Abstract
Vip3A (vegetative insecticidal protein) is a representative member of the Vip3 family, which is widely used for lepidopteran pest control. This Vip3A protein, a non-growth-associated protein, is an effective bioinsecticide against insect pests, but there is relatively little information about its production processes at large scales. Hence, the effects of environmental factors on Vip3A production by Bacillus thuringiensis Bt294 (antifoam agents, shaking speeds, agitation and aeration rates), as well as controlling physical conditions such as the lowest point of dissolved oxygen and controlling of culture pH, were observed in shaking flasks and bioreactors. The results showed that antifoam agents, flask types and shaking speeds had significant effects on Vip3A and biomass production. Cultivation without pH control and DO control in 5 L bioreactors at lower agitation and aeration rates, which was not favorable for biomass production, resulted in a high Vip3A protein production of 5645.67 mg/L. The scale-up studies of the Vip3A protein production in a pilot-scale 750 L bioreactor gave 3750.0 mg/L. Therefore, this study demonstrated the significant effects of agitation, aeration rates and culture pH on Vip3A production by B. thuringiensis Bt294. Balancing of physical conditions was necessary for obtaining the highest yield of Vip3A by slowing down the production rate of biomass. Moreover, this Vip3A protein has high potential as a bioinsecticide for lepidopteran pest control in organic crops. This information will be important for significantly increasing the Vip3A protein concentration by the bacterium and will be useful for field application at a lower cost.
1. Introduction
One of the most successful microbial alternatives to bioinsecticides to replace chemicals is biocontrol agents because of their high host specificity, low lethal dose concentration and natural biodegradability or low harm to consumers. Bacillus thuringiensis is one of the entomopathogenic bacteria that can produce the vegetative insecticidal protein family (Vip) during the vegetative growth phase. It is considered an excellent toxic candidate [1]. These Vip proteins represent the second generation of extracellular insecticidal proteins, which can be used either alone or in complement with Cry proteins for the management of various detrimental pests. According to the new classification system of Crickmore et al., 2020 [2], Vip1 and Vip4, which have similar structures, have been classified as Vpb groups and renamed Vpb1 and Vpb2, respectively. The Vip2 protein has been categorized as the Vpa group. Finally, Vip3 proteins with multidomain structures have been noted as Vip and are currently classified into three subfamilies based on their amino acid identity: Vip3A, Vip3B and Vip3C [3]. Vip3A is the most widely studied Vip toxin so far and is the first protein discovered in this family. This protein shows a strong inhibition of insect larval growth at a low concentration [4] due to a unique protein sequence and distinct receptor binding properties in the insect midgut cell membranes from Cry proteins and ion channel properties [5], which makes them a good alternative or complement to Cry proteins for resistance management. The Vip3A toxin shows a high level of activity against a range of lepidopteran pests, including black cutworm (Agrotis ipsilon), fall armyworm (Spodoptera frugiperda), beet armyworm (Spodoptera exigua), tobacco budworm (Heliothis virescens), corn earworm (Helicoverpa zea) [5], and more, as shown in Milne et al., 2008 [6] and Gupta et al., 2021 [1].
Vip3 proteins are most commonly found in several B. thuringiensis strains. Despite their efficiency, the low quantities of Vip3 toxins secreted by vegetative cells present a limitation for their use as bioinsecticides [7]. In a survey of research data on the production of Vip3A protein from microorganisms, it was found that the maximum production was only 170 μg/mL [8]. The lower yield of Vip3A production is the boundary critical problem caused by the use of this biocontrol agent in the field with low efficacy of insect pest control in comparison with chemicals. Hence, to increase its efficacy in competition with chemicals, Vip3A protein yield at high concentrations needs to be developed by a potent selected bacterial strain of Bacillus thuringiensis. Approaches to improve the production of this Vip3A protein by B. thuringiensis could be achieved by optimization of production processes [9,10,11], media optimization [12,13,14], and strain improvement using mutagenesis [7], adaptation [15] and genetic engineering [16]. The protein production and cell growth of B. thuringiensis are easily affected by physical cultivation parameters such as dissolved oxygen (DO), agitation speeds, aeration rates and culture pH [17,18,19]. Furthermore, the scale-up processes involved with these physical parameters of reproducible production using optimal parameters have to be developed for commercialization. Many studies have reported various aspects of crystal protein (delta-endotoxin) production processes. For example, Vu et al. [20] found that the combination of NaOH and CH3COOH as pH-controlling agents during the growth of B. thuringiensis in wastewater sludge gave the highest delta-endotoxin concentration. NH4OH/H2SO4 and NaOH/H2SO4 were suitable for alkaline protease and amylase production, respectively. The level of dissolved oxygen in liquid media is a crucial factor in the bacterial growth and delta-endotoxin cultivation of B. thuringiensis. Thus, many studies have shown that the level of dissolved oxygen in the cultivation of B. thuringiensis affects cell growth and δ-endotoxin synthesis [10,21,22,23]. Our previous study on the optimization of Vip3A production by B. thuringiensis Bt294 in shake flasks and in a bioreactor produced 300 mg/L of Vip3A in a 5 L bioreactor [24]. The Vip3A protein showed satisfactory product yield and entomotoxicity results. In the present study, we focused on Vip3A production in large-scale production. However, regarding Vip3A production, the effects of environmental factors on Vip3A production remain poorly understood. Therefore, the effects of environmental factors, as well as controlling the cultivation processes on Vip3A production by B. thuringiensis Bt294, were investigated in shaking flasks and bioreactors in this study. This high Vip3A protein production by wild-type B. thuringiensis Bt294 might be useful in organic crops as well as in GMO-prohibited areas.
2. Materials and Methods
2.1. Microorganism Strain, Inoculum Preparation and Culture Media
The potential Vip3A-producing bacterium B. thuringiensis Bt294 (accession number: MK955482) was isolated from paddy soil in Kalasin Province, Thailand. It was maintained in LB (Luria–Bertani) agar (composed (L−1) of 8 g tryptone, 4 g yeast extract, 4 g NaCl and 12 g agar) for approximately 5 days until sporulation. Then, the endospores were harvested and mixed with a cryo-protective agent containing 20% glycerol and stored at −80 °C as the stock culture. The bacterium was activated by defrosting at room temperature and then streaked on LB agar plates. The steaked plates were then incubated at 30 °C for 1–2 days. One colony was then transferred into 50 mL of LB broth in a 250 mL Erlenmeyer flask. The cultures were incubated at 30 °C in a rotary incubator shaker at a speed of 200 rpm for 16 h, and the optical density (OD600 nm) was adjusted to 1.0 for inoculum at 5% (v/v) in further cultivation experiments.
The Vip3A production (VP) medium was the semi-defined medium that was optimized by our previous study [24] containing (L−1) 5.05 g glycerol, 49.17 g soytone, 30.05 g casein hydrolysate, 1.99 g CaCl2·2H2O, 0.0075 g CuSO4, 0.015 g MnSO4, H2O, 9.4 g K2HPO4, 2.2 g KH2PO4, 0.2 g MgSO4·7H2O, 5 g yeast extract, 0.0025 g NiCl2·6H2O, and 3 mL vitamin solution. The initial pH was adjusted to 7.2.
2.2. Effects of Different Physical Factors Which Affect Dissolved Oxygen on Vip3A Production by B. thuringiensis BT294 in Shake Flasks
Dissolved oxygen content affected both the growth and product formation of the bacterium in all liquid cultures. Each cultivation size had different factors that affected the amount of dissolved oxygen. Factors affecting Vip3A production in shake flask cultivation were studied, such as the type and concentration of antifoam agents, type of flask and shaking speed rate. B. thuringiensis Bt294 was cultured in 50 mL of VP medium at an initial pH of 7.2 in 250 mL flasks using a 5% concentration of inoculum in this study. The cultivation was then incubated in a rotary shaker, and the temperature was controlled at 30 °C for 5 days.
