Abstract
Lignocellulose consists of cellulose, hemicellulose, and lignin and is a sustainable feedstock for a biorefinery to generate marketable biomaterials like biofuels and platform chemicals. Enormous tons of lignocellulose are obtained from agricultural waste, but a few tons are utilized due to a lack of awareness of the biotechnological importance of lignocellulose. Underutilizing lignocellulose could also be linked to the incomplete use of cellulose and hemicellulose in biotransformation into new products. Utilizing lignocellulose in producing value-added products alleviates agricultural waste disposal management challenges. It also reduces the emission of toxic substances into the environment, which promotes a sustainable development goal and contributes to circular economy development and economic growth. This review broadly focused on lignocellulose in the production of high-value products. The aspects that were discussed included: (i) sources of lignocellulosic biomass; (ii) conversion of lignocellulosic biomass into value-added products; and (iii) various bio-based products obtained from lignocellulose. Additionally, several challenges in upcycling lignocellulose and alleviation strategies were discussed. This review also suggested prospects using lignocellulose to replace polystyrene packaging with lignin-based packaging products, the production of crafts and interior decorations using lignin, nanolignin in producing environmental biosensors and biomimetic sensors, and processing cellulose and hemicellulose with the addition of nutritional supplements to meet dietary requirements in animal feeding.
1. Introduction
Lignocellulose is a plant biomass available in large amounts, and it is a renewable resource. It is a complex structure primarily composed of the polymers cellulose and hemicellulose (polysaccharides), lignin (a phenolic macromolecule), as shown in Figure 1, and other components, such as proteins, lipids, and inorganic compounds [1,2,3]. These polymers contain cellulose ranging from 35 to 55%, hemicellulose from 20 to 40%, lignin (10–25%) by mass, and other polar and non-polar compounds [4]. The elemental compositions of most lignocellulosic biomass are classified as major elements (e.g., C, H, O, N, K, and Ca), minor elements (Mg, Al, Si, P, Cl, Na, S, and Fe), and trace elements (e.g., Mn and Ti) [5,6].
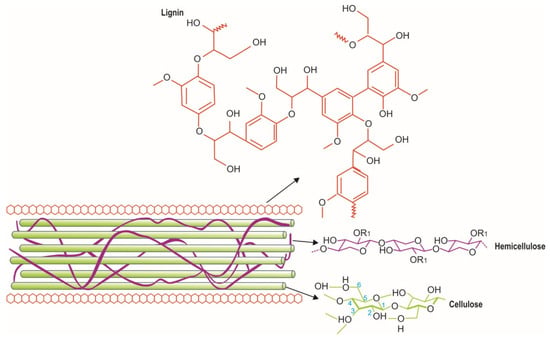
Figure 1.
Lignocellulose composition and organization (adapted from Tran et al., 2019 [2]).
The cellulose in lignocellulose is a homopolysaccharide with chains of D-glucose monomers linked together via β-1-4 glycosyl units, stabilized by hydrogen bonds and van der Waals forces [1]. Cellulose comprises the repetitive structural unit called cellobiose (D-glucopyranosyl-β-1,4-D-glucopyranose) [7], and it is linked to lignin by hemicellulose via hydrogen and covalent bonds [8] (Figure 1). Hemicellulose is a branched heteropolysaccharide with two or more free monosaccharides, such as xylose and arabinose (five-carbon sugars), mannose, glucose, galactose (six-carbon sugars), and carboxylic acids (e.g., mannuronic acid and galacturonic acid) [8,9]. Moreover, hemicellulose bridges the deposition of lignin monomers in the secondary cell wall [9,10]. Meanwhile, lignin is a complex amorphous polymer that contains various monolignols, such as p-coumaryl alcohol, sinapyl alcohol, and coniferyl, and it is a crosslinked macromolecule formed via the polymerization of phenylpropanoid monomers (p-coumaryl alcohol, sinapyl alcohol, and coniferyl) [8,10]. Lignin is hydrophobic and highly resistant to hydrolysis; it binds hemicellulose to cellulose in the cell wall and acts as a barrier that limits cellulose accessibility [11].
Extensive studies on the physicochemical properties of lignocellulose have yet to be conducted; however, literature searches have indicated that lignocellulose physicochemical properties are assessed based on its particle size, density, flowability, moisture sorption, grindability, and thermal properties (physical properties), along with ash, volatile matter, moisture, and fixed carbon (chemical properties) [12].
The world’s food-producing countries are China, the USA, India, Brazil, and Turkey, and they are the largest producers of agricultural waste [13,14]. Although large volumes of lignocellulose are obtained from agricultural waste, a few tons of lignocellulose are utilized. For instance, the recent global annual lignocellulose production is about 181.5 billion tons, and only 8.2 billion tons were used for distinct applications [15]. Noticeably, agricultural waste is burned in some countries with no sustainable management practices, resulting in excessive emissions of gases and aerosols, which causes air pollution that could adversely affect health [16]. In addition, the burning of agricultural waste results in soil fertility deterioration, while frequent burning reduces the soil’s carbon and nitrogen and kills the in-situ microflora and fauna [17].
Stringent measures like government intervention towards regulating crop waste management and adequate awareness of the biotechnological importance of agricultural waste to mitigate the improper management and underutilization of agricultural waste should be implemented. Conversion of lignocellulose into a broad spectrum of marketable bio-based products, such as chemicals and biofuels, aligns with the United Nations Climate Change Conference of the Parties [18] mission, and such a conversion aims at a Sustainable Development Goal (SDG 13) using alternative materials for energy production. The concept of reusing lignocellulose, recycling, and converting it into bio-based products reduces the emission of toxic substances into the environment and contributes to circular economy development [19] and economic growth.
As such, this review paper aimed to raise more awareness of the biotechnological importance of lignocellulose by focusing on lignocellulose sources, steps and processes in utilizing lignocellulose for high-value product production, various bio-based products obtained from lignocellulose, challenges and alleviation strategies in upcycling lignocellulose, and prospects.
2. Lignocellulosic Biomass Sources
Lignocellulosic biomass resources are widely available, and they are agricultural and forestry residues from plant wastes [20]. Industrial and food wastes are also sources of lignocellulose [21]. A number of examples of lignocellulose sources are listed in Table 1. Several harvests from a single planting that reduce the average annual cost of managing energy crops compared to conventional crops make lignocellulosic biomass resources the most promising future resources to generate value-added products [22]. Rice, wheat, sugarcane, and maize are the major crops that generate a large amount of lignocellulosic biomass. The world’s first most important cereal crop is corn. In 2022/2023, around 1.2 billion metric tons of corn and nearly 783.8 million metric tons of wheat were produced, followed by 510 million metric tons of milled rice (the second-most important cereal crop) [23]. Approximately 177.3 million metric tons of sugarcane were also produced in 2022/2023 [23]. China and the United States of America account for more than half of worldwide corn production, while China is the world’s leading rice producer, followed by India and Bangladesh [23]. Similarly, China, followed by India, Russia, and the United States of America are the four largest wheat producers in the world, while India and Brazil are the world’s top two sugar producers [24].
Vast waste, such as rice straw, wheat straw, sugarcane bagasse, corn stover, etc., is generated annually via agricultural crop production. Rice straw (stems, leaf blades, and sheets) is generated from the rice harvest [25,26], and wheat straw is the waste obtained from wheat grain production [22]. Sugarcane waste or bagasse is obtained after sugarcane stalks are crushed for sugar [27], while corn stover (consisting of leaves, cobs, husks, and stalks) is the waste product obtained from corn kernel processing [28] from the maize plants. These wastes constitute a major portion of lignocellulosic biomass. Other agricultural wastes that contribute to a small amount of the total agricultural waste production include barley straw, cotton stalks, sweet sorghum straws, potato haulms (the tops, stems, and foliage of potato plants), and others [22].

Table 1.
Sources and selected examples of lignocellulosic materials.
Table 1.
Sources and selected examples of lignocellulosic materials.
| Sources | Examples | References |
|---|---|---|
| Agricultural residues | Sugarcane bagasse, corn and rice straw, cotton stalk, corn cobs and leaves, wheat straw, barley straw, sweet sorghum straw, potato haulms, and cocoa pods. | [25,29,30,31,32,33,34,35,36,37,38] |
| Forestry residues | Spruce chips, willow, cedars, poplar, and eucalyptus. | [12,39,40,41,42,43,44] |
| Industrial wastes | Brewer’s spent grains, chemical pulps (e.g., waste sulfite liquor from pulp), and waste papers from paper mills. | [22,45,46,47,48] |
| Food wastes | The kitchen remains, such as vegetable peels and fruit waste. | [49] |
| Agro-wastes | Animal manure (e.g., solid cattle, cow, and pig manure). | [50,51] |
3. Conversion of Lignocellulosic Biomass into Value-Added Products
The production of biofuels and platform chemicals (i.e., value-added products) using lignocellulose is a sustainable option that can alleviate challenges associated with agricultural waste management [31]. Lignocellulose undergoes different biorefinery processing stages before it is converted into value-added products. These processing stages include pretreatment, hydrolysis, fermentation, and product purification/recovery [52,53].
3.1. Pretreatment Methods of Lignocellulose
The pretreatment of lignocellulose is a delignification process that makes lignocellulosic materials accessible to generate sugars. In many cases, lignocellulose was reported to be recalcitrant due to the complexity of the cell wall, lignin components, and crystalline structure of cellulose [20]. As such, selecting a suitable pretreatment method to generate sugars for the downstream application is essential. Lignocellulose can be pretreated using physical, chemical, physicochemical, biological, or nanotechnology methods [54,55,56]. As shown in Figure 2, these pretreatment methods have been reported, and specific experimental procedures have been developed to disrupt the lignocellulose structure to liberate sugars for value-added product production [55]. For instance, in physical pretreatments, lignocellulose is ground, milled, chipped, shredded (mechanical comminution), frozen, or pyrolyzed to produce various material sizes, thereby elevating the surface area and decreasing cellulose crystallinity and polymerization [57]. In physical pretreatment methods, lignocellulose can also be thermally degraded at an elevated temperature under non-oxidizing conditions (slow, fast, or flash pyrolysis) [58].
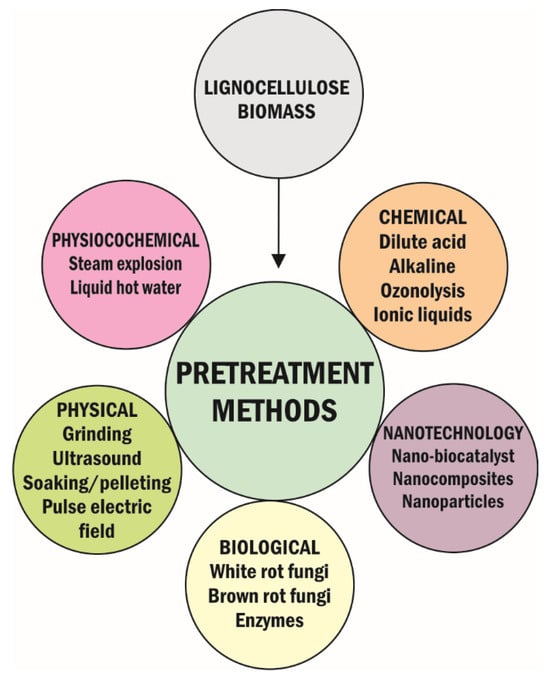
Figure 2.
Pretreatment methods of lignocellulosic materials (modified from Arora et al., 2020 [59]).
Different processes used in chemical pretreatment methods include dilute acid, alkaline, ionic liquid, organosolv process, ozonolysis, and deep eutectic solvents [60]. In the dilute acid pretreatments, inorganic or organic acids, such as HCl, H2SO4, HNO3, and formic acid, break down the hydrogen and glycosidic bonds in cellulose/hemicellulose [61,62]. Bases such as NaOH, NH4OH, Ca(OH)2, and KOH are always used in alkaline pretreatment methods to solubilize lignin [3].
Physicochemical pretreatment methods such as ammonia fiber explosion (AFEX) or carbon dioxide explosion are employed where the milled or ground lignocellulose is treated with ammonia under a high temperature (e.g., 90 °C) or carbon dioxide pressure is released to disrupt the structure of cellulose [57,63]. The disruption of the structure of cellulose reduces cellulose crystallinity, enhances cellulose permeability, and increases its surface area, thereby increasing the accessibility of enzymes [64].
Biological pretreatments also offer capable commercially available microbial enzymes or crude enzymes in the delignification of lignocellulose. The different enzymes used in biological pretreatment include ligninolytic enzymes such as phenol oxidase (e.g., laccase) and heme peroxidase (e.g., lignin peroxidase). Additionally, fungi, such as white rot (e.g., Irpex lacteus, Ceriporiopsis subvermispora, and Lentinus edodes) [65,66,67], red rot (e.g., Fomitopsis annosa) [68], and brown rot (e.g., Neolentinus lepideus and Gloeophyllum trabeum) [69], have been used to attack lignin, hemicellulose and cellulose, lignin and hemicellulose, or cellulose and hemicellulose directly due to the lignolytic enzymes they produce [66,70]. Most of these fungi are Ascomycetes, Deuteromycetes, or Basidiomycetes; they live on wood and degrade the wood components, thereby causing wood rots [71,72]. Brown rot fungi, for instance, constitute around 6–10% of wood decay fungi, and they not only degrade cellulose and hemicellulose but also modify lignin through a demethylation reaction [72,73,74]. It was reported that lignocellulose degradation occurs in the S2 layer of the cell wall via oxidation and hydrolysis mechanisms [75]. White rots are the most effective fungi in degrading the main components of the cell wall (e.g., lignin, hemicellulose, and cellulose) [72,73,74].
Previous studies that had used white rot fungi in lignocellulose pretreatment established that: (i) two strains of Ceriporiopsis subvermispora used to pretreat wheat straw for seven weeks revealed that Ceriporiopsis subvermispora (CS), mostly CS1, showed a higher selectivity in lignin degradation than CS2, with higher laccase activity but lower manganese peroxide than C2 [76]; (ii) there was a selective degradation of lignin wheat straw and lignin oak wood chips when incubated with Ceriporiopsis subvermispora and Lentinus edodes, and alkylitaconic acids for delignification were produced by Ceriporiopsis subvermispora and Lentinus edodes [67]; (iii) there was degradation of 265 g·kg−1 of lignin and 320 g·kg−1 of neutral detergent soluble when eight different cultivars of wheat straw were incubated with Irpex lacteus for 56 days at 28 °C [77]; and (iv) the lignin content of wheat straw pretreated with Ceriporiopsis subvermispora, CS1 (CBS 347.63), at 24 °C reduced by 48.5% [78].
