Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution
Abstract
1. Introduction
2. Bioplastics Production
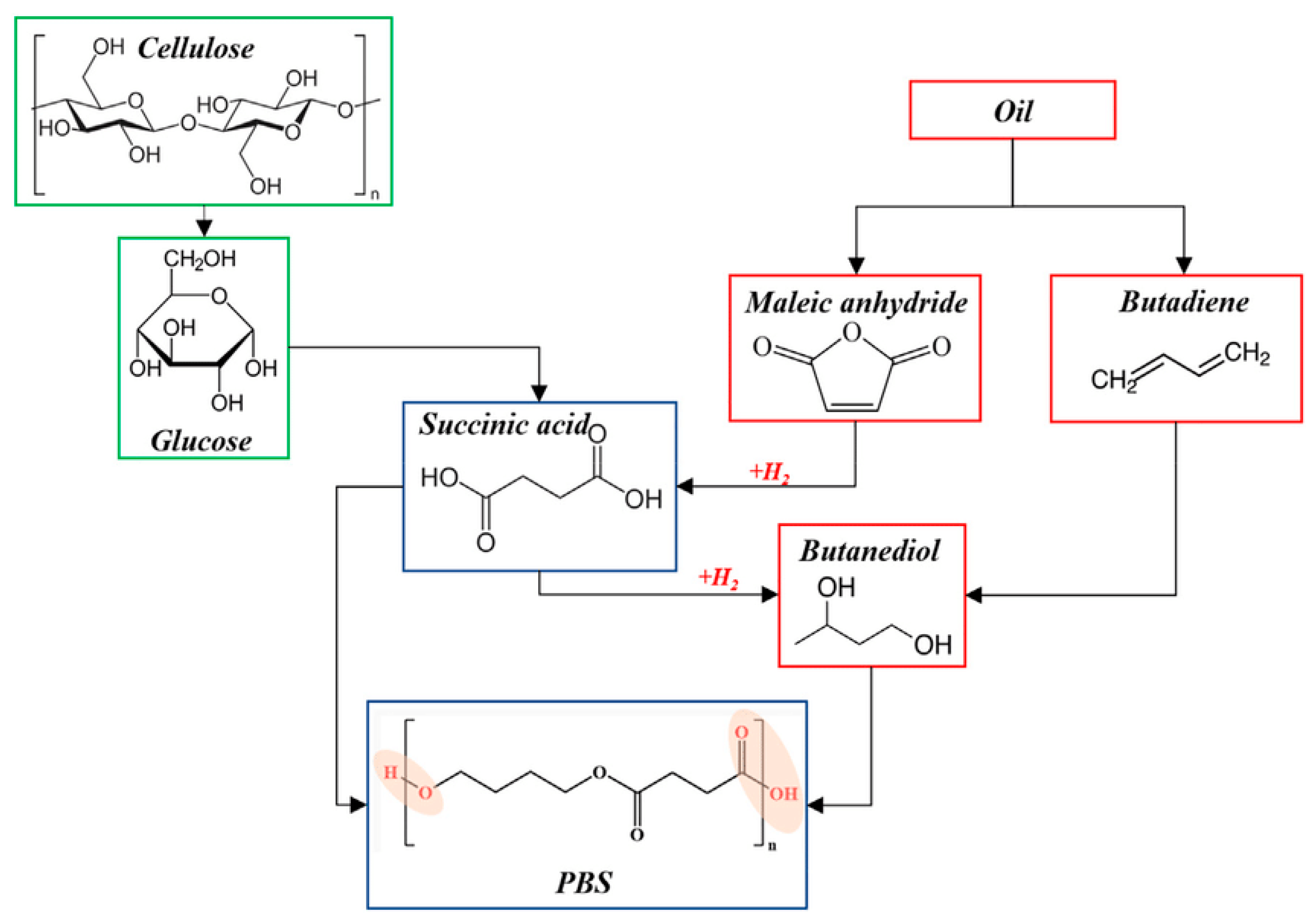
3. Polybutylene Succinate (PBS)
4. Succinic Acid and Its Associated Bioplastics
5. The Palm Oil Industry
5.1. Palm Oil Production By-Products
5.2. Oil Palm Biomass Compositional Attributes
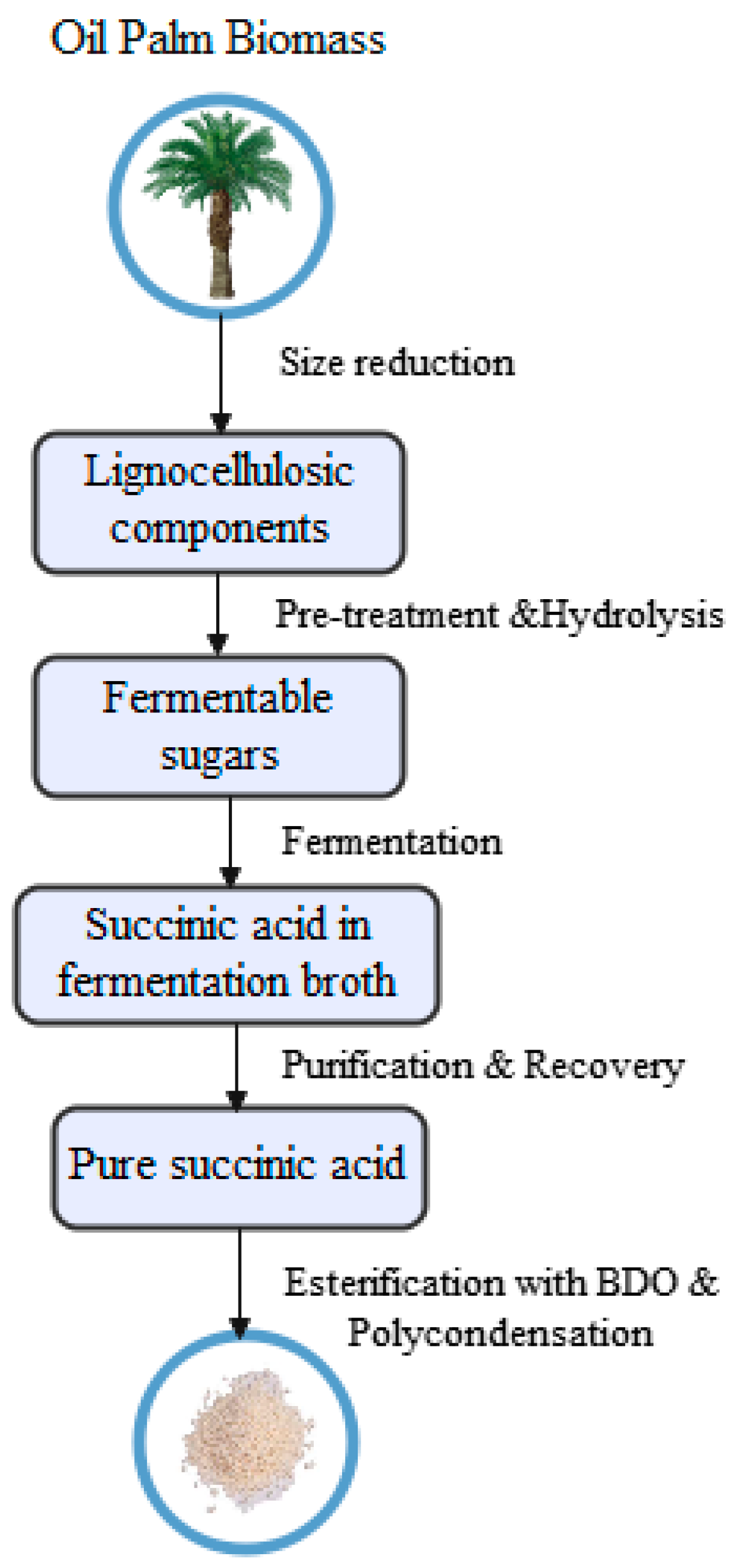
5.3. Pretreatment and Hydrolysis of Fermentable Sugars from Oil Palm Biomass
5.4. Bioplastic Production from Oil Palm Biomass
5.5. Biomass By-Products from the Palm Oil Industry Utilisation for Bioplastic and Succinic Acid Productions
6. Production Parameters and Strategies for Higher Yield of Fermentation-Based Succinic Acid
6.1. Succinic Acid Fermentation Biocatalysts
6.2. pH
6.3. Nitrogen Sources and Additional Supplementations
6.4. Strategies for Increasing Succinic Acid Yield via Fermentation
7. Conclusions, Challenges, and Recommendations
- The potentials of microbial pretreatment and saccharification of oil palm biomass should be explored in great length, as it would be beneficial in establishing a cost effective and clean production process, due to the reduced amount of chemicals utilization;
- More strategies, such as semi-simultaneous enzymatic hydrolysis and fermentation (SSSF) of oil palm biomass should be explored and optimized to increase time efficiency and minimize energy consumption. To date, a limited amount of research has been conducted to merge the processes prior to fermentation of succinic acid from oil palm biomass into a single unit operation. Proper optimization of the simultaneous process for hydrolysis and fermentation will help to confer a faster hydrolytic rate before fermentation in order to achieve a higher succinic acid yield and final titer;
- Detoxification process optimisation by targeting to maximise the removal of inhibitors while minimising sugar loss should be considered to solve the issue of toxic by-product generation during pretreatment. Furthermore, alternative methods of detoxification besides activated carbon treatment require exploration.