2.2.1. Effects of Antifoam Agents and Concentrations on Vip3A Production by B. thuringiensis BT294 in Shake Flasks
An antifoam agent is essential in protein production in submerged fermentation, especially in highly aerobic conditions. Apart from controlling foaming, the antifoam agent also decreases the dissolving of oxygen in the culture medium. The effects of various antifoam agents and concentrations on Vip3A production by B. thuringiensis Bt294 were investigated. Each antifoam agent included polyether-based (Antifoam 204, Sigma-Aldrich, St. Louis, MO, USA)), silicone-based (Antifoam AFE-1520; XIAMETER, Antifoam 1410; Dow, MI, USA) and various vegetable oil groups (palm oil, soybean oil, olive oil, rice bran oil and coconut oil) at different initial concentrations of 0.05, 0.1 and 0.5% (v/v) and was added into a 250 mL Erlenmeyer flask containing 50 mL of VP medium before sterilization. B. thuringiensis Bt294 was inoculated into a sterile medium and incubated at 30 °C, 200 rpm. The Vip3A concentration after 5 days of cultivation was analyzed by SDS-PAGE.
2.2.2. Effects of Different Flask Types on Vip3A Production by B. thuringiensis BT294
B. thuringiensis is a highly aerobic bacteria that requires high levels of dissolved oxygen to grow and produce essential metabolites. Modifications of shaking flasks by the introduction of baffles are frequently necessary to provide sufficient aeration [25]. In this study, baffled flasks were used to increase the degree of aeration. Thus, the effects of aeration on Vip3A production by B. thuringiensis Bt294 were studied in two different types of shaking flask geometries, including Erlenmeyer flasks and baffled flasks. B. thuringiensis BT294 was inoculated in 50 mL of VP medium in two types of 250 mL flasks and incubated at 30 °C at 200 rpm. The cultivation broth was collected and maintained at −20 °C before Vip3A concentration analysis by SDS-PAGE. The Vip3A concentration at 3, 4 and 5 days was analyzed by SDS-PAGE.
2.2.3. Effects of Aeration at the Shake Flask Scale with Shaking Speed Rates on Vip3A Production by B. thuringiensis BT294
The shaking speed rate is a significant factor in the shaking flask cultivation scale, affecting growth and product formation. Therefore, it is an important factor that needs to be studied. B. thuringiensis Bt294 was cultured with VP medium in a 250 mL baffled flask. In this study, different shaking speed rates of 150, 170, 200 and 230 rpm on an incubator shaker were used at 30 °C. Vip3A concentrations at 3, 4 and 5 days were analyzed by SDS-PAGE.
2.3. Effects of pH-Controlled Culture and Dissolved Oxygen on Vip3A Production by B. thuringiensis BT294 in a 5 L Bioreactor
Batch cultivation was carried out in a 5 L bioreactor (Biostat B Plus, Sartorius stedim, Germany) with a working volume of 3 L of VP medium at 30 °C. The inoculum was used at 5% (v/v) of the working volume. The initial culture condition and stirring rate were fixed at 300 rpm with an aeration rate of 1 vvm. The culture pH and DO2 were measured by a pH sensor (EasyFerm Plus K8 325, Hamilton, Bonaduz, Switzerland) and DO2 sensor (OxyFerm FDA 325, Hamilton, Bonaduz, Switzerland), respectively. Samples were taken every 3 h for a 72 h cultivation period.
2.3.1. Effects of the pH-Controlled Culture on Vip3A Production by B. thuringiensis BT294
The effects of pH-controlled culture on the Vip3A production were evaluated by comparison of the Vip3A production in the VP medium with an initial pH of 7.2. During the cultivation, pH was controlled at 7.2 (±0.1) throughout the cultivation time with 4 N NaOH and 2 N H2SO4, and uncontrol pH was demonstrated against the Vip3A production. The supernatant was determined for Vip3A concentration by SDS-PAGE. Cell growth was determined by measuring the optical density at 600 nm using a spectrophotometer. The pH profile was also monitored and recorded.
2.3.2. Effects of Different Dissolved Oxygen Levels on Vip3A Production by B. thuringiensis BT294
The effects of different dissolved oxygen (DO) levels on Vip3A production were studied and compared with uncontrolled pH cultivation in this study. During the cultivation, the lowest point of dissolved oxygen was controlled at above 5, 10, 20 and 40% by cascade controlled of agitation rate at 300–800 rpm and aeration with air. The supernatant was collected every 3 h, and the Vip3A concentration and cell growth were analyzed.
2.3.3. Effect of Agitation Rate on Vip3A Production by B. thuringiensis BT294
In this experiment, Vip3A protein was produced in a 5 L bioreactor with different agitation rates of 300, 400, 500 and 600 rpm using the same aeration rate at 1.33 vvm (4 L/min) without pH control. The supernatant was collected, and the Vip3A concentration and cell growth were measured.
2.3.4. Effects of Aeration Rate on Vip3A Production by B. thuringiensis BT294
The effect of the aeration rate was studied after the appropriate agitation rate was obtained. The different rates of aeration such as 1.00, 1.33 and 1.66 vvm were used with suitable agitation from a previous experiment without pH control. The supernatant during cultivation was collected, and the Vip3A concentration and cell growth were analyzed.
2.4. Scale-up of Vip3A Production by B. thuringiensis BT294 in a 750 L Pilot-Scale Bioreactor
The pilot-scale production was performed in a 750 L bioreactor (B.E. Marubishi, Thailand) of 0.85 m diameter, T, and 1.52 m height, H, fit with 2 Rushton turbine impellers of diameter, D, of 0.28 m, agitating at 180 rpm (equivalent to the power input, −εT of 0.94 W/kg). The bioreactor contained 250 L of VP medium, which was flooded with only one impeller, with 1–1.33 vvm aeration. The power P (W) is calculated from P = nPogρN3D5, where n is the number of impellers, Pog is the power number of 2.4 (no unit or dimensionless) for fluid with air sparging of Rushton turbine impeller in the bioreactor, ρ is the culture broth density (kg/m3), N is the impeller speed (rps), and D is the impeller diameter (m). The power input or energy dissipation rate per unit mass, ε¯T (W/kg) is calculated from ε¯T or , where V is the culture broth volume (m3).
The cultivation medium compositions were similar to the compositions and concentration of the 5 L medium. The cultivation was conducted with the optimized uncontrolled pH and dissolved oxygen (DO). The supernatant was then collected, and the Vip3A concentration and cell growth were analyzed.
2.5. Insect Toxicity Assay
Vip3A bioassays were performed using 3rd instar larvae of Spodoptera litura obtained from the NPV production pilot plant at Thailand Science Park, Thailand. The insect toxicity of Vip3A in culture filtrates of B. thuringiensis Bt294 was analyzed by surface contamination assays. The cultivated medium that contained Vip3A was diluted with distilled water to different concentrations (0, 100, 200, 400, 600 and 1000 ng/cm2). In total, 25 µL of each concentration was dropped and poured on the artificial diet surface (consisting of (L−1) 10 g yeast extract, 1.5 g sorbic acid, 2.5 g ascorbic acid, 20 mL multivitamin stock, 120 g ground mung bean and 12.5 g agar) in a 24-well tissue culture plate. Each treatment was performed with 6 replicates (12 larvae/replicate). After the protein solution was completely absorbed, the larvae were added to each well and incubated at room temperature (30 ± 2 °C). Larvae mortality was recorded for 0–7 days after incubation and calculated to be corrected mortality. In total, 72 S. litura larvae were treated with each concentration, and 3 independent experiments were performed. The LC50 was assessed using probit analysis [14]. The optimal medium was used as a negative control.
