Recent Advancements and Strategies of Improving CO2 Utilization Efficiency in Bio-Succinic Acid Production
Abstract
:1. Introduction
2. Microbial Fixation of CO2 to Synthesize SA
3. CO2 Supply Bottleneck in the Process of Microbial Carbon Sequestration to Synthesize SA
4. Research Progress of Improving the Efficiency of Microbial CO2 Fixation
4.1. Research Progress in Intracellular Regulation to Improve Carbon Sequestration Efficiency
4.2. Research Progress to Promote Extracellular CO2 Supply
5. Potential Strategies to Improve the Efficiency of Microbial CO2 Fixation
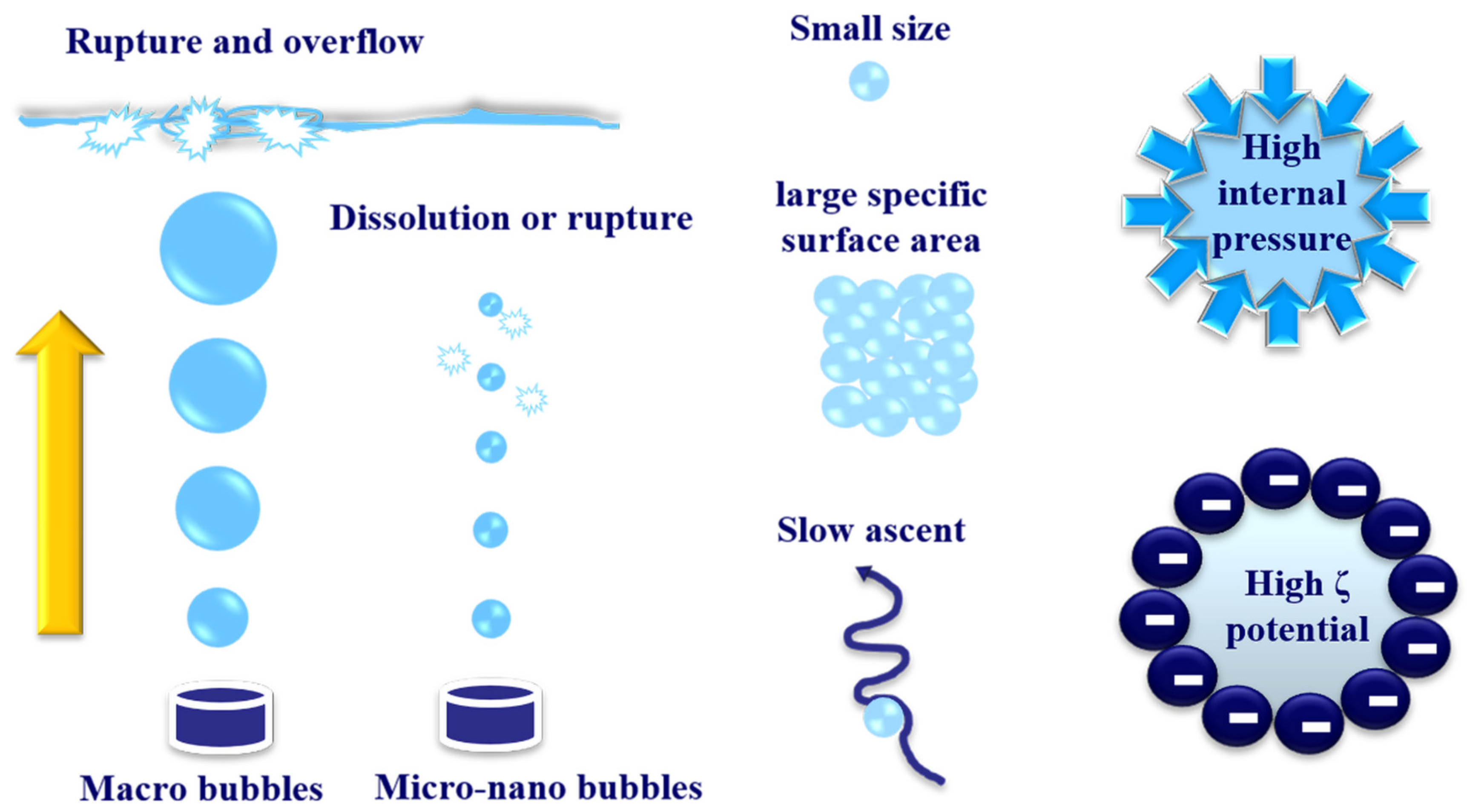
5.1. Micro-Nano Bubbles
5.2. CO2 Adsorption Material
5.3. Biofilm
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Xu, S.; Qiao, Z.; Luo, L.; Sun, Y.; Wong, J.W.-C.; Geng, X.; Ni, J. On-site CO2 bio-sequestration in anaerobic digestion: Current status and prospects. Bioresour. Technol. 2021, 332, 125037. [Google Scholar] [CrossRef] [PubMed]
- Jatain, I.; Dubey, K.K.; Sharma, M.; Usmani, Z.; Sharma, M.; Gupta, V.K. Synthetic biology potential for carbon sequestration into biocommodities. J. Clean. Prod 2021, 323, 129176. [Google Scholar] [CrossRef]
- Amulya, K.; Kopperi, H.; Venkata Mohan, S. Tunable production of succinic acid at elevated pressures of CO2 in a high pressure gas fermentation reactor. Bioresour. Technol. 2020, 309, 123327. [Google Scholar] [CrossRef]
- Jeong, D.; Park, H.; Jang, B.-K.; Ju, Y.; Shin, M.H.; Oh, E.J.; Lee, E.J.; Kim, S.R. Recent advances in the biological valorization of citrus peel waste into fuels and chemicals. Bioresour. Technol. 2021, 323, 124603. [Google Scholar] [CrossRef]
- Lee, S.Y.; Oh, Y.-K.; Lee, S.; Fitriana, H.N.; Moon, M.; Kim, M.-S.; Lee, J.; Min, K.; Park, G.W.; Lee, J.-P.; et al. Recent developments and key barriers to microbial CO2 electrobiorefinery. Bioresour. Technol. 2021, 320, 124350. [Google Scholar] [CrossRef] [PubMed]
- Rin Kim, S.; Kim, S.J.; Kim, S.K.; Seo, S.O.; Park, S.; Shin, J.; Kim, J.S.; Park, B.R.; Jin, Y.S.; Chang, P.S.; et al. Yeast metabolic engineering for carbon dioxide fixation and its application. Bioresour. Technol. 2022, 346, 126349. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Jang, Y.-S.; Lee, S.Y. Production of succinic acid by metabolically engineered microorganisms. Curr. Opin. Biotechnol. 2016, 42, 54–66. [Google Scholar] [CrossRef]
- Zhang, Q.; Nurhayati; Cheng, C.-L.; Nagarajan, D.; Chang, J.-S.; Hu, J.; Lee, D.-J. Carbon capture and utilization of fermentation CO2: Integrated ethanol fermentation and succinic acid production as an efficient platform. Appl. Energy 2017, 206, 364–371. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Laivenieks, M.; Schindler, B.D.; McKinlay, A.A.; Siddaramappa, S.; Challacombe, J.F.; Lowry, S.R.; Clum, A.; Lapidus, A.L.; Burkhart, K.B.; et al. A genomic perspective on the potential of Actinobacillus succinogenes for industrial succinate production. BMC Genom. 2010, 11, 680. [Google Scholar] [CrossRef]
- Meynial-Salles, I.; Dorotyn, S.; Soucaille, P. A new process for the continuous production of succinic acid from glucose at high yield, titer, and productivity. Biotechnol. Bioeng. 2008, 99, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pateraki, C.; Patsalou, M.; Vlysidis, A.; Kopsahelis, N.; Webb, C.; Koutinas, A.A.; Koutinas, M. Actinobacillus succinogenes: Advances on succinic acid production and prospects for development of integrated biorefineries. Biochem. Eng. J. 2016, 112, 285–303. [Google Scholar] [CrossRef]