Lastly, the pretreatment method based on nanotechnology employs the ability of nanoparticles to penetrate the cell membrane of lignocellulose [55]. Recycling and reusing magnetic nanoparticles for subsequent cycles in lignocellulose pretreatment reduces the overall processing cost [20]. Examples of nanotechnology pretreatments are acid-functionalized magnetic nanoparticles and nano-scale shear hybrid alkaline methods. Acid-functionalized magnetic nanoparticles are strong acid nanocatalysts that effectively degrade lignocellulose [59]. In the nano-scale shear hybrid alkaline method, lignocellulose is degraded by combining chemical catalysts and the high-speed shear force [79]. Recent advances in processing lignocellulose for value-added products have been reported. The nanotechnology-based approach in pretreating lignocellulose is a promising application in biorefineries, though there is limited information on using nanoparticles for lignocellulose pretreatment. Recently, nanotechnology applications were reported to enhance the effectiveness of lignocellulose pretreatment, resulting in a cost-effective process. Integration of lignocellulose pretreatment and hydrolysis in one step using enzyme-immobilized functionalized magnetic nanomaterials (nanocatalysts) could present a path-breaking alternative to pretreatment methods. For instance, a cost-effective simultaneous pretreatment and saccharification of lignocellulose using cerium-doped iron oxide nanoparticles (CeFe3O4-NPs) with cellulase and hemicellulase enzymes was reported to effectively generate 20.3 ± 1.01 g·L−1 of glucose and 22.0 ± 2.22 g·L−1 of xylose within 24 h [80]. Sugarcane bagasse pretreated with alkyl sulfonic acid and butyl carboxylic acid functionalized magnetic nanoparticles also yielded 18.83 g·L−1 and 18.67 g·L−1 of sugar concentrations, which were higher than the acid pretreatment (15.40 g·L−1) [81]. Due to nanotechnology prospects in biorefinery sustainability, there should be more focus on using the nanotechnology-based approach in lignocellulose conversion to produce value-added products, such as biohydrogen, biomethane, etc.
Meanwhile, each pretreatment method has its pros and cons. For instance, physical methods do not generate inhibitory compounds. They can offer green pretreatments, in which the product (hydrolyzate) can be directly utilized to generate sugars. Still, physical methods, such as mechanical comminution and pyrolysis, have been considered to be too expensive for a full-scale process due to their high energy consumption; however, the main disadvantage of physical pretreatment methods is their inability to degrade the structure of lignin [56,82].
In chemical pretreatments, the hydrolysis of lignocellulose by acid alters the structure of lignin, thus resulting in high glucose yields and solubilizing hemicellulose to xylose and other sugars. The drawbacks of acid hydrolysis include the high cost of corrosive-resistant equipment and the generation of inhibitors, such as levulinic, formic, and acetic acids. Low inhibitors are produced under alkaline hydrolysis, but this process requires a long residence time and a high cost of alkaline catalysts [83].
Furthermore, steam explosion and carbon dioxide explosion methods are examples of cost-effective physicochemical pretreatments. No inhibitory compounds are generated during carbon dioxide explosion, but the main drawback of this method is that lignin and hemicellulose cannot be modified. Thus, steam explosion is unsuitable for lignocellulosic biomass with high lignin content, and it produces inhibitors such as acetic acid and furan aldehyde [84].
The biological pretreatment method is eco-friendly and cost-effective, as it requires low energy [85,86] and does not generate inhibitors [75]. The main drawbacks of biological pretreatment include its low hydrolysis rate [86], bacterial (e.g., Bacillus sp.) and fungal (e.g., mold) contamination (that can affect lignin degradation) [87,88,89,90], and microbial mutation [88].
Nanotechnology pretreatment methods have been considered the best option for delignification, as these pretreatment methods are cost-effective because the immobilized enzymes are easily retrievable and reusable [91,92]. Depending on the type of nanomaterial used, a few drawbacks of nanotechnology pretreatment methods include their potential poor dispersion abilities of some nanoparticles (due to the difficulty of dispersing in the aqueous solution, where hydronium ions are not effective) [93], and biocatalyst desorption could arise due to the weak bonds [94].
3.2. Hydrolysis of Lignocellulose
The pretreated lignocellulose is then subjected to hydrolysis. Hydrolysis is the process that liberates monomeric sugar molecules, viz. glucose, mannose, galactose, xylose, or arabinose, from structural polysaccharides, such as cellulose and hemicellulose in lignocellulose [95,96]. Cellulose hydrolysis using acids or enzymes has been reported. The first acid hydrolysis technology was developed in 1923, when a sulfuric acid solution was used to hydrolyze white spruce wood; the sugars obtained were glucose, mannose, galactose, xylose, and arabinose [97]. Inorganic acids (e.g., hydrochloric acid and hydrogen fluoride) and organic acids (e.g., citric, oxalic, and maleic acids) were also used in cellulose hydrolysis [44,98,99].
The hydrolysis of cellulose under room temperature using ca. 12 mol·L−1 of hydrochloric acid yielded approximately 32 percent of volume-reducing sugar [100], and cellulose hydrolysis with 6–7 mol·L−1 of hydrochloric acid at 90 °C in the presence of CaCl2 and LiCl as additives resulted in an 85% glucose yield [101]. When cellulose was hydrolyzed using hydrogen fluoride, the sugar yield was approximately 45% at 0 °C [99]. The most notable drawbacks of acid hydrolysis include problems in product/catalyst separation, catalyst recycling, corrosion of reactors, and waste effluent treatment that makes it environmentally unfriendly. In addition, acid hydrolysis necessitates relatively higher temperatures and produces levulinic acid, formic acid, acetic acid, furfural, and other by-products, along with sugars [97], thus reducing sugar yield.
In contrast, enzymatic hydrolysis of cellulose and hemicelluloses (e.g., xylan, galactomannan, and xyloglucan) for producing sugars offers a greater scope of advancement than acid hydrolysis, as it promotes a higher conversion efficiency with no substrate loss. Furthermore, enzymatic hydrolysis employs a non-corrosive operating mode with low process energy [102]. Different enzymes capable of hydrolyzing cellulose and hemicellulose include cellulase and hemicellulase, and they are under the carbohydrate-active enzymes (CAZy) glucoside hydrolase (G.H.) classification system [103] (Table 2). For an effective degradation of cellulose, the cocktail of cellulases, β-1,4-endoglucanase, endoglucanase/cellobiohydrolases, and β-glucosidase are used [104,105]. While xylan is mostly hydrolyzed by β-1,4-endoxylanase and β-1,4-xylosidase, xyloglucan-active β-1,4-endoglucanase and β-1,4-glucosidase hydrolyze xyloglucan, and galactomannan is hydrolyzed by β-1,4-endomannanase and β-1,4-mannosidase [105].

Table 2.
Enzyme families and their lignocellulosic substrates [96,103,105,106,107,108,109].
3.3. Fermentation of Sugars
Fermentation is an enzyme-catalyzed biochemical process in which capable microorganisms convert sugars into new products [110], especially value-added products. Several fermentation products include biofuels like alcohol (e.g., ethanol), gases (such as methane and biogas), and organic acids (e.g., lactic, citric, succinic, and acetic acids) [22,111,112,113,114,115,116]. Producing these new products depends on the selected microorganisms and fermentation conditions. Different fermentation modes and methods have been employed in producing value-added products. These modes include batch fermentation, fed-batch fermentation, repeated-batch fermentation, and continuous fermentation [117].
Importantly, the systems are closed in batch mode, and all the required ingredients and microorganisms are added prior to fermentation. The pH is usually regulated during fermentation via an attached acid or alkaline system [117]. The fed-batch system contains the same required components as in the batch system, but during the fed-batch fermentation process, the depleted required components (e.g., carbon and nitrogen) are sequentially added at regular intervals to actively control microbial growth [118]. In repeated-batch fermentation, microbial cells are increased through repeated re-inoculation of microbial cells from one batch fermentation into the next batch [113,119]. Continuous fermentation is an open system with a continuous influx of fresh media and a continuous efflux of spent media at a constant rate while maintaining a constant internal environment of fermentation [120].
Fermentation methods, such as separate hydrolysis and fermentation (SHF), simultaneous saccharification and fermentation (SSF), separate hydrolysis and co-fermentation (SHcoF), simultaneous saccharification and co-fermentation (SScoF), and consolidated bioprocessing (CBP), have been described in the literature [21,121] as methods that can be used during fermentation tasks. In SHF, lignocellulose is first pretreated, and following the degradation of lignin, the pretreated lignocellulose (hydrolyzate) is subjected to saccharification, followed by fermentation of the simple sugar. In SSF, the pretreated lignocellulose (hydrolyzate) is subjected to simultaneous saccharification and fermentation [21]. Separate hydrolysis and co-fermentation (SHcoF) is similar to SHF; the difference is the presence of at least two sugars for fermentation in SHcoF [122]. Simultaneous saccharification and co-fermentation (SScoF) involves concurrent substrate saccharification and fermentation of at least two sugars in a system [123]. Consolidated bioprocessing consists of a reactor with ligninolytic enzyme production via microorganisms, substrate saccharification, and sugar fermentation in a single process [124].
Numerous capable microorganisms are used to ferment fermentable sugars, and there is an ongoing search for more critical biotechnological microorganisms. The selection of microorganisms for sugar fermentation depends on the product of interest. Microorganisms for sugar fermentation are selected based on their metabolic pathways and optimal environmental conditions, such as pH and temperature. These microorganisms used in fermentation to produce high-value products are divided into three categories: (i) naturally fermenting, (ii) prokaryotic genetically engineered, and (iii) eukaryotic genetically engineered microorganisms [125]. A number of microorganisms have been genetically modified to improve the yield of fermentation products [126,127].
Edible food crops, lignocellulose (non-edible), and algae are the three generations of feedstock sources used to generate sugars for fermentation [128]. However, using edible food crops as feedstock affects the food supply, thus shifting the focus to using non-edible lignocellulose for producing value-added products, as lignocellulose is ubiquitous.
3.4. Purification of Value-Added Product
Purification is one of the most essential stages in value-added product production. Factors that elevate the difficulties of product recovery include, but are not limited to, the low concentration of the product, the presence of impurities, the product produced intracellularly, and heat-labile products. The extraction and purification of fermentation products depend on the specific product. The choice of purification process is based on the concentration of the product, intracellular or extracellular location of the product, physicochemical properties of the product, the impurities in the fermentation broth, acceptable standard of purity, and the product’s intended use.
The stages in the recovery/purification of products, such as organic acids (e.g., citric, lactic, and succinic acids) from fermentation broth (extracellular product), involve the removal of solid particles and microbial cells using filtration and centrifugation followed by broth extraction into different fractions [129]. Ultrafiltration, adsorption, precipitation, distillation, liquid–liquid extraction, supercritical fluid extraction, ion exchange, dialysis, electrodialysis, or membrane separation can be employed for broth extraction [129,130,131,132,133].
Moreover, biofuels can be recovered/purified using different methods. For instance, the conventional distillation process is the first step of ethanol recovery, followed by dehydration using azeotropic distillation, adsorption, pervaporation, or membrane processes [134]. Different methods like equilibrium-based separation (e.g., distillation, liquid–liquid extraction, and supercritical fluid extraction), affinity-based separation (adsorption and ion exchange), solid–liquid separation, and membrane-based separation have been employed in biodiesel purification [135]. Generally, the biodiesel purification process is wet washing using water and dry washing using adsorption, ion exchange, and membrane separation [136]. Membrane separation techniques, pressure swing adsorption, and cryogenic distillation can, therefore, be used for biohydrogen purification [137], while the methods currently used in purifying biogas include biofiltration, adsorption pressurized water scrubbing, and refrigeration [138].
4. Value-Added Products from Lignocellulosic Biomass
A value-added product is obtained by transforming a raw material from its original form into a valuable product. Lignocellulose, considered second-generation feedstock, is a promising raw material for producing a number of value-added products. Recently, the focus has been on efficiently converting lignocellulose into value-added products, as polysaccharides, such as cellulose and hemicellulose, in lignocellulose bio-transformed into new products may be underutilized, resulting in the incomplete use of lignocellulose [46]. Bioconversion strategies that have been intensively used to consume these polysaccharides completely include biochemical and thermochemical approaches [6].
Using lignocellulose as a second-generation producer of bioproducts constitutes a marked improvement over using fossil fuels as sustainable resources. For instance, as an efficient feedstock in producing biomaterials and second-generation biofuel, lignocellulose makes fuel production more sustainable, as it does not compromise global food security [11]. The literature has indicated that lignocellulose, a second-generation producer of biomaterials, has been in existence since 1964, when it was used in textiles [139] and to produce paper from the bleached bark of mulberry in China [140]. Mass production of paper from lignocellulose became cost-effective with the development of pulping and bleaching technologies [141]; lately, its by-products are being used to generate sugars to produce high-value products. Lignocellulose has been used to generate biofuels [22,23] and high-value chemicals [116,142,143,144,145].
4.1. Biofuels
Biofuels are an inexhaustible and biodegradable class of renewable energy obtained from living materials [146]. Biofuels are primarily used as transportation fuels and can be used to generate electricity and heat [147]. The three different generations of biofuel are: (i) first-generation biofuels (produced from edible crops); (ii) second-generation biofuels (produced from lignocellulose); and (iii) third-generation biofuels (produced from algae and microorganisms) [128]. In 2021, the United States of America produced 643,000 barrels of oil equivalent per day, followed by Brazil and Indonesia (which produced 376,000 and 140,000 barrels of oil equivalent per day, respectively) [148,149]. Additionally, biofuels are eco-friendly and capable of eliminating the emission of hazardous gases such as sulfur oxide and carbon monoxide, thereby maintaining a cleaner environment [150]. The most common biofuels include alcohols, biodiesel, biohydrogen, and biogas [22].
4.1.1. Alcohols
Alcohol is often used to denote ethanol or methanol. Since the development of the internal combustion engine, ethanol has been used as a motor fuel [151]. Bioethanol is produced from lignocellulose via pretreatment to break the recalcitrant structure of lignocellulose, followed by the enzymatic saccharification of cellulose and hemicellulose into simple sugars, and, lastly, fermentation of the generated simple sugars by microorganisms such as Saccharomyces cerevisiae, Zymomonas mobilis, and several genetically engineered microorganisms [152,153,154]. Notably, several recombinant microorganisms were developed to ferment hexose and pentose into ethanol [155,156,157]. The concentration and productivity of bioethanol depend on the lignocellulose source, the selected pretreatment method, and the microorganism(s) used in fermentation [157]. Several studies on the production of bioethanol from lignocellulose have been reported. For instance, bioethanol (214.5 g·L−1) was produced from alkali-pretreated rice straw within seven days [158], while 25.3 g·L−1 of ethanol was produced from rice straw [159]. Table 3 presents several studies on the concentrations, yield, and productivity of bioethanol from lignocellulose. Simple sugars are fermented into ethanol in the same or separate reactor. For instance, the production of ethanol in the same reactor takes place in a single step where there is concurrent saccharification of the pretreated lignocellulose and fermentation (SSF) of the generated glucose or xylose, or where there is simultaneous saccharification and co-fermentation (SSCo-F) of sugars (e.g., glucose and xylose). In a separate reactor, ethanol is produced in two steps: saccharification of the pretreated lignocellulose, followed by fermentation of the generated sugar (separate hydrolysis and fermentation) [8,160].