2.6. Analyses and Quantitative Methods
2.6.1. Cell Growth Determination
Cell growth was analyzed by measuring the optical density at 600 nm with a spectrophotometer, and the dried cell weight concentration was calculated from the OD and standard slope.
2.6.2. Soluble Protein Measurement
Before the measurement of VIP3A concentration, the soluble protein in the supernatant was measured by Bradford assay [26]. Bradford dye (Bio-Rad) was added to the supernatant, and the optical density was measured at 595 nm. The concentration of soluble protein from the supernatant was calculated by comparison with a bovine serum albumin (BSA) standard.
2.6.3. Determination of Vip3A Concentration
The concentration of Vip3A protein was measured using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Then, 20 µL of soluble protein from the culture supernatant was mixed with SDS-PAGE sample buffer (consisting of 0.6 mL of 1 M Tris buffer (pH 6.8), 5 mL of 50% glycerol, 2 mL of 10% SDS, 1 mL of 1% bromophenol blue, 50 μL of β-mercaptoethanol, and 0.9 mL of water) and boiled at 100 °C for 10 min. After that, the samples were loaded and separated by SDS-PAGE (ATTO AE-6530 system, Tokyo, Japan) using a continuous gel that included a 12% resolving gel and 4% stacking gel. The gel was stained using Coomassie blue R250 stain [15]. The protein concentration of Vip3A was estimated by a densitometer with Gene Directory software (Syngene, Cambridge, UK) after SDS-PAGE separation. Bovine serum albumin (BSA) was used as a standard.
2.6.4. Calculation of Corrected Mortality
Corrected mortality is the true death percentage of insecticides from the effect of treatment calculated following Abbott’s formula [27].
3. Results and Discussion
3.1. Effects of the Physical Factor Which Affected Dissolved Oxygen on Vip3A Production by B. thuringiensis Bt294 in Shake Flasks
3.1.1. Effects of Types and Concentrations of Antifoam Agents on Vip3A Production by B. thuringiensis Bt294
Foam formation is an important problem for the cultivation processes of microorganisms in liquid media, especially in liquid processes containing an enriched protein content of the growth and production medium (from yeast extract, peptone or fish mill), high aeration, high agitation and protein formation from the microorganism [28,29]. Foaming can lead to reduced productivity since bursting bubbles can damage proteins [30]. This resulted in loss of sterility if foams escaped the bioreactor or led to overpressure by blocking the exit filters. A defoamer or antifoam agents, particularly chemical or biological agents, are routinely employed for foaming solutions. However, the type and concentration of antifoam agent are still necessary factors to study. They affect the KLa, oxygen transfer rate (OTR) and oxygen utilization rate (OUR) of microbial growth in cultivation processes and subsequently affect the growth rate and product formation.
Routledge and Bill, 2012 [31] reported that different antifoam agents had different impacts on microbial physiology and growth. The addition of antifoam agents affects the dissolved oxygen level depending on the dose and type of antifoam agent, which often reduces the dissolved oxygen level of the culture medium, and the choice of the chemical used should take this into consideration. Thus, this research studied the effects of eight antifoam agents at different concentrations in shake flasks on Vip3A production by B. thuringiensis Bt294. The results in Table 1 show that the production with controlled conditions, without antifoam added, produced Vip3A at 1500.00 mg/L. Only Antifoam 204 at 0.5% (v/v), which is classified as polyether-based, had a positive effect on Vip3A production. This antifoam promoted a Vip3A production yield of 1833.00 mg/L. While another antifoam agent of silicone-based and vegetable oils had a slightly negative effect on Vip3A production, Vip3A production of 1050.00–1240.00 mg/L was obtained. Moreover, 0.05% (v/v) palm oil had the greatest effect on Vip3A production, and only 650 mg Vip3A/L was obtained. Vidyarthi and Desrosiers, 2000 [28] reported that natural oils (canola, olive and peanut oils) enhanced the DO2 concentration in the cultivation liquid, which acted as an oxygen vector. Thus, to prevent the formation of foam in bioreactors in this study, Antifoam 204 was employed for further studies.

Table 1.
Effects of antifoam on Vip3A production by B. thuringiensis Bt294.
3.1.2. Effects of Flask Types on Vip3A Production by B. thuringiensis Bt294
Shake flasks are the most applicable laboratory-scale cultivation vessels. However, shake flasks do not provide an ideal environment for protein production due to their relatively low aeration capacity. A simple approach to improve dissolved oxygen levels in shake flask cultures is to use special flasks, such as baffled flasks, which can increase dissolved oxygen through the collision of the culture medium with the bottom baffle. A preliminary study on the effects of aeration on Vip3A production was carried out in baffled flasks compared with normal Erlenmeyer flasks. Figure 1 shows that cultivation in baffled flasks produced higher Vip3A proteins at 2000.00 mg/L after 3 days of cultivation. Cultivation in an Erlenmeyer flask produced a lower concentration of 44.50 mg/L at 3 days of cultivation, and the 1500.00 mg/L Vip3A protein was observed at 5 days. Baffled flasks improved the aeration and mixing of the culture, which directly affected oxygen transfer in the cultivation system [32] and promoted cell and protein production.
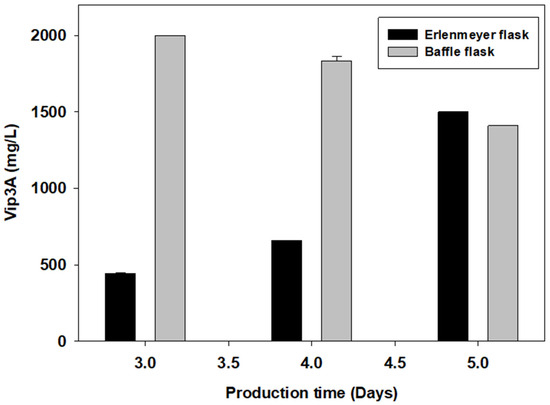
Figure 1.
Changes in Vip3A concentration in different flask cultivation types.
3.1.3. Effects of Shaking Speeds on Vip3A Production by B. thuringiensis Bt294
To study the effects of dissolved oxygen level on Vip3A production, the effects of different shaking speeds at 150, 170, 200 and 230 rpm were determined in baffled flask cultivation. Figure 2 shows the highest protein production at 3 days and then decreased. The different shaking speeds significantly affected protein production, and shaking speeds of 150–200 rpm gave 600.00, 642.00 and 2000.00 mg/L Vip3A protein at 3 days. These results showed that a higher shaking speed directly improved the aeration quality and oxygen transfer in the aerobic cultivation process, in which higher Vip3A production was produced. Many studies have reported that shaking speeds of 0–200 rpm increase both the cell biomass and protein production of Bacillus sp. [33,34]. However, a shaking speed higher than 230 rpm resulted in a lower Vip3A production of 1042.00 mg/L at 3 days, which might be due to shear stress affecting protein synthesis and excretion [35]. At lower speeds, microorganisms and substrates were not well mixed, and the dissolved oxygen content was low. Therefore, this study suggested that a shaking speed of 200 rpm with a baffled flask was the optimal condition for producing Vip3A by B. thuringiensis Bt294.
