Abstract
Pretreatment with the addition of metals to anaerobic digestion in biogas production is crucial to address improper degradation of organic compounds with low methane production. Biogas production from a combination of cassava pulp and cassava wastewater in the batch system under the variation of alkaline and heat conditions as a pretreatment was investigated with the zero-valent iron (ZVI) addition after the pretreatment. It was found that alkaline pretreatment at pH 10 with the heat at 100 °C for 30 min combined with 50 g of ZVI kg of TVS−1 showed the highest methane production up to 4.18 m3 CH4 kg TVS−1. Nevertheless, chemical oxygen demand (COD) and volatile fatty acid (VFA) removals were slightly reduced when ZVI was added to the system. Furthermore, application in the continuous system showed increased COD and VFA removals after applying alkaline and heat pretreatments. On the other hand, additional ZVI in the substrate after the pretreatments in the continuous system increased the methane production from 0.58 to 0.90 and 0.19 to 0.24 of CH4 m3 kg TVS−1 in 20 and 60 days of hydraulic retention times (HRTs), respectively. Thus, a suitable combination of alkaline and heat pretreatments with ZVI is essential for increasing methane production in batch and continuous systems.
1. Introduction
Global energy demand has increased dramatically in recent decades [1]. Renewable and green energy resources play important roles nowadays in supplying global energy demand [2]. Biogas is one of the most developed green energy alternatives since it can be utilized in numerous ways, such as internal combustion for transportation, fuel cell, heat, and power generation [3,4]. It can be generated from numerous substrates to create valuable products and treat waste/wastewater simultaneously. It also provides a sufficient renewable energy supply and removes excess nutrients in the waste/wastewater loads [5,6]. Agricultural wastes such as animal manure, municipal waste such as solid waste leachate, and other organic debris from industrial factories are among the most used substrates for biogas generation [7].
Among the sources of biogas substrates from agricultural products, cassava pulp is one of the most abundant commodities available throughout Asia and Africa [5]. Production of cassava starch also produces pulp and wastewater with high-fiber content, consisting of cellulose, hemicellulose, and lignin [8]. The former two are capable of degradation in the early stages of biogas production (aerobic conditions). On the other hand, lignin cannot easily remove or degrade to release the simple saccharides [9]. The failure of complex saccharide degradation can result in a low amount of available cellulose and hemicellulose for further biogas generation because lignin acts as a protective coating for cellulose and hemicellulose, decreasing the methanogenesis process [5,10,11].
Several studies have been conducted to remove lignin from biogas substrates for higher biogas production. These attempts were included in the pretreatment process before the substrates passed to the anaerobic reactor for methanogenesis. Cheng et al. [12] demonstrated the optimization of lignin elimination at the very first stage by hydrolysis using acid and alkaline conditions. However, Binder and Raines [13] found that acid hydrolysis by adding sulfuric acid and nitric acid negatively affected the environment and required costly acid recovery. In the same study, acid pretreatment was also found to decrease CH4 production since they produce hydrogen sulfide (H2S) and nitrogen (N2) as majority fractions of the gasses. On the other hand, Gaewchingduang and Pengthemkeerati [14] reported that simple saccharides from cellulose or hemicellulose were generated in an optimum amount from 2% of total solid (TS) cassava pulp after sulfuric acid and heat pretreatment. These reducing sugars are essential in anaerobic digestion using cassava waste as a substrate.
Alkaline pretreatment was also commonly employed to address the lignin occurrence in the biogas substrates. The alkaline substance interferes with the cell walls and cellulose membrane in the substrates [15]. A large amount of free hydroxyl groups in the alkaline condition eases the digestion of proteins and fats by incorporating a carbonyl carbon [16]. This pretreatment is usually combined with heat treatment to increase efficiency [7,17,18]. Apart from breaking the lignin structure, hydroxyl groups in the substrate can also reduce the crystallization of cellulose, a process that can limit available cellulose for methanogens [16]. It is also noteworthy that alkaline hydrolysis pretreatment requires less total energy than other pretreatments [15]. A comparison by Sukwanitch [19] also revealed that biogas production in alkaline conditions (pH 13) produced methane three times higher than in non-alkali states.