4.1.2. Biodiesel Production
Biodiesel or fatty acid methyl ester (FAME) with lower alkyl esters and long-chain fatty acids [161]. Biodiesel is a clean-burning, renewable substitute for petroleum diesel, and like petroleum diesel, it is used in diesel engines (e.g., generators and vehicles) and heating oil [162,163,164]. Pure biodiesel is called B100, and the most common blend is B20, which contains 20% biodiesel and 80% petroleum diesel [163]. Biodiesel increases energy security and improves air quality [162]. It was reported that a gallon of biodiesel (B100) produces 74% less carbon dioxide than petroleum diesel [163].
Biodiesel can be produced from second-generation biological materials, such as vegetable waste oil, non-edible vegetables, oleaginous microbes, and jatropha [161,165,166]. In biodiesel synthesis using lignocellulose, the pretreated hydrolyzate is saccharified, and oleaginous microbes such as Rhodosporidium toruloides, Gordonia sp., Yarrowia sp., Rhodotorula sp., etc., convert the generated simple sugar into pyruvate that will be further converted to lipids in the microbes [166,167]. The lipids are extracted via cell disruption using various methods that have been described by Khot (2020) [167]. The extracted lipids are then converted into biodiesel via transesterification, in which the lipid reacts with short-chain alcohols, such as methanol and ethanol, in the presence of a catalyst [161,166]. Different biodiesel production processes have been described [161,166]. Still, supercritical non-catalytic and enzymatic biodiesel production technologies are the best, as these technologies can process low-quality feedstock without pretreatment [168]. The production of biodiesel using second-generation feedstock is underdeveloped; as such, limited information on biodiesel production from lignocellulose is available. Biodiesel production was reported when single-cell oil was grown over sugarcane bagasse using oleaginous Yarrowia lipolytical [169]. It was also established that of six lignocellulosic materials, bagasse had the highest lipid (biodiesel) accumulation using Fusarium oxysporum NRC2017 [170].
4.1.3. Biohydrogen Production
Biohydrogen is an elementary substrate for ammonia, methane, methanol, synthesis gas, and olefin hydrogenation synthesis [38]. Commercial-scale biohydrogen production technologies are yet to be established; as such, more research focus should be directed towards biohydrogen production. Generally, biohydrogen can be produced using thermochemical, photoelectrochemical, electrolysis, and biological technologies, among which the biological method (dark fermentation) is eco-friendly and sustainable [171].
In dark fermentation using pretreated lignocellulose, the cellulose and hemicellulose are saccharified, and the generated sugars are subjected to anaerobic fermentation where hydrogen, carbon dioxide, volatile fatty acids, and alcohols are generated via various pathways. During biohydrogen production, glucose is catabolized to pyruvate, and pyruvate formate lyase or pyruvate ferredoxin oxidoreductase converts pyruvate into acetyl-CoA and formate or reduced ferredoxin [20,171]. Moreover, biohydrogen can be generated from formate and reduced ferredoxin (catalyzed by formate lyase and hydrogenase) [20,171]. Microorganisms involved in biohydrogen production include Caloranaerobacter azorensis, Clostridium butyricum, Clostridium Beijerinckii, Clostridium thermocellum, Escherichia coli WDHL, Enterobacter ludwigii, and Enterobacter aerogenes [172,173,174,175,176,177]. Different studies have been conducted to improve biohydrogen production levels. For instance, levels of biohydrogen production were assessed using different nanomaterials, and a study found that the inclusion of metal oxide nanoparticles (e.g., iron, copper, nickel, silver, etc.) improved biohydrogen production levels using Clostridium sp. during dark fermentation [20]. Table 3 includes selected studies conducted for biohydrogen production.
4.1.4. Biogas Production
Biogas (or biomethane) is a renewable pure energy source generated through biodigestion. Biogas has various applications in cooking, drying, cooling, and generating heat and electricity [178]. Biogas is produced through anaerobic digestion under a naturally occurring biological process that involves five steps. The steps are: (i) pretreatment of lignocellulose for easy accessibility to cellulose and hemicellulose to produce hydrolyzates; (ii) saccharification of hydrolyzate, resulting in monomers such as sugars, amino acids, and fatty acids; (iii) conversion of these monomers by acidogens into short-chain volatile fatty acids (acidogenesis); (iv) conversion of volatile fatty acids by acetogens into acetate, carbon dioxide, and hydrogen (acetogenesis); and (v) acetate, carbon dioxide, and hydrogen are converted into biomethane by methanogens (methanogenesis) [179]. Meanwhile, several studies on biomethane production have been reported (Table 3). The production of biomethane using an alkaline hydrogen peroxide-pretreated organic fraction of municipal solid waste, where 463.7 mL·g−1 of biomethane was produced, was reported [180]. A study on biomethane production using anaerobic co-digestion of sewage sludge and cocoa pod husks was conducted, and the result showed 555.7 mL·L−1 of biomethane production [181].

Table 3.
Biofuel production from selected lignocellulosic hydrolyzates.
Table 3.
Biofuel production from selected lignocellulosic hydrolyzates.
| Substrate | Microorganism | Concentration | Productivity | Yield | Reference |
|---|---|---|---|---|---|
| Teak wood hydrolyzate | E. coli MSO4 | 32.90 BioEtOH(a) | 0.45 BioEtOH(b) | 0.96 BioEtOH(c) | [157] |
| Oil palm empty fruit bunches hydrolyzate | Klyveromyces marxinus | 28.10 BioEtOH(a) | 0.58 BioEtOH(b) | 0.28 BioEtOH(c) | [182] |
| Cellulose-rich corncob hydrolyzate | Saccharomyces cerevisiae TC-5 | 31.96 BioEtOH(a) | 0.22 BioEtOH(b) | 0.40 BioEtOH(c) | [183] |
| Xylose-rich Paulownia hydrolyzate | Saccharomyces cerevisiae MEC1133 | 12.50 BioEtOH(a) | 0.51 BioEtOH(b) | 0.26 BioEtOH(c) | [184] |
| Scheffersomyces stipitis CECT1922 | 14.20 BioEtOH(a) | 0.53 BioEtOH(b) | 0.31 BioEtOH(c) | ||
| Shorea robusta hydrolyzate | Saccharomyces cerevisiae | 9.43 BioEtOH(a) | 0.39 BioEtOH(b) | 0.97 BioEtOH(c) | [122] |
| Cornstalk hydrolyzate | Rhodobacter capsulator JL1601 (cheR2−) | - | - | 224.85 BioH(d) | [185] |
| Corncob hydrolyzate | Clostridium acetobutylicum | - | - | 132.00 BioH(d) | [186] |
| Agave hydrolyzate | Clostridium acetobutylicum | - | - | 150.00 BioH(d) | [187] |
| Wheat straw | Anaerobic sludge | - | - | 250.50 BioMeth(e) | [188] |
| Rice straw | Bovine rumen fluid | - | - | 165.00 BioMeth(e) | [189] |
| Corn stover leaf blade | Co-culture of Pecoramyces sp. and Methanobrevibacter sp. | - | - | 42.4 ± 1.00 BioMeth(e) | [190] |
| Corn stover stem pith | Co-culture of Pecoramyces sp. and Methanobrevibacter sp. | - | - | 40.9 ± 1.35 BioMeth(e) | |
| Wood waste | Anaerobic activated sludge | - | - | 175.81 BioMeth(e) | [191] |
| Pig manure | - | - | 245.09 BioMeth(e) | ||
| Co-digestion of wood waste and pig manure | - | - | 234.88 BioMeth(e) |
BioEtOH(a): bioethanol (g·L−1); BioEtOH(b): bioethanol (g·L−1·h−1); BioEtOH(c): bioethanol (g·g−1); BioH(d): biohydrogen (mL·g−1); and BioMeth(e): biomethane (mL·g−1).
4.2. Platform Chemicals
Several platform chemicals, such as dicarboxylic acids (e.g., succinic, fumaric, glutamic, aspartic, and levulinic acids), organic acids (lactic, citric, and acetic acids), ABE (acetone, butanol, and ethanol), polyol (sorbitol, xylitol, and glycerol), and furan (furfural and 5-hydroxymethylfurfural) [192,193], have been extensively produced on laboratory scales.
4.2.1. Fermentative Production of the Platform Chemicals from Lignocellulose
The traditional fermentative production of lactic, succinic, citric, and acetic acids from lignocellulose is completed through sequential pretreatment steps (to make cellulose and hemicellulose accessible to subsequent enzymatic hydrolysis), followed by enzymatic saccharification or hydrolysis (for the generation of simple sugars), and, finally, fermentation of the simple sugars by capable microorganisms. The microorganisms involved in simple sugar (hexose and pentose) fermentation to lactic acid include bacteria (LAB), Enterococcus faecalis, and Rhizopus sp. (for lactic acid production) [113,114,194]. Actinobaccilus succinogenes, Saccharomyces cerevisiae, and other engineered microorganisms were used to produce succinic acid [146,195]. The fungal and bacterial strains that have been found to produce citric acid include: Aspergillus niger, Candida sp., Bacillus sp. and Pseudomonas sp. [196], and acetic acid bacteria (AAB) such as Acetobacter sp. and Gluconacetobacter sp., and are involved in acetic acid production [116].
Fermentable sugars (hexose and pentose) are metabolized to lactic, succinic, citric, and acetic acids through various microbial metabolic pathways. For instance, lactic acid can be produced via the (i) glycolytic, (ii) phosphoketolase, and (iii) pentose phosphate pathways [197]. In the glycolytic pathway, lactic acid bacteria, under anaerobic conditions, use glucose (a carbon source) to produce pyruvate, and lactate dehydrogenase catalyzes the conversion of pyruvate into lactate [197]. In the phosphoketolase pathway, glucose is converted into lactate, ethanol, and carbon dioxide, while bacteria, such as Leuconostoc sp., metabolize pentose to form lactate and acetate [197,198].
Succinic acid is biosynthesized from simple sugars via (i) reductive tricarboxylic acid (rTCA), (ii) the tricarboxylic acid (TCA) oxidation cycle; or (iii) glyoxylic pathways [125]. The rTCA pathway (the main succinic acid production pathway under anaerobic conditions) occurs by converting the simple sugar (e.g., glucose) into phosphoenolpyruvic (PEP) acid and PEP to oxaloacetic acid by PEP carboxykinase. Oxaloacetic acid is then reduced to succinic acid by malate dehydrogenase, fumarase, and fumarate reductase [111,112]. In the TCA cycle, glucose is converted into acetyl-CoA, citrate, isocitrate, and succinate by succinate dehydrogenase under aerobic conditions. The theoretical succinic acid yield of 1 mol mol−1 glucose with the release of 2 mol carbon dioxide is obtained in the TCA cycle, while in the glyoxylic pathway, the succinic acid yield is 1.71 mol mol−1 glucose due to carbon loss during the oxidative carboxylation reaction [111,112].
Citrate is produced from the aldol condensation of oxaloacetate and acetyl CoA in the Krebs cycle (known as the TCA cycle) by citrate synthase. Acetyl CoA may be derived from oxidative decarboxylation of pyruvate from glycolysis (where there is β-oxidation of fatty acids in the mitochondrial matrix) or by oxidative degradation of certain amino acids (e.g., leucine, isoleucine and threonine) [199].
Acetate can be generated from pyruvate via decarboxylation (catalyzed by pyruvate oxidase) or from acetyl CoA through acetyl phosphate (catalyzed by acetate kinase and phosphotransacetylase) [200]. The platform chemicals meet the global demand, as they are used in making various high-value derivatives. Additionally, they contribute to the global economy and promote innovation.
4.2.2. Global Production and Market Values of the Platform Chemicals
The commercial production of lactic, succinic, citric, and acetic acids from lignocellulose has gained enormous attention. The demand for lactic acid (LA) in the past years has mainly increased due to polylactic acid (PLA) production, as LA serves as a building block for PLA production. Polylactic acid is used for drug delivery systems, prostheses, biodegradable packaging materials, and surgical suture production [201]. Furthermore, lactic acid is also used in the cosmetics, food, pharmaceutical, and chemical industries. For instance, LA is used in producing: (i) parenteral dialysis solutions, (ii) moisturizing and anti-acne creams, (iii) flavoring and preservatives, and (iv) acidulants and pH regulators [201]. In 2022, the lactic acid market volume was approximately 1.5 million metric tons, and its market value reached about USD 1.46 billion [202]. Meanwhile, the global lactic acid market was valued at USD 1.6 billion in 2023, and it is anticipated to grow at a compound annual growth rate (CAGR) of 12.4% between 2023 and 2028 [203]. It is projected to grow to almost 2.8 million metric tons in 2030 [202]. The USA is North America’s largest producer and consumer of lactic acid, with a total consumption of over 800,000 tons/year [204]. Countries such as the U.S., Netherlands, Belgium, Japan, Switzerland, China, India, Switzerland, and Austria are the key players in the global lactic acid market [204].
The demand for succinic acid (a dicarboxylic acid) is due to the global movement towards sustainability. In the organic and natural food industry, succinic acid is frequently used as a taste enhancer and food additive due to its ability to increase flavor and improve shelf life [205]. Succinic acid is also extensively used in the pharmaceutical industry as an excipient medicine formulation, and it is a precursor in the chemical industry to produce resins, polymers, solvents, plastics, fumaric acids, and glyoxylic acids [205,206,207]. In 2022, the succinic acid market value was USD 0.13 billion, and it is projected to grow from USD 0.15 billion in 2023 to USD 0.30 billion by 2030, with a CAGR of 6% in this forecasted period of 2023–2030 [208]. The Asia–Pacific region (China, Japan, and India) has the largest market share of the succinic acid market, and the growth of the succinic acid market in the Asia–Pacific region is due to the increasing demand for bio-based chemicals. Similarly, North America’s (the USA and Canada) succinic acid accounts for the second-largest market share, followed by Europe (the UK, Germany, Italy, France, and Spain) [208].
Citric acid is a weak organic acid commonly found in lemon juice and other citrus fruits. It is one of the most widely used organic acids in the food industries as an acidulant, a flavoring, a coloring agent, and a preservative agent owing to its long shelf life [209]. Citric acid is also used in personal care products, detergents, sealants, pharmaceutical products, plastics, polymers, and animal nutrition [196,209]. The global citric acid market reached approximately 2.59 million tons in 2022 and is expected to reach 3.29 million tons in 2028 from 2023 to 2028 [210]. Relatedly, the global citric acid market size was evaluated at USD 3.52 billion in 2022 and is projected to grow to about USD 5.12 billion in 2032, with a CAGR of 3.82% from 2023 to 2032 [211]. Specifically, China is the highest producer of citric acid, followed by the USA and Europe [212].