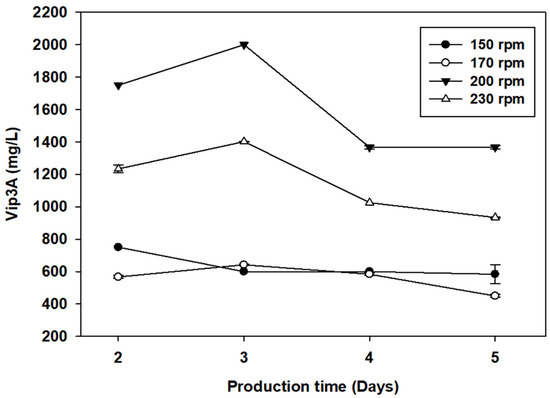
Figure 2.
Vip3A protein production by B. thuringiensis Bt294 at different shaking speeds.
Based on the results of this study, cultivation in baffled flasks at a shaking speed of 200 rpm was the best condition for the shake flask scale. It produced the highest concentration of Vip3A at 2000.00 mg/L. This concentration was higher than that reported in the other research. Boukedi et al., 2018 [36] reported that optimization of the culture conditions in recombinant E. coli MOS Blue cells produced Vip3A protein at 170.00 mg/L.
3.2. Effects of Dissolved Oxygen and pH on Vip3A Production by B. thuringiensis Bt294 in a 5 L Bioreactor
3.2.1. Effects of Different pH-Controlled Culture on Vip3A Production by B. thuringiensis Bt294
The pH-controlled culture affected growth and Vip3A protein production directly, which included respiration and product formation of microorganisms. Microbial cells respond to environmental alterations and maintain the intracellular pH at a constant level. Even with large variations in the pH of the extracellular medium, the pH of the environment has a natural tendency to change along with the bioprocess [37]. The pH in the shake flask could not be controlled throughout the cultivation, but was carried out using buffer chemicals, which was different from the cultivation of bacteria in bioreactors in which the pH can be controlled. The effects of pH-controlled culture on Vip3A production by B. thuringiensis Bt294 was studied in a 5 L bioreactor, and the time profile of Vip3A production, bacterial growth, pH and dissolved oxygen are shown in Figure 3. The highest Vip3A concentration at 1203.00 mg/L under pH uncontrolled culture (Figure 3a) was obtained. Vip3A production of 525.00 mg/L under the pH-controlled culture at 7.2 (Figure 3a) was obtained. This result was consistent with the other previous research. Smith, 1982 [38] and Yousten and Wallis, 1987 [39] demonstrated that the uncontrol pH during the cultivation of B. thuringiensis and B. sphaericus resulted in an increase in insecticidal activity compared with pH control. Furthermore, the highest growth was found when the pH was kept stable in the cultivation process (Figure 3b). The growth rates of the bacterium under uncontrolled pH decreased as a result of the increasing pH during the cultivation. The higher rate of bacterial growth at pH 7.2 might reduce the Vip3A protein, which is a non-growth-associated protein. The higher biomass concentration obtained at a pH of 7.2 compared to uncontrolled pH might be due to the depletion of glycerol that resulted in the pH increasing and was inappropriate for bacterial growth. This resulted in greater Vip3A production.
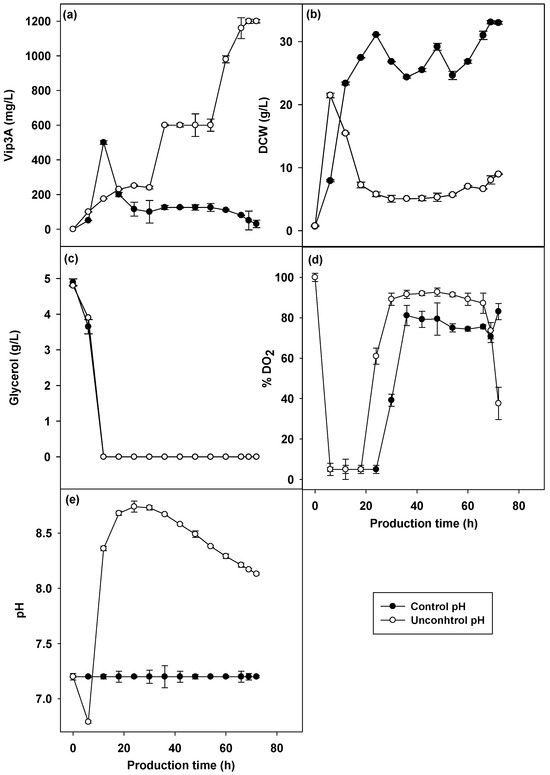
Figure 3.
Vip3A concentration (a), CDW (b), glycerol (c), pH (d) and dissolved oxygen (e) in different pH-controlled cultivations of B. thuringiensis Bt294.
Continuous control of pH during cultivation prevents cell injury and death caused by prolonged exposure to pH fluctuations. However, cultivation under a pH-controlled culture at 7.2 did not favor Vip3A production by B. thuringiensis Bt294. Thus, uncontrolled pH cultures throughout the course of cultivation were chosen for the next experiment to optimize Vip3A protein production by B. thuringiensis Bt294.
3.2.2. Effects of Controlled and Uncontrolled of the Lowest Point of Dissolved Oxygen (DO2) Levels on Vip3A Production by B. thuringiensis Bt294
The dissolved oxygen (DO2) is the amount of oxygen that is present in the culture medium. It is the significant parameter that affects cell growth, metabolite product synthesis, and cell activities maintained in the cultivation process involving aerobic microorganisms. There has also been a direct correlation between aeration rate and agitation rate. There are three methods that are generally used to control the DO2 concentration during the fermentation processes. One of the most frequently employed methods is the manipulation of the agitation speed to promote the mass transfer of the gas bubbles through the gas–liquid (cell culture medium) interface. The second method involves adjustment of the aeration rate, and the third method is based on altering the partial pressure of oxygen in the gas phase [40]. In this research, dissolved oxygen was controlled by adaptation of agitation speed without pure oxygen feed or changing the rate of aeration because the agitation alone can supply enough dissolved oxygen through this controlled cascade.
Microorganisms varied in their oxygen requirement. In particular, oxygen acts as a terminal electron acceptor for oxidative reactions to provide energy for cellular activities. Most Bacillus species are strictly aerobic organisms [41]. Figure 4 shows the production of Vip3A protein (Figure 4a), cell dry weight (Figure 4b), dissolved oxygen (Figure 4d) and pH (Figure 4e) at different controlled DO2 levels (not lower than 5%, 10%, 20% and 40%) compared with uncontrolled DO2. The cultivation with the highest DO2 control at above 40% promoted the highest production of cell biomass of 26.51 g/L but did not promote the production of the Vip3A protein, which gave Vip3A production of 350.00 mg/L at 48 h. The cultivation of Vip3A at above 20% dissolved oxygen control gave the highest Vip3A protein of 1250 mg/L at 36 h compared with the controlled DO2 levels, and the lowest cell concentration of 18.15 g/L was obtained. The controlled DO2 level below 20% gave a higher cell concentration, but the Vip3A protein was not obtained from these conditions. The results suggested that a higher DO2 led to a biosynthetic pathway of cell growth but did not favor Vip3A production, and this result was consistent with other reports [9]. The excessive dissolved oxygen condition improved growth and sporulation, while delta-endotoxin production was reduced. During cultivation, it was observed that the frequency of the increasing agitation rate of 400 rpm to 800 rpm to maintain the dissolved oxygen level within the desired range differed during the log phase of cell growth. Higher dissolved oxygen control conditions resulted in a higher frequency of acceleration. A high agitation speed may cause protein denaturation and damage bacterial cells that reduce Vip3A protein production [42].