Several pretreatments have been applied before the anaerobic digestion in cassava pulp utilization to increase methane yield and removal efficiency. Chemical pretreatments were reported to improve the wastewater and pulp digestibility and removal efficiency [5]. Physical treatment, such as heat pretreatment, was also reported to help disrupt the cell wall structure of cassava pulp and degrade hemicellulose and starch to the smaller form of saccharides [20]. Other studies also suggested the application of biological pretreatment to digest the substrate before anaerobic digestion [21]. These pretreatments have increased the removal efficiency in cassava pulp anaerobic digestion. However, the increase in methane production often faces the cost barrier, feasibility of the advanced technologies, and upscaling process [22].
Similarly, additional substances are mixed in the substrates to optimize the utilization of potential components in biogas production. Enhancement of cellulose and protein breakdown is also often afforded by the addition of zero-valent iron (ZVI). In the ZVI-catalyzed reaction, available electrons (Fe0) are abundant as reducing agents to enhance hydrolysis–acidogenesis processes [23,24]. It is also reported that the addition of ZVI to the wastewater from the secondary sedimentation tanks in the up-flow anaerobic sludge blanket (UASB) tank system showed improvement in protein and cellulose (main constituents of the wastewater) breakdown efficiency [23]. Furthermore, Zhang et al. [25] have also proven that the productivity of CH4 from wastewater from the second stage of the sedimentation tank in the wastewater treatment plan rose up to 91.5% after an additional ZVI 10 mg L−1 together with the alkaline condition (pH 10).
Other advantages of adding ZVI to the biogas substrates have also been reported in the following stage of biogas production. ZVI is found to release the electron that the microorganisms can later carry for CH4 production. The carried electron from the ZVI is proven to catalyze the conversion of the intermediate products after sugar degradation, and volatile fatty acids (VFAs) to acetate by the microorganisms, and eventually increase the CH4 yield [25]. In this reaction, ZVI acts as an electron donor, reacting with single hydrogen ions to become H2, and further, the H2 is utilized by methanogen archaea to produce CH4 [26,27].
Another obstacle in the production of biogas is the low percentage of CH4 in biogas. Some other gases often found in higher amounts than CH4 are hydrogen sulfate (H2S) and carbon dioxide (CO2). The sulfate-reducing reaction to H2S (ΔG° = −47.6 kJ) is more reactive than the CH4 reaction (ΔG° = −31.0 kJ); therefore, H2S is often found in a higher percentage than CH4 in total biogas accumulation [28,29]. H2S is also toxic to the archaea methanogens that produced CH4 [28]. Interestingly, ZVI was proven to reduce H2S, increasing CH4 percentage by removing the inhibition factor [30]. In consequence, Zou et al. [31] also reported that ZVI has successfully captured the majority of the CO2 fraction in biogas by forming Fe3C. The captured CO2 can be utilized optimally to generate CH4 as it is not directly released into the gas phase of the reactor. Thus, adding ZVI to the AD enhances methane production.
Numerous studies have demonstrated the advantages of alkaline pretreatment over acid pretreatment. It was reported that both attempts could reduce total costs and increase productivity. Similarly, ZVI has been added to various substrates to enhance methane production. Nevertheless, a combination of both factors has never been completely explored to increase methane quantity and quality significantly. Thus, this study aimed to investigate the combination of alkaline and heat pretreatments with the addition of ZVI for wastewater treatment and biogas production using cassava pulp as a substrate.
2. Materials and Methods
2.1. Chemical Analysis
Cassava pulp and cassava wastewater used throughout this study were obtained from Korat Flour Industry Co., Ltd. Nakhon Ratchasima, Thailand (N 14°53053″ E 102°04000″). Cassava pulp was the semi-solid waste from the cassava starch extraction in the cassava tuber. Meanwhile, cassava wastewater was the wastewater generated from the wastewater combined from several activities in the flour industry, including cooling machines, washing of tuber, etc. The wastewater and pulp were mixed to obtain the 3% total solid (TS) value. The 3% TS was chosen based on the previously conducted optimization using the same substrate for biogas production [32]. The mixture is then called pulp and wastewater for the following sections.
