Biotechnological Valorization of Cupuaçu By-Products: Solid-State Fermentation for Lipase Production by Yarrowia lipolytica
Abstract
1. Introduction
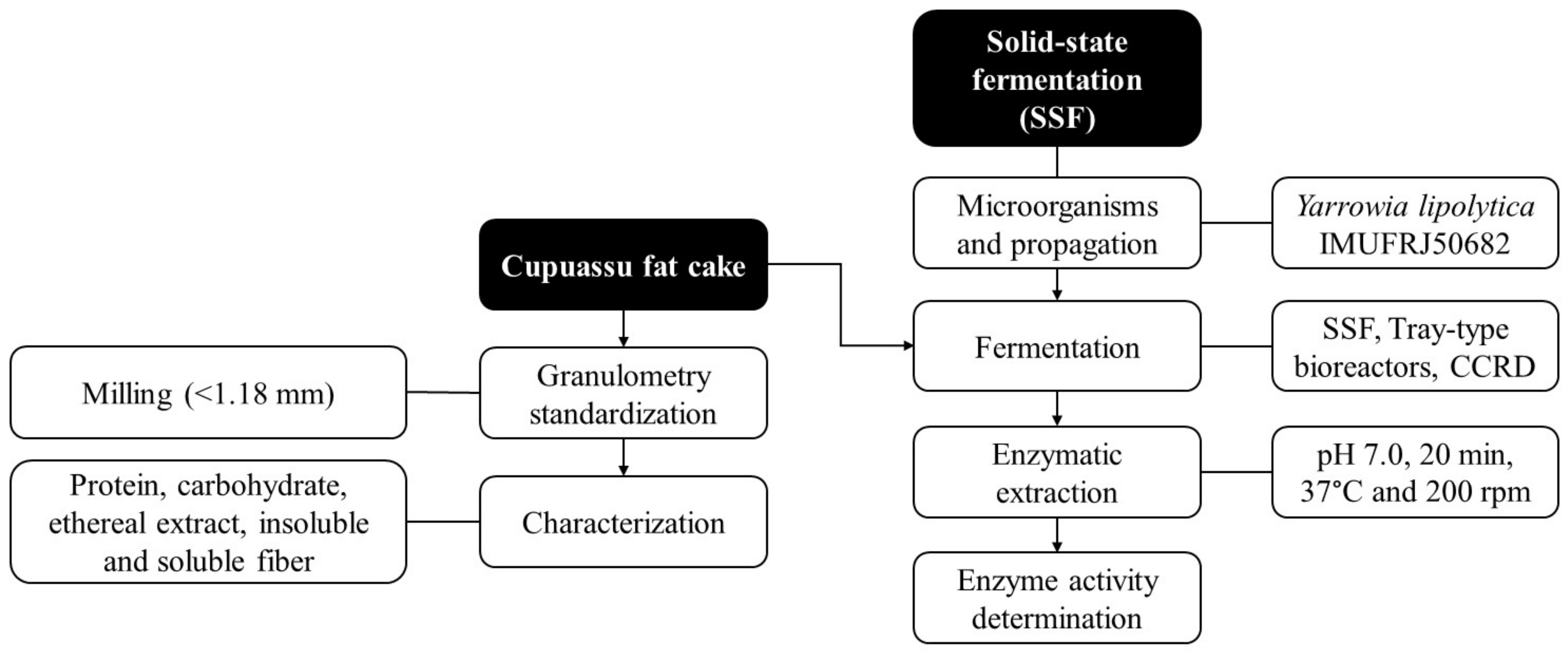
2. Materials and Methods
2.1. Materials
2.2. Characterization of Cupuaçu Fat Cake
2.3. Microorganisms and Propagation
2.4. Solid-State Fermentation
2.5. Central Composite Rotational Design
2.6. Enzyme Extraction
2.7. Screening for Lipase Activity
2.7.1. Spectrophotometric Assay
2.7.2. Titrimetric Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cupuaçu Fat Cake
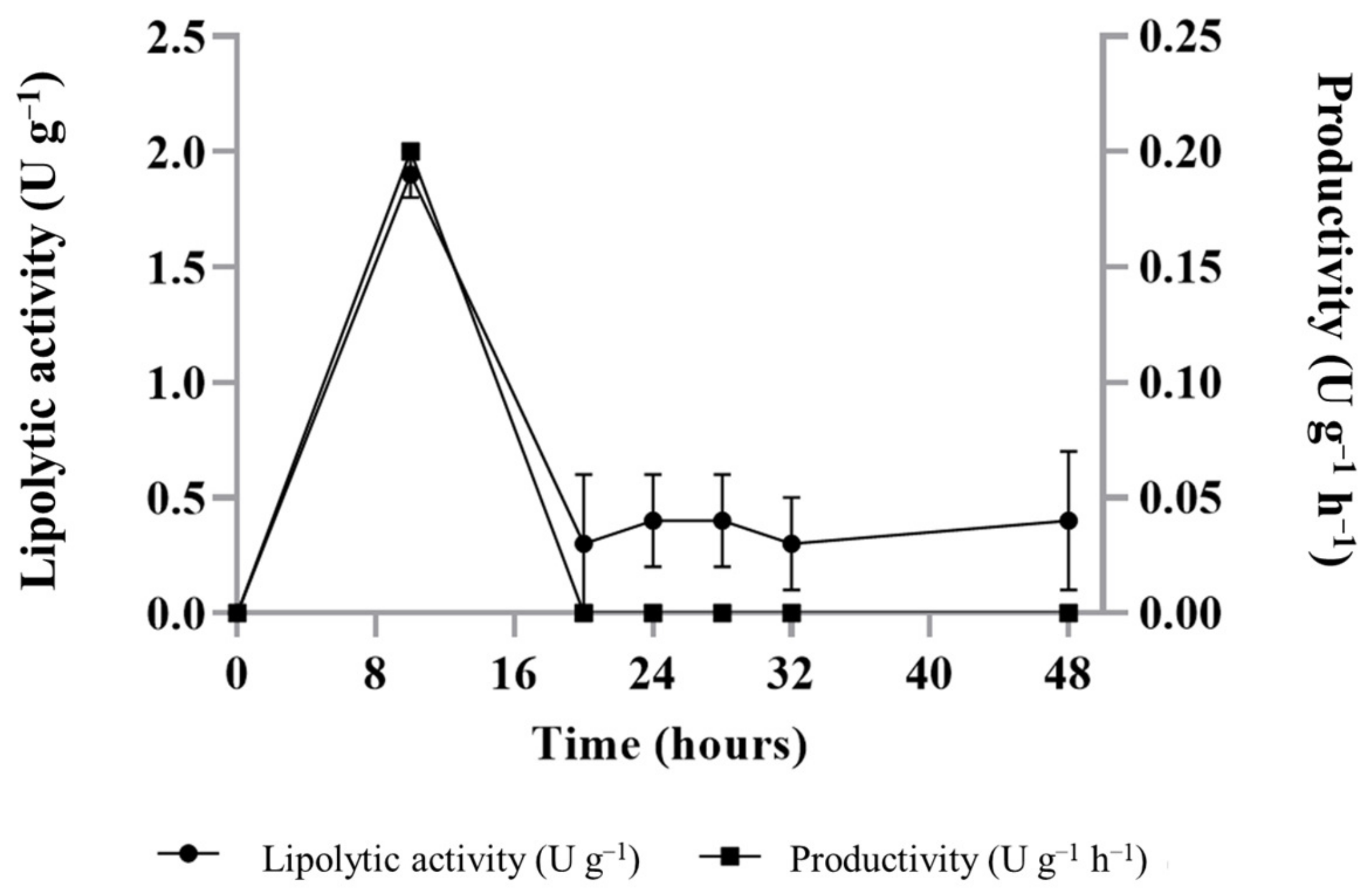
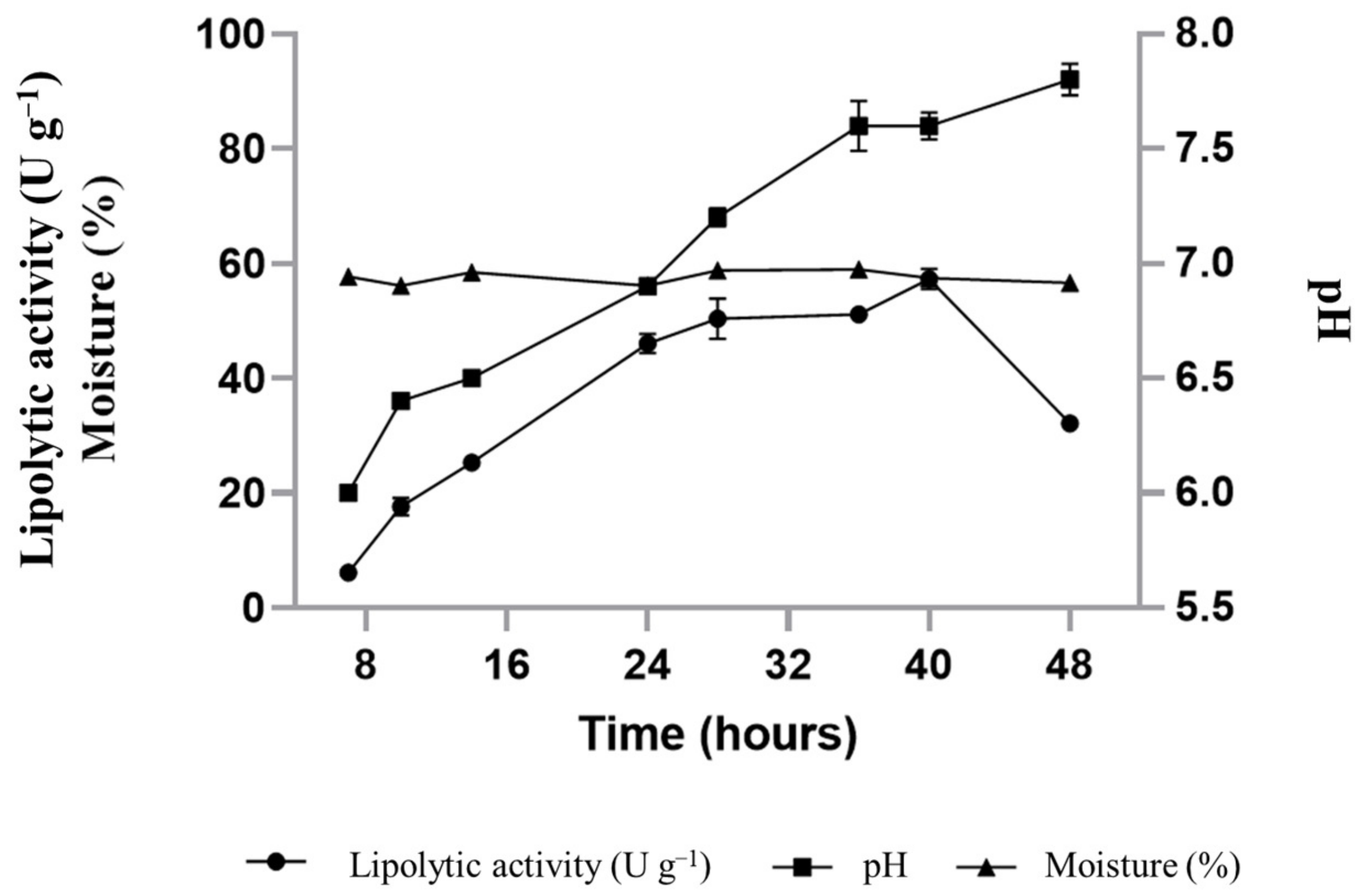
3.2. Lipase Production by Y. lipolytica Using Cupuaçu Fat Cake as Substrate and the Supplementation with Soybean Oil
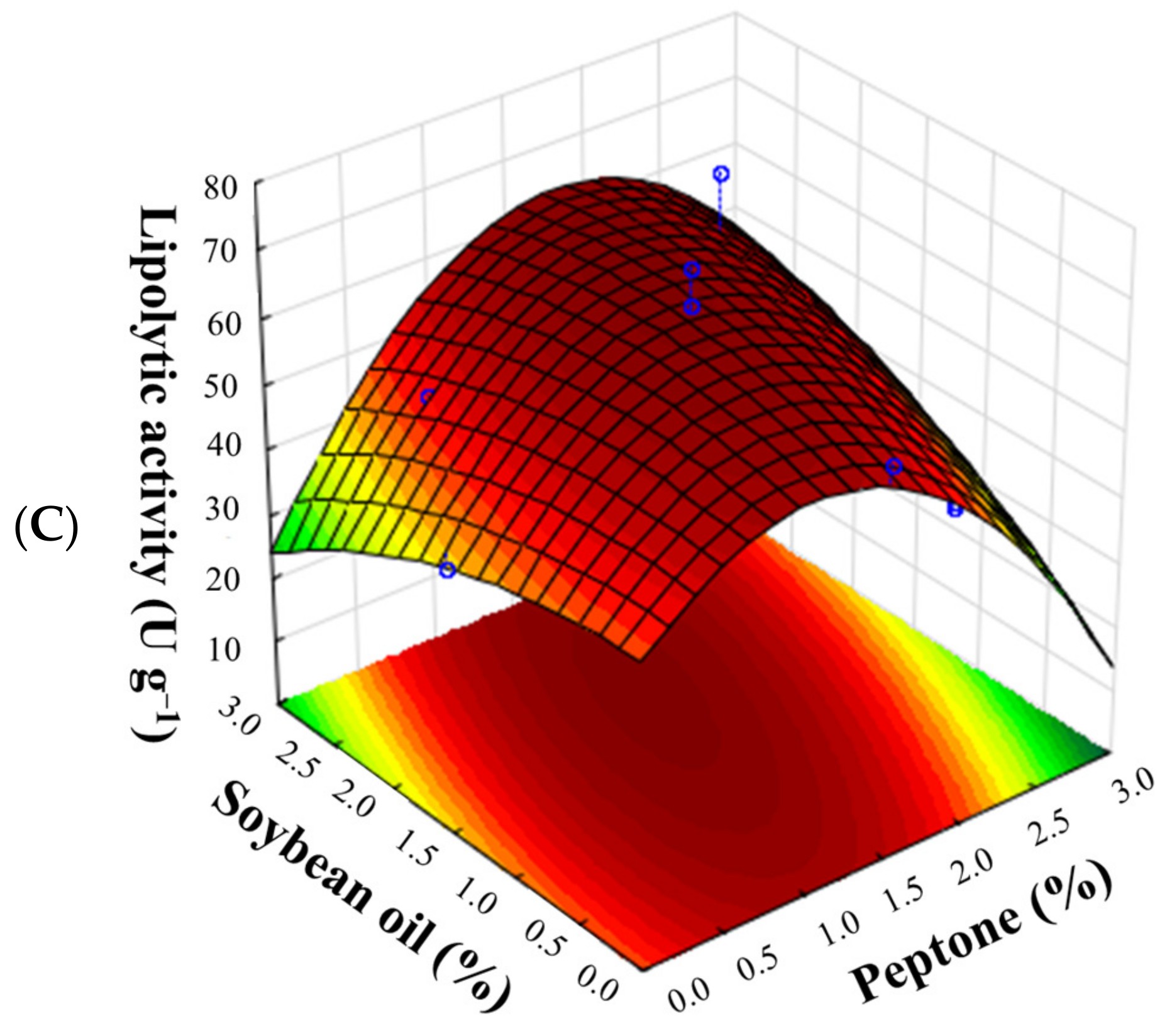
3.3. Optimization of Lipase Production Using a CCRD (23)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Treichel, H.; Oliveira, D.; Mazutti, M.A.; Di Luccio, M.; Oliveira, J.V. A Review on Microbial Lipases Production. Food Bioprocess Technol. 2010, 3, 182–196. [Google Scholar] [CrossRef]
- Ali, S.; Khan, S.A.; Hamayun, M.; Lee, I.-J. The Recent Advances in the Utility of Microbial Lipases: A Review. Microorganisms 2023, 11, 510. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, B.D.; de Castro, A.M.; Coelho, M.A.; Freire, D.M. Production and use of lipases in bioenergy: A review from the feedstocks to biodiesel production. Enzym. Res. 2011, 2011, 615803. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.L.; Sales, M.B.; Castro Bizerra, V.; Nobre, M.M.R.; Sousa Braz, A.K.; Silva Sousa, P.; Cavalcante, A.L.G.; Melo, R.L.F.; Gonçalves De Sousa Junior, P.; Neto, F.S.; et al. Lipase from Yarrowia lipolytica: Prospects as an Industrial Biocatalyst for Biotechnological Applications. Fermentation 2023, 9, 581. [Google Scholar] [CrossRef]
- Santos, L.N.; Perna, R.F.; Vieira, A.C.; Almeida, A.F.; Ferreira, N.R. Trends in the Use of Lipases: A Systematic Review and Bibliometric Analysis. Foods 2023, 12, 3058. [Google Scholar] [CrossRef] [PubMed]
- Ali, U.; Anwar, Z.; Hasan, S.; Zafar, M.; Ain, N.u.; Afzal, F.; Khalid, W.; Rahim, M.A.; Mrabti, H.N.; AL-Farga, A.; et al. Bioprocessing and Screening of Indigenous Wastes for Hyper Production of Fungal Lipase. Catalysts 2023, 13, 853. [Google Scholar] [CrossRef]
- Al Mohaini, M.; Farid, A.; Muzammal, M.; Ghazanfar, S.; Dadrasnia, A.; Alsalman, A.J.; Al Hawaj, M.A.; Alhashem, Y.N.; Ismail, S. Enhancing Lipase Production of Bacillus salmalaya Strain 139SI Using Different Carbon Sources and Surfactants. Appl. Microbiol. 2022, 2, 237–247. [Google Scholar] [CrossRef]
- Nascimento, F.V.d.; Lemes, A.C.; Castro, A.M.d.; Secchi, A.R.; Zarur Coelho, M.A. A Temporal Evolution Perspective of Lipase Production by Yarrowia lipolytica in Solid-State Fermentation. Processes 2022, 10, 381. [Google Scholar] [CrossRef]