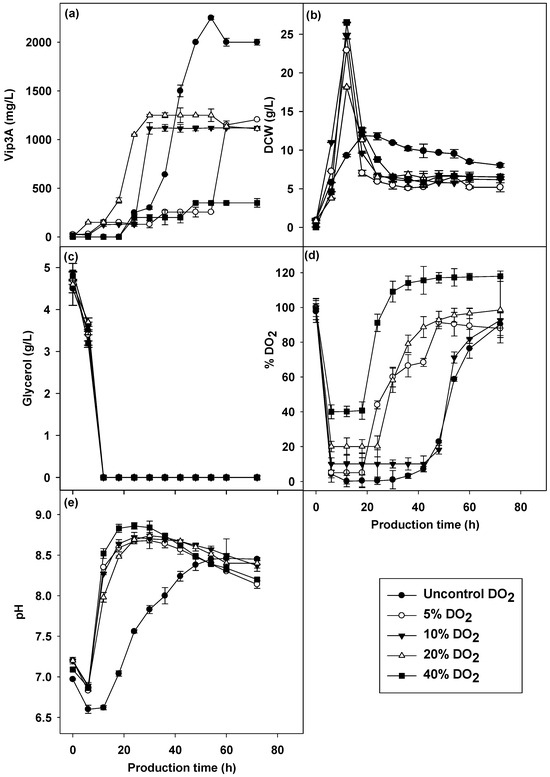
Figure 4.
Vip3A concentration (a), CDW (b), glycerol (c), pH (d) and dissolved oxygen (e) in different dissolved oxygen cultures of B. thuringiensis Bt294.
However, it was clearly seen that the amount of Vip3A protein produced in all lowest point-controlled DO2 was lower than in the uncontrolled DO2 conditions. Cultivation with uncontrolled DO2 at 400 rpm and 4 L aeration gave the highest Vip3A concentration of 2250 mg/L at 54 h among the other condition, while the lowest cell biomass was obtained under this condition. The results suggested that B. thuringiensis Bt294 produced higher Vip3A in the dissolved oxygen limitation and in a biomass growth limitation phase. Furthermore, cultivation under control of the lowest point of DO2 was not an effective process for Vip3A protein production.
3.2.3. Effects of Agitation Rate on Vip3A Production by B. thuringiensis Bt294
Agitation plays an important factor in mixing, oxygen transfer rate and shearing in the cultivation process that directly influences growth and bioproduct formations. To study the effects of agitation on Vip3A production, the cultivations were run at different agitation rates of 300, 400, 500 and 600 rpm at 30 °C with a constant aeration rate of 4 L/min (1.33 vvm) and an initial pH of 7.2. The results showed that the agitation rate directly affected cell mass production (Figure 5b). The cell biomass of B. thuringiensis increased when the agitation rate increased, and the highest values of DCW at 300-600 rpm were 5.59, 9.13, 12.88 and 17.76 g/L at 12 h. Agitation rates affected both Vip3A concentration and production time (Figure 5a). The maximum production of the Vip3A protein was achieved at an agitation rate of 500 rpm (5609.24 mg/L) at 36 h, while the highest Vip3A production at agitation rates of 300, 400 and 600 rpm were 1986.44 (48 h), 4222.55 (42 h) and 4544.98 (24 h), respectively. The increased agitation rate improved the mass transfer and oxygen transfer and maintained homogeneous chemical and physical conditions in the medium that promoted Vip3A protein and cell mass production [43]. The higher agitation rate of more than 500 rpm reduced Vip3A protein production due to oxidative, shear stress and heterogeneous mixing effects. In contrast, a decrease in protein production was also observed below the agitation rate of 500 rpm, resulting from incomplete mixing and/or oxygen transfer resistance at a low agitation rate [44].
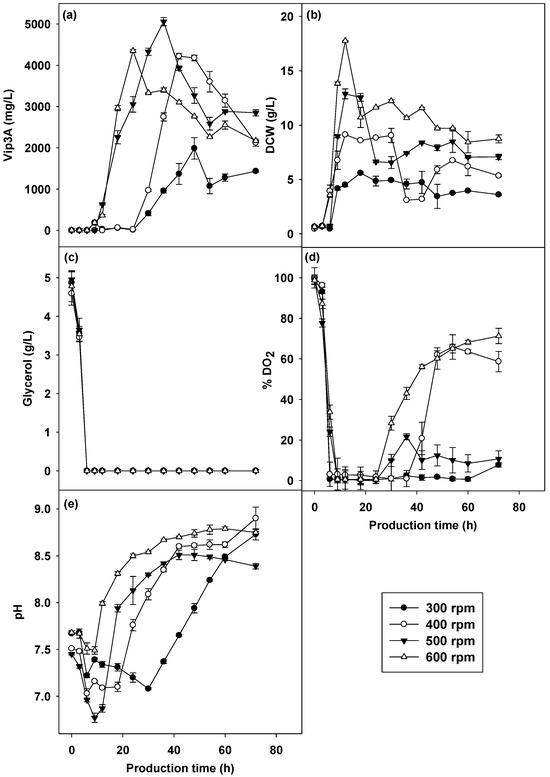
Figure 5.
Vip3A concentration (a), CDW (b), glycerol (c), pH (d) and dissolved oxygen (e) in different agitation rate cultivations of B. thuringiensis Bt294.
The pH change (Figure 5e) during the growth of B. thuringiensis Bt294 showed that the bacterium secreted some organic acids into the cultivation broth, especially in the oxygen limitation state (300 rpm). The pH values at the early phase decreased from the initial pH and increased while the bacterium produced higher Vip3A proteins. All agitation rates (Figure 5d) with dissolved oxygen decreased greatly before 9 h of cultivation and remained continuously close to 0%; then, the DO2 profile changed according to agitation speeds (Figure 5). The DO2 was distinctly lower at an agitation rate of 300 rpm, and the DO2 levels remained around 0% throughout the cultivation times. The DO2 at 400 rpm and 600 rpm slightly increased and remained constant at about ~60%. Thus, the DO2 at 500 rpm was increased to ~10% at 30 h throughout the cultivation period. The results showed that the agitation rate at 500 rpm (equivalent to the power input, −εT of 1.34 W/kg) was the optimal agitation speed for B. thuringiensis Bt294 to produce the Vip3A protein. The bacterium produced a higher concentration of Vip3A protein in a shorter time compared to all agitation rates, which reduced the cost of energy consumption involved in bioreactor manipulation at larger scales.
