Characteristics of Koji Using Liquid Starter for Soy Sauce Production
Abstract
:1. Introduction
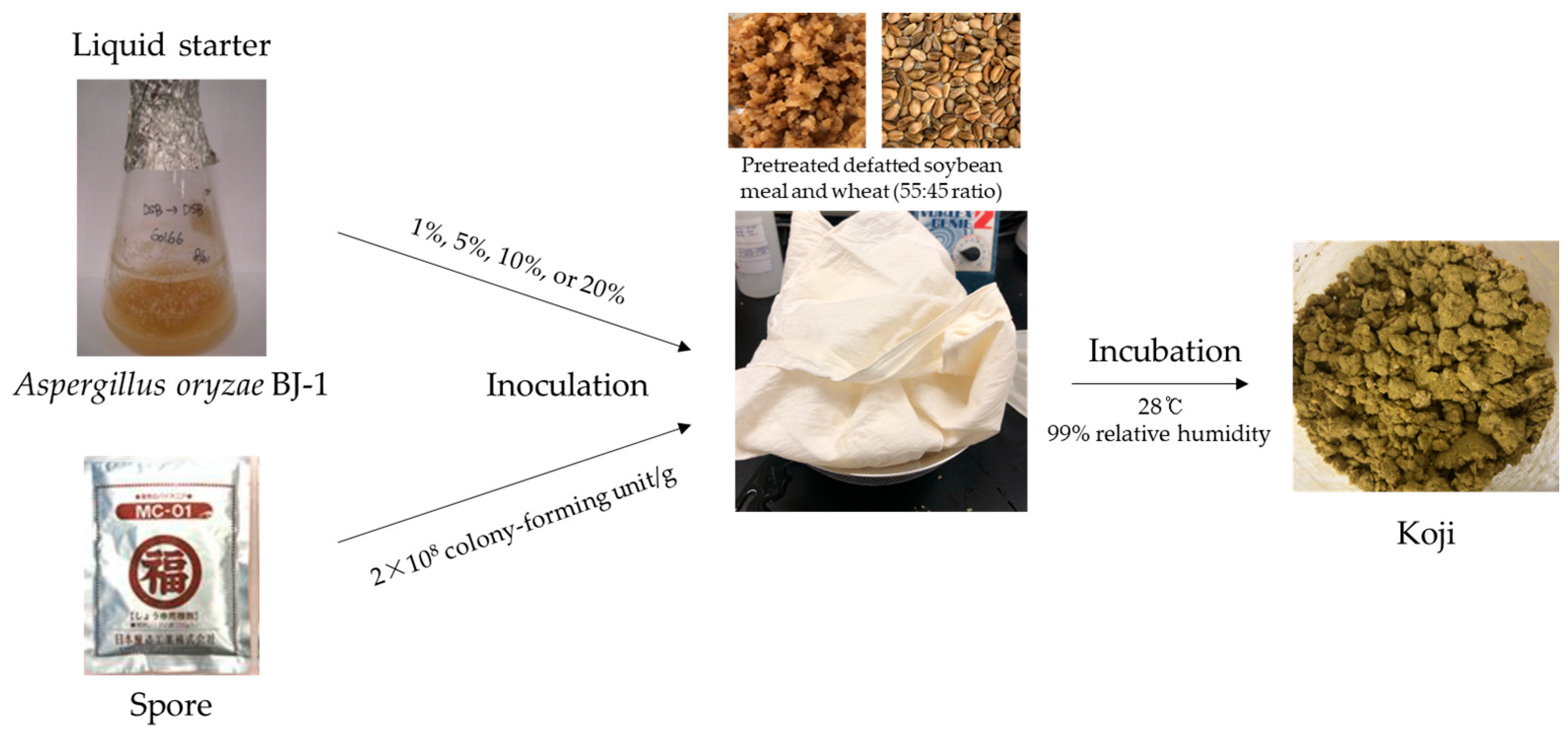
2. Materials and Methods
2.1. Isolation and Identification of Fungi from Koji Starter
2.2. Enzyme Activities and Growth Patterns According to the Medium Compositions in Liquid Starters
- V = Titration volume of 0.1 N sodium hydroxide (mL).
- F = Factor of 0.1 N sodium hydroxide.