- Zieniuk, B.; Fabiszewska, A. Yarrowia lipolytica: A beneficious yeast in biotechnology as a rare opportunistic fungal pathogen: A minireview. World J. Microbiol. Biotechnol. 2018, 35, 10. [Google Scholar] [CrossRef]
- Coelho, M.A.; Amaral, P.; Belo, I. Yarrowia lipolytica: An Industrial Workhorse; Formatex Research Center: Badajoz, Spain, 2010; Volume 2. [Google Scholar]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and valorization of agri-food wastes and by-products using the non-conventional yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Isocitric Acid Production from Ethanol Industry Waste by Yarrowia lipolytica. Fermentation 2021, 7, 146. [Google Scholar] [CrossRef]
- Eszterbauer, E.; Németh, Á. Investigations for a Yarrowia-Based Biorefinery: In Vitro Proof-of-Concept for Manufacturing Sweetener, Cosmetic Ingredient, and Bioemulsifier. Fermentation 2023, 9, 793. [Google Scholar] [CrossRef]
- Brígida, A.I.S.; Amaral, P.F.F.; Coelho, M.A.Z.; Gonçalves, L.R.B. Lipase from Yarrowia lipolytica: Production, characterization and application as an industrial biocatalyst. J. Mol. Catal. B Enzym. 2014, 101, 148–158. [Google Scholar] [CrossRef]
- Pan, D.; Dai, S.; Jiao, L.; Zhou, Q.; Zha, G.; Yan, J.; Han, B.; Yan, Y.; Xu, L. Homologous High-Level Lipase and Single-Cell Protein Production with Engineered Yarrowia lipolytica via Scale-Up Fermentation for Industrial Applications. Fermentation 2023, 9, 268. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Braschi, G.; Rossi, S.; Bains, N.; Vannini, L.; Patrignani, F.; Lanciotti, R. Selection of Yarrowia lipolytica Strains as Possible Solution to Valorize Untreated Cheese Whey. Fermentation 2023, 9, 51. [Google Scholar] [CrossRef]
- Sayın Börekçi, B.; Kaya, M.; Kaban, G. Citric Acid Production by Yarrowia lipolytica NRRL Y-1094: Optimization of pH, Fermentation Time and Glucose Concentration Using Response Surface Methodology. Fermentation 2022, 8, 731. [Google Scholar] [CrossRef]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Costa, A.R.; Fernandes, H.; Salgado, J.M.; Belo, I. Solid State and Semi-Solid Fermentations of Olive and Sunflower Cakes with Yarrowia lipolytica: Impact of Biological and Physical Pretreatments. Fermentation 2023, 9, 734. [Google Scholar] [CrossRef]
- Bulgari, D.; Renzetti, S.; Messgo-Moumene, S.; Monti, E.; Gobbi, E. Optimization of Esterase Production in Solid-State Fermentation of Agricultural Digestate. Fermentation 2023, 9, 524. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-State Fermentation from Organic Wastes: A New Generation of Bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Jain, R.; Naik, S.N. Adding value to the oil cake as a waste from oil processing industry: Production of lipase in solid state fermentation. Biocatal. Agric. Biotechnol. 2018, 15, 181–184. [Google Scholar] [CrossRef]