Acetate is an anion form of acetic acid, and salts are formed by combining acetic acid with alkaline or other bases. Acetate is a vital building block in various industry applications [213]. Acetate is a coating solvent for paints and varnishes, printing inks, and nail polish [214]. It is used in the food industries as a food preservative (e.g., sodium acetate and potassium acetate) and a synthetic flavor enhancer (e.g., ethyl acetate, the ester of ethanol and acetic acid) [213]. Ethyl acetate is also used as a solvent for stains, fat, and dry cleaning [214,215], while vinyl acetate monomer (produced from the combination of acetic acid and ethene in the presence of oxygen), a building block of polyvinyl alcohol and polyvinyl acetate, is used to make packaging materials [216]. The increase in acetic acid demand results from its end-use applications, including vinyl acetate monomer and ethyl acetate. Vinyl acetate monomer accounts for 35% of global acetic acid consumption, and polyvinyl alcohol, polyvinyl acetate, and ethene vinyl acetate are the main downstream markets for vinyl acetate monomer [215]. Meanwhile, the top vinyl acetate monomer-producing countries are China, the USA, Taiwan, Japan, and Germany, and the major ethyl acetate-producing countries are China, India, the United Kingdom, Japan, and Brazil [215].
5. Challenges and Alleviation Strategies in Upcycling Lignocellulose
Several difficulties must be mitigated to be able to fully utilize lignocellulose for economically feasible value-added product production. These difficulties include the high cost of pretreatment technology, production of inhibitors after delignification (which adversely affects the quality of the hydrolyzed sugars for fermentation), feedback inhibition, substrate inhibition, end-product inhibition, the high cost of hydrolytic enzymes, and challenges in developing efficient enzyme cocktails for the effective hydrolysis of cellulose and hemicellulose [15,117].
By-products (inhibitors) such as coumaric acid, acetic acid, formic acid, furfural, levulinic acid, and aldehyde formed during the chemical pretreatment of lignocellulose have been reported to affect microbial growth, substrate utilization, and fermentation adversely [217,218,219]. In saccharification of lignocellulose hydrolyzates, increased cellobiose and glucose concentrations could inhibit cellulase in breaking cellulose to cellobiose and glucose, thereby resulting in feedback inhibition [21]. In fermentation, challenges of substrate and end-product inhibition could occur. Substrate inhibition occurs when the fermentative microorganisms’ growth is inhibited due to a high feedstock concentration (glucose or pentose). Growth inhibition occurs due to low water activity, high osmotic pressure, and cell lysis [21]. Likewise, end-product inhibition occurs when overexposure to the product formed causes the product to penetrate the microbial cell membrane, which results in increased intracellular activity that disrupts the cell, thereby leading to cell death [21].
The cost-effective operation of lignocellulose biorefinery will incline if these challenges are abated and all three constituents (cellulose, hemicellulose, and lignin) of lignocellulose are efficiently converted into value-added products. Nanotechnology, where enzyme immobilization is used in delignification, could be the best pretreatment technology for solving the bottlenecks of by-product inhibition [94]. Reports have shown that feedback inhibition can be minimized by removing sugars during saccharification using electrodialysis, avoiding cellobiose accumulation, and optimizing enzymatic activities during hydrolysis [220,221]. Methods used to reduce substrate inhibition include continuous and semi-continuous fermentation [222], and end-product inhibition can be overcome via fermentation product removal using nanofiltration, ion exchange resin, electrodialysis, or ultrafiltration [21,223,224,225].
Essentially, crude enzymes produced directly from microorganisms can be used in cellulose and hemicellulose saccharification to reduce the cost of hydrolytic enzymes. Efficient enzyme cocktails for effective cellulose and hemicellulose hydrolysis can, therefore, be developed using computer-generated models or simulations [226] before hydrolysis. Process systems engineering is a computer-generated simulation that can offer an efficient and cost-effective approach for designing and optimizing efficient enzyme cocktails for the effective hydrolysis of cellulose and hemicellulose.
6. Prospects of Lignocellulose
Lignocellulose can be used in these ways: (i) directly as raw materials in several chemical and biological treatments, and (ii) in producing value-added products [227]. Barley lignocellulose components, such as straw and hulls, could be applied directly in biological and chemical conversions, such as phytoremediation, composting-based processes [228], and algae growth inhibition [229].
Value-added products are mostly produced from cellulose and hemicellulose, while lignin is underutilized and can be used for various applications. The typical commercial application of lignin includes using lignosulfonate to produce value-added products such as concrete additives, dust control, animal feed pelleting aid, and phenol formaldehyde [10]. Also, lignin has been used to synthesize adsorbents to remove toxic dyes and organic molecules [230]. Furthermore, lignin has been extensively employed in preparing fluorescent probes owing to its aromatic structures and optical properties [231]. It was reported that lignin-based polyurethanes doped with multiwall carbon nanotubes at concentrations above the percolation threshold are suitable for sensor applications [232]. The emerging developments in nanotechnology have led to exploring lignocellulosic bionanomaterials, specifically nanolignin, in developing highly sensitive biosensors [233]. For instance, in a study, gold bare electrodes were modified with organosolv and kraft lignin nanoparticles synthesized from sulfur-free and sulfur lignin, and the modified electrodes were assembled with concanavalin A and glucose oxidase for biosensing glucose. The modified electrode with the gold kraft lignin nanoparticles concanavalin A and glucose oxidase was highly sensitive to glucose [234]. Using lignin as a nanomaterial in developing zinc oxide nanocomposites also showed nanolignin as an excellent antioxidant, UV-blocking, and antimicrobial agent that can be used as a body cream additive [235].
There are more prospects for using lignin to produce value-added products. For instance, lignin can be processed for electronic and other device packaging. Using lignin-based packaging products may likewise reduce the use of polystyrene in the packaging industry, since polystyrene contains styrene that can cause injury to the nervous system after long-term exposure [236]. Lignin can also be used in crafts and interior decorations where natural textures or surfaces must be imitated. Due to lignin’s optical properties, nanolignin can be used in developing environmental biosensors (to monitor actual environmental conditions and detect contaminants) and biomimetic sensors (to simulate the biological organs’ function and performance). Another prospect of lignocellulose is the development of cellulose and hemicellulose in animal feeding. Cellulose and hemicellulose can be processed with nutritional supplements to meet dietary requirements.
7. Conclusions
Lignocellulose is a vital, cheap, and abundantly available biomass feedstock that includes agricultural and forestry residues from plant, industrial, and food wastes. The concept of reusing, recycling, and converting lignocellulose into bio-based products reduces environmental pollution in terms of agricultural waste disposal and the emission of toxic substances into the environment, and it contributes to circular economy development.
Lignocellulose undergoes different biorefinery processing stages, namely pretreatment, hydrolysis, fermentation, and product purification/recovery, before being converted into value-added products. Nanotechnology, physical, chemical, physicochemical, and biological pretreatment methods could be utilized to access cellulose and hemicellulose for sugar generation. Furthermore, cellulose and hemicellulose saccharification is carried out using enzymatic hydrolysis, and batch fermentation, fed-batch fermentation, repeated-batch fermentation, and continuous fermentation modes are deployed to produce value-added products. The fermentation methods include separate hydrolysis and fermentation, simultaneous saccharification and fermentation, separate hydrolysis and co-fermentation, simultaneous saccharification and co-fermentation, and consolidated bioprocessing. The microorganisms that are used to produce high-value products are naturally fermenting, prokaryotic genetically engineered, and eukaryotic genetically engineered microorganisms. It should also be noted that the purification methods depend on the value-added products. The purification methods include, but are not limited to, distillation and dehydration, liquid–liquid extraction, supercritical fluid extraction, adsorption, biofiltration, ion exchange, dialysis, electrodialysis, and membrane separation.
The value-added products from lignocellulose include biofuels and platform chemicals. Challenges in upcycling lignocellulose include the high cost of pretreatment technology, generation of by-product inhibitors after pretreatment, feedback-, substrate-, and end-product inhibition, high cost of hydrolytic enzymes, and development of efficient enzyme cocktails for effective hydrolysis of cellulose and hemicellulose. Nanotechnology could, therefore, be the best pretreatment technology for solving by-product inhibition, and feedback inhibition can be alleviated by optimizing enzymatic activities during hydrolysis and using electrodialysis to remove sugars during saccharification to prevent cellobiose accumulation. Substrate inhibition can be prevented using continuous and semi-continuous fermentation, and end-product inhibition can be overcome by removing fermentation products using nanofiltration, ion exchange resin, or ultrafiltration. Using crude enzymes produced directly from microorganisms in saccharification could reduce hydrolytic enzyme costs. Additionally, efficient enzyme cocktails can be developed using computer-generated models or simulations for effective cellulose and hemicellulose hydrolysis before laboratory or industrial hydrolysis.
Lignocellulose has many prospects. For instance, cellulose and hemicellulose can be used in animal feeding, where they can be processed with nutritional supplements to meet dietary requirements. The lignin obtained can be processed for electronic and other device packaging and used in crafts and interior decorations. Nanolignin can be used to develop environmental biosensors (for environmental monitoring) and biomimetic sensors (for simulation of the biological organs’ function and performance). The integration process that involves the use of cellulose, hemicellulose, and the generated lignin could thereby result in the production of value-added products with the zero-waste concept. This review has the potential to raise more awareness of the biotechnological importance of lignocellulose and its prospects in producing high-value products. It also contributes to alleviating the challenges of lignocellulose conversion into value-added products.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The author declares no conflict of interest.
References
- Brethauer, S.; Shahab, R.L.; Studer, M.H. Impacts of Biofilms on the Conversion of Cellulose. Appl. Microbiol. Biotechnol. 2020, 104, 5201–5212. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.; Le, P.; Mai, P.; Nguyen, Q. Ethanol Production from Lignocellulosic Biomass; IntechOpen: Rijeka, Croatia, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A Review on Alkaline Pretreatment Technology for Bioconversion of Lignocellulosic Biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Tsai, M.-L.; Sharma, V.; Sun, P.-P.; Nargotra, P.; Bajaj, B.K.; Chen, C.-W.; Dong, C.-D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2022, 10, 6. [Google Scholar] [CrossRef]
- Nigam, P.S.; Singh, A. Production of Liquid Biofuels from Renewable Resources. Prog. Energy Combust. Sci. 2011, 37, 52–68. [Google Scholar] [CrossRef]
- Dahman, Y.; Syed, K.; Begum, S.; Roy, P.; Mohtasebi, B. Biofuels: Their Characteristics and Analysis. In Biomass, Biopolymer-Based Materials, and Bioenergy: Construction, Biomedical, and Other Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 277–325. [Google Scholar] [CrossRef]
- Zhao, Y.; Wu, B.; Yan, B.; Gao, P. Mechanism of Cellobiose Inhibition in Cellulose Hydrolysis by Cellobiohydrolase. Sci. China Ser. C Life Sci. 2004, 47, 18–24. [Google Scholar] [CrossRef]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic Biomass for Bioethanol: An Overview on Pretreatment, Hydrolysis and Fermentation Processes. Rev. Environ. Health 2019, 34, 1–12. [Google Scholar] [CrossRef]
- Zhang, W.; Qin, W.; Li, H.; Wu, A.M. Biosynthesis and Transport of Nucleotide Sugars for Plant Hemicellulose. Front. Plant Sci. 2021, 12, 1–13. [Google Scholar] [CrossRef]
- Doherty, W.O.S.; Mousavioun, P.; Fellows, C.M. Value-Adding to Cellulosic Ethanol: Lignin Polymers. Ind. Crops Prod. 2011, 13, 259–276. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paës, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 1–11. [Google Scholar] [CrossRef]
- Cai, J.; He, Y.; Yu, X.; Banks, S.W.; Yang, Y.; Zhang, X.; Yu, Y.; Liu, R.; Bridgwater, A.V. Review of Physicochemical Properties and Analytical Characterization of Lignocellulosic Biomass. Renew. Sustain. Energy Rev. 2017, 76, 309–322. [Google Scholar] [CrossRef]
- Dutta, S. Top 10 Agricultural Producing Countries in the World. 2020. Available online: https://www.insidermonkey.com/blog/top-10-agricultural-producing-countries-in-the-world-885643/6/NEWS (accessed on 19 September 2023).
- Koul, B.; Yakoob, M.; Shah, M.P. Agricultural Waste Management Strategies for Environmental Sustainability. Environ. Res. 2022, 206, 112285. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Korstad, J.; Guldhe, A.; Kothari, R. Editorial: Emerging Feedstocks and Clean Technologies for Lignocellulosic Biofuel. Front. Energy Res. 2022, 10, 1–2. [Google Scholar] [CrossRef]
- Bhuvaneshwari, S.; Hettiarachchi, H.; Meegoda, J.N. Crop Residue Burning in India: Policy Challenges and Potential Solutions. Int. J. Environ. Res. Public Health 2019, 16, 832. [Google Scholar] [CrossRef] [PubMed]
- Porichha, G.K.; Hu, Y.; Rao, K.T.V.; Xu, C.C. Crop Residue Management in India: Stubble Burning vs. Other Utilizations Including Bioenergy. Energies 2021, 14, 4281. [Google Scholar] [CrossRef]
- COP26. Together for Our Planet. 2021. Available online: https://www.un.org/en/climatechange/cop26 (accessed on 21 September 2023).
- Ruensodsai, T.; Sriariyanun, M. Sustainable Development and Progress of Lignocellulose Conversion to Platform Chemicals. J. King Mongkut’s Univ. Technol. North Bangk. 2022, 32, 1–4. [Google Scholar] [CrossRef]
- Singhvi, M.; Zinjarde, S.; Kim, B.S. Sustainable Strategies for the Conversion of Lignocellulosic Materials into Biohydrogen: Challenges and Solutions toward Carbon Neutrality. Energies 2022, 15, 8987. [Google Scholar] [CrossRef]
- Ojo, A.O.; de Smidt, O. Lactic Acid: A Comprehensive Review of Production to Purification. Processes 2023, 11, 688. [Google Scholar] [CrossRef]
- Saini, J.K.; Saini, R.; Tewari, L. Lignocellulosic Agriculture Wastes as Biomass Feedstocks for Second-Generation Bioethanol Production: Concepts and Recent Developments. 3 Biotech 2015, 5, 337–353. [Google Scholar] [CrossRef] [PubMed]
- Shahbandeh, M. Rice—Statistics and Facts. 2023. Available online: https://www.statista.com/topics/1443/rice/#topicOverview (accessed on 29 August 2023).
- World Population Review. Wheat Production by Country. 2023. Available online: https://worldpopulationreview.com/country (accessed on 29 August 2023).