- This review only included the generation of PHA and PHB bioplastics through fermentation from OPT sap and EFB hydrolysate in oil palm biomass. Consequently, future studies should consider different types of bioplastic production from oil palm biomass. The possibility of directly generating PBS from the succinic acid downstream processing unit in tandem through process integration should also be examined.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ford, H.V.; Jones, N.H.; Davies, A.J.; Godley, B.J.; Jambeck, J.R.; Napper, I.E.; Suckling, C.C.; Williams, G.J.; Woodall, L.C.; Koldewey, H.J. The Fundamental Links between Climate Change and Marine Plastic Pollution. Sci. Total Environ. 2022, 806, 150392. [Google Scholar] [CrossRef] [PubMed]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, 25–29. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, P.; Schartup, A.T.; Zhang, Y. Plastic Waste Release Caused by COVID-19 and Its Fate in the Global Ocean. Proc. Natl. Acad. Sci. USA 2021, 118, e2111530118. [Google Scholar] [CrossRef]
- Zheng, J.; Suh, S. Strategies to Reduce the Global Carbon Footprint of Plastics. Nat. Clim. Chang. 2019, 9, 374–378. [Google Scholar] [CrossRef]
- World Wide Fund-Australia. The Lifecycle of Plastics. Available online: https://www.wwf.org.au/news/blogs/the-lifecycle-of-plastics (accessed on 30 October 2021).
- Tenenbaum, L.; These Three Plastic Recycling Myths Will Blow Your Mind. SCIENCE. Available online: https://www.forbes.com/sites/lauratenenbaum/2019/05/15/these-three-plastic-recycling-myths-will-blow-your-mind/?sh=5de570f975f0 (accessed on 10 November 2021).
- Leslie, H.A.; van Velzen, M.J.M.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and Quantification of Plastic Particle Pollution in Human Blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Stanton, L.; 100 Ocean Plastic Pollution Statistics & Facts. FISHKEEPING. 2022. Available online: https://www.hepper.com/marine-ocean-plastic-pollution-statistics/ (accessed on 30 October 2021).
- F. & F International. Removing or Restricting Microplastic Ingredients or “Microbeads ” From Consumer and Industrial Products; Fauna & Flora International: Cambridge, UK, 2017. [Google Scholar]
- Rani, G.U.; Sharma, S. Biopolymers, Bioplastics and Biodegradability: An Introduction. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Szczerba, H.; Komon-Janczara, E.; Dudziak, K.; Wasko, A.; Targonski, Z. A Novel Biocatalyst, Enterobacter Aerogenes LU2, for Efficient Production of Succinic Acid Using Whey Permeate as a Cost-Effective Carbon Source. Biotechnol. Biofuels 2020, 13, 96. [Google Scholar] [CrossRef]
- Yang, Q.; Wu, M.; Dai, Z.; Xin, F.; Zhou, J.; Dong, W.; Ma, J.; Jiang, M.; Zhang, W. Comprehensive Investigation of Succinic Acid Production by Actinobacillus Succinogenes: A Promising Native Succinic Acid Producer. Biofuels Bioprod. Biorefin. 2020, 14, 950–964. [Google Scholar] [CrossRef]
- Hossain, N.; Zaini, J.H.; Mahlia, T.M.I. A Review of Bioethanol Production from Plant-Based Waste Biomass by Yeast Fermentation. Int. J. Technol. 2017, 8, 5–18. [Google Scholar] [CrossRef]
- Shah, T.V.; Vasava, D.V. A Glimpse of Biodegradable Polymers and Their Biomedical Applications. E-Polymers 2019, 19, 385–410. [Google Scholar] [CrossRef]
- Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.J. Bio-Based Plastics—A Review of Environmental, Social and Economic Impact Assessments. J. Clean. Prod. 2018, 185, 476–491. [Google Scholar] [CrossRef]
- Jabeen, N.; Majid, I.; Nayik, G.A. Bioplastics and Food Packaging: A Review. Cogent Food Agric. 2015, 1, 1117749. [Google Scholar] [CrossRef]
- Renee, C.; The Truth About Bioplastics. Sustainability. Available online: https://news.climate.columbia.edu/2017/12/13/the-truth-about-bioplastics/ (accessed on 2 November 2022).
- Markets, R.; Global Bioplastics Market (2022 to 2029)-by Type, Application and Geography. GLOBE NEWSWIRE. Available online: https://www.globenewswire.com/news-release/2022/03/15/2403399/28124/en/Global-Bioplastics-Market-2022-to-2029-by-Type-Application-and-Geography.html (accessed on 4 November 2022).