- Thuy, N.T.H.; Kongkaew, A.; Flood, A.; Boontawan, A. Fermentation and crystallization of succinic acid from Actinobacillus succinogenes ATCC55618 using fresh cassava root as the main substrate. Bioresour. Technol. 2017, 233, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Seo, H.; Park, W.; Seok, J.; Lee, J.A.; Kim, W.J.; Kim, G.B.; Kim, K.-J.; Lee, S.Y. Enhanced succinic acid production by Mannheimia employing optimal malate dehydrogenase. Nat. Commun. 2020, 11, 1970. [Google Scholar] [CrossRef]
- Stylianou, E.; Pateraki, C.; Ladakis, D.; Vlysidis, A.; Koutinas, A. Optimization of fermentation medium for succinic acid production using Basfia succiniciproducens. Environ. Technol. Innov. 2021, 24, 101914. [Google Scholar] [CrossRef]
- Wang, J.; Wang, H.; Yang, L.; Lv, L.; Zhang, Z.; Ren, B.; Dong, L.; Li, N. A novel riboregulator switch system of gene expression for enhanced microbial production of succinic acid. J. Ind. Microbiol. Biotechnol. 2018, 45, 253–269. [Google Scholar] [CrossRef]
- Yu, J.H.; Zhu, L.W.; Xia, S.T.; Li, H.M.; Tang, Y.L.; Liang, X.H.; Chen, T.; Tang, Y.J. Combinatorial optimization of CO2 transport and fixation to improve succinate production by promoter engineering. Biotechnol. Bioeng. 2016, 113, 1531–1541. [Google Scholar] [CrossRef]
- Li, K.; Li, C.; Zhao, X.-Q.; Liu, C.-G.; Bai, F.-W. Engineering Corynebacterium glutamicum for efficient production of succinic acid from corn stover pretreated by concentrated-alkali under steam-assistant conditions. Bioresour. Technol. 2023, 378, 128991. [Google Scholar] [CrossRef]
- Molina Grima, E.; Belarbi, E.H.; Acién Fernández, F.G.; Robles Medina, A.; Chisti, Y. Recovery of microalgal biomass and metabolites: Process options and economics. Biotechnol. Adv. 2003, 20, 491–515. [Google Scholar] [CrossRef]
- Van Den Hende, S.; Vervaeren, H.; Boon, N. Flue gas compounds and microalgae: (Bio-)chemical interactions leading to biotechnological opportunities. Biotechnol. Adv. 2012, 30, 1405–1424. [Google Scholar] [CrossRef]
- Chiu, S.Y.; Kao, C.Y.; Huang, T.T.; Lin, C.J.; Ong, S.C.; Chen, C.D.; Chang, J.S.; Lin, C.S. Microalgal biomass production and on-site bioremediation of carbon dioxide, nitrogen oxide and sulfur dioxide from flue gas using Chlorella sp. cultures. Bioresour. Technol. 2011, 102, 9135–9142. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, W.; Ji, Y.; Yi, X.; Ma, J.; Wu, H.; Jiang, M. Coupled CO2 fixation from ethylene oxide off-gas with bio-based succinic acid production by engineered recombinant Escherichia coli. Biochem. Eng. J. 2017, 117, 1–6. [Google Scholar] [CrossRef]
- He, A.; Kong, X.; Wang, C.; Wu, H.; Jiang, M.; Ma, J.; Ouyang, P. Efficient carbon dioxide utilization and simultaneous hydrogen enrichment from off-gas of acetone–butanol–ethanol fermentation by succinic acid producing Escherichia coli. Bioresour. Technol. 2016, 214, 861–865. [Google Scholar] [CrossRef]
- Tan, J.P.; Luthfi, A.A.I.; Manaf, S.F.A.; Wu, T.Y.; Jahim, J.M. Incorporation of CO2 during the production of succinic acid from sustainable oil palm frond juice. J. CO2 Util. 2018, 26, 595–601. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Li, W.; Xing, J.; Su, Z.G. Improvement of succinate production by overexpression of a cyanobacterial carbonic anhydrase in Escherichia coli. Enzym. Microb. Technol. 2009, 45, 491–497. [Google Scholar] [CrossRef]