- Goodman, B.A. Utilization of Waste Straw and Husks from Rice Production: A Review. J. Bioresour. Bioprod. 2020, 5, 143–162. [Google Scholar] [CrossRef]
- Singh, R.B.; Sana, R.C.; Singh, M.; Chandra, D.; Shukla, S.G.; Walli, T.K.; Pradhan, P.K.; Kessels, H.P.P. Rice Straw—Its Production and Utilization in India. In Handbook for Straw Feeding Systems; Indian Council of Agricultural Research: New Delhi, Indian, 1995; pp. 325–337. [Google Scholar]
- Ajala, E.O.; Ighalo, J.O.; Ajala, M.A.; Adeniyi, A.G.; Ayanshola, A.M. Sugarcane Bagasse: A Biomass Sufficiently Applied for Improving Global Energy, Environment and Economic Sustainability. Bioresour. Bioprocess. 2021, 8, 87. [Google Scholar] [CrossRef]
- Pennington, D. Corn Stover: What Is Its Worth? 2013. Available online: https://www.canr.msu.edu/news/corn_stover_what_is_its_worth (accessed on 27 August 2023).
- Yang, J.; Ching, Y.C.; Chuah, C.H. Applications of Lignocellulosic Fibers and Lignin in Bioplastics: A Review. Polymers 2019, 11, 751. [Google Scholar] [CrossRef] [PubMed]
- Yetri, Y.; Hoang, A.T.; Mursida; Dahlan, D.; Muldarisnur; Taer, E.; Chau, M.Q. Synthesis of Activated Carbon Monolith Derived from Cocoa Pods for Supercapacitor Electrodes Application. Energy Sources Part A Recover. Util. Environ. Eff. 2020, 1–15. [Google Scholar] [CrossRef]
- Yadav, N.; Nain, L.; Khare, S.K. One-Pot Production of Lactic Acid from Rice Straw Pretreated with Ionic Liquid. Bioresour. Technol. 2021, 323, 124563. [Google Scholar] [CrossRef] [PubMed]
- Luque, L.; Oudenhoven, S.; Westerhof, R.; Van Rossum, G.; Berruti, F.; Kersten, S.; Rehmann, L. Comparison of Ethanol Production from Corn Cobs and Switchgrass Following a Pyrolysis-Based Biorefinery Approach. Biotechnol. Biofuels 2016, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A. Underutilized Lignocellulosic Waste as Sources of Feedstock for Biofuel Production in Developing Countries. Front. Energy Res. 2022, 10, 1–21. [Google Scholar] [CrossRef]
- Borrega, M.; Hinkka, V.; Hörhammer, H.; Kataja, K.; Kenttä, E.; Ketoja, J.A.; Palmgren, R.; Salo, M.; Sundqvist-Andberg, H.; Tanaka, A. Utilizing and Valorizing Oat and Barley Straw as an Alternative Source of Lignocellulosic Fibers. Materials 2022, 15, 7826. [Google Scholar] [CrossRef] [PubMed]
- Keshav, P.K.; Banoth, C.; Kethavath, S.N.; Bhukya, B. Lignocellulosic Ethanol Production from Cotton Stalk: An Overview on Pretreatment, Saccharification and Fermentation Methods for Improved Bioconversion Process. Biomass Convers. Biorefinery 2021, 13, 4477–4493. [Google Scholar] [CrossRef]
- Dong, M.; Wang, S.; Xu, F.; Wang, J.; Yang, N.; Li, Q.; Chen, J.; Li, W. Pretreatment of Sweet Sorghum Straw and Its Enzymatic Digestion: Insight into the Structural Changes and Visualization of Hydrolysis Process. Biotechnol. Biofuels 2019, 12, 276. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of Lignocellulosic Materials as Substrates for Fermentation Processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef]
- Díaz-González, A.; Luna, M.Y.P.; Morales, E.R.; Saldaña-Trinidad, S.; Blanco, L.R.; de la Cruz-Arreola, S.; Pérez-Sariñana, B.Y.; Robles-Ocampo, J.B. Assessment of the Pretreatments and Bioconversion of Lignocellulosic Biomass Recovered from the Husk of the Cocoa Pod. Energies 2022, 15, 3544. [Google Scholar] [CrossRef]
- Azhar, S.; Wang, Y.; Lawoko, M.; Henriksson, G.; Lindström, M.E. Extraction of Polymers from Enzyme-Treated Softwood. BioResources 2011, 6, 4606–4614. [Google Scholar] [CrossRef]
- Dou, J.; Chandgude, V.; Vuorinen, T.; Bankar, S.; Hietala, S.; Lê, H.Q. Enhancing Biobutanol Production from Biomass Willow by Pre-Removal of Water Extracts or Bark. J. Clean. Prod. 2021, 327, 129432. [Google Scholar] [CrossRef]
- Lempiäinen, H.; Lappalainen, K.; Haverinen, J.; Tuuttila, T.; Hu, T.; Jaakkola, M.; Lassi, U. The Effect of Mechanocatalytic Pretreatment on the Structure and Depolymerization of Willow. Catalysts 2020, 10, 255. [Google Scholar] [CrossRef]
- Fellak, S.; Rafik, M.; Haidara, H.; Boukir, A.; Lhassani, A. Study of Natural Degradation Effect on Lignocellulose Fibers of Archaeological Cedar Wood: Monitoring by Fourier Transform Infrared (FTIR) Spectroscopy. MATEC Web Conf. 2022, 360, 6. [Google Scholar] [CrossRef]
- Rego, F.; Soares Dias, A.P.; Casquilho, M.; Rosa, F.C.; Rodrigues, A. Fast Determination of Lignocellulosic Composition of Poplar Biomass by Thermogravimetry. Biomass Bioenergy 2019, 122, 375–380. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, K.; Xiao, L.P.; Sun, R.C.; Song, G. Total Utilization of Lignin and Carbohydrates in Eucalyptus Grandis: An Integrated Biorefinery Strategy towards Phenolics, Levulinic Acid, and Furfural. Biotechnol. Biofuels 2020, 13, 2. [Google Scholar] [CrossRef]
- Pejin, J.; Radosavljević, M.; Kocić-Tanackov, S.; Djukić-Vuković, A.; Mojović, L. Lactic Acid Fermentation of Brewer’s Spent Grain Hydrolysate by Lactobacillus Rhamnosus with Yeast Extract Addition and pH Control. J. Inst. Brew. 2017, 123, 98–104. [Google Scholar] [CrossRef]
- Mathews, S.L.; Pawlak, J.; Grunden, A.M. Bacterial Biodegradation and Bioconversion of Industrial Lignocellulosic Streams. Appl. Microbiol. Biotechnol. 2015, 99, 2939–2954. [Google Scholar] [CrossRef] [PubMed]
- Rueda, C.; Calvo, P.A.; Moncalián, G.; Ruiz, G.; Coz, A. Biorefinery Options to Valorize the Spent Liquor from Sulfite Pulping. J. Chem. Technol. Biotechnol. 2015, 90, 2218–2226. [Google Scholar] [CrossRef]
- Ojewumi, M.E.; Emetere, M.E.; Obanla, O.R.; Babatunde, D.E.; Adimekwe, E.G. Bio-Conversion of Waste Paper Into Fermentable Sugars—A Review. Front. Chem. Eng. 2022, 4, 926400. [Google Scholar] [CrossRef]
- Verma, M.; Mishra, V. Utilization of Fruit-Vegetable Waste as Lignocellulosic Feedstocks for Bioethanol Fermentation. In Food Waste to Green Fuel: Trend & Development. Clean Energy Production Technologies; Srivastava, N., Malik, M., Eds.; Springer: Singapore, 2022; pp. 189–211. [Google Scholar]
- Yan, Q.; Liu, X.; Wang, Y.; Li, H.; Li, Z.; Zhou, L.; Qu, Y.; Li, Z.; Bao, X. Cow Manure as a Lignocellulosic Substrate for Fungal Cellulase Expression and Bioethanol Production. AMB Express 2018, 8, 190. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Awasthi, M.K.; Zhao, J.; Ren, X.; Li, R.; Wang, Z.; Wang, M.; Zhang, Z. Improvement of Pig Manure Compost Lignocellulose Degradation, Organic Matter Humification and Compost Quality with Medical Stone. Bioresour. Technol. 2017, 243, 771–777. [Google Scholar] [CrossRef]
- Inyang, V.; Laseinde, O.T.; Kanakana, G.M. Techniques and Applications of Lignocellulose Biomass Sources as Transport Fuels and Other Bioproducts. Int. J. Low-Carbon Technol. 2022, 17, 900–909. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Alriksson, B.; Nilvebrant, N.O. Bioconversion of Lignocellulose: Inhibitors and Detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Nauman Aftab, M.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different Pretreatment Methods of Lignocellulosic Biomass for Use in Biofuel Production. In Biomass for Bioenergy—Recent Trends and Future Challenges; IntechOpen: Rijeka, Croatia, 2019; pp. 1–208. [Google Scholar] [CrossRef]
- Chandel, H.; Kumar, P.; Chandel, A.K.; Verma, M.L. Biotechnological Advances in Biomass Pretreatment for Bio-Renewable Production through Nanotechnological Intervention. Biomass Convers. Biorefinery 2022, 4, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, J. Hydrolysis of Lignocellulosic Materials for Ethanol Production: A Review. Bioresour. Technol. 2002, 83, 1–11. [Google Scholar] [CrossRef]
- Yogalakshmi, K.; Poornima, D.T.; Sivashanmugam, P.; Kavitha, S.; Yukesh, K.R.; Varjani, S.; AdishKumar, S.; Kumar, G.; Rajesh, B.J. Lignocellulosic Biomass-Based Pyrolysis: A Comprehensive Review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Arora, A.; Nandal, P.; Singh, J.; Verma, M.L. Nanobiotechnological Advancements in Lignocellulosic Biomass Pretreatment. Mater. Sci. Energy Technol. 2020, 3, 308–318. [Google Scholar] [CrossRef]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent Trends in the Pretreatment of Lignocellulosic Biomass for Value-Added Products. Front. Energy Res. 2018, 6, 1–19. [Google Scholar] [CrossRef]
- Sarip, H.; Sohrab Hossain, M.; Mohd Azemi, M.N.; Allaf, K. A Review of the Thermal Pretreatment of Lignocellulosic Biomass towards Glucose Production: Autohydrolysis with DIC Technology. BioResources 2016, 11, 10625–10653. [Google Scholar] [CrossRef]
- Rasri, W.; Thu, V.T.; Corpuz, A.; Nguyen, L.T. Preparation and Characterization of Cellulose Nanocrystals from Corncob via Ionic Liquid [Bmim][HSO4] Hydrolysis: Effects of Major Process Conditions on Dimensions of the Product. RSC Adv. 2023, 13, 19020–19029. [Google Scholar] [CrossRef] [PubMed]
- Sahay, S. Impact of Pretreatment Technologies for Biomass to Biofuel Production. In Clean Energy Production Technologies: Substrate Analysis for Effective Biofuels Production; Springer Nature: New York, NY, USA, 2020; pp. 173–216. [Google Scholar]
- Morais, A.R.C.; Da Costa Lopes, A.M.; Bogel-Łukasik, R. Carbon Dioxide in Biomass Processing: Contributions to the Green Biorefinery Concept. Chem. Rev. 2014, 115, 3–27. [Google Scholar] [CrossRef]
- Niu, D.; Zuo, S.; Jiang, D.; Tian, P.; Zheng, M.; Xu, C. Treatment Using White Rot Fungi Changed the Chemical Composition of Wheat Straw and Enhanced Digestion by Rumen Microbiota in Vitro. Anim. Feed Sci. Technol. 2018, 237, 46–54. [Google Scholar] [CrossRef]
- Wan, C.; Li, Y. Fungal Pretreatment of Lignocellulosic Biomass. Biotechnol. Adv. 2012, 30, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- van Kuijk, S.J.A.; Sonnenberg, A.S.M.; Baars, J.J.P.; Hendriks, W.H.; del Río, J.C.; Rencoret, J.; Gutiérrez, A.; de Ruijter, N.C.A.; Cone, J.W. Chemical Changes and Increased Degradability of Wheat Straw and Oak Wood Chips Treated with the White Rot Fungi Ceriporiopsis Subvermispora and Lentinula Edodes. Biomass Bioenergy 2017, 105, 381–391. [Google Scholar] [CrossRef]
- Fan, L.T.; Gharpuray, M.M.; Lee, Y.-H. Cellulose Hydrolysis; Aiba, S., Fan, L.T., Fiechter, A., Schiigerl, K.K., Eds.; Springer: Berlin, Germany, 1987. [Google Scholar]
- Zabel, R.A.; Morrell, J.J. Chemical Changes in Wood Caused by Decay Fungi. In Wood Microbiology; Academic Press: Cambridge, MA, USA, 2020; pp. 215–244. [Google Scholar] [CrossRef]
- Abdel-Hamid, A.M.; Solbiati, J.O.; Cann, I.K.O. Insights into Lignin Degradation and Its Potential Industrial Applications. Adv. Appl. Microbiol. 2013, 82, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Eriksson, K.-E.; Blanchette, R.; Ander, P. Biodegradation of Hemicelluloses. In Microbial and Enzymatic Degradation of Wood and Wood Components; Springer: Berlin, Germany, 1990; pp. 252–333. [Google Scholar]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef] [PubMed]
- Canam, T.; Town, J.; Iroba, K.; Tabil, L.; Dumonceaux, T. Pretreatment of Lignocellulosic Biomass Using Microorganisms: Approaches, Advantages, and Limitations. In New Microbial Technologies for Advanced Biofuels: Toward More Sustainable Production Methods; Apple Academic Press: Florida, FL, USA, 2015; pp. 145–175. [Google Scholar] [CrossRef]
- Peralta, R.M.; da Silva, B.P.; Gomes Côrrea, R.C.; Kato, C.G.; Vicente Seixas, F.A.; Bracht, A. Enzymes from Basidiomycetes-Peculiar and Efficient Tools for Biotechnology. In Biotechnology of Microbial Enzymes: Production, Biocatalysis and Industrial Applications; Academic Press: Cambridge, MA, USA, 2017; pp. 119–149. [Google Scholar] [CrossRef]
- Wu, Z.; Peng, K.; Zhang, Y.; Wang, M.; Yong, C.; Chen, L.; Qu, P.; Huang, H.; Sun, E.; Pan, M. Lignocellulose Dissociation with Biological Pretreatment towards the Biochemical Platform: A Review. Mater. Today Bio 2022, 16, 100445. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Differences between Two Strains of Ceriporiopsis Subvermispora on Improving the Nutritive Value of Wheat Straw for Ruminants. J. Appl. Microbiol. 2017, 123, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zuo, S.; Ren, J.; Li, C.; Zheng, M.; Jiang, D.; Xu, C. Effect of Wheat Straw Types on Biological Delignification and in Vitro Rumen Degradability of Wheat Straws during Treatment with Irpex Lacteus. Anim. Feed Sci. Technol. 2020, 267, 114558. [Google Scholar] [CrossRef]