Several critical parameters were measured based on APHA methods [33]. Total solids (TS) and total dissolved solids (TDS) were measured by drying the samples at 103–105 °C. Total volatile solids (TVS) were measured by combusting the sample into the nickel crucible at 550 °C in a furnace (Muffle, Nabertherm, Germany). Volatile fatty acids (VFAs) were measured using the direct titration method. Total Kjeldahl nitrogen (TKN) was measured by the digestion method using a block digestor (BD50, SEAL Analytical GmbH, Germany). Total phosphorous (TP) was measured by the wet digestion method. Chemical oxygen demand (COD) was measured by closed reflux titrimetric method in the COD reactor (DB-1602, MLAB, Thailand). Alkalinity (Alk) was measured using the titration method, and mixed liquor volatile suspended solids (MLVSS) were measured by incineration at 550 °C to measure the concentration of microbial biomass in the sample. pH was measured using a YSI 556 MPS Multiprobe System (Xylem, OH, USA).
2.2. Substrate Preparation and Reactor Design
The experiment to obtain the most suitable condition was conducted in the 6-L batch reactors with 5-L working volume consisting of three tanks: mixing, storage, and water tanks (Figure 1a). In the first tank, the anaerobic digestion took place, the second tank contained the water and connected hose from the upper (air) phase of the first tank, and the third tank acted as the holder of the second tank water that flowed from the second tank due to the air pressure that came from the first tank indicating the biogas generation. This reactor was used to obtain the optimum conditions to later be applied in the continuous system. The batch experiments were conducted at room temperature (27–35 °C).
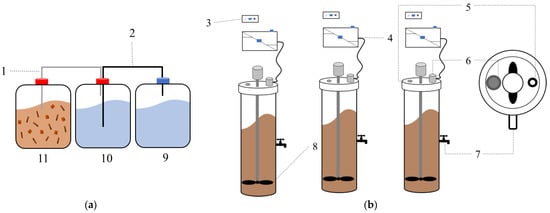
Figure 1.
The reactor used in this study. (a) Batch reactor; (b) continuous stirred tank reactor (CSTR); (1) biogas hose; (2) water hose; (3) power supply; (4) gas measuring device; (5) motor for the mixing pad; (6) inlet; (7) outlet; (8) mixing tank; (9) water tank; (10) air tank; and (11) mixed tank.
Meanwhile, after obtaining the most suitable conditions, the comparison between the control and formulated pretreatment was conducted in the continuous stirred tank reactor (CSTR), guaranteeing the complete mixing with no sediment with a total volume of 12 L and a working volume of 10 L. The food/microbe (F/M) ratio, which implies the amount of incoming food per amount of microorganism in the system, was adjusted to be 0.5 at the beginning of the experiments. Food value was based on the organic matter load from the pulp and wastewater (COD), while the microbe ratio was based on the MLVSS concentration in the pulp and cassava [34]. The MLVSS was measured from the seed sampled from anaerobic digestion sludge in the industry where the pulp and wastewater were collected, as previously demonstrated in the literature [35]. The concentration of F/M was used based on the previous optimization experiment by Yingchon [32].
2.3. Zero-Valent Iron Preparation
The process of zero-valent iron (ZVI) synthesis was conducted based on Lavine et al. [36] and Bang et al. [37]. Briefly, steel scraps from the industrial plant Siam Fastener Co., Ltd. (Nakhon Ratchasima, Thailand) were collected and sieved to the size 0.15–0.25 mm. Furthermore, 100 g of iron powder was collected into a 250 mL flask and washed with 200 mL of 3% hydrochloric acid (HCl). The washing process was further processed by shaker for 30 min at 150 rpm. After the washing process, the scrap was then rinsed with deionized water to remove dirt. Acetone was applied to the iron to ensure the removal of residual acid and water. The iron powder was then dried using an oven at 65 °C and stored in a zip-lock bag for further experiments. The formation of ZVI was confirmed observation under XRD machine (XRD-6100, Shimadzu, Japan) by comparing the XRD pattern of synthesized ZVI with the previous studies.
2.4. Pretreatment and ZVI Level Formulation in the Batch System
A combination of alkaline and heat pretreatments with the addition of ZVI was conducted to obtain the optimum condition in each pretreatment (Figure 2). The alkaline pretreatment was performed by adjusting the pH of the wastewater to be 8, 9, 10, and 11 with a non-adjusted pH of 5.45 as the control. The pH of the wastewater was adjusted by adding NaOH 5 N [38]. After changing the pH, the wastewater was incubated in the jar for 100 min before it was neutralized to pH 7. The wastewater was then incubated in the 5-L reactor to produce biogas (Figure 1a). All the biogas produced was measured daily.