- Sharma, T.; Sharma, S.; Kamyab, H.; Kumar, A. Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. J. Clean. Prod. 2020, 247, 119138. [Google Scholar] [CrossRef]
- Hou, J.; Li, X.; Kaczmarek, M.B.; Chen, P.; Li, K.; Jin, P.; Liang, Y.; Daroch, M. Accelerated CO2 Hydration with Thermostable Sulfurihydrogenibium azorense Carbonic Anhydrase-Chitin Binding Domain Fusion Protein Immobilised on Chitin Support. Int. J. Mol. Sci. 2019, 20, 1494. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Chua, T.K.; Pinard, M.A.; Szebenyi, D.M.; McKenna, R. Carbon Dioxide “Trapped” in a β-Carbonic Anhydrase. Biochemistry 2015, 54, 6631–6638. [Google Scholar] [CrossRef] [PubMed]
- Alber, B.E.; Ferry, J.G. A carbonic anhydrase from the archaeon Methanosarcina thermophila. Proc. Natl. Acad. Sci. USA 1994, 91, 6909–6913. [Google Scholar] [CrossRef]
- Yeates, T.O.; Kerfeld, C.A.; Heinhorst, S.; Cannon, G.C.; Shively, J.M. Protein-based organelles in bacteria: Carboxysomes and related microcompartments. Nat. Rev. Microbiol. 2008, 6, 681–691. [Google Scholar] [CrossRef]
- Cronk, J.D.; Endrizzi, J.A.; Cronk, M.R.; O’Neill, J.W.; Zhang, K.Y.J. Crystal structure of E. coli β–carbonic anhydrase, an enzyme with an unusual pH–dependent activity. Protein Sci. 2001, 10, 911–922. [Google Scholar] [CrossRef]
- Clapero, V.; Arrivault, S.; Stitt, M. Natural variation in metabolism of the Calvin-Benson cycle. Semin. Cell Dev. Biol. 2024, 155, 23–36. [Google Scholar] [CrossRef]
- Strauss, G.; Fuchs, G. Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle. Eur. J. Biochem. 1993, 215, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, A.; Okada, S.; Hols, P.; Satoh, E. Metabolic engineering of Lactobacillus plantarum for succinic acid production through activation of the reductive branch of the tricarboxylic acid cycle. Enzym. Microb. Technol. 2013, 53, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Berg Ivan, A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-Hydroxypropionate/4-Hydroxybutyrate Autotrophic Carbon Dioxide Assimilation Pathway in Archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef]
- Goetzl, S.; Jeoung, J.-H.; Hennig, S.E.; Dobbek, H. Structural Basis for Electron and Methyl-Group Transfer in a Methyltransferase System Operating in the Reductive Acetyl-CoA Pathway. J. Mol. Biol. 2011, 411, 96–109. [Google Scholar] [CrossRef]
- Huber, H.; Gallenberger, M.; Jahn, U.; Eylert, E.; Berg Ivan, A.; Kockelkorn, D.; Eisenreich, W.; Fuchs, G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 2008, 105, 7851–7856. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Andrea, I.; Guedes, I.A.; Hornung, B.; Boeren, S.; Lawson, C.E.; Sousa, D.Z.; Bar-Even, A.; Claassens, N.J.; Stams, A.J.M. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 2020, 11, 5090. [Google Scholar] [CrossRef]
- Blombach, B.; Takors, R. CO2—Intrinsic Product, Essential Substrate, and Regulatory Trigger of Microbial and Mammalian Production Processes. Front. Bioeng. Biotechnol. 2015, 3, 108. [Google Scholar] [CrossRef]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Effectively converting carbon dioxide into succinic acid under mild pressure with Actinobacillus succinogenes by an integrated fermentation and membrane separation process. Bioresour. Technol. 2018, 266, 26–33. [Google Scholar] [CrossRef]
