FadE expression levels to promote the β-oxidation reaction, which promoted the large-scale synthesis of product intermediates and increased the production of trans-2-decenoic acid. A series of catalytic fermentation reactions were performed to determine which recombinant engineered bacteria were successfully constructed, and the protein expression levels before and after optimization were compared. The expression levels of FadD, FadE, and YdiI were used to determine whether the protein expression was significantly improved after optimizing the fermentation process and in order to verify the fermentation optimization results.
3. Results
3.1. Single-Factor Analysis
3.1.1. Effect of Seed Culture Temperature on Trans-2-Decenoic Acid Production
The quality of the seed culture is crucial in the fermentation process. To ensure desirable characteristics like balanced cell growth, uniform bacteria composition, active enzyme system, and strong metabolism, it is important to select an appropriate temperature for cultivating the seed. Deviating from the optimal temperature range can negatively impact cell growth, plasmid stability, and product potency. As shown in Figure 3A, in terms of the seed culture temperature, the titer of trans-2-decenoic acid was highest when the seeds were cultured at 37 °C, with a titer of 1.01 ± 0.03 g/L, which is consistent with the optimal temperature for E. coli growth; therefore, we considered 37 °C as the optimal seed cultivation temperature.
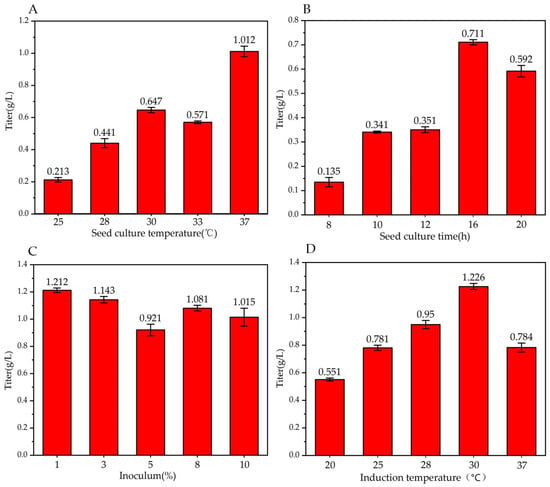
Figure 3.
Effect of different factors on trans-2-decenoic acid production. Fermentation for 72 h. (A) seed culture temperature, control, and other fermentation conditions; seed culture time is 12 h, inoculum is 5%, induction temperature is 37 °C, solvent is ethanol, fed concentration is 0, and inducer concentration is 5 g/L without metal compounds. (B) Seed culture time, control, and other fermentation conditions: Seed culture temperature is 37 °C, inoculum is 5%, induction temperature is 37 °C, solvent is ethanol, fed concentration is 0, and inducer concentration is 5 g/L without metal compounds. (C) Inoculum, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, induction temperature is 37 °C, solvent is ethanol, fed concentration is 0, inducer concentration is 5 g/L without metal compounds. (D) Induction temperature, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, inoculum is 1%, solvent is ethanol, fed concentration is 0, and inducer concentration is 5 g/L without metal compounds.
3.1.2. Effect of Seed Culture Time on Trans-2-Decenoic Acid Production
Seed activity plays a decisive role in reaction systems. Strains in the exponential phase are typically used for the reaction; therefore, a reasonable seed growth time is a key factor in the reaction efficiency. As shown in Figure 3B, when the seed culture time was 16 h, the titer of trans-2-decenoic acid was highest at 0.711 ± 0.011 g/L. Therefore, 16 h was used as the optimal seed culture time.
3.1.3. Effect of Inoculation Amount on Trans-2-Decenoic Acid Production
A low inoculation level can lead to slow bacteria growth and low metabolic efficiency. A high inoculation level can inhibit the enzyme production in bacteria, decreasing the metabolic reaction efficiency. As shown in Figure 3C, when the inoculation amount was 1%, the titer of the trans-2-decenoic acid was highest at 1.212 ± 0.017 g/L. Therefore, we selected 1% as the optimal inoculation amount.
3.1.4. Effect of Induction Temperature on Trans-2-Decenoic Acid Production
The key to this reaction is using inducers of bacterial enzyme production. An appropriate induction timing and temperature can increase the accuracy and efficiency of enzyme production. Early induction may result in a metabolic burden related to protein overexpression in the host strains. Cell product formation occurs at high cell densities after the exponential phase. However, after this phase, the metabolic state of cells may not be conducive to protein expression because of the stressful conditions, triggering reactions that increase protease levels [40,41]. Li et al. [35] used whole cell catalysis production experiments to select an OD600 = 0.8–1.2 range for induction; as shown in Figure 3D. When the induction temperature was 30 °C, the trans-2-decenoic acid titer was highest at 1.226 ± 0.022 g/L. Thus, we chose 30 °C as the optimal temperature.