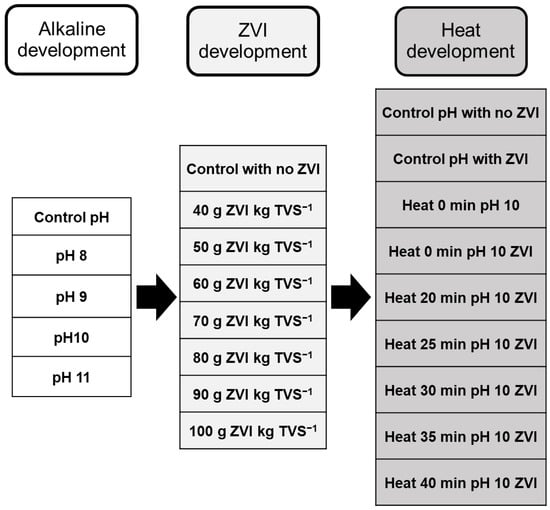
Figure 2.
Experimental design of alkaline and heat pretreatment with zero valent iron (ZVI) in the batch system.
Optimum pH was then applied in the same system to optimize the ZVI level. After adjusting the pH and treating the wastewater with heat for 30 min, the ZVI level was varied. The concentration ranged from 0 g (without ZVI), 40 g, 50 g, 60 g, 70 g, 80 g, 90 g, and 100 g of ZVI gr TVS −1. The biogas was produced after alkaline pretreatment and the addition of ZVI. The most optimum condition of ZVI was then noted and then we proceeded to the heat pretreatment. Heat pretreatment was conducted by boiling the wastewater to 100 °C with different durations (0 min, 10 min, 20 min, 25 min, 30 min, 35 min, and 40 min). The heat pretreatment was optimized after the optimum conditions of pH and ZVI concentration were obtained from the experiment. The heat pretreatment was combined before the anaerobic digestion started by adjusting the pH and heating the substrate in different durations.
2.5. Continuous System
After obtaining the most suitable conditions for the alkaline, heat, and ZVI levels, a continuous system was employed to produce methane on the larger reactor scale. Two hydraulic retention times (HRTs), 20 and 60 days, were used to determine the difference between the pretreated and non-pretreated substrates in the actual condition. These HRTs were chosen based on the operational parameters in the Korat Flour Industry Co., Ltd., biogas reactor. The 20-day HRT was the maximum HRT the reactor could perform. The 60-day HRT was based on the site’s daily operation. The HRTs were used to calculate the flowrate of daily substrate volume that was pumped into the reactor, which also reflected the volume of daily effluent. Furthermore, based on the flowrate, the organic loading rates (OLRs) were calculated based on the value of COD that was analyzed from the substrate.
Briefly, similar wastewater was treated using a formulated pretreatment and combined with ZVI at the beginning of the stage. The reactor was then operated using two HRTs in parallel by pumping the wastewater continuously into the CSTR using metallic pumps according to the manufacturer’s instructions (Figure 1b). Daily and cumulative biogas composition were documented each day. The continuous experiment was carried out for 60 days at room temperature (27–35 °C) to ensure the sustainability of methane production, and the experiment ended when there was no significant methane produced from the system (<1% of generated biogas).
2.6. Effluent and Biogas Analyses
The composition of the emission gas was measured using a gas analyzer (Geotech GA 5000, QED Environmental Systems, Ltd., Coventry, UK). The effluent from the reactor was analyzed to obtain the characteristics of COD, TKN, TP, VFAs, TS, and TVS. Biogas amount and composition were also analyzed daily. The effluent analysis was conducted by the same method used in the substrate analysis (Section 2.1). Furthermore, X-ray diffraction (XRD) patterns were recorded using the Bruker D8 Advanced Diffracto Meter (Bruker, Germany). Similarly, morphological characteristics were also observed using scanning electron microscope (SEM) imagery (Auriga, Zeiss, Germany). The analysis step was based on Xi et al. [39].
2.7. Biogas Calculation
The volume of biogas was calculated using the equation based on Lomwongsopon and Aramrueang (2022) (Equation (1)). It was based on the ideal gas law with an adjustment to the volume at standard temperature (273 K) and pressure (101.3 kPa):
where Vbiogas means the biogas volume in the given time (L), P means the absolute pressure difference (kPa), Vhead implies the volume of the water that is moved by the gas produced (L), C means the molar volume (22.41 L mol−1), R was the universal gas constant (8.314 L kPa K−1 mol−1), and T was the absolute temperature (K).