- Sudesh, K.; Iwata, T. Sustainability of Biobased and Biodegradable Plastics. Clean Soil Air Water 2008, 36, 433–442. [Google Scholar] [CrossRef]
- Changwichan, K.; Silalertruksa, T.; Gheewala, S. Eco-Efficiency Assessment of Bioplastics Production Systems and End-of-Life Options. Sustainability 2018, 10, 952. [Google Scholar] [CrossRef]
- Aliotta, L.; Seggiani, M.; Lazzeri, A.; Gigante, V.; Cinelli, P. A Brief Review of Poly (Butylene Succinate) (PBS) and Its Main Copolymers: Synthesis, Blends, Composites, Biodegradability, and Applications. Polymers 2022, 14, 844. [Google Scholar] [CrossRef]
- Gironi, F.; Piemonte, V. Bioplastics and Petroleum-Based Plastics: Strengths and Weaknesses. Energy Sources Part A Recover. Util. Environ. Eff. 2011, 33, 1949–1959. [Google Scholar] [CrossRef]
- Ishioka, R.; Kitakuni, E.; Ichikawa, Y. Aliphatic Polyesters: “Bionolle”. In Biopolymers; Doi, Y., Steinbüchel, A., Eds.; Wiley VCH: New York, NY, USA, 2022; pp. 275–297. [Google Scholar]
- Rafiqah, S.A.; Khalina, A.; Harmaen, A.S.; Tawakkal, I.A.; Zaman, K.; Asim, M.; Nurrazi, M.N.; Lee, C.H. A Review on Properties and Application of Bio-based Poly(Butylene Succinate). Polymers 2021, 13, 1436. [Google Scholar] [CrossRef]
- Charlon, S.; Marais, S.; Dargent, E.; Soulestin, J.; Sclavons, M.; Follain, N. Structure-Barrier Property Relationship of Biodegradable Poly(Butylene Succinate) and Poly[(Butylene Succinate)-Co-(Butylene Adipate)] Nanocomposites: Influence of the Rigid Amorphous Fraction. Phys. Chem. Chem. Phys. 2015, 17, 29918–29934. [Google Scholar] [CrossRef]
- Barletta, M.; Aversa, C.; Ayyoob, M.; Gisario, A.; Hamad, K.; Mehrpouya, M.; Vahabi, H. Progress in Polymer Science Poly (Butylene Succinate) (PBS): Materials, Processing, and Industrial Applications. Prog. Polym. Sci. 2022, 132, 101579. [Google Scholar] [CrossRef]
- Dickson, R.; Mancini, E.; Garg, N.; Woodley, J.M.; Gernaey, K.V.; Pinelo, M.; Liu, J.; Mansouri, S.S. Sustainable Bio-Succinic Acid Production: Superstructure Optimization, Techno-Economic, and Lifecycle Assessment. Energy Environ. Sci. 2021, 14, 3542–3558. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Jahim, J.M.; Harun, S.; Tan, J.P.; Manaf, S.F.A.; Shah, S.S.M. Kinetics of the Bioproduction of Succinic Acid by Actinobacillus Succinogenes from Oil Palm Lignocellulosic Hydrolysate in a Bioreactor. Bioresources 2018, 13, 8279–8294. [Google Scholar] [CrossRef]
- Nghiem, N. Production of Succinic Acid by Anaerobiospirillum Succiniciproducens. Appl. Biochem. Biotechnol. Part Enzym. Eng. Biotechnol. 1997, 63–65, 565–576. [Google Scholar] [CrossRef]
- Song, H.; Jang, S.; Park, J.; Lee, S. Modeling of Batch Fermentation Kinetics for Succinic Acid Production by Mannheimia Succiniciproducens. Biochem. Eng. J. 2008, 40, 107–115. [Google Scholar] [CrossRef]
- Andersson, C. Succinic Acid Production Using Metabolically Engineered Escherichia Coli. Ph.D. Thesis, Luleå Tekniska Universitet, Luleå, Sweden, 2007. [Google Scholar]
- Okino, S.; Noburyu, R.; Suda, M.; Jojima, T.; Inui, M.; Yukawa, H. An Efficient Succinic Acid Production Process in a Metabolically Engineered Corynebacterium Glutamicum Strain. Appl. Microbiol. Biotechnol. 2008, 81, 459–464. [Google Scholar] [CrossRef]
- Xu, J.; Guo, B.H. Poly(Butylene Succinate) and Its Copolymers: Research, Development and Industrialization. Biotechnol. J. 2010, 5, 1149–1163. [Google Scholar] [CrossRef]
- Stepien, K.; Miles, C.; McClain, A.; Wisniewska, E.; Sobolewski, P.; Kohn, J.; Puskas, J.; Wagner, H.D.; Fray, M.E. Bio-Copolyesters of Poly (Butylene Succinate)(PBS) Containing Long Chain Bio-Based Glycol. ACS Sustain. Chem. Eng. 2019, 7, 10623–10632. [Google Scholar] [CrossRef]
- Research. Global Succinic Acid Market Size By Type (Petro-based and Bio-based), By End-User (Industrial, Coating, Food & Beverage, Cosmetics, and Pharmaceuticals), By Geographic Scope and Forecast. Available online: https://www.verifiedmarketresearch.com/product/succinic-acid-market/ (accessed on 5 November 2022).
- Saxena, R.K.; Saran, S.; Isar, J.; Kaushik, R. Production and Applications of Succinic Acid. In Current Developments in Biotechnology and Bioengineering; Elsevier: New Delhi, India, 2017; pp. 601–630. [Google Scholar] [CrossRef]
- Nghiem, N.P.; Kleff, S.; Schwegmann, S. Succinic Acid: Technology Development and Commercialization. Fermentation 2017, 3, 26. [Google Scholar] [CrossRef]
- Baidya, P.K.; Sarkar, U.; Villa, R.; Sadhukhan, S. Liquid-Phase Hydrogenation of Bio-Refined Succinic Acid to 1,4-Butanediol Using Bimetallic Catalysts. BMC Chem. Eng. 2019, 1, 10. [Google Scholar] [CrossRef]
- Kuglarz, M.; Rom, M. Influence of Carbon Dioxide and Nitrogen Source on Sustainable Production of Succinic Acid from Miscanthus Hydrolysates. Int. J. Environ. Sci. Dev. 2019, 10, 362–367. [Google Scholar] [CrossRef]
- Sadare, O.O.; Ejekwu, O.; Moshokoa, M.F.; Jimoh, M.O.; Daramola, M.O. Membrane Purification Techniques for Recovery of Succinic Acid Obtained from Fermentation Broth during Bioconversion of Lignocellulosic Biomass: Current Advances and Future Perspectives. Sustainability 2021, 13, 6794. [Google Scholar] [CrossRef]
- Kidwell, H. Bio-Succinic Acid to Go Commercial. Available online: http://www.in-pharmatechnologist.com/Materials-Formulation/Bio-succinic-acid-to-go-commercial (accessed on 6 November 2022).
- MarketWatch. Bio-Based Succinic Acid Market Size, Share 2022. Press Release. Available online: https://www.marketwatch.com/press-release/bio-based-succinic-acid-market-sizeshare-2022-global-development-strategy-explosive-factors-of-revenue-by-key-vendors-demand-future-trends-and-industry-growth-research-report-2022-03-15 (accessed on 7 November 2022).
- Research, G.V.; Bio-succinic Acid Market Size Worth $272.4 Million by 2030: Grand View Research, Inc. NEWS. Available online: https://www.prnewswire.com/news-releases/bio-succinic-acid-market-size-worth-272-4-million-by-2030-grand-view-research-inc-301522567.html (accessed on 8 November 2022).
- Kumar, R.; Basak, B.; Jeon, B.H. Sustainable Production and Purification of Succinic Acid: A Review of Membrane-Integrated Green Approach. J. Clean. Prod. 2020, 277, 123954. [Google Scholar] [CrossRef]
- Wang, C.; Ming, W.; Yan, D.; Zhang, C.; Yang, M.; Liu, Y.; Zhang, Y.; Guo, B.; Wan, Y.; Xing, J. Novel Membrane-Based Biotechnological Alternative Process for Succinic Acid Production and Chemical Synthesis of Bio-Based Poly (Butylene Succinate). Bioresour. Technol. 2014, 156, 6–13. [Google Scholar] [CrossRef]
- Dirkes, R.; Neubauer, P.R.; Rabenhorst, J. Pressed Sap from Oil Palm (Elaeis Guineensis) Trunks: A Revolutionary Growth Medium for the Biotechnological Industry? Biofuels, Bioprod. Biorefin. 2021, 15, 931–944. [Google Scholar] [CrossRef]
- GoldenAgri. What Are the Palm Oil Trends to Watch in 2022? Environment. Available online: https://www.goldenagri.com.sg/palm-oil-trends-in-2022/ (accessed on 9 November 2022).