3.1.5. Effects of Different Solvents Dissolving Substrates on Production of Trans-2-Decenoic Acid
Decanoic acid crystals are white with a unique odor. They are insoluble in water and soluble in most organic solvents. Thus, it is particularly important to choose an organic solvent that brings decanoic acid into the reaction system and allows it to fully react. As shown in Figure 4A, in the reactions performed using five different solvents, the titer of trans-2-decenoic acid was highest when absolute ethanol was used, showing a value of 1.28 ± 0.03 g/L. This result may be attributed to the unique characteristics of absolute ethanol; the bactericidal properties reduce the risk of bacterial contamination, and the high compatibility of the decanoic acid substrate with absolute ethanol ensures that the substrate can carry out the transformation and fermentation reactions normally. Therefore, we used absolute ethanol as the solvent to dissolve the substrate.
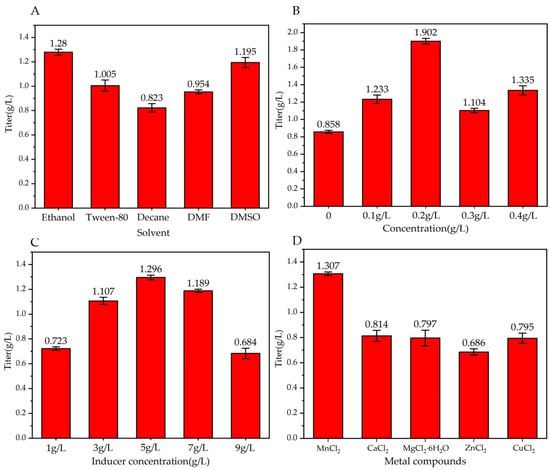
Figure 4.
Effect of different factors on trans-2-decenoic acid production. Fermentation for 72 h. (A) solvent, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, inoculum is 1%, induction temperature is 30 °C, fed concentration is 0, and inducer concentration is 5 g/L without metal compounds. (B) Fed concentration, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, inoculum is 1%, induction temperature is 30 °C, solvent is ethanol, and inducer concentration is 5 g/L without metal compounds. (C) Inducer concentration, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, inoculum is 1%, induction temperature is 30 °C, solvent is ethanol, and fed concentration is 0.2 g/L without metal compounds. (D) Metal compound, control, and other fermentation conditions; seed culture temperature is 37 °C, seed culture time is 16 h, inoculum is 1%, induction temperature is 30 °C, solvent is ethanol, fed concentration is 0.2 g/L, and inducer concentration is 5 g/L.
3.1.6. Effect of Feeding Different Concentrations of Substrate on Production of Trans-2-Decenoic Acid
To ensure continuous trans-2-decenoic acid production, the product production and substrate consumption must maintain a high dynamic equilibrium; therefore, the substrate consumption rate should be considered during the fermentation process. Continuity of the fermentation process was ensured via the fed substrate. As shown in Figure 4B, the titer of trans-2-decenoic acid was highest when the fed concentration was 0.2 g/L, showing a value of 1.902 ± 0.032 g/L. Therefore, a fed concentration of 0.2 g/L was used as the bottom stream plus the concentration.
3.1.7. Effect of Inducer Concentration on Trans-2-Decenoic Acid Production
The concentration of the inducer is an important factor affecting the expression of recombinant proteins from inducible promoters. If the concentration is too low, the amount of inducer may be insufficient to bind to the repressor protein, reducing the expression level. If the concentration of the inducer is too high, the bacteria may be damaged. Growth is limited and inclusion bodies are produced if the recombinant protein is expressed too quickly; therefore, it is particularly important to choose an appropriate inducer concentration. As shown in Figure 4C, when the inducer concentration was 5 g/L, the titer of trans-2-decenoic acid was highest at 1.296 ± 0.019 g/L. Thus, 5 g/L was considered as the optimal inducer concentration.