2.8. Data Analysis
All the experiments were conducted in triplicate. For the physicochemical characteristics, the replication of analysis was five. The data are shown in mean values and analyzed using t-test and ANOVA analyses using SPSS Statistical Software version 26 (IBM, Armonk, NY, USA). Duncan’s multiple range test was also conducted to determine the difference among the groups based on the results of the ANOVA tests.
3. Results and Discussion
3.1. Characterization of Biogas Substrate, Inoculum, and Synthesized ZVI
Characteristics of the substrate prepared for biogas were then analyzed. The substrate of wastewater and pulp showed relatively high COD nitrogen (TKN) and phosphorous (TP) concentrations (Table 1). Commonly, the raw pulp contains high COD from the debris of cassava tuber.

Table 1.
Composition of substrate containing cassava pulp and cassava wastewater with TS 3%.
The literature reported a high percentage of cellulose and hemicellulose as the main constituents with considerable amounts of lignin in cassava waste [5,20,40]. The high COD indicates a high possibility of methane production only if hydrolysis can change these structural saccharides [40]. Thus, pretreatment was conducted to provide efficient conversion from the possible substrate concentration into the biogas [41].
Another critical parameter to consider was the COD:N:P ratio. As it is known, C will be the source of energy and is converted to biogas (including CH4), while N is used for cell proliferation. High C content can limit the proper function of the cells, while high N can potentially reduce VFAs and increase the ammonia generation [42]. As is widely known, C in the wastewater was often expressed in COD since this is the only form of C that the methanogens can utilize (biodegradable carbon) [43,44]. Optimum COD:N:P ratios were varied under different types of wastewater. In cassava wastewater, the COD:N:P ratios ranged between 250:8:0.55 and 250:0.007:0.12 [45]. Here, the total COD:N:P ratio was relatively lower than the usual concentration of cassava wastewater
Interestingly, the COD:N ratio was higher than the typical cassava wastewater values reported previously. The optimum range of the COD:N ratio was reported to be 86 [46]. Differently, using modified kinetics models, it has been found that the suitable production of biogas can be obtained using a COD:N ratio of 393. However, in other literature, the optimal ratio for wastewater biogas production was found in 100 [47]. Here, both COD:N:P and COD:N ratios of the cassava wastewater exceeded the optimum condition for biogas production. The values were lower than the optimum condition, so incomplete carbon degradation with high COD emission was expected [48].
The results of cassava wastewater combined with the cassava pulp have similar characteristics to the previous study. Cassava wastewater has been known for its high content of nitrogen and phosphorus. It has been reported that similar wastewater contained 156 mg L−1 of TKN and 61.8 mg L−1 of TP [49]. Similarly, cassava pulp, the colloidal substance from tapioca starch production, usually contains a high amount of COD and VFAs. Literature has shown cassava pulp contains 2.1 g kg−1 VS of COD and 1.73 g kg−1 TS of VFA with 305 ± 2 g kg−1 fresh pulp of TS [50]. Similarly, TS of the cassava wastewater that was combined with other waste in the tapioca industry showed a high amount of TVS (17.3 g kg−1 of TVS) with 33.7 ± 0.8 g L−1 of COD in the relatively acidic condition (pH of 5.53) [51]. Moreover, MLVSS, which indicates the inoculum for the anaerobic digestion, was found to have a concentration of 22,644 ± 0.13 mg L−1.
ZVI was provided by synthesizing it from scrap iron. Iron particles were analyzed using an XRD machine after synthesizing to ensure the occurrence of ZVI in the treated scrap iron. The XDR diffractogram was obtained at 2θ (Coupled TwoTheta/Theta) at the wavelength of 1.54060 in the range of 10–90°. The clear peaks from the XRD diffractogram were observed at 45 and 65° (Figure S1). These peaks were also detected in other attempts to synthesize ZVI from iron scrap [52,53,54,55]. The patterns are mostly stated as the iron is found mainly in the Fe0 state. Among the indicator peaks under the XRD diffractogram, peaks of a 2θ value at 44.9° and additional peaks at 65.22° indicated the basic reflection of Fe0 [56]. Thus, the synthesis of ZVI successfully produced iron ions that mainly occurred in the state of Fe0.