- Costa, A.R.; Salgado, J.M.; Lopes, M.; Belo, I. Valorization of by-products from vegetable oil industries: Enzymes production by Yarrowia lipolytica through solid state fermentation. Front. Sustain. Food Syst. 2022, 6, 1006467. [Google Scholar] [CrossRef]
- Uscátegui, Y.L.; Jimenez-junca, C.A.; Suárez, C.; Prieto-Correa, E. Evaluation of the induction of lipolytic enzymes from a Pseudomona aeruginosa isolated from african palm fruit (Elaeis guineensis). Vitae 2012, 19, 280–286. [Google Scholar] [CrossRef]
- Cano y Postigo, L.O.; Jacobo-Velázquez, D.A.; Guajardo-Flores, D.; Garcia Amezquita, L.E.; García-Cayuela, T. Solid-state fermentation for enhancing the nutraceutical content of agrifood by-products: Recent advances and its industrial feasibility. Food Biosci. 2021, 41, 100926. [Google Scholar] [CrossRef]
- Lemes, A.C.; Egea, M.B.; Oliveira Filho, J.G.d.; Gautério, G.V.; Ribeiro, B.D.; Coelho, M.A.Z. Biological Approaches for Extraction of Bioactive Compounds from Agro-industrial By-products: A Review. Front. Bioeng. Biotechnol. 2022, 9, 802543. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.-L.; Nargotra, P.; Chen, C.-W.; Kuo, C.-H.; Sun, P.-P.; Dong, C.-D. Agro-Industrial Food Waste as a Low-Cost Substrate for Sustainable Production of Industrial Enzymes: A Critical Review. Catalysts 2022, 12, 1373. [Google Scholar] [CrossRef]
- Souza, C.E.C.; Farias, M.A.; Ribeiro, B.D.; Coelho, M.A.Z. Adding Value to Agro-industrial Co-products from Canola and Soybean Oil Extraction through Lipase Production Using Yarrowia lipolytica in Solid-State Fermentation. Waste Biomass Valorization 2017, 8, 1163–1176. [Google Scholar] [CrossRef]
- Putri, D.N.; Khootama, A.; Perdani, M.S.; Utami, T.S.; Hermansyah, H. Optimization of Aspergillus niger lipase production by solid state fermentation of agro-industrial waste. Energy Rep. 2020, 6, 331–335. [Google Scholar] [CrossRef]
- Prabaningtyas, R.K.; Putri, D.N.; Utami, T.S.; Hermansyah, H. Production of immobilized extracellular lipase from Aspergillus niger by solid state fermentation method using palm kernel cake, soybean meal, and coir pith as the substrate. Energy Procedia 2018, 153, 242–247. [Google Scholar] [CrossRef]
- Sales, J.C.S.; Castro, A.M.; Ribeiro, B.D.; Coelho, M.A.Z. Supplementation of watermelon peels as an enhancer of lipase and esterase production by Yarrowia lipolytica in solid-state fermentation and their potential use as biocatalysts in poly(ethylene terephthalate) (PET) depolymerization reactions. Biocatal. Biotransformation 2020, 38, 457–468. [Google Scholar] [CrossRef]
- Carvalho, A.S.S.; Sales, J.C.S.; Nascimento, F.V.d.; Ribeiro, B.D.; Souza, C.E.C.d.; Lemes, A.C.; Coelho, M.A.Z. Lipase Production by Yarrowia lipolytica in Solid-State Fermentation Using Amazon Fruit By-Products and Soybean Meal as Substrate. Catalysts 2023, 13, 289. [Google Scholar] [CrossRef]
- Silva, J.N.; Godoy, M.G.; Gutarra, M.L.E.; Freire, D.M.G. Impact of Extraction Parameters on the Recovery of Lipolytic Activity from Fermented Babassu Cake. PLoS ONE 2014, 9, e103176. [Google Scholar] [CrossRef] [PubMed]