3.1.8. Effects of Adding Different Metal Ions to the Culture Medium on the Production of Trans-2-Decenoic Acid
Mn2+ is crucial for enhancing the activities of various enzymes involved in sugar metabolism and nucleotide processing. It also facilitates the adenylation of amino acids in antibiotics, a prerequisite for producing many peptide antibiotics. Consequently, the Mn2+ content significantly influences the population size of organisms and the titer of products. However, adding Mg2+ boosts the activity of essential enzymes such as hexose phosphorylase, citrate dehydrogenase, and carboxylase. This, in turn, affects processes such as matrix oxidation and protein synthesis. Additionally, Mg2+ is necessary for the proper functioning of most enzymes that act on nucleotides. Mn2+ enhances the tolerance of certain antibiotic-producing strains to the generated antibiotics. As shown in Figure 4D, trans-2-decenoic acid production was highest when MnCl2 was added to the reaction system, reaching 1.307 ± 0.014 g/L. Therefore, we incorporated MnCl2 into the culture medium for further experiments.
3.2. Response Surface Testing
A BBD was used based on a single-factor experiment, with the flow rate, inducer concentration, and MnCl2 concentration as independent variables. The response value was the titer of trans-2-decenoic acid. The results are presented in Table 2. Multiple regression analysis was performed on Table 2 using Design Expert 8.0 software (Minneapolis, MN, USA) to obtain the regression equation.
Y = +1.77 + 0.19A + 0.032B − 0.059C − 0.017AB + 0.028AC − 0.026BC − 0.083A2 − 0.090B2 + 0.066C2

Table 2.
Response surface test design and results.
Seventeen experiments were performed using three independent variables at three levels (−1, 0, and +1). Table 2 lists the results of the 17 experiments, which were analyzed using Design Expert 8.0 software (Minneapolis, MN, USA). Analysis of variance was used to test the statistical significance of the quadratic regression equations (Table 3). The p-value of the regression model was <0.0001, indicating that the regression equation obtained using this model was extremely significant. The p-value for the missing FT was 0.2998, and the difference was not significant. The regression coefficient (R2) was used to determine the model fitting efficiency. R2 and the corrected quadratic model were 0.9963 and 0.9916, respectively, which were very close to 1. Thus, the model fits the data well, indicating that 99% of the variation in response can be described by this polynomial model.

Table 3.
Response surface regression model analysis of variance.
This equation explained more than 99.63% of the experimental values. Each factor exerted a different effect on trans-2-decenoic acid production. The factors influenced production in the order of A (<0.0001) > C (<0.0001) > B (0.0005). The interaction effects among AB, AC, and BC were not significant. Items affected by second factors, A2, B2, and C2, showed an extremely significant impact (p < 0.001).
3.3. Response Surface Interaction
To further examine the effects of the substrate feeding concentration, inducer concentration, and MnCl2 concentration on the production of trans-2-decenoic acid, the response surface and contours were drawn according to the regression equation, as shown in Figure 5. A steeper curve trend indicates a stronger the interaction and greater impact on trans-2-decenoic acid production. The contour plot intuitively reflects the significance of the interactions between various factors. As any two variables increased, the production of trans-2-decenoic acid also increased. After the interaction between the two reached a peak, the surface area decreased. The interaction between the substrate-feeding concentration and inducer concentration showed the highest significance among the factors evaluated, with the steepest curve and an elliptical contour.
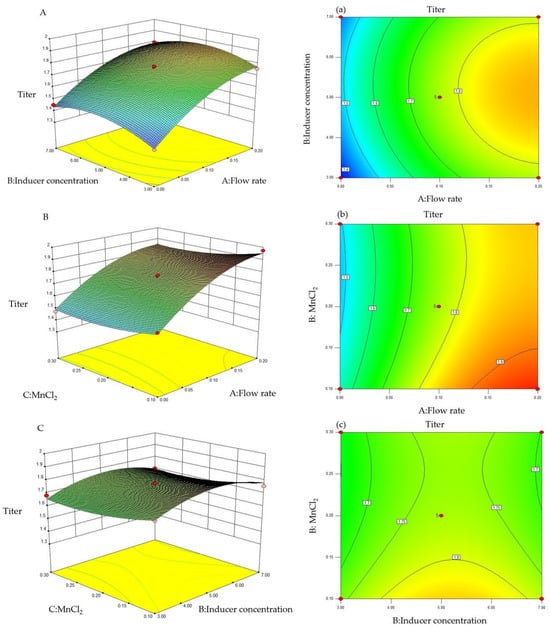
Figure 5.
Response surface and contour plots for trans-2-decenoic acid production. (A,a) Effect of flow rate and inducer concentration. (B,b) Effect of flow rate and MnCl2 concentration. (C,c) Effect of inducer concentration and MnCl2.