- Corley, R.; Tinker, P. The Oil Palm, 4th Editio; Wiley Blackwell: Hoboken, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Descals, A.; Wich, S.; Meijaard, E.; Gaveau, D.L.A.; Peedell, S.; Szantoi, Z. High-Resolution Global Map of Smallholder and Industrial Closed-Canopy Oil Palm Plantations. Earth Syst. Sci. Data 2021, 13, 1211–1231. [Google Scholar] [CrossRef]
- Malaysian Palm Oil Board (MPOB). Malaysian Oil Palm Statistics 2019; Malaysian Palm Oil Board: Bandar Baru Bangi, Malaysia, 2020. [Google Scholar]
- Kadir, A.P.G. Malaysia’s Oil Palm Industry Celebrates 105th Anniversary. New Straits Times Press. Available online: https://www.nst.com.my/business/2022/05/800724/malaysias-oil-palm-industry-celebrates-105th-anniversary (accessed on 9 November 2022).
- Nambiappan, B.; Ismail, A.; Hashim, N.; Ismail, N.; Shahari, D.N.; Idris, N.A.N.; Omar, N.; Salleh, K.M.; Hassan, N.A.M.; Kushairi, A. Malaysia: 100 Years of Resilient Palm Oil Economic Performance. J. Oil Palm Res. 2018, 30, 13–25. [Google Scholar] [CrossRef]
- Ofori-Boateng, C.; Lee, K.T. Sustainable Utilization of Oil Palm Wastes for Bioactive Phytochemicals for the Benefit of the Oil Palm and Nutraceutical Industries. Phytochem. Rev. 2013, 12, 173–190. [Google Scholar] [CrossRef]
- Parveez, G.K.A.; Tarmizi, A.H.A.; Sundram, S.; Loh, S.K.; Ong-Abdullah, M.; Palam, K.D.P.; Salleh, K.M.; Ishak, S.M.; Idris, Z. Oil Palm Economic Performance in Malaysia and R&D Progress in 2020. J. Oil Palm Res. 2021, 33, 181–214. [Google Scholar] [CrossRef]
- Zakria, R.M.; Gimbun, J.; Asras, M.F.F.; Chua, G.K. Magnesium Sulphate and Β-Alanine Enhanced the Ability of Kluyveromyces Marxianus Producing Bioethanol Using Oil Palm Trunk Sap. Biofuels 2016, 8, 595–603. [Google Scholar] [CrossRef]
- Loh, S.K. The Potential of the Malaysian Oil Palm Biomass as a Renewable Energy Source. Energy Convers. Manag. 2017, 141, 285–298. [Google Scholar] [CrossRef]
- Norhazimah, H.A.; Siti, F.M.; Aida, M.; Dilaeleyana, A.; Nur Shahirah, M. Direct Fermentation of Oil Palm (Elaeis Guineensis) Trunk Sap to Bioethanol by Saccharomyces Cerevisiae. IOP Conf. Ser. Mater. Sci. Eng. 2020, 943, 012012. [Google Scholar] [CrossRef]
- Saleh, S.H.; Noor, M.A.M.; Rosma, A. Fractionation of Oil Palm Frond Hemicelluloses by Water or Alkaline Impregnation and Steam Explosion. Carbohydr. Polym. 2015, 115, 533–539. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Manaf, S.F.A.; Illias, R.M.; Harun, S.; Mohammad, A.W.; Jahim, J.M. Biotechnological Route for Sustainable Succinate Production Utilizing Oil Palm Frond and Kenaf as Potential Carbon Sources. Appl. Microbiol. Biotechnol. 2017, 101, 3055–3075. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, B.E.; Hamid, Z.A.A.; Arai, T.; Kosugi, A.; Murata, Y.; Hashim, R.; Sulaiman, O.; Mori, Y.; Sudesh, K. Potential of Oil Palm Trunk Sap as a Novel Inexpensive Renewable Carbon Feedstock for Polyhydroxyalkanoate Biosynthesis and as a Bacterial Growth Medium. Clean Soil Air Water 2012, 40, 310–317. [Google Scholar] [CrossRef]
- Mostapha, M.; Jahar, N.A.; Zakaria, S.; Aizat, W.M.; Azizan, K.A.; Jaafar, S.N.S. Metabolite Profiling of Core Oil Palm Trunk (COPT) Sap: The Effects of Different Storage Durations, Conditions and Temperatures. J. Oil Palm Res. 2018, 30, 111–120. [Google Scholar] [CrossRef]
- Dessie, W.; Xin, F.; Zhang, W.; Jiang, Y.; Wu, H.; Ma, J.; Jiang, M. Opportunities, Challenges, and Future Perspectives of Succinic Acid Production by Actinobacillus Succinogenes. Appl. Microbiol. Biotechnol. 2018, 102, 9893–9910. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, N.A.; Loh, S.K.; Nasrin, A.B.; Luthfi, A.A.I.; Harun, S.; Abdul, P.M.; Jahim, J.M. Compatibility of Utilising Nitrogen-Rich Oil Palm Trunk Sap for Succinic Acid Fermentation by Actinobacillus Succinogenes 130Z. Bioresour. Technol. 2019, 293, 122085. [Google Scholar] [CrossRef] [PubMed]
- Abdul, P.M.; Jahim, J.M.; Harun, S.; Markom, M.; Lutpi, N.A.; Hassan, O.; Balan, V.; Dale, B.E.; Mohd, N.M.T. Effects of Changes in Chemical and Structural Characteristic of Ammonia Fibre Expansion (AFEX) Pretreated Oil Palm Empty Fruit Bunch Fibre on Enzymatic Saccharification and Fermentability for Biohydrogen. Bioresour. Technol. 2016, 211, 200–208. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Hassan, M.A. Hydrothermal Pretreatment Enhanced Enzymatic Hydrolysis and Glucose Production from Oil Palm Biomass. Bioresour. Technol. 2015, 176, 142–148. [Google Scholar] [CrossRef]
- Santos, R.B.; Hart, P.; Jameel, H.; Chang, H.M. Wood Based Lignin Reactions Important to the Biorefinery and Pulp and Paper Industries. BioResources 2013, 8, 1456–1477. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hagerdal, B. Fermentation of Lignocellulosic Hydrolysates. 1: Inhibition and Detoxification. Bioresour. Technol. 2000, 74, 17–24. [Google Scholar] [CrossRef]
- Kunasundari, B.; Arai, T.; Sudesh, K.; Hashim, R.; Sulaiman, O.; Stalin, N.J.; Kosugi, A. Detoxification of Sap from Felled Oil Palm Trunks for the Efficient Production of Lactic Acid. Appl. Biochem. Biotechnol. 2017, 183, 412–425. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Tan, J.P.; Harun, S.; Manaf, S.F.A.; Jahim, J.M. Homogeneous Solid Dispersion (HSD) System for Rapid and Stable Production of Succinic Acid from Lignocellulosic Hydrolysate. Bioprocess Biosyst. Eng. 2018, 42, 117–130. [Google Scholar] [CrossRef]