- Santos, R.R.d.; Muruci, L.N.M.; Santos, L.O.; Antoniassi, R.; Silva, J.P.L.d.; Damaso, M.n.C.T. Characterization of Different Oil Soapstocks and Their Application in the Lipase Production by Aspergillus niger under Solid State Fermentation. J. Food Nutr. Res. 2014, 2, 561–566. [Google Scholar] [CrossRef]
- Damaso, M.C.T.; Passianoto, M.A.; Freitas, S.C.d.; Freire, D.M.G.; Lago, R.C.A.; Couri, S. Utilization of agroindustrial residues for lipase production by solid-state fermentation. Braz. J. Microbiol. 2008, 39, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Guedes, E.H.S.; Santos, A.L.d.; Ibiapina, A.; Aguiar, A.O.; Soares, C.M.d.S.; Vellano, P.O.; Santos, L.S.S.d.; Chagas Junior, A.F. Agro-industrial waste as a substrate for the production of microbial lipases: A review. Res. Soc. Dev. 2021, 10, e30710212537. [Google Scholar] [CrossRef]
- Araujo, N.M.P.; Arruda, H.S.; Marques, D.R.P.; Oliveira, W.Q.; Pereira, G.A.; Pastore, G.M. Functional and nutritional properties of selected Amazon fruits: A review. Food Res. Int. 2021, 147, 110520. [Google Scholar] [CrossRef]
- Garrett, R.D.; Cammelli, F.; Ferreira, J.; Levy, S.A.; Valentim, J.; Vieira, I. Forests and Sustainable Development in the Brazilian Amazon: History, Trends, and Future Prospects. Annu. Rev. Environ. Resour. 2021, 46, 625–652. [Google Scholar] [CrossRef]
- Oliveira, F.; Souza, C.E.; Peclat, V.R.O.L.; Salgado, J.M.; Ribeiro, B.D.; Coelho, M.A.Z.; Venâncio, A.; Belo, I. Optimization of lipase production by Aspergillus ibericus from oil cakes and its application in esterification reactions. Food Bioprod. Process. 2017, 102, 268–277. [Google Scholar] [CrossRef]
- Venturieri, G.A. Flowering levels, harvest season and yields of cupuassu (Theobroma grandiflorum). Acta Amaz. 2011, 41, 143–151. [Google Scholar] [CrossRef]
- Costa, R.S.d.; Santos, O.V.d.; Lannes, S.C.d.S.; Casazza, A.A.; Aliakbarian, B.; Perego, P.; Ribeiro-Costa, R.M.; Converti, A.; Silva JÚNior, J.O.C. Bioactive compounds and value-added applications of cupuassu (Theobroma grandiflorum Schum.) agroindustrial by-product. Food Sci. Technol. 2020, 40, 401–407. [Google Scholar] [CrossRef]
- Said, M.M.; Rivas, A.A.F.; Oliveira, L.A.d. Cupuassu plant management and the market situation of Itacoatiara, Manacapuru and Presidente Figueiredo counties, Amazonas State, Brazil. Res. Soc. Dev. 2021, 10, e15110313109. [Google Scholar] [CrossRef]
- Pereira, A.L.F.; Abreu, V.K.G.; Rodrigues, S. Cupuassu—Theobroma grandiflorum. In Exotic Fruits; Rodrigues, S., de Oliveira Silva, E., de Brito, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 159–162. [Google Scholar]
- Gondim, T.M.S.; Thomazini, M.J.; Cavalcante, M.J.B.; Souza, J.M.L. Aspectos da Produção de Cupuaçu; Embrapa: Rio Branco, Brazil, 2001; 43p. [Google Scholar]