- Zou, W.; Zhu, L.W.; Li, H.M.; Tang, Y.J. Significance of CO2 donor on the production of succinic acid by Actinobacillus succinogenes ATCC 55618. Microb. Cell Fact. 2011, 10, 87. [Google Scholar] [CrossRef]
- Samuelov, N.S.; Lamed, R.; Lowe, S.; Zeikus, J.G. Influence of CO2-HCO3− Levels and pH on Growth, Succinate Production, and Enzyme Activities of Anaerobiospirillum succiniciproducens. Appl. Environ. Microbiol. 1991, 57, 3013–3019. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.C.; Tran, L.M.; Liao, J.C. A Global Regulatory Role of Gluconeogenic Genes in Escherichia coli Revealed by Transcriptome Network Analysis. J. Biol. Chem. 2005, 280, 36079–36087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jantama, K.; Moore, J.C.; Jarboe, L.R.; Shanmugam, K.T.; Ingram, L.O. Metabolic evolution of energy-conserving pathways for succinate production in Escherichia coli. Proc. Natl. Acad. Sci. USA 2009, 106, 20180–20185. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Laivenieks, M.; Vieille, C.; Zeikus, J.G. Effect of overexpression of Actinobacillus succinogenes phosphoenolpyruvate carboxykinase on succinate production in Escherichia coli. Appl. Environ. Microbiol. 2004, 70, 1238–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, L.; Wu, M.; Chen, K.; Jiang, M.; Ma, J.; Wei, P.; Ouyang, P. CO2 fixation for succinic acid production by engineered Escherichia coli co-expressing pyruvate carboxylase and nicotinic acid phosphoribosyltransferase. Biochem. Eng. J. 2013, 79, 77–83. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Vieille, C. 13C-metabolic flux analysis of Actinobacillus succinogenes fermentative metabolism at different NaHCO3 and H2 concentrations. Metab. Eng. 2008, 10, 55–68. [Google Scholar] [CrossRef]
- Mulana, F.; Munawar, E.; Heldiana, H.; Rahmi, M. The effect of carbon dioxide gas pressure on solubility, density and pH of carbon dioxide—Water mixtures. Mater. Today Proc. 2022, 63, S46–S49. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.W.; Choi, S.; You, J.K.; Hong, W.H.; Lee, S.Y. Effects of dissolved CO2 levels on the growth of Mannheimia succiniciproducens and succinic acid production. Biotechnol. Bioeng. 2007, 98, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.-l.; Chen, K.-q.; Li, J.; Fang, X.-j.; Zheng, X.-y.; Sui, S.-s.; Jiang, M.; Wei, P. Optimization of culture conditions in CO2 fixation for succinic acid production using Actinobacillus succinogenes. J. Ind. Microbiol. Biotechnol. 2011, 38, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, Q.; Li, Z.-m.; Ye, Q. Succinic acid production and CO2 fixation using a metabolically engineered Escherichia coli in a bioreactor equipped with a self-inducing agitator. Bioresour. Technol. 2012, 107, 376–384. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, M.; Su, L.; Wei, P. CO2 fixation by Actinobacillus succinogenes in succinic acid production. Chem. Eng. 2009, 37, 49–52. [Google Scholar]
- Lee, P.C.; Lee, W.G.; Kwon, S.; Lee, S.Y.; Chang, H.N. Succinic acid production by Anaerobiospirillum succiniciproducens: Effects of the H2/CO2 supply and glucose concentration. Enzym. Microb. Technol. 1999, 24, 549–554. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Song, Z.; Zhou, W.; Su, Z.; Xing, J. High cell density fermentation via a metabolically engineered Escherichia coli for the enhanced production of succinic acid. J. Chem. Technol. Biotechnol. 2011, 86, 512–518. [Google Scholar] [CrossRef]