- Manaf, S.F.A.; Jahim, J.M.; Harun, S.; Luthfi, A.A.I. Fractionation of Oil Palm Fronds (OPF) Hemicellulose Using Dilute Nitric Acid for Fermentative Production of Xylitol. Ind. Crops Prod. 2018, 115, 6–15. [Google Scholar] [CrossRef]
- Tan, J.P.; Luthfi, A.A.I.; Manaf, S.F.A.; Wu, T.Y.; Jahim, J.M. Incorporation of CO2 during the Production of Succinic Acid from Sustainable Oil Palm Frond Juice. J. CO2 Util. 2018, 26, 595–601. [Google Scholar] [CrossRef]
- Saleh, S.; Shah, S.N.M.; Khalil, K.; Bujang, A. Xylooligosaccharides Production from Oil Palm Frond by Trichoderma Longibrachiatumxylanase. Malaysian J. Anal. Sci. 2016, 20, 525–530. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Jahim, J.M.; Harun, S.; Tan, J.P.; Mohammad, A.W. Biorefinery Approach towards Greener Succinic Acid Production from Oil Palm Frond Bagasse. Process Biochem. 2016, 51, 1527–1537. [Google Scholar] [CrossRef]
- Luthfi, A.A.I.; Jahim, J.M.; Harun, S.; Tan, J.P.; Mohammad, A.W. Potential Use of Coconut Shell Activated Carbon as an Immobilisation Carrier for High Conversion of Succinic Acid from Oil Palm Frond Hydrolysate. RSC Adv. 2017, 7, 49480–49489. [Google Scholar] [CrossRef]
- Tan, J.P.; Jahim, J.M.; Harun, S.; Wu, T.Y.; Mumtaz, T. Utilization of Oil Palm Fronds as a Sustainable Carbon Source in Biorefineries. Int. J. Hydrogen Energy 2016, 41, 4896–4906. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Loh, S.K.; Luthfi, A.A.I.; Abdul, P.M.; Harun, S.; Jahim, J.M. Effect of Neutralizing Agents in the Preparation of Succinic Acid from Oil Palm Trunk. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1195, 012032. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Jahim, J.M.; Loh, S.K.; Nasrin, A.B.; Harun, S.; Abdul, P.M. Organic Acid Pretreatment of Oil Palm Trunk Biomass for Succinic Acid Production. Waste Biomass Valorization 2020, 11, 5549–5559. [Google Scholar] [CrossRef]
- Komonkiat, I.; Cheirsilp, B. Felled Oil Palm Trunk as a Renewable Source for Biobutanol Production by Clostridium spp. Bioresour. Technol. 2013, 146, 200–207. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Jahim, J.M.; Loh, S.K.; Bakar, N.A.; Luthfi, A.A.I. Response Surface Optimisation of Enzymatically Hydrolysed and Dilute Acid Pretreated Oil Palm Trunk Bagasse for Succinic Acid Production. Bioresources 2019, 14, 1679–1693. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Loh, S.K.; Luthfi, A.A.I.; Abdul, P.M.; Bakar, N.A.; Harun, S.; Jahim, J.M. Whole Slurry Saccharification of Mild Oxalic Acid-Pretreated Oil Palm Trunk Biomass Improves Succinic Acid Production. Ind. Crop. Prod. 2021, 171, 113854. [Google Scholar] [CrossRef]
- Bukhari, N.A.; Loh, S.K.; Luthfi, A.A.I.; Abdul, P.M.; Jahim, J.M. Low Cost Nutrient-Rich Oil Palm Trunk Bagasse Hydrolysate for Bio-Succinic Acid Production by Actinobacillus Succinogenes. Prep. Biochem. Biotechnol. 2021, 52, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, J.; Idris, A. Oil Palm Empty Fruit Bunches a Promising Substrate for Succinic Acid Production via Simultaneous Saccharification and Fermentation. Renew. Energy 2017, 114, 917–923. [Google Scholar] [CrossRef]
- Akhtar, J.; Hassan, N.; Idris, A.; Ngadiman, N.H.A. Optimization of Simultaneous Saccharification and Fermentation Process Conditions for the Production of Succinic Acid from Oil Palm Empty Fruit Bunches. J. Wood Chem. Technol. 2020, 40, 136–145. [Google Scholar] [CrossRef]
- Cimini, D.; Argenzio, O.; Ambrosio, S.; Lama, L.; Finore, I.; Finamore, R.; Pepe, O.; Faraco, V.; Schiraldi, C. Production of Succinic Acid from Basfia Succiniciproducens up to the Pilot Scale from Arundo Donax Hydrolysate. Bioresour. Technol. Technol. 2016, 222, 355–360. [Google Scholar] [CrossRef]
- Ferone, M.; Raganati, F.; Ercole, A.; Olivieri, G.; Salatino, P.; Marzocchella, A. Continuous Succinic Acid Fermentation by Actinobacillus Succinogenes in a Packed-Bed Biofilm Reactor. Biotechnol. Biofuels 2018, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.K.; Goyal, V.; Saini, A.; Yadav, A.; Gupta, R. Enzymatic Saccharification of Pretreated Rice Straw by Cellulases from Aspergillus Niger BK01. 3 Biotech. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Nisha, M.; Saranyah, K.; Shankar, M.; Saleena, L.M. Enhanced Saccharification of Lignocellulosic Agricultural Biomass and Increased Bioethanol Titre Using Acclimated Clostridium Thermocellum DSM1313. 3 Biotech. 2017, 7, 35. [Google Scholar] [CrossRef]
- Yustinah; Hidayat, N.; Alamsyah, R.; Roslan, A.M.; Hermansyah, H.; Gozan, M. Production of Polyhydroxybutyrate from Oil Palm Empty Fruit Bunch (OPEFB) Hydrolysates by Bacillus Cereus Suaeda B-001. Biocatal. Agric. Biotechnol. 2019, 18, 101019. [Google Scholar] [CrossRef]
- Takamitsu, A.; Kosugi, A.; Mori, Y.; Murata, Y.; Lokesh, B.E. Production of Biodegradable Plastic from Oil Palm Sap; Universiti Sains Malaysia: Gelugor, Malaysia, 2011; pp. 2–3. [Google Scholar]