- A = Amount of organic acid equivalent to 1 mL of 0.1 N sodium hydroxide (lactic acid: 0.0090).
- D = Dilution factor.
- S = Amount of sample (g).
2.3. Characterization of Koji by Assessing Inoculation Rate of Liquid Starter
2.4. Determination of Enzyme Activities
2.4.1. α-Amylase Activity
2.4.2. Total Endo-Protease Activity
2.4.3. Acidic Protease Activity
2.4.4. Neutral Protease Activity
2.4.5. Alkaline Protease Activity
2.4.6. Glutaminase Activity
2.5. Statistical Analysis
3. Results and Discussion
3.1. Isolation and Identification of Fungi from Commercial Koji Starter
3.2. Proximate Compositions of Defatted Soybean Meal and Wheat
3.3. Optimization of Medium Composition for Liquid Starters
3.4. Characterization of Koji by Assessing Inoculation Ratio of Liquid Starter
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, N.D. Trend of research papers on the soy sauce tastes in Japan. Food Ind. Nutr. 2007, 12, 40–50. [Google Scholar]
- Lioe, H.N.; Apriyantono, A.; Yasuda, M. Soy sauce: Typical aspects of Japanese Shoyu and Indonesian kecap. In Handbook of Plant-Based Fermented Food and Beverage Technology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2012; p. 821. [Google Scholar]
- Hui, Y.H.; Meunier-Goddik, L.; Josephsen, J.; Nip, W.K.; Stanfield, P.S. (Eds.) Handbook of Food and Beverage Fermentation Technology; CRC Press: Boca Raton, FL, USA, 2004; Volume 134. [Google Scholar]
- Hoang, N.X.; Ferng, S.; Ting, C.H.; Huang, W.H.; Chiou, R.Y.Y.; Hsu, C.K. Optimizing the initial moromi fermentation conditions to improve the quality of soy sauce. LWT 2016, 74, 242–250. [Google Scholar] [CrossRef]
- Robinson, R.K. Encyclopedia of Food Microbiology; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Chung, D.H.; Lee, H.C.; Shim, S.K.; Han, B.R. Soybean Femented Foods; Ik Jae, H., Ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Kaewkrod, A.; Niamsiri, N.; Likitwattanasade, T.; Lertsiri, S. Activities of macerating enzymes are useful for selection of soy sauce koji. LWT 2018, 89, 735–739. [Google Scholar] [CrossRef]
- Sulaiman, J.; Gan, H.M.; Yin, W.F.; Chan, K.G. Microbial succession and the functional potential during the fermentation of Chinese soy sauce brine. Front. Microbiol. 2014, 5, 556. [Google Scholar] [CrossRef]
- Elhalis, H.; Chin, X.H.; Chow, Y. Soybean fermentation: Microbial ecology and starter culture technology. Crit. Rev. Food Sci. Nutr. 2023, 1–23. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and sensory characteristics of soy sauce: A review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, C.; Zhao, H.; Gao, X.; Zhao, M.; Sun, W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Zhong, Y.; Lu, X.; Xing, L.; Ho, S.W.A.; Kwan, H.S. Genomic and transcriptomic comparison of Aspergillus oryzae strains: A case study in soy sauce koji fermentation. J. Ind. Microbiol. Biotechnol. 2018, 45, 839–853. [Google Scholar] [CrossRef]