3.2.4. Effects of Aeration Rates on Vip3A Production by B. thuringiensis Bt294
Aeration is another essential parameter for the growth of aerobic microorganisms. It plays an important role in carbon dioxide removal and temperature and moisture control [45]. It affected the dissolved oxygen concentration in the culture medium and enhanced both growth and bioproduct formation [46]. Batch cultivations were performed using three different aeration rates at 1.00, 1.33 and 1.66 vvm at the same agitation rate of 500 rpm, and the cultivation temperature was controlled at 30 °C. Figure 6 shows the effects of aeration rate on Vip3A protein production (Figure 6a), CDW (Figure 6b), DO2 concentration (Figure 6d) and pH values (Figure 6e) during the cultivation process. The results showed that higher aeration levels resulted in a higher cell biomass and Vip3A protein concentration; moreover, higher aeration reduced the Vip3A production period. The cultivation performed at 1.66 vvm had the highest growth (15.46 g/L at 18 h) and Vip3A production (5645.67 mg/L at 30 h) compared to other cultivations carried out at different aeration rates. The cultivation performed with lower aeration rates of 1.33 and 1.00 vvm had lower growth (13.39 g/L at 12 h and 14.18 g/L at 18 h) and Vip3A concentrations (4978.71 mg/L at 36 h and 2864.76 mg/L at 42 h). This might be because an increase in aeration provides more oxygen for microbial respiration, particularly in the oxidative phosphorylation pathway, which potentially improves cell growth rates, biomass formation, and the production titer of target compounds [47]. DO2 concentration profiles were greatly different under three levels of aeration rates. The DO2 concentration of 1.66 vvm was lower than 10% (but not below 1%) at 6–18 h. These aeration rates gave the highest Vip3A protein concentration at a shorter time of 30 h of cultivation. The cultivation performed at 1.33 vvm produced the second-highest Vip3A concentration at 36 h, even when the DO2 concentration was below 10% at 9–36 h. However, the following two-phase DO2 profile was observed: below 1% at 9–12 h and above 1%, but lower than 10% at 18–36 h. The cultivation at 1.00 vvm produced the lowest Vip3A protein with a longer time of 42 h. The DO2 level was observed to be below 1% at 6–24 h due to the short supply of oxygen. From the results, it was found that prolonged cultivation under 1% DO2 resulted in a decrease in Vip3A protein yield and a longer production time. The aeration rate of 1.66 vvm was the optimal aeration for the Vip3A protein production by the wild-type strain of B. thuringiensis BT 294, which increased the protein yield and reduced the production period.
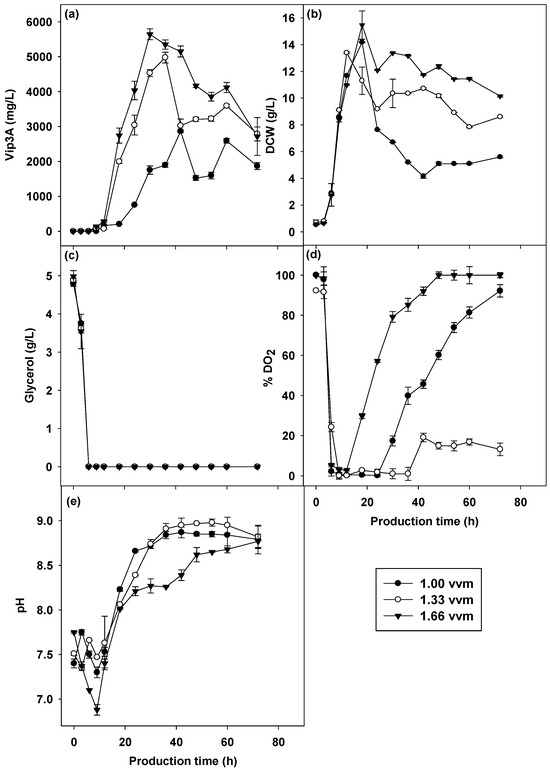
Figure 6.
Vip3A concentration (a), CDW (b), glycerol (c), pH (d) and dissolved oxygen (e) in different aeration cultivations by B. thuringiensis Bt294.
3.3. Vip3A Production by B. thuringiensis Bt294 in 750 L Bioreactor
The necessary scaling up of the fermentation process demonstrates the cultivation production at a larger scale, resulting in consistency of productivity and quality compared with smaller scales. The keys to scaling up are to achieve project goals, produce desired products with acceptable quality and yield, and fully understand and control operational risks at large-scale manufacturing [48]. This research produced a bioinsecticide, Vip3A protein, at a higher concentration in a pilot-scale bioreactor (750 L) of 3750.00 mg/L at 42 h (Figure 7a). From these results, the cultivation in the 750 L bioreactor slightly increased in the production of Vip3A at 18 h, which was slower than that in the 5 L bioreactor. The growth profile (Figure 7a) showed a slight increase in the lag phase, resulting in a shift in Vip3A production to 24 h with a lower CDW of 13.85 g/L. Regarding the dissolved oxygen concentration (Figure 7b), it was shown that the dissolved oxygen concentration during cultivation decreased to 0% at 12 h. A productivity of 89.29 mg/L/h Vip3A protein was calculated. This was lower than that in the 5 L laboratory-scale bioreactor due to the different operational parameters. The power input and aeration rate of the pilot-scale were only 0.93 W/kg (agitation speed of 180 rpm with 1 rushton turbine) and 1.00 vvm (0–6 h and 36–72 h) and 1.33 vvm (12–30 h). A 5 L bioreactor was used with a higher power input and agitation rate of 1.34 w/kg and 1.66 vvm, respectively.
Figure 7.
Vip3A concentration, CDW and glycerol (a), pH and dissolved oxygen (b) in 750 L cultivation by B. thuringiensis Bt294.
3.4. Insect Toxicity Assay of Vip3A Produced by B. thuringiensis Bt294
Vip3A proteins were evaluated with the lepidopteran insect pest S. litura. For all treatments, death was observed at 48 h. An accumulative mortality of more than 80% was observed at 72 h in the highest concentration treatment. After 7 days, 89.8% corrected mortality was observed in the 1000 ng/cm2 treatment. Meanwhile, 72.40%, 45.00%, 22.20%, 13.00% and 0.00% of corrected mortality were obtained in the lower concentration treatments, respectively. From the result of corrected mortality, Vip3A was highly active against third instar larvae of S. litura, with LC50 values of 472.5 ng/cm2 at 7 days (Table 2). Compared to Song et al., 2016 [49], LC50 of Vip3Aa against third instar larvae of S. litura was shown at 200.627 ng/cm2. Another recombinant Vip3A protein production was tested against S. litura, which was 200 ng/cm2 of LC50 [50].

Table 2.
Insecticidal activity of culture supernatant containing Vip3A of B. thuringiensis Bt294 against S. litura.
4. Conclusions
From all experiments in this study, it can be concluded that the dissolved oxygen content in the medium broth was a very important factor for Vip3A protein production by B. thuringiensis Bt294 at both the shake flask and bioreactor scales. The production of Vip3A protein at the shake flask level in baffled flasks with a shaking speed of 200 rpm increased the dissolved oxygen and mixing efficiency without cell damage by shear stress compared to the Erlenmeyer flask. The production of Vip3A in 5 L laboratory bioreactors showed that dissolved oxygen had significant effects on protein production by B. thuringiensis Bt294. However, the oxygen controlled by the cascade system was ineffective due to the culture favoring producing biomass in conditions with plenty of oxygen and shear stress at a high agitation rate at 800 rpm. Agitation rates lower than 500 rpm and aeration rates of 1.66 vvm resulted in higher Vip3A protein production by B. thuringiensis Bt294. These results suggested that production of the Vip3A protein by B. thuringiensis Bt294 required the proper dissolved oxygen limitation phase and pH to shift the metabolic flux to Vip3A instead of biomass production. The larger-scale production of Vip3A gave lower productivity of Vip3A production in comparison with laboratory scales due to the limitations of some parameters, such as power input and agitation rate. The obtained Vip3A was highly active against third instar larvae of S. litura, with LC50 values of 472.50 ng/cm2 at 7 days. From these results, the Vip3A protein has high potential for use as a bioinsecticide to control lepidopteran pests on organic farms.