- Liu, Y.-P.; Zheng, P.; Sun, Z.-H.; Ni, Y.; Dong, J.-J.; Wei, P. Strategies of pH control and glucose-fed batch fermentation for production of succinic acid by Actinobacillus succinogenes CGMCC1593. J. Chem. Technol. Biotechnol. 2008, 83, 722–729. [Google Scholar] [CrossRef]
- Vigato, F.; Angelidaki, I.; Woodley, J.M.; Alvarado-Morales, M. Dissolved CO2 profile in bio-succinic acid production from sugars-rich industrial waste. Biochem. Eng. J. 2022, 187, 108602. [Google Scholar] [CrossRef]
- Kumar, R.; Basak, B.; Jeon, B.-H. Sustainable production and purification of succinic acid: A review of membrane-integrated green approach. J. Clean. Prod. 2020, 277, 123954. [Google Scholar] [CrossRef]
- Cui, Z.; Gao, C.; Li, J.; Hou, J.; Lin, C.S.K.; Qi, Q. Engineering of unconventional yeast Yarrowia lipolytica for efficient succinic acid production from glycerol at low pH. Metab. Eng. 2017, 42, 126–133. [Google Scholar] [CrossRef]
- Razzak, M.A.; Lee, D.W.; Lee, J.; Hwang, I. Overexpression and Purification of Gracilariopsis chorda Carbonic Anhydrase (GcCAα3) in Nicotiana benthamiana, and Its Immobilization and Use in CO2 Hydration Reactions. Front. Plant Sci. 2020, 11, 563721. [Google Scholar] [CrossRef]
- Effendi, S.S.W.; Tan, S.-I.; Ting, W.-W.; Ng, I.S. Genetic design of co-expressed Mesorhizobium loti carbonic anhydrase and chaperone GroELS to enhancing carbon dioxide sequestration. Int. J. Biol. Macromol. 2021, 167, 326–334. [Google Scholar] [CrossRef]
- Wang, D.; Li, Q.; Li, W.; Liu, Q.; Xing, J.; Su, Z. Overexpression of a cyanobacterial carbonic anhydrase in Escherichia coli enhances succinic acid production. J. Biotechnol. 2008, 136, S26–S27. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.-u.; Cho, S.; Kim, H.; Oh, H.B.; Pack, S.P.; Lee, J. Increased incorporation of gaseous CO2 into succinate by Escherichia coli overexpressing carbonic anhydrase and phosphoenolpyruvate carboxylase genes. J. Biotechnol. 2017, 241, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, Y.; Ai, M.; Jia, X. Surface display of carbonic anhydrase on Escherichia coli for CO2 capture and mineralization. Synth. Syst. Biotechnol. 2022, 7, 460–473. [Google Scholar] [CrossRef] [PubMed]
- Van Hecke, W.; Bockrath, R.; De Wever, H. Effects of moderately elevated pressure on gas fermentation processes. Bioresour. Technol. 2019, 293, 122129. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Simões, M.M.Q.; Delgadillo, I.; Saraiva, J.A. Effect of High Pressure on Paracoccus denitrificans Growth and Polyhydroxyalkanoates Production from Glycerol. Appl. Biochem. Biotechnol. 2019, 188, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Forrester, S.E.; Rielly, C.D.; Carpenter, K.J. Gas-inducing impeller design and performance characteristics. Chem. Eng. Sci. 1998, 53, 603–615. [Google Scholar] [CrossRef]
- Forrester, S.E.; Rielly, C.D. Modelling the increased gas capacity of self-inducing impellers. Chem. Eng. Sci. 1994, 49, 5709–5718. [Google Scholar] [CrossRef]
- Cai, M.; Zhou, X.; Lu, J.; Fan, W.; Niu, C.; Zhou, J.; Sun, X.; Kang, L.; Zhang, Y. Enhancing aspergiolide A production from a shear-sensitive and easy-foaming marine-derived filamentous fungus Aspergillus glaucus by oxygen carrier addition and impeller combination in a bioreactor. Bioresour. Technol. 2011, 102, 3584–3586. [Google Scholar] [CrossRef]