3.4. Response Surface Results’ Optimization
After the response surface analysis, the optimal fermentation conditions were considered as a substrate flow addition amount of 0.2 g/L, an inducer concentration of 4.72 g/L, and an MnCl2 concentration of 0.15 mM. Fermentation verification was performed in a 1 L fermentation tank under optimal conditions in three sets of parallel experiments in Table 4.

Table 4.
Optimal fermentation conditions.
By analyzing the regression model equation, we determined that the optimal process conditions for trans-2-decenoic acid were a substrate flow addition amount of 0.14 g/L, an inducer concentration of 5.63 g/L, and an MnCl2 concentration of 0.11 mM. Under these conditions, the titer of trans-2-decenoic acid was 1.9 g/L. The actual operation feasibility was improved, and the fermentation process was determined as follows: an inoculum amount of 1%, fermentation temperature of 30 °C, seed liquid culture time of 20 h, substrate flow addition amount of 0.15 g/L, inducer concentration of 5.60 g/L, and MnCl2 concentration of 0.10 mm. Three repeated experiments were performed under these conditions, and the results are shown in Figure 6B. The average titer of trans-2-decenoic acid after optimization was 1.982 ± 0.110 g/L, which differs from the theoretical value by 0.082 g/L, indicating that the model is reliable and that the regression equation can be used for fermentative production via a biocatalytic synthesis of trans-2-decenoic acid from decanoic acid.
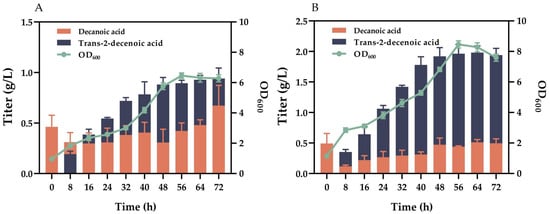
Figure 6.
Comparison of trans-2-decenoic acid production and OD600 before and after optimization. (A) Titer and OD600 before optimization. (B) Titer and OD600 after optimization.
We also compared trans-2-decenoic acid production before and after fermentation optimization. Production using LB medium before optimization was 0.940 ± 0.104 g/L (Figure 6A), whereas this value was 1.982 ± 0.110 g/L (Figure 6B) after optimization. Optimization increased the production of trans-2-decenoic acid by 1.04 g/L. We also monitored the OD600 during fermentation. As shown in Figure 6A, the highest OD600 before optimization was 6.46. After optimization, the maximum OD600 was 8.46 (Figure 6B). Optimization promoted the growth of bacteria in the reaction system.
3.5. Comparison of SDS-PAGE Protein Expression in Engineered E. coli before and after Optimization
The samples were comparatively analyzed before and after the optimization of trans-2-decenoic acid fermentation using sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Lane M represents the protein marker, and lanes 1, 2, and 3 are three sets of parallel bands in Figure 7A. Figure 7B shows a protein gel image of the fermentation broth sample under optimal fermentation conditions; the protein size of the FadD was 59 kDa, FadE was 45 kDa, and YdiI was 30 kDa. A comparison of the two images showed that the protein levels of the three bands in Figure 7A were significantly lower than those in Figure 7B. These results demonstrate that protein expression in the reaction system was higher after fermentation optimization compared to before optimization, indicating that the improved reaction system was more efficient.
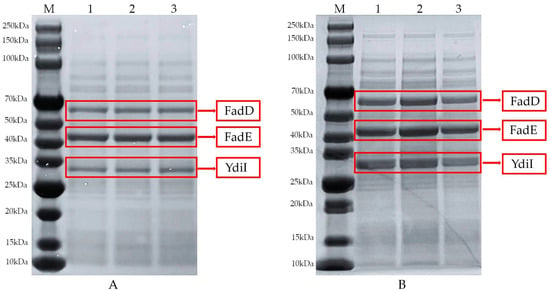
Figure 7.
Comparison of protein expression in samples before and after optimization. (A) Protein expression before optimization. (B) Protein expression after optimization.
4. Discussion
Based on the E. coli BL21(DE3)-△fadB-△fadJ-△fadR/pET28a-sumo-CtydiI/pCDFDuet-1-MaMACS-PpfadE) designed previously, we used the biological fermentation method to catalyze the synthesis of trans-2-decenoic acid from decanoic acid. The reaction system was placed in a fermentation tank for amplification. Based on the aerobic characteristics of E. coli, better ventilation and stirring of the fermentation tank were used to improve the reaction system. The substrate concentration and inoculum volume were controlled at 0.5 g/L and 1%, respectively, to enable efficient bacteria growth in the system and to maintain strong metabolic activity to increase biocatalytic reactions [35].