- Ferreira, M.G.R.; Nogueira, A.E.; Damião-Filho, C.F. Estudo Morfológico de Folhas de Cupuaçu (Theobroma grandiflorum Schum.); Embrapa: Porto Velho, Brazil, 2006; 12p. [Google Scholar]
- Matos, C.B. Caracterização Fisica, Química, Físico-Química de Cupuaçus (Theobroma grandiflorum (willd Ex. Spreng) Schum) com Diferentes Formatos; Universidade Estadual de Santa Cruz: Ilhéus, Brazil, 2007. [Google Scholar]
- Alves, R.M.; Filgueiras, G.C.; Homma, A.K.O. Aspectos socioeconômicos do cupuaçuzeiro na Amazônia: Do extrativismo a domesticação. In Mercado, Cadeias Produtivas e Desenvolvimento Rural na Amazônia; Santana, A.C., Ed.; UFRA: Belém, Brazil, 2014; pp. 197–223. [Google Scholar]
- Costa, C.M.d.; Silva, K.A.d.; Santos, I.L.; Yamaguchi, K.K.d.L. Integral use of cupuassu in baking. Res. Soc. Dev. 2022, 11, e34711528176. [Google Scholar] [CrossRef]
- Hagler, A.N.; Mendonça-Hagler, L.C. Yeasts from marine and estuarine waters with different levels of pollution in the state of Rio de Janeiro, Brazil. Appl. Environ. Microbiol. 1981, 41, 173–178. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995. [Google Scholar]
- Rocha, R.C.P.; Carvalho, A.S.S.; Sales, J.C.S.; Souza, C.E.C.; Lemes, A.C.; Coelho, M.A.Z. Production of lipase from Yarrowia lipolytica with cupuaçu fat cake using a fractional factorial desing. In Proceedings of the XXIII National Bioprocess Symposium—SINAFERM, Búzio, Brazil, 28–31 August 2022; pp. 1–2. [Google Scholar]
- Pereira-Meirelles, F.V.; Rocha-Leão, M.H.M.; Sant’Anna, G.L. A Stable Lipase from Candida lipolytica. In Biotechnology for Fuels and Chemicals: Proceedings of the Eighteenth Symposium on Biotechnology for Fuels and Chemicals Held 5–9 May 1996, at Gatlinburg, Tennessee; Davison, B.H., Wyman, C.E., Finkelstein, M., Eds.; Humana Press: Totowa, NJ, USA, 1997; pp. 73–85. [Google Scholar]
- Freire, D.M.; Teles, E.M.; Bon, E.P.; Sant’Anna, G.L., Jr. Lipase production by Penicillium restrictum in a bench-scale fermenter: Effect of carbon and nitrogen nutrition, agitation, and aeration. Appl. Biochem. Biotechnol. 1997, 63–65, 409–421. [Google Scholar] [CrossRef]
- Barbosa, M.M.; Detmann, E.; Valadares, S.C.; Detmann, K.S.C.; Franco, M.O.; Batista, E.D.; Rocha, G.C. Evaluation of methods for the quantification of ether extract contents in forage and cattle feces. An. Da Acad. Bras. De Ciênc. 2017, 89, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Meirelles, F.V.; Rocha-Leão, M.H.M.; Sant’Anna, G.L. Lipase location in Yarrowia lipolytica cells. Biotechnol. Lett. 2000, 22, 71–75. [Google Scholar] [CrossRef]
- Salihu, A.; Alam, M.Z.; AbdulKarim, M.I.; Salleh, H.M. Lipase production: An insight in the utilization of renewable agricultural residues. Resour. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Almeida, A.F.; Taulk-Tornisielo, S.M.; Carmona, E.C. Influence of carbon and nitrogen sources on lipase production by a newly isolated Candida viswanathii strain. Ann. Microbiol. 2013, 63, 1225–1234. [Google Scholar] [CrossRef]