3.2. Nutrient Removals
Nutrient removal is essential to determine the successful process of biogas generation. It shows the flow of the substrate conversion and wastewater treatment efficiency simultaneously. Different pretreatments showed different results in the nutrient removal efficiency. Removal of COD was noticeably high (>90%) in all treatments of alkaline pretreatments, including the control pretreatment (Figure 3a). It showed that microorganisms consumed the COD an adequate amount. Nevertheless, additional ZVI in some concentrations reduced the removal of COD (Figure 3b). Moreover, the removal of COD in the various heat pretreatments showed a relatively low reduction in COD (Figure 3c).
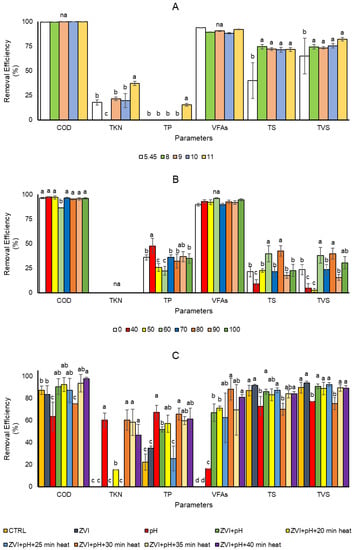
Figure 3.
The removal efficiency of the wastewater content after a 60-day period; (A) different alkaline pretreatments; (B) different zero-valent iron (ZVI) concentrations after pretreatment (g ZVI kg TVS−1); and (C) different pretreatment with different boiling times. Different letters indicate significant differences among treatments using Duncan’s multiple range test (DMRT) with α = 0.05 and n = 3.
Alkaline pretreatment destroys substrate complex bonds with hydroxyl radicals. It increases simple molecules’ availability through several degradation reactions from complex molecules such as lignin and lignocellulose [7,57,58]. It is important to note that higher SCOD was available in the wastewater after the pretreatment than in the non-alkaline condition. The higher COD content demands high removal efficiency and uptake for high removal.
Additionally, trace metal occurrence in the proper amount tends to impact biogas production positively. Among the trace metals, iron (Fe) was used in this study in the form of ZVI. Fe was involved in anaerobic metabolisms as a critical factor for metabolic intermediates and enzyme activity in hydrolysis, acidification, and methanogenesis [59]. Meanwhile, nanoparticle sizes ease the particles to penetrate through living membranes, and later it involves the uptake, absorption, distribution, and metabolism processes in living organisms [60].
COD was removed efficiently (>90%) in almost all treatments involving ZVI except the 60 g ZVI kg TVS−1 (Figure 3b). It might be due to increased hydrolysis activity being enhanced in all concentrations where available SCOD became higher than only alkaline pretreatment [61]. However, the high removal efficiency of COD indicated that hydrolysis rates were higher than the conversion to VFAs by ZVI [62]. It was in line with the VFA and TP removal efficiencies that increased with the addition of ZVI, while the removal of TKN, TS, and TVS were reduced (Figure 3b). It has previously been reported that additional iron in the system could cause the enhancement of phosphorous removal through precipitation processes [63]. The increase in VFA removal indicates the balance of the prior process of hydrolysis with the subsequent fermentation of sugars, amino acids, and fatty acids that can cause the accumulation of VFA and other intermediate metabolites [62].
On the other hand, heat pretreatment was also used for wastewater pretreatment to hydrolyze starch and disrupt the cell wall to provide possible sugar recovery and low inhibitor production to enhance biogas generation [20]. It is widely used to treat high-lignin substrates for higher bioproducts [64]. Variation of heat pretreatments showed increases in TS, TVS, and TP removal efficiencies while reducing the removal of COD and VFAs (Figure 3c). TS and TVS were reported to decrease significantly as the COD was removed from the wastewater [50]. Barua et al. [65] reported significant increases in COD and VFAs after heat pretreatment in anaerobic digestion. This result may explain the decrease in COD and VFA removal efficiencies in the heat pretreatments. As mentioned earlier, the balance of hydrolysis and acidogenesis with the following processes, such as methanogenesis, may be imbalanced. Rates of complex and macromolecule hydrolysis and their degradation into acids are slower than the acetogenesis and methanogenesis processes [62]. The rate of hydrolytic and acid bacteria activities may be faster than in acetogenesis and methanogenesis [66]. The microorganisms involved in acetogenesis and methanogenesis may also be interrupted by the imbalance in the concentration of substrates, such as N [67] or pH [68].