- Nayan, N.; Sonnenberg, A.S.M.; Hendriks, W.H.; Cone, J.W. Variation in the Solubilization of Crude Protein in Wheat Straw by Different White-Rot Fungi. Anim. Feed Sci. Technol. 2018, 242, 135–143. [Google Scholar] [CrossRef]
- Sowmya Dhanalakshmi, C.; Madhu, P. Biofuel Production of Neem Wood Bark (Azadirachta Indica) through Flash Pyrolysis in a Fluidized Bed Reactor and Its Chromatographic Characterization. In Energy Sources, Part A: Recovery, Utilization and Environmental Effects; Bellwether Publishing, Ltd.: Columbia, MD, USA, 2021; Volume 43, pp. 428–443. [Google Scholar] [CrossRef]
- Kim, M.; Singhvi, M.S.; Kim, B.S. Eco-Friendly and Rapid One-Step Fermentable Sugar Production from Raw Lignocellulosic Biomass Using Enzyme Mimicking Nanomaterials: A Novel Cost-Effective Approach to Biofuel Production. Chem. Eng. J. 2023, 465, 142879. [Google Scholar] [CrossRef]
- Ingle, A.P.; Philippini, R.R.; Silvério da Silva, S. Pretreatment of Sugarcane Bagasse Using Two Different Acid-Functionalized Magnetic Nanoparticles: A Novel Approach for High Sugar Recovery. Renew. Energy 2020, 150, 957–964. [Google Scholar] [CrossRef]
- Aslanzadeh, S.; Ishola, M.M.; Richards, T.; Taherzadeh, M.J. An Overview of Existing Individual Unit Operations. In Biorefineries: Integrated Biochemical Processes for Liquid Biofuels; Elsevier: Amsterdam, The Netherlands, 2014; pp. 3–36. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.S.; Silva, A.S.; Moutta, R.O.; Ferreira-Leitão, V.S.; Barros, R.R.; Ferrara, M.A.; Bon, E.P. Biomass Pretreatment: A Critical Choice for Biomass Utilization via Biotechnological Routes. BMC Proc. 2014, 8, 34. [Google Scholar] [CrossRef]
- Akhtar, N.; Gupta, K.; Goyal, D.; Goyal, A. Recent Advances in Pretreatment Technologies for Efficient Hydrolysis of Lignocellulosic Biomass. Environ. Prog. Sustain. Energy 2016, 35, 489–511. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A. Lata Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Antunes, F.A.F.; Chandel, A.K.; Terán-Hilares, R.; Ingle, A.P.; Rai, M.; dos Santos Milessi, T.S.; da Silva, S.S.; dos Santos, J.C. Overcoming Challenges in Lignocellulosic Biomass Pretreatment for Second-Generation (2G) Sugar Production: Emerging Role of Nano, Biotechnological and Promising Approaches. 3 Biotech 2019, 9, 230. [Google Scholar] [CrossRef]
- Niu, D.; An, W.; Yu, C.; Zhu, P.; Li, C.; Yin, D.; Zhi, J.; Jiang, X.; Ren, J. Pre-Pasteurization Enhances the Fermentation of Wheat Straw by Irpex Lacteus: Chemical Composition, Enzymatic Hydrolysis, and Microbial Community. Ind. Crops Prod. 2023, 202, 116962. [Google Scholar] [CrossRef]
- González-Bautista, E.; Alarcón-Gutiérrez, E.; Dupuy, N.; Gaime-Perraud, I.; Ziarelli, F.; Foli, L.; Farnet-Da-Silva, A.M. Preparation of a Sugarcane Bagasse-Based Substrate for Second-Generation Ethanol: Effect of Pasteurisation Conditions on Dephenolisation. Renew. Energy 2020, 157, 859–866. [Google Scholar] [CrossRef]
- Sasaki, C.; Yamanaka, S. Novel Sterilization Method Combining Food Preservative Use and Low Temperature Steaming for Treatment of Lignocellulosic Biomass with White Rot Fungi. Ind. Crops Prod. 2020, 155, 112765. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, J.; Martínez-Hernández, J.L.; Segura-Ceniceros, P.; López, G.; Saade, H.; Medina-Morales, M.A.; Ramos-González, R.; Aguilar, C.N.; Ilyina, A. Cellulases Immobilization on Chitosan-Coated Magnetic Nanoparticles: Application for Agave Atrovirens Lignocellulosic Biomass Hydrolysis. Bioprocess Biosyst. Eng. 2017, 40, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.; Singh, R.; Srivastava, M.; Mohammad, A.; Harakeh, S.; Pratap Singh, R.; Pal, D.B.; Haque, S.; Tayeb, H.H.; Moulay, M.; et al. Impact of Nanomaterials on Sustainable Pretreatment of Lignocellulosic Biomass for Biofuels Production: An Advanced Approach. Bioresour. Technol. 2023, 369, 128471. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.S.; Chia, W.Y.; Tang, D.Y.Y.; Show, P.L.; Chew, K.W.; Chen, W.H. Nanomaterials Utilization in Biomass for Biofuel and Bioenergy Production. Energies 2020, 13, 892. [Google Scholar] [CrossRef]
- Tan, W.Y.; Gopinath, S.C.B.; Anbu, P.; Yaakub, A.R.W.; Subramaniam, S.; Chen, Y.; Sasidharan, S. Bio-Enzyme Hybrid with Nanomaterials: A Potential Cargo as Sustainable Biocatalyst. Sustainability 2023, 15, 7511. [Google Scholar] [CrossRef]
- Xiros, C.; Topakas, E.; Christakopoulos, P. Hydrolysis and Fermentation for Cellulosic Ethanol Production. Wiley Interdiscip. Rev. Energy Environ. 2013, 2, 633–654. [Google Scholar] [CrossRef]
- Stickel, J.J.; Elander, R.T.; Mcmillan, J.D.; Brunecky, R. Enzymatic Hydrolysis of Lignocellulosic Biomass. In Bioprocessing of Renewable Resources to Commodity Bioproducts; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 77–103. [Google Scholar]
- Sherrard, E.C.; Blanco, G.W. Some of the Products Obtained in the Hydrolysis of White Spruce Wood with Dilute Sulfuric Acid under Steam Pressure. Ind. Eng. Chem. 1923, 15, 611–616. [Google Scholar] [CrossRef]
- Lin, Q.; Li, H.; Ren, J.; Deng, A.; Li, W.; Liu, C.; Sun, R. Production of Xylooligosaccharides by Microwave-Induced, Organic Acid-Catalyzed Hydrolysis of Different Xylan-Type Hemicelluloses: Optimization by Response Surface Methodology. Carbohydr. Polym. 2017, 157, 214–225. [Google Scholar] [CrossRef]
- Erickel, R.; Franz, R.; Woernle, R.; Riehm, T. Process for Hydrolyzing Cellulose-Material with Gaseous Hydrogen Fluoride. 1985. Available online: https://patentimages.storage.googleapis.com/7c/c3/7c/06812146dfef03/US4556431.pdf (accessed on 19 July 2023).
- Bergius, F. Conversion of Wood to Carbohydrates and Problems in the Industrial Use of Concentrated Hydrochloric Acid. Ind. Eng. Chem. 1937, 29, 247–253. [Google Scholar] [CrossRef]
- Ragg, P.L.; Fields, P.R. The Development of a Process for the Hydrolysis of Lignocellulosic Waste. Math. Phys. Sci. 1987, 321, 537–547. [Google Scholar]
- Bon, E.P.S.; Ferrara, M.A. Bioethanol Production via Enzymatic Hydrolysis of Cellulosic Biomass. In The Role of Agricultural Biotechnologies for Production of Bioenergy in Developing Countries; FAO: Rome, Italy, 2007. [Google Scholar]
- Aymé, L.; Hébert, A.; Henrissat, B.; Lombard, V.; Franche, N.; Perret, S.; Jourdier, E.; Heiss-Blanquet, S. Characterization of Three Bacterial Glycoside Hydrolase Family 9 Endoglucanases with Different Modular Architectures Isolated from a Compost Metagenome. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129848. [Google Scholar] [CrossRef]
- Laca, A.; Laca, A.; Díaz, M. Hydrolysis: From Cellulose and Hemicellulose to Simple Sugars. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 213–240. [Google Scholar] [CrossRef]
- Van Den Brink, J.; De Vries, R.P. Fungal Enzyme Sets for Plant Polysaccharide Degradation. Appl. Microbiol. Biotechnol. 2011, 91, 1477–1492. [Google Scholar] [CrossRef]
- Gavande, P.V.; Goyal, A. Endo-β-1,4-Glucanase. In Foundations and Frontiers in Enzymology, Glycoside Hydrolases: Biochemistry, Biophysics, and Biotechnology; Elsevier: Amsterdam, The Netherlands, 2023; pp. 55–76. [Google Scholar] [CrossRef]
- Weignerová, L.; Simerská, P.; Křen, V. α-Galactosidases and Their Applications in Biotransformations. Biocatal. Biotransform. 2009, 27, 79–89. [Google Scholar] [CrossRef]
- Wang, J.; Cao, X.; Chen, W.; Xu, J.; Wu, B. Identification and Characterization of a Thermostable Gh36 α-Galactosidase from Anoxybacillus Vitaminiphilus Wmf1 and Its Application in Synthesizing Isofloridoside by Reverse Hydrolysis. Int. J. Mol. Sci. 2021, 22, 10778. [Google Scholar] [CrossRef] [PubMed]
- Komiya, D.; Hori, A.; Ishida, T.; Igarashi, K.; Samejima, M.; Koseki, T.; Fushinobu, S. Crystal Structure and Substrate Specificity Modification of Acetyl Xylan Esterase from Aspergillus Luchuensis. Appl. Environ. Microbiol. 2017, 83, e01251. [Google Scholar] [CrossRef] [PubMed]
- Taveira, I.C.; Nogueira, K.M.V.; De Oliveira, D.L.G.; Silva, R.D.N. Preservation and Fermentation: Past, Present and Future. Front. Young Minds 2021, 79, 3–16. [Google Scholar] [CrossRef]
- Dai, Z.; Guo, F.; Zhang, S.; Zhang, W.; Yang, Q.; Dong, W.; Jiang, M.; Ma, J.; Xin, F. Bio-Based Succinic Acid: An Overview of Strain Development, Substrate Utilization, and Downstream Purification. Biofuels Bioprod. Biorefining 2019, 14, 965–985. [Google Scholar] [CrossRef]
- Cheng, K.K.; Wang, G.Y.; Zeng, J.; Zhang, J.A. Improved Succinate Production by Metabolic Engineering. Biomed Res. Int. 2013, 2013, 538790. [Google Scholar] [CrossRef] [PubMed]
- Wee, Y.J.; Yun, J.S.; Kim, D.; Ryu, H.W. Batch and Repeated Batch Production of L(+)-Lactic Acid by Enterococcus Faecalis RKY1 Using Wood Hydrolyzate and Corn Steep Liquor. J. Ind. Microbiol. Biotechnol. 2006, 33, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Takano, M.; Hoshino, K. Lactic Acid Production from Paper Sludge by SSF with Thermotolerant Rhizopus Sp. Bioresour. Bioprocess. 2016, 3, 29. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J.K.; Yang, Z.X.; Han, L.; Tan, T. Utilization of White Rice Bran for Production of L-Lactic Acid. Biomass Bioenergy 2012, 39, 53–58. [Google Scholar] [CrossRef]
- Kim, H.M.; Choi, I.S.; Lee, S.; Yang, J.E.; Jeong, S.G.; Park, J.H.; Ko, S.H.; Hwang, I.M.; Chun, H.H.; Wi, S.G.; et al. Biorefining Process of Carbohydrate Feedstock (Agricultural Onion Waste) to Acetic Acid. ACS Omega 2019, 4, 22438–22444. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic Acid Production from Lignocellulose-Derived Sugars Using Lactic Acid Bacteria: Overview and Limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Paulova, L.; Chmelik, J.; Branska, B.; Patakova, P.; Drahokoupil, M.; Melzoch, K. Comparison of Lactic Acid Production by L. Casei in Batch, Fed-Batch and Continuous Cultivation, Testing the Use of Feather Hydrolysate as a Complex Nitrogen Source. Braz. Arch. Biol. Technol. 2020, 63, 1–12. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, L.; Ma, C.; Yang, C.; Xu, P.; Ma, Y. Repeated Open Fermentative Production of Optically Pure L-Lactic Acid Using a Thermophilic Bacillus Sp. Strain. Bioresour. Technol. 2010, 101, 6494–6498. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; Usmanbaha, N.; Khaonuan, S.; Jariyaboon, R.; O-Thong, S.; Reungsang, A. Butanol Production from Algal Biomass by Acetone-Butanol-Ethanol Fermentation Process. In Clean Energy and Resources Recovery: Biomass Waste Based Biorefineries; Elsevier: Amsterdam, The Netherlands, 2021; Volume 1, pp. 421–446. [Google Scholar] [CrossRef]
- Rawoof, S.A.A.; Kumar, P.S.; Vo, D.-V.N.; Devaraj, K.; Mani, Y.; Devaraj, T.; Subramanian, S. Production of Optically Pure Lactic Acid by Microbial Fermentation: A Review. Environ. Chem. Lett. 2020, 19, 539–556. [Google Scholar] [CrossRef]
- Raina, N.; Slathia, P.S.; Sharma, P. Experimental Optimization of Thermochemical Pretreatment of Sal (Shorea Robusta) Sawdust by Central Composite Design Study for Bioethanol Production by Co-Fermentation Using Saccharomyces Cerevisiae (MTCC-36) and Pichia Stipitis (NCIM-3498). Biomass Bioenergy 2020, 143, 105819. [Google Scholar] [CrossRef]
- Qin, L.; Zhao, X.; Li, W.C.; Zhu, J.Q.; Liu, L.; Li, B.Z.; Yuan, Y.J. Process Analysis and Optimization of Simultaneous Saccharification and Co-Fermentation of Ethylenediamine-Pretreated Corn Stover for Ethanol Production. Biotechnol. Biofuels 2018, 11, 118. [Google Scholar] [CrossRef] [PubMed]
- Minty, J.J.; Lin, X.N. Engineering Synthetic Microbial Consortia for Consolidated Bioprocessing of Lignocellulosic Biomass into Valuable Fuels and Chemicals. In Direct Microbial Conversion of Biomass to Advanced Biofuels; Elsevier: Amsterdam, The Netherlands, 2015; pp. 365–381. [Google Scholar] [CrossRef]
- Zhou, S.; Zhang, M.; Zhu, L.; Zhao, X.; Chen, J.; Chen, W.; Chang, C. Hydrolysis of Lignocellulose to Succinic Acid: A Review of Treatment Methods and Succinic Acid Applications. Biotechnol. Biofuels Bioprod. 2023, 16, 1. [Google Scholar] [CrossRef]
- Dien, B.S.; Cotta, M.A.; Jeffries, T.W. Bacteria Engineered for Fuel Ethanol Production: Current Status. Appl. Microbiol. Biotechnol. 2003, 63, 258–266. [Google Scholar] [CrossRef]