Author Contributions
Experimental investigation, methodology, data curation, formal analysis and writing—original draft preparation were performed by P.N.; investigation and methodology were performed by B.W., S.K., N.P., C.T., A.R. and K.M.; supervision was performed by B.P.; and conceptualization, funding acquisition, project administration, resources, supervision, writing were performed by W.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was financially supported by the Cluster Program Management Office (CPMO), the National Science and Technology Development Agency (NSTDA), Thailand, (P18-50649).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Review Board (or Ethics Committee) of National Center for Genetic Engineering and Biotechnology, National Science and Technology Development Agency (protocol code BT-Animal 31/61 on 14 September 2018).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to acknowledge the NXPO via the Program Management Unit for Human Resources & Institutional Development, Research and Innovation, Thailand.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, M.; Kumar, H.; Kaur, S. Vegetative Insecticidal Protein (Vip): A Potential Contender from Bacillus thuringiensis for Efficient Management of Various Detrimental Agricultural Pests. Front. Microbiol. 2021, 12, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Crickmore, N.; Berry, C.; Panneerselvam, S.; Mishra, R.; Connor, T.R.; Bonning, B.C. A Structure-Based Nomenclature for Bacillus thuringiensis and Other Bacteria-Derived Pesticidal Proteins. J. Invertebr. Pathol. 2020, 186, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ruiz de Escudero, I.; Banyuls, N.; Bel, Y.; Maeztu, M.; Escriche, B.; Muñoz, D.; Caballero, P.; Ferré, J. A Screening of Five Bacillus thuringiensis Vip3A Proteins for Their Activity against Lepidopteran Pests. J. Invertebr. Pathol. 2014, 117, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Syed, T.; Askari, M.; Meng, Z.; Li, Y.; Abid, M.; Wei, Y.; Guo, S.; Liang, C.; Zhang, R. Current Insights on Vegetative Insecticidal Proteins (Vip) as next Generation Pest Killers. Toxins 2020, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.K.; Miles, P.J.; Chen, J.S. Brush Border Membrane Binding Properties of Bacillus thuringiensis Vip3A Toxin to Heliothis virescens and Helicoverpa zea Midguts. Biochem. Biophys. Res. Commun. 2006, 339, 1043–1047. [Google Scholar] [CrossRef]
- Milne, R.; Liu, Y.; Gauthier, D.; van Frankenhuyzen, K. Purification of Vip3Aa from Bacillus thuringiensis HD-1 and Its Contribution to Toxicity of HD-1 to Spruce budworm (Choristoneura fumiferana) and Gypsy moth (Lymantria dispar) (Lepidoptera). J. Invertebr. Pathol. 2008, 99, 166–172. [Google Scholar] [CrossRef]
- Hmani, M.; Boukedi, H.; Ben Khedher, S.; Elleuch, A.; Tounsi, S.; Abdelkefi-Mesrati, L. Improvement of Vip3Aa16 Toxin Production and Efficiency through Nitrous Acid and UV Mutagenesis of Bacillus thuringiensis (Bacillales: Bacillaceae). J. Econ. Entomol. 2018, 111, 108–111. [Google Scholar] [CrossRef]
- Boukedi, H.; Ben Khedher, S.; Triki, N.; Kamoun, F.; Saadaoui, I.; Chakroun, M.; Tounsi, S.; Abdelkefi-Mesrati, L. Overproduction of the Bacillus thuringiensis Vip3Aa16 Toxin and Study of Its Insecticidal Activity against the Carob Moth Ectomyelois Ceratoniae. J. Invertebr. Pathol. 2015, 127, 127–129. [Google Scholar] [CrossRef]
- Zouari, N.; Achour, O.; Jaoua, S. Production of Delta-Endotoxin by Bacillus thuringiensis subsp kurstaki and Overcoming of Catabolite Repression by Using Highly Concentrated Gruel and Fish Meal Media in 2- and 20-Dm3 Fermenters. J. Chem. Technol. Biotechnol. 2002, 77, 877–882. [Google Scholar] [CrossRef]
- Ghribi, D.; Zouari, N.; Trabelsi, H.; Jaoua, S. Improvement of Bacillus thuringiensis Delta-Endotoxin Production by Overcome of Carbon Catabolite Repression through Adequate Control of Aeration. Enzym. Microb. Technol. 2007, 40, 614–622. [Google Scholar] [CrossRef]
- Masri, M.M.M.; Ariff, A.B. Effect of Different Fermentation Strategies on Bacillus thuringiensis Cultivation and Its Toxicity towards the Bagworm, Metisa plana Walker (Lepidoptera: Psychidae). Egypt. J. Biol. Pest Control 2020, 30, 2. [Google Scholar] [CrossRef]
- Zou, H.; Ding, S.; Zhang, W.; Yao, J.; Jiang, L.; Liang, J. Study on Influence Factors in Bacillus thuringiensis Production by Semi-Solid State Fermentation Using Food Waste. Procedia Environ. Sci. 2016, 31, 127–135. [Google Scholar] [CrossRef]
- Ndao, A.; Sellamuthu, B.; Gnepe, J.R.; Tyagi, R.D.; Valero, J.R. Pilot-Scale Biopesticide Production by Bacillus thuringiensis subsp. kurstaki Using Starch Industry Wastewater as Raw Material. J. Environ. Sci. Health Part B 2017, 52, 623–630. [Google Scholar] [CrossRef]
- Rojas, N.L.; Lewkowicz, E.S.; Nobile, M.L. Alternative Low-Cost Process for Large-Scale Production of Bacillus thuringiensis in a Simple and Novel Culture System. J. Environ. Sci. Health Part B 2018, 53, 719–728. [Google Scholar] [CrossRef]
- Ghribi, D.; Zouari, N.; Jaoua, S. Improvement of Bioinsecticides Production through Adaptation of Bacillus thuringiensis Cells to Heat Treatment and NaCl Addition. J. Appl. Microbiol. 2005, 98, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Lone, S.A.; Malik, A.; Padaria, J.C. Molecular Cloning and Characterization of a Novel Vip3-Type Gene from Bacillus thuringiensis and Evaluation of Its Toxicity against Helicoverpa Armigera. Microb. Pathog. 2018, 114, 464–469. [Google Scholar] [CrossRef]
- Boniolo, F.S.; Rodrigues, R.C.; Prata, A.M.R.; López, M.L.; Jacinto, T.; da Silveira, M.M.; Berbert-Molina, M.A. Oxygen Supply in Bacillus thuringiensis Fermentations: Bringing New Insights on Their Impact on Sporulation and δ-Endotoxin Production. Appl. Microbiol. Biotechnol. 2012, 94, 625–636. [Google Scholar] [CrossRef]
- Mounsef, J.R.; Salameh, D.; Louka, N.; Brandam, C.; Lteif, R. The Effect of Aeration Conditions, Characterized by the Volumetric Mass Transfer Coefficient KLa, on the Fermentation Kinetics of Bacillus thuringiensis kurstaki. J. Biotechnol. 2015, 210, 100–106. [Google Scholar] [CrossRef]
- Holmberg, A.; Sievänen, R.; Carlberg, G. Fermentation of Bacillus thuringiensis for Exotoxin Production: Process Analysis Study. Biotechnol. Bioeng. 1980, 22, 1707–1724. [Google Scholar] [CrossRef]
- Vu, K.D.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Impact of Different PH Control Agents on Biopesticidal Activity of Bacillus thuringiensis during the Fermentation of Starch Industry Wastewater. Bioprocess Biosyst. Eng. 2008, 32, 511–519. [Google Scholar] [CrossRef]