- Mumtaz, T.; Yahaya, N.A.; Abd-Aziz, S.; Rahman, N.A.; Yee, P.L.; Shirai, Y.; Hassan, M.A. Turning Waste to Wealth-Biodegradable Plastics Polyhydroxyalkanoates from Palm Oil Mill Effluent-a Malaysian Perspective. J. Clean. Prod. 2010, 18, 1393–1402. [Google Scholar] [CrossRef]
- Sangkharak, K.; Prasertsan, P. The Production of Polyhydroxyalkanoate by Bacillus Licheniformis Using Sequential Mutagenesis and Optimization. Biotechnol. Bioprocess Eng. 2013, 18, 272–279. [Google Scholar] [CrossRef]
- Pasma, S.A.; Daik, R.; Maskat, M.Y. Production of Succinic Acid from Oil Palm Empty Fruit Bunch Cellulose Using Actinobacillus Succinogenes Production of Succinic Acid from Oil Palm Empty Fruit Bunch Cellulose Using Actinobacillus Succinogenes. AIP Conf. Proc. 2013, 1571, 753. [Google Scholar] [CrossRef]
- Pereira, B.; Miguel, J.; Vilaça, P.; Soares, S.; Rocha, I.; Carneiro, S. Reconstruction of a Genome-Scale Metabolic Model for Actinobacillus Succinogenes 130Z. BMC Syst. Biol. 2018, 12, 61. [Google Scholar] [CrossRef] [PubMed]
- Carlson, A.; Coggio, B.; Lau, K.; Mercogliano, C.; Millis, J. Industrial Production of Succinic Acid. In Chemicals and Fuels from Bio Based Building Blocks; Wiley-VCH Verlag GmbH & Co., KGaA: Weinheim, Germany, 2016; pp. 173–190. [Google Scholar] [CrossRef]
- Engel, C.A.R.; Straathof, A.J.J.; Zijlmans, T.W.; Van Gulik, W.M.; Van Der Wielen, L.A.M. Fumaric Acid Production by Fermentation. Appl. Microbiol. Biotechnol. 2008, 78, 379–389. [Google Scholar] [CrossRef]
- Omwene, P.I.; Yagcioglu, M.; Ocal-Sarihan, Z.B.; Ertan, F.; Keris-Sen, U.D.; Karagunduz, A.; Keskinler, B. Batch Fermentation of Succinic Acid from Cheese Whey by Actinobacillus Succinogenes under Variant Medium Composition. 3 Biotech. 2021, 11, 389. [Google Scholar] [CrossRef]
- McKinlay, J.B.; Vieille, C. 13C-Metabolic Flux Analysis of Actinobacillus Succinogenes Fermentative Metabolism at Different NaHCO3 and H2 Concentrations. Metab. Eng. 2008, 10, 55–68. [Google Scholar] [CrossRef]
- Salma, A.; Abdallah, R.; Fourcade, F.; Amrane, A.; Djelal, H. A New Approach to Produce Succinic Acid Through a Co-Culture System. Appl. Biochem. Biotechnol. 2021, 193, 2872–2892. [Google Scholar] [CrossRef]
- Choi, S.; Song, H.; Lim, S.W.; Kim, T.Y.; Ahn, J.H.; Lee, J.W.; Lee, M.H.; Lee, S.Y. Highly Selective Production of Succinic Acid by Metabolically Engineered Mannheimia Succiniciproducens and Its Efficient Purification. Biotechnol. Bioeng. 2016, 113, 2168–2177. [Google Scholar] [CrossRef]
- Samuelov, N.S.; Datta, R.; Jain, M.K.; Zeikus, J.G. Whey Fermentation by Anaerobiospirillum Succiniciproducens for Production of a Succinate-based Animal Feed Additive. Appl. Environ. Microbiol. 1999, 65, 2260–2263. [Google Scholar] [CrossRef]
- Lopez-Garzon, C.S.; Straathof, A.J.J. Recovery of Carboxylic Acids Produced by Fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef]
- Zhu, L.W.; Wang, C.C.; Liu, R.S.; Li, H.M.; Wan, D.J.; Tang, Y.J. Actinobacillus Succinogenes ATCC 55618 Fermentation Medium Optimization for the Production of Succinic Acid by Response Surface Methodology. J. Biomed. Biotechnol. 2012, 2012, 626137. [Google Scholar] [CrossRef]
- Chan, S.; Kanchanatawee, S.; Jantama, K. Production of Succinic Acid from Sucrose and Sugarcane Molasses by Metabolically Engineered Escherichia Coli. Bioresour. Technol. 2012, 103, 329–336. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Wang, Y.; Gao, B.; Chang, J.; Zhu, D. Two-Stage Crystallization Combining Direct Succinimide Synthesis for the Recovery of Succinic Acid From Fermentation Broth. Front. Bioeng. Biotechnol. 2020, 7, 471. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yan, L.; Fu, H.; Xiu, Z. Salting-out Extraction and Crystallization of Succinic Acid from Fermentation Broths. Process Biochem. 2014, 49, 506–511. [Google Scholar] [CrossRef]
- Pal, P.; Kumar, R.; Banerjee, S. Manufacture of Gluconic Acid: A Review towards Process Intensification for Green Production. Chem. Eng. Process 2016, 104, 160–171. [Google Scholar] [CrossRef]
- Putri, D.N.; Sahlan, M.; Montastruc, L.; Meyer, M.; Negny, S.; Hermansyah, H. Progress of Fermentation Methods for Bio-Succinic Acid Production Using Agro-Industrial Waste by Actinobacillus Succinogenes. In Proceedings of the 6th International Conference on Energy and Environment Research (ICEER 2019), Aveiro, Portugal, 22–25 July 2019; pp. 234–239. [Google Scholar] [CrossRef]
- Shen, N.; Zhang, H.; Qin, Y.; Wang, Q.; Zhu, J.; Li, Y.; Jiang, M.G.; Huang, R. Efficient Production of Succinic Acid from Duckweed (Landoltia Punctata) Hydrolysate by Actinobacillus Succinogenes GXAS137. Bioresour. Technol. 2018, 250, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Wang, Y.; Luo, J.; Yin, J.; Xing, J.; Wan, Y. Succinic Acid Biosynthesis from Cane Molasses under Low PH by Actinobacillus Succinogenes Immobilized in Luffa Sponge Matrices. Bioresour. Technol. 2018, 268, 45–51. [Google Scholar] [CrossRef]
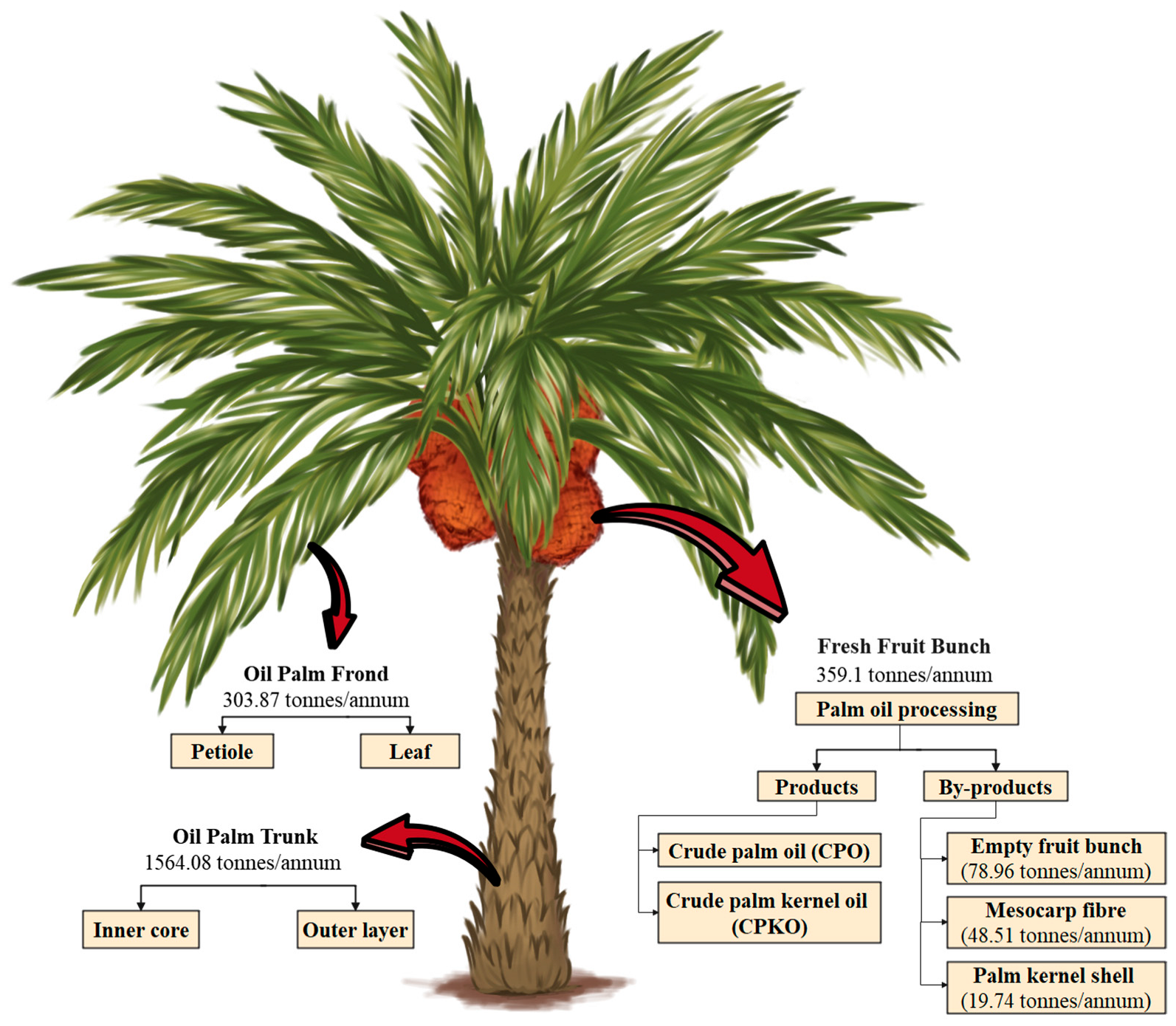
| Type of Biomass | Amount Generated (Tonnes/Hectare) | Amount Generated in Malaysia (Tonnes/Annum) | The Total Amount Generated Globally (Tonnes/Annum) |
|---|---|---|---|
| Oil Palm Frond (OPF) | 14.47 | 86.82 | 303.87 |