- Joshi, A.; Verma, K.K.; D Rajput, V.; Minkina, T.; Arora, J. Recent Advances in Metabolic Engineering of Microorganisms for Advancing Lignocellulose-Derived Biofuels. Bioengineered 2022, 13, 8135–8163. [Google Scholar] [CrossRef]
- Bardhan, S.K.; Gupta, S.; Gorman, M.E.; Haider, M.A. Biorenewable Chemicals: Feedstocks, Technologies and the Conflict with Food Production. Renew. Sustain. Energy Rev. 2015, 51, 506–520. [Google Scholar] [CrossRef]
- Mores, S.; Vandenberghe, L.P.d.S.; Magalhães Júnior, A.I.; de Carvalho, J.C.; de Mello, A.F.M.; Pandey, A.; Soccol, C.R. Citric Acid Bioproduction and Downstream Processing: Status, Opportunities, and Challenges. Bioresour. Technol. 2021, 320, 124426. [Google Scholar] [CrossRef] [PubMed]
- Alexandri, M.; Schneider, R.; Mehlmann, K.; Venus, J. Recent Advances in D-Lactic Acid Production from Renewable Resources: Case Studies on Agro-Industrial Waste Streams. Food Technol. Biotechnol. 2019, 57, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Thakur, A.; Panesar, P.S. Lactic Acid and Its Separation and Purification Techniques: A Review. Rev. Environ. Sci. Biotechnol. 2019, 18, 823–853. [Google Scholar] [CrossRef]
- Pleissner, D.; Neu, A.K.; Mehlmann, K.; Schneider, R.; Puerta-Quintero, G.I.; Venus, J. Fermentative Lactic Acid Production from Coffee Pulp Hydrolysate Using Bacillus Coagulans at Laboratory and Pilot Scales. Bioresour. Technol. 2016, 218, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Glassner, D.A.; Elankovan, P.; Beacom, D.R.; Berglund, K.A. Purification Process for Succinic Acid Produced by Fermentation. Appl. Biochem. Biotechnol. 1995, 51–52, 73–82. [Google Scholar] [CrossRef]
- Saini, S.; Chandel, A.K.; Sharma, K.K. Past Practices and Current Trends in the Recovery and Purification of First Generation Ethanol: A Learning Curve for Lignocellulosic Ethanol. J. Clean. Prod. 2020, 268, 122357. [Google Scholar] [CrossRef]
- Bateni, H.; Saraeian, A.; Able, C. A Comprehensive Review on Biodiesel Purification and Upgrading. Biofuel Res. J. 2017, 15, 668–690. [Google Scholar] [CrossRef]
- Mma, S.; Jaber, R.; Shirazi, M.; Toufaily, J.; Hamieh, A.; Noureddin, A.; Ghanavati, H.; Ghaffari, A.; Zenouzi, A.; Karout, A.; et al. Biodiesel Wash-Water Reuse Using Microfiltration: Toward Zero-Discharge Strategy for Cleaner and Economized Biodiesel Production. Biofuel Res. J. 2015, 5, 148–151. [Google Scholar]
- Adhikari, S.; Fernando, S. Hydrogen Membrane Separation Techniques. Ind. Eng. Chem. Res. 2006, 45, 875–881. [Google Scholar] [CrossRef]
- Zăbavă, B.-Ș; Voicu, G.; Ungureanu, N.; Dincă, M.; Paraschin, G.; Munteanu, M.; Ferdes, M. Methods of Biogas Purification—A Review. Acta Tech. Corviniensis Bull. Eng. 2019, XII, 65–68. [Google Scholar]
- Poot, A.A.H. Chemical Bleaching of Ancient Textiles. Stud. Conserv. 1964, 9, 53–64. [Google Scholar] [CrossRef]
- Bajpai, P. Introduction and the Literature. In Biermann’s Handbook of Pulp and Paper; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–18. [Google Scholar] [CrossRef]
- Eugenia Eugenio, M.; Ibarra, D.; Martín-Sampedro, R.; Espinosa, E.; Bascón, I.; Rodríguez, A. Alternative Raw Materials for Pulp and Paper Production in the Concept of a Lignocellulosic Biorefinery. In Cellulose; IntechOpen: Rijeka, Croatia, 2019; pp. 1–34. [Google Scholar] [CrossRef]
- Yankov, D. Fermentative Lactic Acid Production from Lignocellulosic Feedstocks: From Source to Purified Product. Front. Chem. 2022, 10, 823005. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Li, J.; Gao, H.; Zhou, D.; Xu, H.; Cong, Y.; Zhang, W.; Xin, F.; Jiang, M. Recent Progress on Bio-Succinic Acid Production from Lignocellulosic Biomass. World J. Microbiol. Biotechnol. 2021, 37, 16. [Google Scholar] [CrossRef]
- Lee, J.S.; Lin, C.J.; Lee, W.C.; Teng, H.Y.; Chuang, M.H. Production of Succinic Acid through the Fermentation of Actinobacillus Succinogenes on the Hydrolysate of Napier Grass. Biotechnol. Biofuels Bioprod. 2022, 15, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.P.; Meng, J.; Bao, J. Fermentative Production of High Titer Citric Acid from Corn Stover Feedstock after Dry Dilute Acid Pretreatment and Biodetoxification. Bioresour. Technol. 2017, 224, 563–572. [Google Scholar] [CrossRef] [PubMed]
- BYJU’S. Types of Fermentation: Definition, Process, Advantages. 2023. Available online: https://byjus.com/biology/biofuel (accessed on 5 July 2023).
- U.S. Energy Information Administration. Biofuel Explained. 2021. Available online: https://www.eia.gov/energyexplained/biofuel/biodiesel-rd-other-use-supply.php (accessed on 15 September 2023).
- Aizarani, J. Fuel Ethanol Production Worldwide in 2022, by Country. 2023. Available online: https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries/Source:https://www.statista.com/statistics/281606/ethanol-production-in-selected-countries/ (accessed on 25 August 2023).
- Gitnux. Biofuel Production: Statistics and Trends. 2023. Available online: https://blog.gitnux.com/biofuel-production-statistics/ (accessed on 28 August 2023).
- Suhud Shote, A. Biofuel: An Environmental Friendly Fuel. In Anaerobic Digestion; IntechOpen: Rijeka, Croatia, 2019; pp. 1–13. [Google Scholar] [CrossRef]
- Ghosh, B.B.; Ahindra, N. Biofuels Refining and Performance. In Biofuels Refining and Performance, 1st ed.; McGraw-Hill: New York, NY, USA, 2008. [Google Scholar]
- Park, J.M.; Oh, B.R.; Seo, J.W.; Hong, W.K.; Yu, A.; Sohn, J.H.; Kim, C.H. Efficient Production of Ethanol from Empty Palm Fruit Bunch Fibers by Fed-Batch Simultaneous Saccharification and Fermentation Using Saccharomyces Cerevisiae. Appl. Biochem. Biotechnol. 2013, 170, 1807–1814. [Google Scholar] [CrossRef]
- Braga, A.; Gomes, D.; Rainha, J.; Amorim, C.; Cardoso, B.B.; Gudiña, E.J.; Silvério, S.C.; Rodrigues, J.L.; Rodrigues, L.R. Zymomonas Mobilis as an Emerging Biotechnological Chassis for the Production of Industrially Relevant Compounds. Bioresour. Bioprocess. 2021, 8, 128. [Google Scholar] [CrossRef]
- Piriya, P.S.; Vasan, P.T.; Padma, V.S.; Vidhyadevi, U.; Archana, K.; Vennison, S.J. Cellulosic Ethanol Production by Recombinant Cellulolytic Bacteria Harbouring Pdc and Adh II Genes of Zymomonas Mobilis. Biotechnol. Res. Int. 2012, 2012, 817549. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fernández-Sandoval, M.T.; Galíndez-Mayer, J.; Bolívar, F.; Gosset, G.; Ramírez, O.T.; Martinez, A. Xylose-Glucose Co-Fermentation to Ethanol by Escherichia coli Strain MS04 Using Single- and Two-Stage Continuous Cultures under Micro-Aerated Conditions. Microb. Cell Factories 2019, 18, 145. [Google Scholar] [CrossRef]
- Saha, B.C.; Cotta, M.A. Ethanol Production from Lignocellulosic Biomass by Recombinant Escherichia coli Strain FBR5. Bioengineered 2012, 3, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Ibarra, E.; Alcaraz-Cienfuegos, J.; Vargas-Tah, A.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martinez, A. Ethanol Production by Escherichia coli from Detoxified Lignocellulosic Teak Wood Hydrolysates with High Concentration of Phenolic Compounds. J. Ind. Microbiol. Biotechnol. 2022, 49, 77. [Google Scholar] [CrossRef]
- Ningthoujam, R.; Jangid, P.; Yadav, V.K.; Sahoo, D.K.; Patel, A.; Dhingra, H.K. Bioethanol Production from Alkali-Pretreated Rice Straw: Effects on Fermentation Yield, Structural Characterization, and Ethanol Analysis. Front. Bioeng. Biotechnol. 2023, 11, 1–9. [Google Scholar] [CrossRef]
- Prasad, S.; Kumar, S.; Yadav, K.K.; Choudhry, J.; Kamyab, H.; Bach, Q.V.; Sheetal, K.R.; Kannojiya, S.; Gupta, N. Screening and Evaluation of Cellulytic Fungal Strains for Saccharification and Bioethanol Production from Rice Residue. Energy 2020, 190, 116422. [Google Scholar] [CrossRef]
- Hernawan, H.; Maryana, R.; Pratiwi, D.; Wahono, S.K.; Darsih, C.; Hayati, S.N.; Poeloengasih, C.D.; Nisa, K.; Indrianingsih, A.W.; Prasetyo, D.J.; et al. Bioethanol Production from Sugarcane Bagasse by Simultaneous Sacarification and Fermentation Using Saccharomyces Cerevisiae. Am. Inst. Phys. Conf. Proc. 2017, 1823, 1–5. [Google Scholar] [CrossRef]
- Neupane, D. Biofuels from Renewable Sources, a Potential Option for Biodiesel Production. Bioengineering 2023, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Alternative Fuels Data Centre (AFDC). Biodiesel Benefits and Considerations. 2020. Available online: https://afdc.energy.gov/fuels/biodiesel_benefits.html (accessed on 29 September 2023).
- Smoot, G. What Is the Carbon Footprint of Biofuel? A Life-Cycle Assessment. 2020. Available online: https://impactful.ninja/the-carbon-footprint-of-biofuel/ (accessed on 13 August 2023).
- Integrated Flow Solutions (IFS). Biodiesel Guide-Sources, Production, Uses, and Regulations. 2020. Available online: https://ifsolutions.com/category/power-generation/ (accessed on 29 September 2023).
- Climate Technology Centre and Network. Second and Third Generation of Biofuels. 2011. Available online: https://www.ctc-n.org/files/UNFCCC_docs/ref17x03_3.pdf (accessed on 18 September 2023).
- Chintagunta, A.D.; Zuccaro, G.; Kumar, M.; Kumar, S.P.J.; Garlapati, V.K.; Postemsky, P.D.; Kumar, N.S.S.; Chandel, A.K.; Simal-Gandara, J. Biodiesel Production From Lignocellulosic Biomass Using Oleaginous Microbes: Prospects for Integrated Biofuel Production. Front. Microbiol. 2021, 12, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Khot, M.; Raut, G.; Ghosh, D.; Alarcón-Vivero, M.; Contreras, D.; Ravikumar, A. Lipid Recovery from Oleaginous Yeasts: Perspectives and Challenges for Industrial Applications. Fuel 2020, 259, 116292. [Google Scholar] [CrossRef]
- Pasha, M.K.; Dai, L.; Liu, D.; Guo, M.; Du, W. An Overview to Process Design, Simulation and Sustainability Evaluation of Biodiesel Production. Biotechnol. Biofuels 2021, 14, 192. [Google Scholar] [CrossRef] [PubMed]
- Vasaki, M.; Sithan, M.; Ravindran, G.; Paramasivan, B.; Ekambaram, G.; Karri, R.R. Biodiesel Production from Lignocellulosic Biomass Using Yarrowia Lipolytica. Energy Convers. Manag. X 2022, 13, 100167. [Google Scholar] [CrossRef]
- Sayeda, A.A.; Mohsen, S.A.; Osama, H.E.S.; Azhar, A.H.; Saher, S.M. Biodiesel Production from Egyptian Isolate Fusarium Oxysporum NRC2017. Bull. Natl. Res. Cent. 2019, 43, 210. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Rajendran, K.; Pugazhendhi, A.; Rao, C.V.; Atabani, A.E.; Kumar, G.; Yang, Y.H. Renewable Biohydrogen Production from Lignocellulosic Biomass Using Fermentation and Integration of Systems with Other Energy Generation Technologies. Sci. Total Environ. 2021, 765, 144429. [Google Scholar] [CrossRef]
- Lopez-Hidalgo, A.M.; Sánchez, A.; De León-Rodríguez, A. Simultaneous Production of Bioethanol and Biohydrogen by Escherichia coli WDHL Using Wheat Straw Hydrolysate as Substrate. Fuel 2017, 188, 19–27. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J. Isolation and Characterization of a Novel Strain Clostridium Butyricum INET1 for Fermentative Hydrogen Production. Int. J. Hydrogen Energy 2017, 42, 12173–12180. [Google Scholar] [CrossRef]
- Jiang, L.; Long, C.; Wu, X.; Xu, H.; Shao, Z.; Long, M. Optimization of Thermophilic Fermentative Hydrogen Production by the Newly Isolated Caloranaerobacter Azorensis H53214 from Deep-Sea Hydrothermal Vent Environment. Int. J. Hydrogen Energy 2014, 39, 14154–14160. [Google Scholar] [CrossRef]
- Hu, C.C.; Giannis, A.; Chen, C.L.; Qi, W.; Wang, J.Y. Comparative Study of Biohydrogen Production by Four Dark Fermentative Bacteria. Int. J. Hydrogen Energy 2013, 38, 15686–15692. [Google Scholar] [CrossRef]
- Reyes, L.H.; Xiong, W.; Michener, W.; Maness, P.C.; Chou, K. Engineering Cellulolytic Bacterium Clostridium Thermocellum to Co-Ferment Cellulose- and Hemicellulose-Derived Sugars Simultaneously. In Proceedings of the 2018 AIChE Annual Meeting, Pittsburgh, PA, USA, 27–30 October 2018. [Google Scholar]
- Luthfi, A.A.I.; Abdul, P.M.; Jahim, J.M.; Engliman, N.S.; Jamali, N.S.; Tan, J.P.; Manaf, S.F.A.; Sajab, M.S.; Bukhari, N.A. Isolation and Characterization of Biohydrogen-Producing Bacteria for Biohydrogen Fermentation Using Oil Palm Biomass-Based Carbon Source. Appl. Sci. 2023, 13, 656. [Google Scholar] [CrossRef]
- Environmental and Energy Study Institute (EESI). Biogas: Converting Waste to Energy. 2017. Available online: www.eesi.org (accessed on 23 August 2023).