- Fukushima, D. Industrialization of Fermented Soy Sauce Production Centering Around Japanese Shoyu; Food Science & Technology New York-Marcel Dekker: New York, NY, USA, 2004; pp. 1–88. [Google Scholar]
- Kobayashi, T.; Abe, K.; Asai, K.; Gomi, K.; Juvvadi, P.R.; Kato, M.; Kitamoto, K.; Takeuchi, M.; Machida, M. Genomics of Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2007, 71, 646–670. [Google Scholar] [CrossRef]
- Ito, K.; Matsuyama, A. Koji molds for Japanese soy sauce brewing: Characteristics and key enzymes. J. Fungi 2021, 7, 658. [Google Scholar] [CrossRef]
- Yasui, M.; Oda, K.; Masuo, S.; Hosoda, S.; Katayama, T.; Maruyama, J.I.; Takaya, N.; Takeshita, N. Invasive growth of Aspergillus oryzae in rice koji and increase of nuclear number. Fungal Biol. Biotechnol. 2020, 7, 8. [Google Scholar] [CrossRef]
- Kang, S.G.; Park, I.B.; Jung, S.T. Characteristics of fermented hot pepper soybean paste (kochujang) prepared by liquid beni-koji. Korean J. Food Sci. Technol. 1997, 29, 82–89. [Google Scholar]
- Kwon, Y.A.; Chun, J.K. Microcomputer-controlled Koji Incubation System and Its Application to Barley Koji Manufacture. Korean J. Food Sci. Technol. 1988, 20, 326–330. [Google Scholar]
- Park, S.; Gong, J.W.; Lee, K.S. Calcium absorption and growth characteristics of Agrocybe cylindracea mycelia in submerged culture. Korean J. Food Sci. Technolgy 2008, 40, 419–423. [Google Scholar]
- Sandhya, C.; Sumantha, A.; Szakacs, G.; Pandey, A. Comparative evaluation of neutral protease production by Aspergillus oryzae in submerged and solid-state fermentation. Process Biochem. 2005, 40, 2689–2694. [Google Scholar] [CrossRef]
- Imanaka, H.; Tanaka, S.; Feng, B.; Imamura, K.; Nakanishi, K. Cultivation characteristics and gene expression profiles of Aspergillus oryzae by membrane-surface liquid culture, shaking-flask culture, and agar-plate culture. J. Biosci. Bioeng. 2010, 109, 267–273. [Google Scholar] [CrossRef]
- Hata, Y.; Ishida, H.; Kojima, Y.; Ichikawa, E.; Kawato, A.; Suginami, K.; IMayasu, S. Comparison of two glucoamylases produced by Aspergillus oryzae in solid-state culture (koji) and in submerged culture. J. Ferment. Bioeng. 1997, 84, 532–537. [Google Scholar] [CrossRef]
- Devanthi, P.V.P.; Gkatzionis, K. Soy sauce fermentation: Microorganisms, aroma formation, and process modification. Food Res. Int. 2019, 120, 364–374. [Google Scholar] [CrossRef]
- Mack, S.M.; Madl, A.K.; Pinkerton, K.E. Respiratory health effects of exposure to ambient particulate matter and bioaerosols. Compr. Physiol. 2019, 10, 1. [Google Scholar]