| Oil Palm Trunks (OPT) | 74.48 | 446.88 | 1564.08 |
| Empty Fruit Bunches (EFB) | 3.76 | 22.56 | 78.96 |
| Palm Kernel Shells (PKS) | 0.94 | 5.64 | 19.74 |
| Oil Palm Mesocarp Fibre | 2.31 | 13.86 | 48.51 |
| Total | 95.96 | 575.76 | 2015.16 |
| Pretreatment Condition | Hydrolysis Condition | Findings | References | |
|---|---|---|---|---|
| OPF Bagasse | Alkaline Chemical: 3 M KOH Temperature: 40 °C Agitation speed: 400 rpm Time: 4 h Solid to liquid ratio: 1:10 (w/v) | Enzymatic Hydrolysis Enzyme: Xylanase (2 U/mL) Hemicellulase, HTec2 concentration: 2% (w/v) Temperature: 40 °C pH: 4.6, Time: 48 h Buffer: 0.05 M citrate buffer |
| [72] |
| OPF Bagasse | Alkaline Chemical: 4.42% (w/v) NaOH Temperature: 100 °C Pressure: 1 atm Time: 58 min Solid to liquid ratio: 1:10 (w/v) | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (15 FPU/g cellulose) and Hemicellulase, HTec2 (25 U/g) Temperature: 50 °C pH: 4.8, Time: 72 h Agitation speed: 150 rpm |
| [73] |
| OPF Bagasse | Alkaline Chemical: 1.2 M NaOH Temperature: 100 °C Time: 58 min Autohydrolysis: 121 °C for 20 min | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (30 FPU/g cellulose) and Hemicellulase, HTec2 (50 U/g) 6% glucan loading |
| [74] |
| OPF Bagasse | Alkaline Chemical: 4% (w/v) NaOH Temperature: 100 °C Time: 58 min Solid to liquid ratio: 1:10 (w/v) Autohydrolysis: 121 °C for 20 min | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (50 FPU/g cellulose) and Hemicellulase, HTec2 (75 FXU/g) Temperature: 50 °C pH: 4.8, Time: 72 h Buffer: 0.05 M citrate buffer | Total fermentable sugar: 72.7 g/L | [28] |
| OPF Bagasse | Alkaline and water Chemical: 1.2 M NaOH Temperature: 100 °C Time: 58 min Autohydrolysis: 121 °C for 20 min | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (30 FPU/g cellulose) and Hemicellulase, HTec2 (7.5 FXU/g) 6% glucan loading Temperature: 50 °C pH: 4.8, Time: 72 h Agitation speed: 150 rpm |
| [69] |
| OPF Bagasse | N/A | Acid Hydrolysis Chemical: 3 mL of 72% of sulphuric acid H2SO4 into 0.3 g of dried samples Temperature: 30 °C Agitation speed: 150 rpm Time: 1 h Autohydrolysis: 121 °C for 1 h |
| [75] |
| OPF Bagasse | Acid Chemical: 4% (v/v) HNO3 Temperature: 130 °C Time: 20 min Solid to liquid ratio: 1:8 (mL/g) Autohydrolysis: 121 °C for 20 min | N/A |
| [70] |
| OPT Bagasse | Dilute acid Chemical: 5% (w/v) Oxalic acid Temperature: 120 °C Time: 3 h Solid to liquid ratio: 1:10 (w/v) | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (15 FPU/g cellulose) 0.5% (v/v) Triton X-100 Temperature: 50 °C Agitation speed: 150 rpm pH: 5, Time: 72 h |
| [76] |
| OPT Bagasse | Dilute acid Chemical: 5% (w/v) Oxalic acid Temperature: 120 °C Time: 2 h Solid to liquid ratio: 1:10 (w/v) | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (30 FPU/g cellulose) Temperature: 50 °C Agitation speed: 155 rpm pH: 4.8, Time: 48 h | Total xylose: 61.2% (w/w) | [77] |
| OPT Bagasse | N/A | Acid Hydrolysis Chemical: 500 mL of 1% (v/v) of sulphuric acid H2SO4 into 50 g of OPT fiber Agitation speed: 150 rpm Time: 15 min Microwave heating: 700 W for 10 min | Total fermentable sugars of 30 g/L | [78] |
| OPT Bagasse | Acid Chemical: 1.0% (v/v) H2SO4 Temperature: 120 °C Time: 90 min Solid to liquid ratio: 1:10 (w/v) Autohydrolysis: 121 °C for 20 min | Enzymatic Hydrolysis Enzyme: UKM-enzyme Formulation-3 (49.9 U/g) 0.123% (v/v) Triton X-100 Temperature: 50 °C pH: 5, Time: 48 h Buffer: 0.05 M citrate buffer Agitation speed: 155 rpm |
| [79] |
| OPT Bagasse | Dilute acid Chemical: 5% (w/v) Oxalic acid Temperature: 120 °C Time: 2 h Solid to liquid ratio: 1:10 (w/v) Autohydrolysis: 121 °C for 4 h | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (15 FPU/g cellulose) Temperature: 50 °C Agitation speed: 150 rpm Time: 72 h |
For pretreated solids:
For the whole slurry:
| [80] |
| OPT Bagasse | Dilute acid Chemical: 1% (w/v) Oxalic acid Temperature: 120 °C Time: 180 min Solid to liquid ratio: 1:10 (w/v) | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (15 FPU/g cellulose) Triton X-100 at 0.5% (v/v) Temperature: 50 °C Agitation speed: 150 rpm Time: 72 h |
| [81] |
| EFB | Autoclave alkali (AA) Chemical: (20% w/v) 2.5 M NaOH Temperature: 121 °C Time: 2 h Pressure: 0.12 MPa Sequential dilute acid microwave alkali (DA-MWA) Chemical: 8.0% (v/v) H2SO4 Temperature: 121 °C Time: 1 h Pressure: 0.12 MPa Followed by: Chemical: 10.0% (w/v) 2.5 N NaOH Microwave heating: Power of 900 W for 20 min | Enzymatic Hydrolysis Enzyme: Cellulase, CTec2 (25 FPU/g cellulose): Cellobiase (10 CBU/g) with ratio of 7:1 Buffer: 50 mL citrate buffer Temperature: 50 °C Agitation speed: 180 rpm Time: 48 h |
| [82] |
| EFB | Dilute acid microwave Chemical: H2SO4 Temperature: 121 °C Time: 1 h Followed by: Chemical: 10.0% (w/v) 2.5 N NaOH Microwave heating: 900 W for 20 min | Simultaneous saccharification and fermentation Enzyme: Cellulase, CTec2 (40 FPU/g cellulose) Temperature: 36 °C pH: 5, Time: 48 h Agitation speed: 210 rpm |
| [83] |
| Substrate | Bioplastics | Microorganism | Fermentation Conditions | Substrate Concentration (%) | Product Titer (g/L) | Reference |
|---|---|---|---|---|---|---|