For reaction optimization, we conducted eight single-factor experiments to evaluate the seed culture temperature, seed liquid culture time, inoculum amount, induction temperature, solvent type, feed concentration, inducer concentration, and metal compound type. Based on the influence of different factors on the production of trans-2-decenoic acid, the three factors with the greatest influence on trans-2-decenoic acid were identified as the response variables: feed, inducer, and MnCl2 concentrations. The BBD was used to optimize the fermentation conditions. These three factors influenced trans-2-decenoic acid production in the following order of significance: fed concentration > inducer concentration > MnCl2. After optimizing each factor, the predicted value of the optimal fermentation conditions was 1.9 g/L, fed concentration was 0.14 g/L, inducer concentration was 5.63 g/L, and MnCl2 concentration was 0.11 mM. We conducted three repeated experiments based on the optimized optimal conditions and found that the titer of trans-2-decenoic acid was 1.982 ± 0.110 g/L, which was 1.042 g/L higher than that in the LB medium before optimization. Using a substrate concentration of 0.5 g/L, the titer was 0.479 g/L higher than the average titer achieved by Li et al. [35]. The value was increased by 1.503 ± 0.110 g/L. Optimizing the fermentation conditions significantly affected trans-2-decenoic acid production.
During the catalytic reaction of engineered E. coli, fadD encodes acyl-CoA synthase, which has broad substrate specificity and catalyzes the conversion of free fatty acids into highly metabolically active coenzyme A thioesters. Through the action of CoA synthase, free fatty acids form fatty acyl-CoAs with high metabolic activity [42,43,44]. Subsequently, FadE catalyzes the oxidation of acyl-CoA to trans-2-enoyl CoA [27] and then removes CoA to form α, β-unsaturated fatty acids through the catalysis of acyl-CoA thioesterase YdiI. Therefore, the three enzymes FadD, FadE, and YdiI play vital roles in this reaction process. While optimizing the fermentation conditions, we explored the changes in the expression levels of the three enzymes before and after fermentation. The bands in the optimized samples were more apparent than those in the unoptimized samples, confirming the necessity of optimizing the fermentation conditions.
One issue that arose during fermentation was that the amount of trans-2-decenoic acid did not increase in a regular or stable manner. In the early stages of the reaction, the level of trans-2-decenoic acid gradually increased. As the reaction continued, the levels of products and substrates gradually decreased or were not present. This may be because of the continuous growth and metabolism of the bacteria during the reaction process, which required a large amount of nutrients, including carbon sources. Various substances, such as nitrogen-containing substances and biotin, are gradually consumed or even exhausted as bacterial cells grow, after which the culture medium cannot provide sufficient nutrients to the cells. Therefore, bacterial cells may have been preferentially consumed at this stage. Substrates and products were used as carbon sources to ensure growth and metabolic activity. A more accurate and efficient plan is needed to add nutrients and energy during the reaction process to maintain a stable biological reaction while also considering that long-term fermentation leads to bacterial aging. The aging of bacterial cells decreases enzyme production because the metabolic capacity is decreased. Therefore, after the reaction has been allowed to proceed for a certain period, new culture media must be added to dilute the bacterial cells and allow for the growth of additional bacterial cells.
Due to the various drawbacks of the chemical synthesis of α, β-unsaturated fatty acids currently available on the market, their biosynthesis has gradually gained attention, and alternatives to traditional chemical methods have been explored. There have been few studies of trans-2-decenoic acid. This limitation is reflected in the instability during the fermentation process and low conversion rate and titer; the efficient mass production of trans-2-decenoic acid in an industrial environment remains difficult. At present, the synthesis of trans-2-decenoic acid relies mainly on chemical synthesis; however, this method involves a long process, has low reaction controllability, and can easily cause environmental pollution. Therefore, biosynthetic methods with high safety, strong specificity, and low environmental pollution to catalyze the synthesis of trans-2-decenoic acid are needed.