- Silva, D.A.d.; Manoel da Cruz Rodrigues, A.; Oliveira dos Santos, A.; Salvador-Reyes, R.; Meller da Silva, L.H. Physicochemical and technological properties of pracaxi oil, cupuassu fat and palm stearin blends enzymatically interesterified for food applications. LWT 2023, 184, 114961. [Google Scholar] [CrossRef]
- Fickers, P.; Marty, A.; Nicaud, J.M. The lipases from Yarrowia lipolytica: Genetics, production, regulation, biochemical characterization and biotechnological applications. Biotechnol. Adv. 2011, 29, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.O.; Farias, M.A.; Belo, I.M.P.; Coelho, M.A.Z. Nitrogen sources on tpomw valorization through solid state fermentation performed by Yarrowia lipolytica. Braz. J. Chem. Eng. 2016, 33, 261–270. [Google Scholar] [CrossRef]
- Vargas, G.D.L.P.; Treichel, H.; de Oliveira, D.; Beneti, S.C.; Freire, D.M.G.; Di Luccio, M. Optimization of lipase production by Penicillium simplicissimum in soybean meal. J. Chem. Technol. Biotechnol. 2008, 83, 47–54. [Google Scholar] [CrossRef]
- Azevedo, W.M.; Oliveira, L.F.R.; Alcântara, M.A.; Cordeiro, A.; Damasceno, K.; Assis, C.F.; Sousa Junior, F.C. Turning cacay butter and wheat bran into substrate for lipase production by Aspergillus terreus NRRL-255. Prep. Biochem. Biotechnol. 2020, 50, 689–696. [Google Scholar] [CrossRef] [PubMed]
- Edwinoliver, N.G.; Thirunavukarasu, K.; Naidu, R.B.; Gowthaman, M.K.; Kambe, T.N.; Kamini, N.R. Scale up of a novel tri-substrate fermentation for enhanced production of Aspergillus niger lipase for tallow hydrolysis. Bioresour. Technol. 2010, 101, 6791–6796. [Google Scholar] [CrossRef] [PubMed]
- Mala, J.G.; Edwinoliver, N.G.; Kamini, N.R.; Puvanakrishnan, R. Mixed substrate solid state fermentation for production and extraction of lipase from Aspergillus niger MTCC 2594. J. Gen. Appl. Microbiol. 2007, 53, 247–253. [Google Scholar] [CrossRef]
- Nascimento, F.V.; Castro, A.M.; Secchi, A.R.; Coelho, M.A.Z. Insights into media supplementation in solid-state fermentation of soybean hulls by Yarrowia lipolytica: Impact on lipase production in tray and insulated packed-bed bioreactors. Biochem. Eng. J. 2021, 166, 107866. [Google Scholar] [CrossRef]
- Imandi, S.B.; Garapati, H.R. Lipase production by Yarrowia lipolytica NCIM 3589 in solid state fermentation using mixed substrate. Res. J. Microbiol. 2007, 2, 469–474. [Google Scholar]
- Im, S.B.; Karanam, S.K.; Garapati, H.R. Optimization of Process Parameters for the Production of Lipase in Solid State Fermentation by Yarrowia lipolytica from Niger Seed Oil Cake (Guizotia abyssinica). J. Microb. Biochem. Technol. 2010, 2, 028–033. [Google Scholar]
- Imandi, S.; Karanam, S.; Rao, G. Optimization of media constituents for the production of lipase in solid state fermentation by Yarrowia lipolytica from palm Kernal cake (Elaeis guineensis). Adv. Biosci. Biotechnol. 2010, 1, 115–121. [Google Scholar] [CrossRef][Green Version]