- Jin, N.; Zhang, F.; Cui, Y.; Sun, L.; Gao, H.; Pu, Z.; Yang, W. Environment-friendly surface cleaning using micro-nano bubbles. Particuology 2022, 66, 1–9. [Google Scholar] [CrossRef]
- Jia, J.; Zhu, Z.; Chen, H.; Pan, H.; Jiang, L.; Su, W.-H.; Chen, Q.; Tang, Y.; Pan, J.; Yu, K. Full life circle of micro-nano bubbles: Generation, characterization and applications. Chem. Eng. J. 2023, 471, 144621. [Google Scholar] [CrossRef]
- Sakr, M.; Mohamed, M.M.; Maraqa, M.A.; Hamouda, M.A.; Aly Hassan, A.; Ali, J.; Jung, J. A critical review of the recent developments in micro–nano bubbles applications for domestic and industrial wastewater treatment. Alex. Eng. J. 2022, 61, 6591–6612. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G. Mass transfer of nanobubble aeration and its effect on biofilm growth: Microbial activity and structural properties. Sci. Total Environ. 2020, 703, 134976. [Google Scholar] [CrossRef] [PubMed]
- Tekile, A.; Kim, I.; Lee, J.-Y. Extent and persistence of dissolved oxygen enhancement using nanobubbles. Environ. Eng. Res. 2016, 21, 427–435. [Google Scholar] [CrossRef]
- Patel, A.K.; Singhania, R.R.; Chen, C.-W.; Tseng, Y.-S.; Kuo, C.-H.; Wu, C.-H.; Dong, C.D. Advances in micro- and nano bubbles technology for application in biochemical processes. Environ. Technol. Innovation 2021, 23, 101729. [Google Scholar] [CrossRef]
- Ye, S.; Geng, J.; Zhang, H.; Hu, J.; Zou, X.; Li, J. Boosting oxygen diffusion by micro-nano bubbles for highly-efficient H2O2 generation on air-calcining graphite felt. Electrochim. Acta 2023, 439, 141708. [Google Scholar] [CrossRef]
- Guo, J.; Wang, X.Y.; Li, T.; Gao, M.-t.; Hu, J.; Li, J. Effect of micro-nanobubbles with different gas sources on the growth and metabolism of chemoautotrophic microorganisms. Process Biochem. 2023, 126, 117–124. [Google Scholar] [CrossRef]
- Liu, S.; Oshita, S.; Kawabata, S.; Makino, Y.; Yoshimoto, T. Identification of ROS Produced by Nanobubbles and Their Positive and Negative Effects on Vegetable Seed Germination. Langmuir 2016, 32, 11295–11302. [Google Scholar] [CrossRef]
- Haapala, A.; Honkanen, M.; Liimatainen, H.; Stoor, T.; Niinimäki, J. Hydrodynamic drag and rise velocity of microbubbles in papermaking process waters. Chem. Eng. J. 2010, 162, 956–964. [Google Scholar] [CrossRef]
- Díaz, E.; Muñoz, E.; Vega, A.; Ordóñez, S. Enhancement of the CO2 Retention Capacity of Y Zeolites by Na and Cs Treatments: Effect of Adsorption Temperature and Water Treatment. Ind. Eng. Chem. Res. 2008, 47, 412–418. [Google Scholar] [CrossRef]
- Yazaydın, A.Ö.; Benin, A.I.; Faheem, S.A.; Jakubczak, P.; Low, J.J.; Willis, R.R.; Snurr, R.Q. Enhanced CO2 Adsorption in Metal-Organic Frameworks via Occupation of Open-Metal Sites by Coordinated Water Molecules. Chem. Mater. 2009, 21, 1425–1430. [Google Scholar] [CrossRef]
- Xu, D.; Xiao, P.; Zhang, J.; Li, G.; Xiao, G.; Webley, P.A.; Zhai, Y. Effects of water vapour on CO2 capture with vacuum swing adsorption using activated carbon. Chem. Eng. J. 2013, 230, 64–72. [Google Scholar] [CrossRef]
- Qian, Z.; Wei, L.; Mingyue, W.; Guansheng, Q. Application of amine-modified porous materials for CO2 adsorption in mine confined spaces. Colloids Surf. A 2021, 629, 127483. [Google Scholar] [CrossRef]