- Zouari, N.; Ali, S.B.S.; Jaoua, S. Production of Delta-Endotoxins by Bacillus thuringiensis Strains Exhibiting Various Insecticidal Activities towards Lepidoptera and Diptera in Gruel and Fish Meal Media. Enzym. Microb. Technol. 2002, 31, 411–418. [Google Scholar] [CrossRef]
- Atehortúa, P.; Álvarez, H.; Orduz, S. Modeling of Growth and Sporulation of Bacillus thuringiensis in an Intermittent Fed Batch Culture with Total Cell Retention. Bioprocess Biosyst. Eng. 2007, 30, 447–456. [Google Scholar] [CrossRef] [PubMed]
- Amicarelli, A.; di Sciascio, F.; Toibero, J.M.; Álvarez, H. Including Dissolved Oxygen Dynamics into the Bt δ-Endotoxins Production Process Model and Its Application to Process Control. Braz. J. Chem. Eng. 2010, 27, 41–62. [Google Scholar] [CrossRef]
- Nutaratat, P.; Werapan, B.; Phosrithong, N.; Trakulnaleamsai, C.; Rungrod, A.; Utamatho, M.; Soonsanga, S.; Promdonkoy, B.; Malairuang, K.; Prathumpai, W. Vegetative Insecticidal Protein (Vip3A) Production by Bacillus Thuringiensis Bt294 and Its Efficacy against Lepidopteran Pests (Spodoptera exigua). Biotechnol. Rep. 2023, 40, e00812. [Google Scholar] [CrossRef]
- Büchs, J. Introduction to Advantages and Problems of Shaken Cultures. Biochem. Eng. J. 2001, 7, 91–98. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Vidyarthi, A.S.; Desrosiers, M.; Tyagi, R.D.; Valero, J.R. Foam Control in Biopesticide Production from Sewage Sludge. J. Ind. Microbiol. Biotechnol. 2000, 25, 86–92. [Google Scholar] [CrossRef]
- Junker, B. Foam and Its Mitigation in Fermentation Systems. Biotechnol. Prog. 2007, 23, 767–784. [Google Scholar] [CrossRef]
- Holmes, W.; Smith, R.; Bill, R. Evaluation of Antifoams in the Expression of a Recombinant FC Fusion Protein in Shake Flask Cultures of Saccharomyces cerevisiae & Pichia pastoris. Microb. Cell Factories 2006, 5, 1–3. [Google Scholar]
- Routledge, S.J.; Bill, R.M. The Effect of Antifoam Addition on Protein Production Yields. In Methods in Molecular Biology; Bill, R.M., Ed.; Humana Press: Totowa, NJ, USA, 2012; pp. 87–97. [Google Scholar]
- Kaku, V.J.; Boufadel, M.C.; Venosa, A.D. Evaluation of Mixing Energy in the Swirling and Baffled Flasks. In IUTAM Symposium on Reynolds Number Scaling in Turbulent Flow; Smits, A.J., Ed.; Springer: Dordrecht, The Netherlands, 2002; pp. 211–218. [Google Scholar]
- Sun, D.; Liao, J.; Sun, L.; Wang, Y.; Liu, Y.; Deng, Q.; Zhang, N.; Xu, D.; Fang, Z.; Wang, W.; et al. Effect of Media and Fermentation Conditions on Surfactin and Iturin Homologues Produced by Bacillus natto NT-6: LC–MS Analysis. AMB Express 2019, 9, 120. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, D. Effect of Agitation Speed on the Morphology of Aspergillus niger HFD5A-1 Hyphae and Its Pectinase Production in Submerged Fermentation. World J. Biol. Chem. 2015, 6, 265. [Google Scholar] [CrossRef]
- Pissuwan, D.; Suntornsuk, W. Production of Keratinase by Bacillus sp. FK 28 Isolated in Thailand. Kasetsart J.-Nat. Sci. 2001, 35, 171–178. [Google Scholar]
- Boukedi, H.; Ben Khedher, S.; Abdelkefi-Mesrati, L.; Van Rie, J.; Tounsi, S. Comparative Analysis of the Susceptibility/Tolerance of Spodoptera littoralis to Vip3Aa, Vip3Ae, Vip3Ad and Vip3Af Toxins of Bacillus thuringiensis. J. Invertebr. Pathol. 2018, 152, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, X.; Cheng, Y.; Zhang, X. Manipulation of pH Shift to Enhance the Growth and Antibiotic Activity of Xenorhabdus nematophila. J. Biomed. Biotechnol. 2011, 2011, 672369. [Google Scholar] [CrossRef]
- Smith, R. Effect of Strain and Medium Variation on Mosquito Toxin Production by Bacillus thuringiensis var. Israelensis. Can. J. Microbiol. 1982, 28, 1089–1092. [Google Scholar] [CrossRef]
- Yousten, A.A.; Wallis, D.J. Batch and Continuous Culture Production of the Mosquito Larval Toxin of Bacillus sphaericus 2362. J. Ind. Microbiol. 1987, 2, 277–283. [Google Scholar] [CrossRef]
- Zheng, R.; Pan, F. On-Line Tendency Control of Dissolved Oxygen Concentration during Aerobic Fed-Batch Fermentations. Appl. Sci. 2019, 9, 5232. [Google Scholar] [CrossRef]
- McBride, B.W.; Turnbull, P.C.B. Bacillus, Infection and Immunity. In Encyclopedia of Immunology, 2nd ed.; Delves, P.J., Ed.; Academic Press: Cambridge, MA, USA, 1998; pp. 311–315. [Google Scholar]
- Akhavan Sepahy, A.; Jabalameli, L. Effect of Culture Conditions on the Production of an Extracellular Protease by Bacillus sp. Isolated from Soil Sample of Lavizan Jungle Park. Enzym. Res. 2011, 2011, 219628. [Google Scholar] [CrossRef]
- Zhou, Y.; Han, L.-R.; He, H.-W.; Sang, B.; Yu, D.-L.; Feng, J.-T.; Zhang, X. Effects of Agitation, Aeration and Temperature on Production of a Novel Glycoprotein GP-1 by Streptomyces kanasenisi ZX01 and Scale-up Based on Volumetric Oxygen Transfer Coefficient. Molecules 2018, 23, 125. [Google Scholar] [CrossRef]
- Iqbal Qazi, J.; Nadeem, M.; Syed, Q.; Baig, S. Effect of Aeration and Agitation Rates on Alkaline Protease Production by Bacillus licheniformis UV-9 Mutant. Turk. J. Biochem. 2009, 34, 89–96. [Google Scholar]
- Chisti, Y. Fermentation (Industrial); Basic Considerations. In Encyclopedia of Food Microbiology; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Palmerston North, New Zealand, 2014; pp. 413–420. [Google Scholar]
- Kamble, A.L.; Meena, V.S.; Banerjee, U.C. Effect of Agitation and Aeration on the Production of Nitrile Hydratase by Rhodococcus erythropolis MTCC 1526 in a Stirred Tank Reactor. Lett. Appl. Microbiol. 2010, 51, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Ng, W. Effect of Different Aeration and Polyethylene Glycol Concentration on Growth of Escherichia coli DH5α in a 1L Bioreactor. Can. J. Microbiol. 2018, 5, 1–10. [Google Scholar]
- Yang, X. Scale-up of Microbial Fermentation Process. In Manual of Industrial Microbiology and Biotechnology, 3rd ed.; Bull, A.T., Junker, B., Katz, L., Lynd, L.R., Masurekar, P., Reeves, C.D., Zhao, H., Eds.; Wiley Online Library: Hoboken, NJ, USA, 2010; pp. 669–675. [Google Scholar]
- Song, F.; Lin, Y.; Chen, C.; Shao, E.; Guan, X.; Huang, Z. Insecticidal Activity and Histopathological Effects of Vip3Aa Protein from Bacillus thuringiensis on Spodoptera litura. J. Microbiol. Biotechnol. 2016, 26, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Boonyos, P.; Trakulnalueamsai, C.; Rungrod, A.; Chongthammakun, S.; Promdonkoy, B. Antagonistic Effect of Truncated Fragments of Bacillus thuringiensis Vip3Aa on the Larvicidal Activity of Its Full-Length Protein. Protein Pept. Lett. 2021, 28, 131–139. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).