- Imandi, S.B.; Karanam, S.K.; Garapati, H.R. Use of Plackett-Burman design for rapid screening of nitrogen and carbon sources for the production of lipase in solid state fermentation by Yarrowia lipolytica from mustard oil cake (Brassica napus). Braz. J. Microbiol. 2013, 44, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Moftah, A.; Grbavcic, Z.S.; Moftah, A.; Lukovic, D.; Prodanovic, L.; Jakovetic, M.S.; Knezevic-Jugovic, D.Z. Lipase production by Yarrowia lipolytica using olive oil processing wastes as substrates. J. Serbian Chem. Soc. 2013, 78, 781–794. [Google Scholar] [CrossRef]
- Farias, M.A.; Valoni, E.A.; Castro, A.M.; Coelho, M.A.Z. Lipase Production by Yarrowia Lipolytica in Solid State Fermentation Using Different Agro Industrial Residues. Chem. Eng. Trans. 2014, 38, 301–306. [Google Scholar]
- Fickers, P.; Nicaud, J.M.; Gaillardin, C.; Destain, J.; Thonart, P. Carbon and nitrogen sources modulate lipase production in the yeast Yarrowia lipolytica. J. Appl. Microbiol. 2004, 96, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Turki, S.; Kraeim, I.B.; Weeckers, F.; Thonart, P.; Kallel, H. Isolation of bioactive peptides from tryptone that modulate lipase production in Yarrowia lipolytica. Bioresour. Technol. 2009, 100, 2724–2731. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.; Han, P.C.; Kasim, F.H.; Gopinath, S.C.B.; Ahmad, M.A. Turning oil palm empty fruit bunch waste into substrate for optimal lipase secretion on solid state fermentation by Trichoderma strains. Process Biochem. 2017, 63, 35–41. [Google Scholar] [CrossRef]
- Eichler, P.; Bastiani, D.C.; Santos, F.A.; Ayub, M.A.Z. Lipase production by Aspergillus brasiliensis in solid-state cultivation of malt bagasse in different bioreactors configurations. Acad. Bras. Cienc. 2020, 92, e20180856. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Bolzani, V.S. The potential contribution of the natural products from Brazilian biodiversity to bioeconomy. An. Da Acad. Bras. De Ciênc. 2018, 90, 763–778. [Google Scholar] [CrossRef]
- Teixeira, G.L.; Ibañez, E.; Block, J.M. Emerging Lipids from Arecaceae Palm Fruits in Brazil. Molecules 2022, 27, 4188. [Google Scholar] [CrossRef]
- Torres-León, C.; Ramírez-Guzman, N.; Londoño-Hernandez, L.; Martinez-Medina, G.A.; Díaz-Herrera, R.; Navarro-Macias, V.; Alvarez-Pérez, O.B.; Picazo, B.; Villarreal-Vázquez, M.; Ascacio-Valdes, J.; et al. Food Waste and Byproducts: An Opportunity to Minimize Malnutrition and Hunger in Developing Countries. Front. Sustain. Food Syst. 2018, 2, 52. [Google Scholar] [CrossRef]
- Almaraz-Sánchez, I.; Amaro-Reyes, A.; Acosta-Gallegos, J.A.; Mendoza-Sánchez, M. Processing Agroindustry By-Products for Obtaining Value-Added Products and Reducing Environmental Impact. J. Chem. 2022, 2022, 3656932. [Google Scholar] [CrossRef]