- Blake, C.; Latimer, G.W., Jr. (Eds.) Vitamins and other Nutrients Chapter. In AOAC Official Methods of Analysis, 21st ed.; AOAC International: Rockville, MA, USA, 1990; Volume 45. [Google Scholar]
- Jünger, M.; Mittermeier-Kleßinger, V.K.; Farrenkopf, A.; Dunkel, A.; Stark, T.; Fröhlich, S.; Somoza, V.; Dawid, C.; Hofmann, T. Sensoproteomic discovery of taste-modulating peptides and taste re-engineering of soy sauce. J. Agric. Food Chem. 2002, 70, 6503–6518. [Google Scholar] [CrossRef]
- Uchida, H.; Kondo, D.; Yamashita, S.; Tanaka, T.; Tran, L.H.; Nagano, H.; Uwajima, T. Purification and properties of a protease produced by Bacillus subtilis CN2 isolated from a Vietnamese fish sauce. World J. Microbiol. Biotechnol. 2004, 20, 579–582. [Google Scholar] [CrossRef]
- Katikala, P.K.; Bobbarala, V.; Tadimalla, P.; Guntuku, G.S. Screening of L-glutaminase producing marine bacterial cultures for extracellular production of L-glutaminase. Int. J. ChemTech Res. 2009, 1, 1232–1235. [Google Scholar]
- Goldman, G.H.; Osmani, S.A. (Eds.) The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hajeb, P.; Jinap, S. Umami taste components and their sources in Asian foods. Crit. Rev. Food Sci. Nutr. 2015, 55, 778–791. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Defatted Soybean | Wheat |
|---|---|---|
| Moisture | 9.97 ± 3.34 | 10.64 ± 0.42 |
| Crude protein | 42.99 ± 0.12 | 10.56 ± 0.70 |
| Crude fat | 2.73 ± 0.01 | 1.69 ± 0.041 |
| Crude ash | 5.67 ± 0.21 | 1.55 ± 0.11 |
| Carbohydrate * | 39.74 ± 4.37 | 75.46 ± 0.87 |
| Parameters | Samples * | Incubation Time (h) | ||||
|---|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | 96 | ||
| Mycelium (mg/mL) | Control | 0.50 ± 0.00 a | 2.26 ± 0.00 d | 4.77 ± 0.02 d | 5.04 ± 0.06 d | 5.31 ± 0.16 d |
| T1 | 0.50 ± 0.00 a | 7.03 ± 0.11 c | 11.47 ± 0.02 c | 13.18 ± 0.03 c | 14.05 ± 0.05 b | |
| T2 | 0.50 ± 0.00 a | 9.38 ± 0.03 b | 12.37 ± 0.02 b | 15.04 ± 0.05 b | 16.47 ± 0.03 c | |
| T3 | 0.50 ± 0.01 a | 13.04 ± 0.05 a | 13.94 ± 0.06 a | 16.55 ± 0.05 a | 17.95 ± 0.05 d | |
| Glucose (g/L) | Control | 20.19 ± 0.75 a | 15.93 ± 0.10 a | 10.13 ± 0.11 a | 11.64 ± 0.06 a | 9.75 ± 0.22 a |
| T1 | 1.49 ± 0.01 b | 0.02 ± 0.01 d | 0.04 ± 0.01 c | N.D. ** | N.D. | |
| T2 | 1.62 ± 0.01 b | 0.19 ± 0.01 c | 0.29 ± 0.01 b | 0.20 ± 0.01 c | 0.09 ± 0.01 bc | |
| T3 | 1.84 ± 0.01 b | 0.32 ± 0.01 b | 0.21 ± 0.01 b | 0.36 ± 0.01 b | 0.23 ± 0.01 b | |
| pH | Control | 4.35 ± 0.00 c | 4.45 ± 0.01 d | 4.54 ± 0.01 d | 4.88 ± 0.01 d | 5.09 ± 0.01 d |
| T1 | 6.26 ± 0.01 a | 5.46 ± 0.01 c | 5.97 ± 0.01 a | 7.01 ± 0.01 a | 7.59 ± 0.01 a | |
| T2 | 6.26 ± 0.01 a | 5.66 ± 0.01 b | 5.49 ± 0.01 c | 5.93 ± 0.01 b | 5.70 ± 0.01 b | |
| T3 | 6.25 ± 0.01 b | 5.80 ± 0.01 a | 5.77 ± 0.01 b | 5.32 ± 0.01 c | 5.50 ± 0.01 c | |
| Acidity (%) | Control | 0.07 ± 0.00 a | 0.06 ± 0.00 d | 0.05 ± 0.00 d | 0.05 ± 0.00 d | 0.05 ± 0.00 d |