5. Conclusions
We used engineered E. coli constructed in the laboratory and decanoic acid as a substrate for biological fermentation to catalyze the synthesis of trans-2-decenoic acid from decanoic acid. First, the peak times of decanoic acid and trans-2-decenoic acid production were determined. Three factors showing the greatest impact on the titer of trans-2-decenoic acid were identified. We examined the feed concentration, inducer concentration, and MnCl2 concentration for further optimization. Our results showed that the optimal process conditions for trans-2-decenoic acid are a substrate flow addition of 0.14 g/L, an inducer concentration of 5.63 g/L, and an MnCl2 concentration of 0.11 mm (1 L under these conditions). Under these fermentation tank conditions, 1.98 g/L trans-2-decenoic acid was produced. Compared with that in LB medium before optimization, production was increased by 1.04 g/L; compared with trans-2-decenoic acid, the output of -2-decenoic acid increased by 1.501 g/L. Finally, we compared protein expression levels before and after optimization; enzyme expression in the optimized samples was significantly higher than that in the non-optimized samples. We improved the production of trans-2-decenoic acid and provide an important reference for further studies of biosynthetic fatty acid technology.
Author Contributions
Conceptualization, P.L. and J.S.; methodology, S.N.; software, S.N., K.L. and B.L.; validation, P.L. and J.S.; formal analysis, S.N. and K.L.; investigation, S.N., P.L. and J.S.; writing—original draft preparation, S.N.; writing—review and editing, S.N., P.L. and J.S.; supervision, P.L. and J.S.; project administration, P.L. and J.S.; funding acquisition, P.L. and J.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by The Science Foundation of Shandong Province (grant no. ZR2023MC089), the Focuson Research and Development Plan in Shandong Province (grant no.2023CXGC010714) and Qilu University of Technology (Shandong Academy of Sciences) (grant no 2022JBZ01-06).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are contained within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, Q.J.; Tochtrop, G.P. New methodology toward α,β-unsaturated carboxylic acids from saturated acids. J. Org. Lett. 2014, 16, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Hirao, T. Synthetic Strategy: Palladium-Catalyzed Dehydrogenation of Carbonyl Compounds. J. Org. Chem. 2019, 84, 1687–1692. [Google Scholar] [CrossRef] [PubMed]
- Perrin, C.L.; Chang, K.L. The complete mechanism of an aldol condensation. J. Org. Chem. 2016, 81, 5631–5635. [Google Scholar] [CrossRef] [PubMed]
- Farfan, P.; Gomez, S.; Restrepo, A. Dissection of the mechanism of the Wittig reaction. J. Org. Chem. 2019, 84, 14644–14658. [Google Scholar] [CrossRef]
- Bonnard, I.; Rolland, M.; Salmon, J.M.; Debiton, E.; Barthomeuf, C.; Banaigs, B. Total structure and inhibition of tumor cell proliferation of laxaphycins. J. Med. Chem. 2017, 50, 1266–1279. [Google Scholar] [CrossRef]
- Wang, L.H.; He, Y.; Gao, Y.; Wu, J.E.; Dong, Y.H.; He, C.; Wang, S.X.; Weng, L.X.; Xu, J.L.; Tay, L.; et al. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol. Microbiol. 2004, 51, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Wang, F.; Wang, L.; Wang, L.; Xu, Z.; Yuan, H.; Yang, X.; Li, P.; Su, J.; et al. Production of 10-hydroxy-2-decenoic acid from decanoic acid via whole-cell catalysis in engineered Escherichia coli. ChemSusChem 2022, 15, e202102152. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.P.; Moraes, L.R.; Ferreira, N.U.; Moreno, G.; Uahib, F.M.; Barizon, E.A.; Berretta, A.A. The lyophilization process maintains the chemical and biological characteristics of royal jelly. Evid. Based Complement. Alter. Med. 2015, 2015, 825068. [Google Scholar] [CrossRef]