| Components (%) | Cupuaçu Fat Cake |
|---|---|
| Proteins | 14.93 ± 0.49 |
| Ether extract | 21.45 ± 0.44 |
| Carbohydrates—Nifext | 21.85 ± 0.32 |
| Insoluble fibers—ADF * | 23.45 ± 1.74 |
| Insoluble fibers—NDF ** | 29.82 ± 0.79 |
| Assay | Peptone (%) | Urea (%) | Soybean Oil (%) | Lipase Activity (U g−1) |
|---|---|---|---|---|
| 1 | (−1) 0.58 | (−1) 0.58 | (−1) 0.58 | 47.38 |
| 2 | (+1) 0.58 | (−1) 0.58 | (−1) 2.42 | 43.08 |
| 3 | (−1) 0.58 | (+1) 2.42 | (−1) 0.58 | 51.72 |
| 4 | (+1) 0.58 | (+1) 2.42 | (−1) 2.42 | 49.54 |
| 5 | (−1) 2.42 | (−1) 0.58 | (+1) 0.58 | 37.10 |
| 6 | (+1) 2.42 | (−1) 0.58 | (+1) 2.42 | 43.51 |
| 7 | (−1) 2.42 | (+1) 2.42 | (+1) 0.58 | 37.70 |
| 8 | (+1) 2.42 | (+1) 2.42 | (+1) 2.42 | 66.68 |
| 9 | (−1.68) 0 | (0) 1.50 | (0) 1.50 | 40.61 |
| 10 | (+1.68) 3.05 | (0) 1.50 | (0) 1.50 | 30.48 |
| 11 | (0) 1.50 | (−1.68) 0 | (0) 1.50 | 45.05 |
| 12 | (0) 1.50 | (+1.68) 3.05 | (0) 1.50 | 44.43 |
| 13 | (0) 1.50 | (0) 1.50 | (−1.68) 0 | 59.53 |
| 14 | (0) 1.50 | (0) 1.50 | (+1.68) 3.05 | 55.28 |
| 15 | (0) 1.50 | (0) 1.50 | (0) 1.50 | 58.32 |
| 16 | (0) 1.50 | (0) 1.50 | (0) 1.50 | 70.64 |
| 17 | (0) 1.50 | (0) 1.50 | (0) 1.50 | 65.08 |
| Variation Source | Sum of Squares | Degrees of Freedom | Means Square | F-Value | F-Critical | Adj. R2 |
|---|---|---|---|---|---|---|
| Regression | 1764.90 | 9 | 196.10 | 4.63 | 2.73 | 0.85617 |
| Residual | 296.49 | 7 | 42.36 | |||
| Lack of fit | 220.27 | 5 | 44.05 | 1.16 | 9.29 | |
| Pure error | 76.22 | 2 | 38.11 | |||
| SQ total | 2061.39 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, A.S.S.; Rocha, R.d.C.P.e.; Sales, J.C.S.; Souza, C.E.C.d.; Lemes, A.C.; Coelho, M.A.Z. Biotechnological Valorization of Cupuaçu By-Products: Solid-State Fermentation for Lipase Production by Yarrowia lipolytica. Fermentation 2023, 9, 989. https://doi.org/10.3390/fermentation9110989
Carvalho ASS, Rocha RdCPe, Sales JCS, Souza CECd, Lemes AC, Coelho MAZ. Biotechnological Valorization of Cupuaçu By-Products: Solid-State Fermentation for Lipase Production by Yarrowia lipolytica. Fermentation. 2023; 9(11):989. https://doi.org/10.3390/fermentation9110989
Chicago/Turabian StyleCarvalho, Aparecida Selsiane Sousa, Raíssa de Carvalho Pinto e Rocha, Júlio Cesar Soares Sales, Carlos Eduardo Conceição de Souza, Ailton Cesar Lemes, and Maria Alice Zarur Coelho. 2023. "Biotechnological Valorization of Cupuaçu By-Products: Solid-State Fermentation for Lipase Production by Yarrowia lipolytica" Fermentation 9, no. 11: 989. https://doi.org/10.3390/fermentation9110989
APA StyleCarvalho, A. S. S., Rocha, R. d. C. P. e., Sales, J. C. S., Souza, C. E. C. d., Lemes, A. C., & Coelho, M. A. Z. (2023). Biotechnological Valorization of Cupuaçu By-Products: Solid-State Fermentation for Lipase Production by Yarrowia lipolytica. Fermentation, 9(11), 989. https://doi.org/10.3390/fermentation9110989