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane Production from Lignocellulose: Biomass Recalcitrance and Its Impacts on Anaerobic Digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, A.; Limonti, C.; Curcio, G.M. Improvement of Biomethane Production from Organic Fraction of Municipal Solid Waste (Ofmsw) through Alkaline Hydrogen Peroxide (Ahp) Pretreatment. Fermentation 2021, 7, 197. [Google Scholar] [CrossRef]
- Mora-Cortés, D.; Garcés-Gómez, Y.A.; Pacheco, S.I. Improvement of Biomethane Potential by Anaerobic Co-Digestion of Sewage Sludge and Cocoa Pod Husks. Int. J. Technol. 2020, 11, 482–491. [Google Scholar] [CrossRef]
- Sukhang, S.; Choojit, S.; Reungpeerakul, T.; Sangwichien, C. Bioethanol Production from Oil Palm Empty Fruit Bunch with SSF and SHF Processes Using Kluyveromyces Marxianus Yeast. Cellulose 2020, 27, 301–314. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Srisupa, S.; Chaiyaso, T. Bioethanol Production from Cellulose-Rich Corncob Residue by the Thermotolerant Saccharomyces Cerevisiae TC-5. J. Fungi 2021, 7, 547. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, E.; Del Río, P.G.; Romaní, A.; Garrote, G.; Domingues, L. Hemicellulosic Bioethanol Production from Fast-Growing Paulownia Biomass. Processes 2021, 9, 173. [Google Scholar] [CrossRef]
- Feng, J.; Li, Q.; Zhang, Y.; Yang, H.; Guo, L. High NH3-N Tolerance of a CheR2-Deletion Rhodobacter Capsulatus Mutant for Photo-Fermentative Hydrogen Production Using Cornstalk. Int. J. Hydrogen Energy 2019, 44, 15833–15841. [Google Scholar] [CrossRef]
- Morales-Martínez, T.K.; Medina-Morales, M.A.; Ortíz-Cruz, A.L.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, M.; López-Badillo, C.M.; Ríos-González, L. Consolidated Bioprocessing of Hydrogen Production from Agave Biomass by Clostridium Acetobutylicum and Bovine Ruminal Fluid. Int. J. Hydrogen Energy 2020, 45, 13707–13716. [Google Scholar] [CrossRef]
- Medina-Morales, M.A.; De la Cruz-Andrade, L.E.; Paredes-Peña, L.A.; Morales-Martínez, T.K.; Rodríguez-De la Garza, J.A.; Moreno-Dávila, I.M.; Tamayo-Ordóñez, M.C.; Rios-González, L.J. Biohydrogen Production from Thermochemically Pretreated Corncob Using a Mixed Culture Bioaugmented with Clostridium Acetobutylicum. Int. J. Hydrogen Energy 2021, 46, 25974–25984. [Google Scholar] [CrossRef]
- Schroyen, M.; Vervaeren, H.; Vandepitte, H.; Van Hulle, S.W.H.; Raes, K. Effect of Enzymatic Pretreatment of Various Lignocellulosic Substrates on Production of Phenolic Compounds and Biomethane Potential. Bioresour. Technol. 2015, 192, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, S.; Yukesh Kannah, R.; Kasthuri, S.; Gunasekaran, M.; Pugazhendi, A.; Rene, E.R.; Pant, D.; Kumar, G.; Rajesh Banu, J. Profitable Biomethane Production from Delignified Rice Straw Biomass: The Effect of Lignin, Energy and Economic Analysis. Green Chem. 2020, 22, 8024–8035. [Google Scholar] [CrossRef]
- Li, Y.; Hou, Z.; Shi, Q.; Cheng, Y.; Zhu, W. Methane Production From Different Parts of Corn Stover via a Simple Co-Culture of an Anaerobic Fungus and Methanogen. Front. Bioeng. Biotechnol. 2020, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Tan, W.; Zhao, X.; Dang, Q.; Song, Q.; Xi, B.; Zhang, X. Evaluation on the Methane Production Potential of Wood Waste Pretreated with NaOH and Co-Digested with Pig Manure. Catalysts 2019, 9, 539. [Google Scholar] [CrossRef]
- Anyasi, T.A.; Jideani, A.I.O.; Edokpayi, J.N.; Anokwuru, C.P. Application of Organic Acids in Food Preservation. In Biochemistry Research Trends: Organic Acids Characteristics, Properties and Synthesis; Springer: Berlin, Germany, 2017; pp. 1–45. [Google Scholar]
- Chheda, J.N.; Román-Leshkov, Y.; Dumesic, J.A. Production of 5-Hydroxymethylfurfural and Furfural by Dehydration of Biomass-Derived Mono- and Poly-Saccharides. Green Chem. 2007, 9, 342–350. [Google Scholar] [CrossRef]
- Liu, W.; Bao, Q.; Jirimutu; Qing, M.; Siriguleng; Chen, X.; Sun, T.; Li, M.; Zhang, J.; Yu, J.; et al. Isolation and Identification of Lactic Acid Bacteria from Tarag in Eastern Inner Mongolia of China by 16S RRNA Sequences and DGGE Analysis. Microbiol. Res. 2012, 167, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Hassan, N.; Idris, A.; Ngadiman, N.H.A. Optimization of Simultaneous Saccharification and Fermentation Process Conditions for the Production of Succinic Acid from Oil Palm Empty Fruit Bunches. J. Wood Chem. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Tong, Z.; Zheng, X.; Tong, Y.; Shi, Y.C.; Sun, J. Systems Metabolic Engineering for Citric Acid Production by Aspergillus Niger in the Post-Genomic Era. Microb. Cell Factories 2019, 18, 28. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-Q.; Holland, R. LEUCONOSTOC spp. In Encyclopedia of Dairy Sciences; Elsevier: Amsterdam, The Netherlands, 2002; pp. 1539–1543. [Google Scholar] [CrossRef]
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs Cycle Intermediate Citrate in Metabolic Reprogramming in Innate Immunity and Inflammation. Front. Immunol. 2018, 9, 141. [Google Scholar] [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; De Jong, H. Acetate Metabolism and the Inhibition of Bacterial Growth by Acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef]
- Cubas-Cano, E.; González-Fernández, C.; Ballesteros, M.; Tomás-Pejó, E. Biotechnological Advances in Lactic Acid Production by Lactic Acid Bacteria: Lignocellulose as Novel Substrate. Biofuels Bioprod. Biorefining 2018, 12, 290–303. [Google Scholar] [CrossRef]
- Statista Research Department. Lactic Acid Global Market Volume. 2023. Available online: https://www.statista.com/statistics/1310495/lactic-acid-market-volume-worldwide/Source:https://www.statista.com/statistics/1310495/lactic-acid-market-volume-worldwide/ (accessed on 30 July 2023).
- MARKET AND MARKET. Lactic Acid Market and Industry Analysis. 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/polylacticacid-387.html (accessed on 18 September 2023).
- Hancock, A. Renewable Chemicals Market Size, Share & Trends Analysis Report by 2030. 2023. Available online: https://www.linkedin.com/pulse/renewable-chemicals-market-size-share-trends-analysis-ashley-hancock/ (accessed on 28 September 2023).
- Market.us Study. Succinic Acid Market Size and Value to Reach USD 359.8 Million in 2032. 2023. Available online: https://www.globenewswire.com/en/news-release/2023/05/09/2664413/0/en/Succinic-Acid-Market-Size-and-Value-to-Reach-USD-359-8-Million-in-2032-Growing-at-CAGR-of-7-3-Market-us-Study.html (accessed on 10 September 2023).
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly(Lactic Acid)-Based Electrospun Fibrous Structures for Biomedical Applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Grand View Research. Succinic Acid Market Size, Share & Trends Analysis Report. 2021. Available online: https://www.grandviewresearch.com/industry-analysis/succinic-acid-market (accessed on 30 September 2023).
- Jaiswal, C. Global Succinic Acid Market Overview. 2023. Available online: https://www.marketresearchfuture.com/reports/succinic-acid-market-1914 (accessed on 26 September 2023).
- Research and Markets. Citric Acid Market. 2023. Available online: https://www.researchandmarkets.com/reports/5732513/citric-acid-market-global-industry-trends (accessed on 1 September 2023).
- Expert Market Research. Global Citric Acid Market Size, Share, Analysis Report 2023–2028. 2023. Available online: https://www.expertmarketresearch.com/reports/citric-acid-market (accessed on 15 September 2023).
- Precedence Research. Citric Acid Market Size 2023–2032. 2023. Available online: https://www.precedenceresearch.com/citric-acid-market (accessed on 4 September 2023).
- Market Insider. Global Citric Acid Market 2018–2023. 2018. Available online: https://markets.businessinsider.com/news/stocks/global-citric-acid-market-2018-2023-1002001216 (accessed on 30 July 2023).
- Harahap, B.M.; Ahring, B.K. Acetate Production from Syngas Produced from Lignocellulosic Biomass Materials along with Gaseous Fermentation of the Syngas: A Review. Microorganisms 2023, 11, 995. [Google Scholar] [CrossRef]
- Marino, D.J. Ethyl Acetate. 2005. Available online: https://www.sciencedirect.com/science/article/pii/B0123694000003902 (accessed on 14 September 2023).
- MC GROUP. Ethyl Acetate (ETAC): 2023 World Market Outlook and Forecast up to 2032. 2023. Available online: https://mcgroup.co.uk/researches/acetic-acid (accessed on 25 September 2023).
- McKeen, L.W. Polyolefins, Polyvinyls, and Acrylics. In Permeability Properties of Plastics and Elastomers; Elsevier: Amsterdam, The Netherlands, 2012; pp. 145–193. [Google Scholar] [CrossRef]
- Zaldivar, J.; Martinez, A.; Ingram, L.O. Effect of Selected Aldehydes on the Growth and Fermentation of Ethanologenic Escherichia coli. Biotechnol. Bioeng. 1999, 65, 24–33. [Google Scholar] [CrossRef]
- Van Der Pol, E.C.; Eggink, G.; Weusthuis, R.A. Production of L(+)-Lactic Acid from Acid Pretreated Sugarcane Bagasse Using Bacillus Coagulans DSM2314 in a Simultaneous Saccharification and Fermentation Strategy. Biotechnol. Biofuels 2016, 9, 248. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Xia, L. Production and Immobilization of Cellobiase from Aspergillus Niger ZU-07. Process Biochem. 2004, 39, 1363–1367. [Google Scholar] [CrossRef]
- Ou, M.S.; Mohammed, N.; Ingram, L.O.; Shanmugam, K.T. Thermophilic Bacillus Coagulans Requires Less Cellulases for Simultaneous Saccharification and Fermentation of Cellulose to Products than Mesophilic Microbial Biocatalysts. Appl. Biochem. Biotechnol. 2009, 155, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Amrane, A.; Prigent, Y. A Novel Concept of Bioreactor: Specialized Function Two-Stage Continuous Reactor, and Its Application to Lactose Conversion into Lactic Acid. J. Biotechnol. 1996, 45, 195–203. [Google Scholar] [CrossRef]
- Bouchoux, A.; Roux-De Balmann, H.; Lutin, F. Nanofiltration of Glucose and Sodium Lactate Solutions: Variations of Retention between Single- and Mixed-Solute Solutions. J. Memb. Sci. 2005, 258, 123–132. [Google Scholar] [CrossRef]
- Min-Tian, G.; Hirata, M.; Koide, M.; Takanashi, H.; Hano, T. Production of L-Lactic Acid by Electrodialysis Fermentation (EDF). Process Biochem. 2004, 39, 1903–1907. [Google Scholar] [CrossRef]
- Kim, J.; Kim, Y.M.; Lebaka, V.R.; Wee, Y.J. Lactic Acid for Green Chemical Industry: Recent Advances in and Future Prospects for Production Technology, Recovery, and Applications. Fermentation 2022, 8, 609. [Google Scholar] [CrossRef]
- Han, J. Process Systems Engineering Studies for Catalytic Production of Bio-Based Platform Molecules from Lignocellulosic Biomass. Energy Convers. Manag. 2017, 138, 511–517. [Google Scholar] [CrossRef]
- Hicks, K.B.; Montanti, J.; Nghiem, N.P. Use of Barley Grain and Straw for Biofuels and Other Industrial Uses. In Barley: Chemistry and Technology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 269–291. [Google Scholar] [CrossRef]
- Haddad, M.; Nassar, D.; Shtaya, M. Heavy Metals Accumulation in Soil and Uptake by Barley (Hordeum Vulgare) Irrigated with Contaminated Water. Sci. Rep. 2023, 13, 4121. [Google Scholar] [CrossRef]
- Gibson, M.T.; Welch, I.M.; Barrett, P.R.F.; Ridge, I. Barley Straw as an Inhibitor of Algal Growth II: Laboratory Studies. J. Appl. Phycol. 1990, 2, 241–248. [Google Scholar] [CrossRef]
- Supanchaiyamat, N.; Jetsrisuparb, K.; Knijnenburg, J.T.N.; Tsang, D.C.W.; Hunt, A.J. Lignin Materials for Adsorption: Current Trend, Perspectives and Opportunities. Bioresour. Technol. 2019, 272, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Lv, S.; Zuo, J.; Zhang, S.; Liang, S. Recent Advances Research and Application of Lignin-Based Fluorescent Probes. React. Funct. Polym. 2022, 178, 105354. [Google Scholar] [CrossRef]
- Faria, F.A.C.; Evtuguin, D.V.; Rudnitskaya, A.; Gomes, M.T.S.R.; Oliveira, J.A.B.P.; Graça, M.P.F.; Costa, L.C. Lignin-Based Polyurethane Doped with Carbon Nanotubes for Sensor Applications. Polym. Int. 2012, 61, 788–794. [Google Scholar] [CrossRef]
- Durmaz, E.; Sertkaya, S.; Yilmaz, H.; Olgun, C.; Ozcelik, O.; Tozluoglu, A.; Candan, Z. Lignocellulosic Bionanomaterials for Biosensor Applications. Micromachines 2023, 14, 1450. [Google Scholar] [CrossRef] [PubMed]
- Tortolini, C.; Capecchi, E.; Tasca, F.; Pofi, R.; Venneri, M.A.; Saladino, R.; Antiochia, R. Novel Nanoarchitectures Based on Lignin Nanoparticles for Electrochemical Eco-friendly Biosensing Development. Nanomaterials 2021, 11, 718. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Thakur, N.S.; Chandna, S.; Bhaumik, J. Development of Agri-Biomass Based Lignin Derived Zinc Oxide Nanocomposites as Promising UV Protectant-Cum-Antimicrobial Agents. J. Mater. Chem. B 2020, 8, 260–269. [Google Scholar] [CrossRef]
- Wakefield, J.C. Styrene Toxicological Overview. 2007. Available online: https://assets.publishing.service.gov.uk/media/5a7e4e80ed915d74e33f163f/HPA_STYRENE_Toxicological_Overview_v1.pdf (accessed on 23 August 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).