| T1 | 0.06 ± 0.00 c | 0.07 ± 0.00 c | 0.09 ± 0.00 c | 0.10 ± 0.00 c | 0.11 ± 0.00 c | |
| T2 | 0.06 ± 0.00 bc | 0.08 ± 0.00 b | 0.28 ± 0.00 b | 0.25 ± 0.00 b | 0.24 ± 0.00 b | |
| T3 | 0.06 ± 0.00 b | 0.09 ± 0.00 a | 0.35 ± 0.00 a | 0.51 ± 0.01 a | 0.50 ± 0.00 a | |
| Protease activity (mUnit/mL) | Control | 0.00 ± 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d | 0.00 ± 0.00 d |
| T1 | 0.00 ± 0.00 | 16.67 ± 0.10 b | 17.22 ± 0.04 b | 17.45 ± 0.06 a | 13.58 ± 0.04 c | |
| T2 | 0.00 ± 0.00 | 21.25 ± 0.05 a | 23.21 ± 0.12 a | 10.57 ± 0.08 c | 19.12 ± 0.44 a | |
| T3 | 0.00 ± 0.00 | 10.34 ± 0.07 c | 6.15 ± 0.14 c | 11.70 ± 0.07 b | 16.13 ± 0.06 b | |
| α-Amylase activity (unit/mL) | Control | 0.00 ± 0.00 | 0.00 ± 0.00 d | 0.00 ± 0.00 c | 135.69 ± 9.50 c | 160.27 ± 16.98 c |
| T1 | 0.00 ± 0.00 | 95.30 ± 0.71 c | 187.83 ± 5.29 b | 171.51 ± 3.54 b | 168.38 ± 2.22 b | |
| T2 | 0.00 ± 0.00 | 167.99 ± 2.64 b | 224.50 ± 3.44 a | 214.29 ± 4.97 a | 185.10 ± 4.01 a | |
| T3 | 0.00 ± 0.00 | 199.05 ± 3.52 a | 100.48 ± 2.37 c | 99.62 ± 4.35 d | 90.63 ± 1.88 d | |
| Glutaminase (unit/mL) | Control | 0.00 ± 0.00 | 8.53 ± 0.04 d | 12.31 ± 0.03 d | 13.60 ± 0.51 d | 22.87 ± 0.20 d |
| T1 | 0.00 ± 0.00 | 12.92 ± 0.04 c | 22.95 ± 0.06 c | 19.45 ± 0.10 c | 24.17 ± 0.09 c | |
| T2 | 0.00 ± 0.00 | 24.18 ± 0.08 b | 30.61 ± 1.45 b | 41.95 ± 0.88 b | 59.83 ± 0.85 a | |
| T3 | 0.00 ± 0.00 | 55.37 ± 0.54 a | 39.51 ± 0.94 a | 74.80 ± 0.74 a | 52.43 ± 1.24 b | |
| Parameters | Samples * | Incubation Time (h) | |||
|---|---|---|---|---|---|
| 0 | 24 | 48 | 72 | ||
| Moisture content ** (%) | Control | 48.18 ± 0.03 d | - | - | 40.23 ± 0.12 b |
| K1 | 48.18 ± 0.01 d | - | - | 36.07 ± 0.06 e | |
| K5 | 48.52 ± 0.02 c | - | - | 38.43 ± 0.06 d | |
| K10 | 49.23 ± 0.01 b | - | - | 38.67 ± 0.06 c | |
| K20 | 49.43 ± 0.16 a | - | - | 46.87 ± 0.12 a | |
| pH | Control | 6.37 ± 0.01 a | 6.25 ± 0.00 b | 6.68 ± 0.01 a | 7.07 ± 0.03 a |
| K1 | 6.37 ± 0.00 a | 6.29 ± 0.01 a | 5.89 ± 0.00 b | 6.73 ± 0.01 c | |
| K5 | 6.23 ± 0.01 d | 6.12 ± 0.00 c | 5.24 ± 0.00 d | 6.95 ± 0.01 b | |
| K10 | 6.34 ± 0.01 b | 6.10 ± 0.00 d | 5.17 ± 0.00 e | 6.97 ± 0.01 b | |
| K20 | 6.28 ± 0.01 c | 5.98 ± 0.00 e | 5.46 ± 0.00 c | 5.66 ± 0.02 d | |
| Glucose content (g/L) | Control | 0.36 ± 0.01 e | 3.61 ± 0.02 d | 10.63 ± 0.55 c | 21.23 ± 0.15 b |
| K1 | 0.49 ± 0.00 d | 6.21 ± 0.02 c | 9.11 ± 0.04 d | 19.23 ± 0.06 c | |
| K5 | 1.02 ± 0.04 c | 6.62 ± 0.05 b | 15.30 ± 0.30 b | 24.43 ± 0.50 a | |
| K10 | 1.71 ± 0.01 b | 6.56 ± 0.00 b | 17.40 ± 0.00 a | 21.73 ± 0.25 b | |
| K20 | 3.41 ± 0.03 a | 10.33 ± 0.15 a | 15.60 ± 0.44 b | 13.47 ± 0.49 d | |
| α-Amylase activity (unit/mL) | Control | 22.33 ± 0.58 d | 63.67 ± 0.58 e | 6570.00 ± 115.33 d | 9210.00 ± 5.57 d |
| K1 | 1.83 ± 0.76 e | 497.67 ± 4.04 d | 3769.67 ± 60.58 e | 10,416.33 ± 29.16 bc | |