- Qi, G.; Wang, Y.; Estevez, L.; Duan, X.; Anako, N.; Park, A.H.A.; Li, W.; Jones, C.W.; Giannelis, E.P. High efficiency nanocomposite sorbents for CO2 capture based on amine-functionalized mesoporous capsules. Energy Environ. Sci. 2011, 4, 444–452. [Google Scholar] [CrossRef]
- Luo, S.; Chen, S.; Chen, S.; Zhuang, L.; Ma, N.; Xu, T.; Li, Q.; Hou, X. Preparation and characterization of amine-functionalized sugarcane bagasse for CO2 capture. J. Environ. Manag. 2016, 168, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Chen, S.; Lin, R.; Xu, X. Preparation of a solid amine adsorbent based on polypropylene fiber and its performance for CO2 capture. J. Mater. Res. 2013, 28, 2881–2889. [Google Scholar] [CrossRef]
- Gunathilake, C.; Manchanda, A.S.; Ghimire, P.; Kruk, M.; Jaroniec, M. Amine-modified silica nanotubes and nanospheres: Synthesis and CO2 sorption properties. Environ. Sci. Nano 2016, 3, 806–817. [Google Scholar] [CrossRef]
- Elrhayam, Y.; Elharfi, A. 3D-QSAR studies of the chemical modification of hydroxyl groups of biomass (cellulose, hemicelluloses and lignin) using quantum chemical descriptors. Heliyon 2019, 5, e02173. [Google Scholar] [CrossRef]
- Zafari, R.; Mendonça, F.G.; Tom Baker, R.; Fauteux-Lefebvre, C. Efficient SO2 capture using an amine-functionalized, nanocrystalline cellulose-based adsorbent. Sep. Purif. Technol. 2023, 308, 122917. [Google Scholar] [CrossRef]
- Petrovic, B.; Gorbounov, M.; Masoudi Soltani, S. Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater. 2021, 312, 110751. [Google Scholar] [CrossRef]
- Bonenfant, D.; Mimeault, M.; Hausler, R. Determination of the Structural Features of Distinct Amines Important for the Absorption of CO2 and Regeneration in Aqueous Solution. Ind. Eng. Chem. Res. 2003, 42, 3179–3184. [Google Scholar] [CrossRef]
- Høiby, N. A personal history of research on microbial biofilms and biofilm infections. Pathog. Dis. 2014, 70, 205–211. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Guo, X.; Zhang, L.; Zhang, W.; Man, C.; Jiang, Y. Characterization and transcriptomic basis of biofilm formation by Lactobacillus plantarum J26 isolated from traditional fermented dairy products. LWT 2020, 125, 109333. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting microbial biofilms: Current and prospective therapeutic strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhang, D.; Sha, Y.; Wang, F.; Liang, C.; Chen, T.; Sun, W.; Zhuang, W.; Yu, B.; Liu, D.; et al. Efficient preparation of phytase from genetically modified Pichia pastoris in immobilised fermentation biofilms adsorbed on surface-modified cotton fibres. Process Biochem. 2021, 111, 69–78. [Google Scholar] [CrossRef]
- Zhuang, W.; Yang, J.; Wu, J.; Liu, D.; Zhou, J.; Chen, Y.; Ying, H. Extracellular polymer substances and the heterogeneity of Clostridium acetobutylicum biofilm induced tolerance to acetic acid and butanol. RSC Adv. 2016, 6, 33695–33704. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Yu, L.; Gao, F.; Jiang, Y.; Xu, X. Resistance of biofilm formation and formed-biofilm of Escherichia coli O157:H7 exposed to acid stress. LWT 2020, 118, 108787. [Google Scholar] [CrossRef]
- Antunes, F.A.F.; Santos, J.C.; Chandel, A.K.; Carrier, D.J.; Peres, G.F.D.; Milessi, T.S.S.; da Silva, S.S. Repeated batches as a feasible industrial process for hemicellulosic ethanol production from sugarcane bagasse by using immobilized yeast cells. Cellulose 2019, 26, 3787–3800. [Google Scholar] [CrossRef]