| EFB Hydroly-sate | Polyhydroxy-butyrate (PHB) | Bacillus cereus suaeda B-001 | Batch T: 30 °C P: 6.8 Speed: 150 rpm Media:Luria-Bertani (LB) broth | Sugar: 1.44 | 0.99 | [88] |
| OPT sap | Poly-3-hydroxy-butyrate (PHB) | Bacillus megaterium MC1 | Batch T: 30 °C Speed: 200 rpm Media: Mineral Medium (MM) | Sugar: 2.5 | 3.28 | [89] |
| OPT sap | Polyhydroxy-alkanoate (PHA) | Bacillus megaterium MC1 | Batch T: 30 °C Speed: 200 rpm Media: Mineral Medium (MM) | Sugar: 2.5 | 1.91 | [60] |
| POME | Polyhydroxy-alkanoate (PHA) | Comamonas sp. EB 172 | Batch T: 30 °C pH: 7 Speed: 200–800 rpm Time: 70 h DO: 30% of air saturation Aeration rate: 1 vvm | POME: 0.1 | 2.57 | [90] |
| POME | Polyhydroxy-alkanoate (PHA) | Bacillus licheniformis HAs-007mutant M2-12 | Batch T: 37 °C pH: 7 Speed: 150 rpm Time: 48 h Aeration rate: 1 vvm | POME: 3 Sugar: 4 | 7.35 | [91] |
| Oil Palm Biomass | Pretreatment Process | Mode | Microbes | Sugar Consumed (g/L) | SA Titer (g/L) | Yield (g/g) | Productivity (g/L. h) | Reference |
|---|---|---|---|---|---|---|---|---|
| OPF bagasse hydrolysate | Alkaline pretreatment, Enzymatic Hydrolysis | Batch | A. succinogenes 130Z | 51.55 | 36.6 | 0.71 | 0.61 | [73] |
| OPF bagasse Hydrolysate | Alkaline pretreatment, Enzymatic Hydrolysis | Repeated batch pH: 6.5 Temperature: 39 °C Agitation speed: 200 rpm Fermentation period: 32 h (batch), 180 h (repeated batch of 5 runs) | A. succinogenes 130Z | 50.69 | 44.1 | 0.87 | 1.34 | [74] |
| OPF bagasse Hydrolysate | Alkaline pretreatment, Enzymatic Hydrolysis | Batch pH: 6.8 Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 50 h | A. succinogenes 130Z | 61.29 | 38.0 | 0.62 | 1.95 | [28] |
| OPF juice | Alkaline pretreatment, Enzymatic Hydrolysis | Batch pH: 6.8 Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 50 h | A. succinogenes 130Z | 51.36 | 33.9 | 0.66 | 6.58 | [69] |
| OPF juice | - | Batch pH: 6.8 Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 50 h | A. succinogenes DSM 22257 | 43.24 | 30.7 | 0.71 | n/a | [71] |
| OPF juice | Acid Hydrolysis | Batch pH: 6.8 Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 60 h | A. succinogenes 130Z | 28.77 | 21 | 0.73 | n/a | [75] |
| OPT bagasse Hydrolysate | Dilute acid pretreatment, Enzymatic Hydrolysis | Batch Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 72 h | A. succinogenes 130Z | 39.77 | 17.5 | 0.44 | 0.36 | [80] |
| OPT bagasse Hydrolysate | Acid, Enzymatic Hydrolysis | Batch Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 72 h | A. succinogenes 130Z | 28.08 | 7.30 | 0.26 | 0.30 | [79] |
| OPT bagasse hydrolysate | Dilute acid pretreatment, Enzymatic Hydrolysis | Batch pH:6.8 Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 60 h | A. succinogenes 130Z | 33.95 | 13.92 ± 0.85 | 0.41 ± 0.03 | 0.23 ± 0.01 | [77] |
| OPT bagasse hydrolysate | Dilute acid pretreatment, Enzymatic Hydrolysis | Batch Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 72 h | A. succinogenes 130Z | 36.82 | 13.99 ± 0.16 | 0.38 | n/a | [76] |
| OPT bagasse Hydrolysate | Dilute acid pretreatment, Enzymatic Hydrolysis | Batch Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 72 h | A. succinogenes 130Z | 36.38 | 21.1 | 0.58 | 0.37 ± 0.03 | [81] |
| OPT sap | - | Batch Temperature: 37 °C Agitation speed: 200 rpm Fermentation period: 60 h | A. succinogenes 130Z | 14.48 | 7.82 | 0.54 | 0.36 | [63] |
| EFB | Dilute acid-microwave-alkaline pretreatment, Enzymatic Hydrolysis | Batch Temperature: 38 °C Agitation speed: 210 rpm Fermentation period: 48 h | A. succinogenes 130Z | 71.06 | 33.4 | 0.47 | 1.69 | [82] |
| EFB | Dilute acid-microwave-alkaline pretreatment, simultaneous saccharification and fermentation (SSF) | Batch Temperature: 38–40 °C Agitation speed: 210 rpm Fermentation period: 48 h | A. succinogenes ATCC 55618 | 70.33 | 42.9 | 0.61 | n/a | [83] |
| EFB | Autohydrolysis and cellulase | Batch Temperature: 37 °C Agitation speed: 120 rpm Fermentation period: 38 h | A. succinogenes 130Z ATCC 5618 | 71.21 | 23.5 | 0.33 | 0.62 | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hariz, H.B.; Zaidi, S.A.S.; Luthfi, A.A.I.; Bukhari, N.A.; Sajab, M.S.; Markom, M.; Harun, S.; Tan, J.-P.; Ding, G.-T.; Abdul, P.M. Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution. Fermentation 2023, 9, 46. https://doi.org/10.3390/fermentation9010046
Hariz HB, Zaidi SAS, Luthfi AAI, Bukhari NA, Sajab MS, Markom M, Harun S, Tan J-P, Ding G-T, Abdul PM. Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution. Fermentation. 2023; 9(1):46. https://doi.org/10.3390/fermentation9010046
Chicago/Turabian StyleHariz, Hikmah Bajunaid, Siti Aisyah Syazwani Zaidi, Abdullah Amru Indera Luthfi, Nurul Adela Bukhari, Mohd Shaiful Sajab, Masturah Markom, Shuhaida Harun, Jian-Ping Tan, Gong-Tao Ding, and Peer Mohamed Abdul. 2023. "Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution" Fermentation 9, no. 1: 46. https://doi.org/10.3390/fermentation9010046
APA StyleHariz, H. B., Zaidi, S. A. S., Luthfi, A. A. I., Bukhari, N. A., Sajab, M. S., Markom, M., Harun, S., Tan, J.-P., Ding, G.-T., & Abdul, P. M. (2023). Succinic Acid Production from Oil Palm Biomass: A Prospective Plastic Pollution Solution. Fermentation, 9(1), 46. https://doi.org/10.3390/fermentation9010046