| K5 | 25.33 ± 0.76 c | 985.67 ± 4.04 c | 6936.33 ± 84.56 c | 10,603.67 ± 300.07 ab | |
| K10 | 60.43 ± 0.51 b | 1044.33 ± 33.56 b | 8033.00 ± 22.52 b | 10,813.00 ± 32.51 a | |
| K20 | 69.90 ± 1.15 a | 4832.00 ± 22.07 a | 9181.00 ± 16.82 a | 10,179.67 ± 38.21 c | |
| Glutaminase (unit/mL) | Control | N.D. *** | 322.33 ± 1.15 b | 520.33 ± 10.50 b | 1296.33 ± 31.64 a |
| K1 | N.D. | 156.33 ± 1.53 e | 363.67 ± 12.22 d | 675.00 ± 8.66 d | |
| K5 | N.D. | 218.33 ± 1.15 d | 373.67 ± 2.52 cd | 725.00 ± 3.00 d | |
| K10 | N.D. | 284.00 ± 1.73 c | 385.67 ± 5.86 c | 965.33 ± 5.69 c | |
| K20 | N.D. | 402.00 ± 8.19 a | 812.67 ± 1.15 a | 1099.00 ± 59.81 b | |
| Acidic protease activity (pH 5.0) | Control | N.D. | 155.33 ± 2.31 c | 2456.33 ± 58.48 b | 2667.00 ± 59.91 a |
| K1 | N.D. | 101.00 ± 2.65 d | 1748.67 ± 61.85 c | 2636.67 ± 77.69 a | |
| K5 | N.D. | 188.00 ± 7.00 c | 2464.00 ± 29.55 b | 2663.33 ± 54.99 a | |
| K10 | N.D. | 454.00 ± 10.00 b | 2421.00 ± 49.43 b | 2685.33 ± 64.27 a | |
| K20 | N.D. | 2033.33 ± 59.50 a | 2762.00 ± 75.74 a | 2652.00 ± 112.21 a | |
| Neutral protease activity (pH 7.0) | Control | N.D. | 110.33 ± 3.06 c | 2634.33 ± 39.88 c | 2595.33 ± 4.04 d |
| K1 | N.D. | 57.00 ± 1.00 c | 1949.67 ± 5.51 e | 2522.00 ± 0.00 e | |
| K5 | N.D. | 136.07 ± 115.95 c | 2571.33 ± 0.58 d | 2680.33 ± 0.58 c | |
| K10 | N.D. | 442.67 ± 2.52 b | 2682.67 ± 11.02 b | 2776.33 ± 1.53 a | |
| K20 | N.D. | 2146.67 ± 38.81 a | 2752.00 ± 3.00 a | 2711.33 ± 11.50 b | |
| Alkaline protease activity (pH 8.0) | Control | N.D. | 165.00 ± 4.58 d | 2699.33 ± 11.50 c | 2821.33 ± 8.96 ab |
| K1 | N.D. | 110.33 ± 0.58 e | 1932.00 ± 13.53 e | 2769.67 ± 60.58 b | |
| K5 | N.D. | 228.00 ± 1.00 c | 2692.00 ± 13.00 d | 2860.00 ± 4.58 a | |
| K10 | N.D. | 479.33 ± 4.51 b | 2741.00 ± 7.00 b | 2814.33 ± 6.66 ab | |
| K20 | N.D. | 2258.67 ± 8.14 a | 2971.67 ± 25.58 a | 2817.67 ± 7.77 ab | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, J.; Kwon, T.; Park, Y.; Kim, A.Y. Characteristics of Koji Using Liquid Starter for Soy Sauce Production. Fermentation 2023, 9, 979. https://doi.org/10.3390/fermentation9110979
Choi J, Kwon T, Park Y, Kim AY. Characteristics of Koji Using Liquid Starter for Soy Sauce Production. Fermentation. 2023; 9(11):979. https://doi.org/10.3390/fermentation9110979
Chicago/Turabian StyleChoi, Jonghoon, Taeeun Kwon, Yeongbin Park, and Augustine Yonghwi Kim. 2023. "Characteristics of Koji Using Liquid Starter for Soy Sauce Production" Fermentation 9, no. 11: 979. https://doi.org/10.3390/fermentation9110979
APA StyleChoi, J., Kwon, T., Park, Y., & Kim, A. Y. (2023). Characteristics of Koji Using Liquid Starter for Soy Sauce Production. Fermentation, 9(11), 979. https://doi.org/10.3390/fermentation9110979