- Townsend, G.F.; Morgan, J.F.; Hazlett, B. Activity of 10-hydroxydecenoic acid from royal jelly against experimental leukemia and ascitic tumours. Nature 1959, 183, 1270–1271. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Yu, N.; Liu, Z.; Gu, J.; Lu, J.; Ma, W. Electronic supplementary information facile synthesis of ultra-small pbse nanorods for photovoltaic application. Nanoscale 2015, 7, 2461–2470. [Google Scholar] [CrossRef]
- Pavel, A.C.I.; Mărghitaş, L.; Bobiş, O.; Mădaş, M.N. Lucrari Stiintifice. Biological activities of royal jelly—Review. Zootehnie Biotecnol. 2011, 44, 1. [Google Scholar]
- Koh, W.K.; Bartnik, A.C.; Wise, F.W.; Murray, C.B. Synthesis of monodisperse pbse nanorods: A case for oriented attachment. J. Am. Chem. Soc. 2010, 132, 3909–3913. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.N.; Davies, D.G.; Sauer, K. Control of biofilms with the fatty acid signaling molecule cis-2-decenoic acid. Pharmaceuticals 2015, 8, 816–835. [Google Scholar] [CrossRef]
- Cai, P.J.; Xiao, X.; He, Y.R.; Li, W.W.; Yu, L.; Yu, H.Q. Disintegration of aerobic granules induced by trans-2-decenoic acid. Bioresour. Technol. 2013, 128, 823–826. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Iinuma, M.; Fukumitsu, H. Anxiolytic-like effect of trans-2-decenoic acid ethyl ester in stress-induced anxiety-like model mice. Biomed. Res. 2013, 34, 259–267. [Google Scholar] [CrossRef]
- Liu, X.; Yu, H.; Jiang, X.; Ai, G.; Yu, B.; Zhu, K. Biosynthesis of butenoic acid through fatty acid biosynthesis pathway in Escherichia coli. Appl. Microbiol. Biotechnol. 2015, 99, 1795–1804. [Google Scholar] [CrossRef]
- Sarkar, A.; Middya, T.R.; Jana, A.D. A qsar study of radical scavenging antioxidant activity of a series of flavonoids using dft based quantum chemical descriptors—the importance of group frontier electron density. J. Mol. Model. 2012, 6, 2621–2631. [Google Scholar] [CrossRef]
- Wei, D.X.; Dao, J.W.; Chen, G.Q. A micro-ark for cells: Highly open porous polyhydroxyalkanoate microspheres as injectable scaffolds for tissue regeneration. Adv. Mater. 2018, 31, e1802273. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Buijs, N.A.; Zhu, Z.; Qin, J.; Siewers, V.; Nielsen, J. Production of fatty acid-derived oleochemicals and biofuels by synthetic yeast cell factories. Nat. Commun. 2016, 7, 11709. [Google Scholar] [CrossRef]
- Kim, S.; Cheong, S.; Gonzalez, R. Engineering Escherichia coli for the synthesis of short- and medium-chain α,β-unsaturated carboxylic acids. Metab. Eng. 2016, 36, 90–98. [Google Scholar] [CrossRef]
- Cronan, J.E., Jr.; Rock, C.O. Biosynthesis of membrane lipids. EcoSal Plus 2008, 3, a004713. [Google Scholar] [CrossRef]
- Jawed, K.; Mattam, A.J.; Fatma, Z.; Wajid, S.; Abdin, M.Z.; Yazdani, S.S. Engineered production of short chain fatty acid in Escherichia coli using fatty acid synthesis pathway. PLoS ONE 2016, 11, e0160035. [Google Scholar] [CrossRef] [PubMed]
- Iram, S.H.; Cronan, J.E. The beta-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J. Bacteriol. 2006, 188, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Krupa, R.A.; Zhang, F.; Hajimorad, M.; Holtz, W.J.; Prasad, N.; Lee, S.K.; Keasling, J.D. Bglbrick vectors and datasheets: A synthetic biology platform for gene expression. J. Biol. Eng. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Morsczeck, C.; Berger, S.; Plum, G. The macrophage-induced gene (mig) of mycobacterium avium encodes a medium-chain acyl-coenzyme a synthetase—sciencedirect. Biochim. Biophys. Acta 2001, 1521, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Guzik, M.W.; Narancic, T.; Ilic-Tomic, T.; Vojnovic, S.; Kenny, S.T.; Casey, W.T.; Duane, G.F.; Casey, E.; Woods, T.; Babu, R.P.; et al. Identification and characterization of an acyl-CoA dehydrogenase from Pseudomonas putida KT2440 that shows preference towards medium to long chain length fatty acids. Microbiology 2014, 160, 1760–1771. [Google Scholar] [CrossRef]
- Campbell, J.W.; Cronan, J.E. The enigmatic Escherichia coli fade gene is yafh. J. Bacteriol. 2002, 184, 3759–3764. [Google Scholar] [CrossRef]