- Chen, P.-C.; Zheng, P.; Ye, X.-Y.; Ji, F. Preparation of A. succinogenes immobilized microfiber membrane for repeated production of succinic acid. Enzym. Microb. Technol. 2017, 98, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzocchella, A. Continuous succinic acid fermentation by Actinobacillus succinogenes in a packed-bed biofilm reactor. Biotechnol. Biofuels 2018, 11, 138. [Google Scholar] [CrossRef]
- Xiao, W.; Xu, G.; Li, G. Effect of nanobubble application on performance and structural characteristics of microbial aggregates. Sci. Total Environ. 2021, 765, 142725. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Huang, Y.; Liao, Q.; Fu, Q.; Xia, A.; Zhu, X. Impact of the accumulation and adhesion of released oxygen during Scenedesmus obliquus photosynthesis on biofilm formation and growth. Bioresour. Technol. 2017, 244, 198–205. [Google Scholar] [CrossRef]
- Chen, B.; Zhou, S.; Zhang, N.; Liang, H.; Sun, L.; Zhao, X.; Guo, J.; Lu, H. Micro and nano bubbles promoted biofilm formation with strengthen of COD and TN removal synchronously in a blackened and odorous water. Sci. Total Environ. 2022, 837, 155578. [Google Scholar] [CrossRef] [PubMed]
- Harun, M.H.C.; Zimmerman, W.B. Membrane defouling using microbubbles generated by fluidic oscillation. Water Supply 2018, 19, 97–106. [Google Scholar] [CrossRef]
- Agarwal, A.; Xu, H.; Ng, J.; Liu, Y. Biofilm detachment by self-collapsing air microbubbles: A potential chemical-free cleaning technology for membrane biofouling. J. Mater. Chem. 2012, 22, 2203–2207. [Google Scholar] [CrossRef]


| Microorganism | Fermentation type | Titer (g·L−1) | Productivity (g·L−1·h−1) | Reference |
|---|---|---|---|---|
| A. succiniciproducens | Anaerobic, continuous culture | 83 | 10.4 | [10] |
| A. succinogenes FZ53 | Anaerobic batch | 105.8 | 1.36 | [11] |
| A. succinogenes ATCC 55618 | Anaerobic fed-batch | 151.44 | 3.22 | [12] |
| Engineered M. succiniciproducens | Fed-batch | 134.25 | 21.3 | [13] |
| B. succiniciproducens JF4016 | Anaerobic batch | 19 | 1.9 | [14] |
| E. coli JW1021 | Dual-phase, fed-batch | 114.0 | 3.25 | [15] |
| E. coli (Tang1527) | Dual-phase batch | 89.4 | 1.24 | [16] |
| C. glutamicum | Anaerobic batch | 64.16 | 1.07 | [17] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Wu, H.; Chen, Y.; Liao, J.; Zhang, W.; Jiang, M. Recent Advancements and Strategies of Improving CO2 Utilization Efficiency in Bio-Succinic Acid Production. Fermentation 2023, 9, 967. https://doi.org/10.3390/fermentation9110967
Chen X, Wu H, Chen Y, Liao J, Zhang W, Jiang M. Recent Advancements and Strategies of Improving CO2 Utilization Efficiency in Bio-Succinic Acid Production. Fermentation. 2023; 9(11):967. https://doi.org/10.3390/fermentation9110967
Chicago/Turabian StyleChen, Xin, Hao Wu, Ying Chen, Jingwen Liao, Wenming Zhang, and Min Jiang. 2023. "Recent Advancements and Strategies of Improving CO2 Utilization Efficiency in Bio-Succinic Acid Production" Fermentation 9, no. 11: 967. https://doi.org/10.3390/fermentation9110967
APA StyleChen, X., Wu, H., Chen, Y., Liao, J., Zhang, W., & Jiang, M. (2023). Recent Advancements and Strategies of Improving CO2 Utilization Efficiency in Bio-Succinic Acid Production. Fermentation, 9(11), 967. https://doi.org/10.3390/fermentation9110967