- Poirier, Y.; Antonenkov, V.D.; Glumoff, T.; Hiltunen, J.K. Peroxisomal beta-oxidation--a metabolic pathway with multiple functions. Biochim. Biophys. Acta 2006, 1763, 1413–1426. [Google Scholar] [CrossRef]
- Yan, Q.; Simmons, T.R.; Cordell, W.T.; Hernández Lozada, N.J.; Breckner, C.J.; Chen, X.; Jindra, M.A.; Pfleger, B.F. Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone, and 2-undecanone. Metab. Eng. 2020, 61, 335–343. [Google Scholar] [CrossRef]
- Tao, A.; Feng, X.; Sheng, Y.; Song, Z. Optimization of the Artemisia polysaccharide fermentation process by Aspergillus niger. Front. Nutr. 2022, 9, 842766. [Google Scholar] [CrossRef]
- Kuo, C.H.; Hsiao, F.W.; Chen, J.H.; Hsieh, C.W.; Liu, Y.C.; Shieh, C.J. Kinetic aspects of ultrasound-accelerated lipase catalyzed acetylation and optimal synthesis of 4′-acetoxyresveratrol. Ultrason. Sonochem. 2013, 20, 546–552. [Google Scholar] [CrossRef]
- Gu, F.; Xu, F.; Tan, L.; Wu, H.; Chu, Z.; Wang, Q. Optimization of enzymatic process for vanillin extraction using response surface methodology. Molecules 2012, 17, 8753–8761. [Google Scholar] [CrossRef]
- Wang, Y.H.; Xuan, Z.H.; Tian, S.; Du, G.H. Echinacoside protects against 6-hydroxydopamine-induced mitochondrial dysfunction and inflammatory responses in PC12 cells via reducing ROS production. Evid. Based Complement. Altern. Med. 2015, 2015, 189239. [Google Scholar] [CrossRef] [PubMed]
- Peiran, L.; Ying, L.; Mingzhuo, Z.; Ye, Y.; Xiuming, C. The development of a Panax notoginseng medicinal liquor processing technology using the response surface method and a study of its antioxidant activity and its effects on mouse melanoma B16 cells. Food Funct. 2017, 8, 4251–4264. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, L.; Wang, R.; Wang, Z.; Wang, J.; Yuan, H.; Su, J.; Li, J.; Ynag, S.; Han, T. Efficient biosynthesis of 10-hydroxy-2-decenoic acid using a nad(p)h regeneration p450 system and whole-cell catalytic biosynthesis. ACS Omega 2022, 7, 17774–17783. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.J.; Shin, K.C.; Oh, D.K. Production of 10-hydroxy-12,15(z,z)-octadecadienoic acid from α-linolenic acid by permeabilized cells of recombinant Escherichia coli expressing the oleate hydratase gene of stenotrophomonas maltophilia. Biotechnol. Lett. 2013, 35, 1487–1493. [Google Scholar] [CrossRef]
- Jung, D.H.; Jung, J.H.; Seo, D.H.; Ha, S.J.; Kweon, D.K.; Park, C.S. One-pot bioconversion of sucrose to trehalose using enzymatic sequential reactions in combined cross-linked enzyme aggregates. Bioresour. Technol. 2013, 130, 801–804. [Google Scholar] [CrossRef]
- Rajendran, V.; Simab, K.; Aran, I. Non-ionic surfactant integrated extraction of exopolysaccharides from engineered Synechocystis sp. PCC 6803 under fed-batch mode facilitates the sugar-rich syrup production for ethanol fermentation. Algal Res. 2022, 66, 102772. [Google Scholar]
- Mackenzie, S.; Zachary, W.; Lauren, W. Manganese homeostasis in bacteria: Interaction of the small protein MntS and manganese exporter MntP in E. coli. FASEB J. 2022, 36. [Google Scholar] [CrossRef]
- Liszkowska, W.; Berlowska, J. Yeast fermentation at low temperatures: Adaptation to changing environmental conditions and formation of volatile compounds. Molecules 2021, 26, 1035. [Google Scholar] [CrossRef]
- Carneiro, S.; Ferreira, E.C.; Rocha, I. Metabolic responses to recombinant bioprocesses in Escherichia coli. J. Biotechnol. 2013, 164, 396–408. [Google Scholar] [CrossRef]
- Ford, T.J.; Way, J.C. Enhancement of E. coli acyl-coa synthetase fadd activity on medium chain fatty acids. PeerJ 2015, 3, e1040. [Google Scholar] [CrossRef]
- Black, P.N.; Dirusso, C.C.; Metzger, A.K.; Heimert, T.L. Cloning, sequencing, and expression of the fadd gene of 2scherichia coli encoding acyl coenzyme a synthetase. J. Biol. Chem. 1992, 267, 25513–25520. [Google Scholar] [CrossRef]
- Kameda, K.; Nunn, W.D. Purification and characterization of acyl coenzyme a synthetase from Escherichia coli. J. Biol. Chem. 1981, 256, 5702–5707. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).