The combination of alkaline, ZVI addition, and heat treatments showed the fluctuation of the wastewater removals in all parameters. The efficiency seems to be lower after combining pretreatments and additional ZVI. It may be due to the increase in available nutrients in the wastewater after the pretreatments and the addition of ZVI. However, it is still rare to find that the high loading rate of organic and inorganic substances in the wastewater can be completely removed in the anaerobic digestion to the level that fulfills the discharging standard of the effluent to the environment [69]. Thus, enhancement of the efficiencies may still need to improve, and secondary or tertiary treatments may be required before the effluent can be discharged.
Interestingly, the removal of TKN and TP was found to be higher at pH 11 than at the other pH conditions in the alkaline pretreatment (Figure 3a). The high pH condition may cause the N and P to precipitate in the pretreatment process [70,71]. Nevertheless, N and P removals were also considered low in the pretreatment of alkaline and additional ZVI (Figure 3a,b). The result was improved in the combination of alkaline and heat pretreatment with additional ZVI (Figure 3c). However, the removal was still considerably low in all combinations of pretreatment and ZVI. This low removal of N and P may have resulted from the low COD:N:P values. Low COD content may cause nutrient removal failure as the removal of N and P requires a high amount of COD to incorporate them in the metabolisms [50]. The improvement that occurred in the presence of ZVI can cause the optimization of the removal by the presence of the ZVI-P binding process [72] and upregulation of the related genes in the related microbial in the system that enhanced the nutrient removal and metabolism activity for stabilization of the removal of nutrients [73,74]. Apart from that, the TS and TVS removals that increased in the combination of ZVI with alkaline and heat pretreatment were in line with the N and P removals (Figure 3c). The TS and TVS removals may indicate that the precipitation did not cause the nutrient removals in ZVI addition with alkaline and heat pretreatments. Still, the nutrient may be removed by the conversion to organic matter [73]. The reduction in COD removal in the combination of pretreatments and ZVI supported this assumption.
3.3. Methane Production
Methane production was observed as the effect of different pretreatments. Total biogas was measured, and methane fraction in the biogas was calculated based on methane production. The methane fraction was found to range from 68 to 71%, with additional gasses such as CO2 and H2S for about 29% and <1%, respectively. The results showed that the pretreatment significantly affected methane production in all treatments. Alkaline pretreatment showed significant increases in all treatments (Figure 4a). Alkaline pretreatment in pH 9 and 10 showed the highest methane production, followed by pH 11 and 8, respectively. This result was consistent with the previous study of Pedersen, Johansen, and Meyer [9], who reported that the optimum alkaline pretreatment for methane production was between pH 10 and 13.
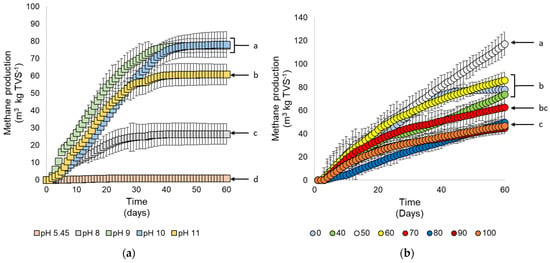
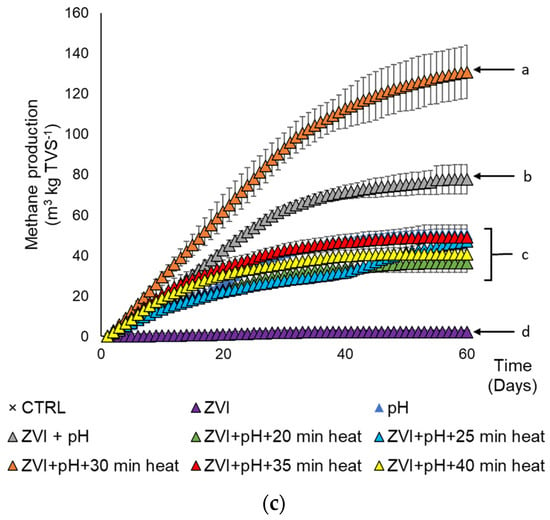
Figure 4.
Cumulative methane yield from several pretreatment and treatment conditions on methane production of cassava wastewater. (a) Effect of pH, (b) effect of ZVI concentrations (g ZVI kgTVS−1), and (c) effect of the duration of heat pretreatment at 100 °C (min). Different letters indicate significant differences among treatments using Duncan’s multiple range test (DMRT) with α = 0.05 and n = 3.
Interestingly, methane was not detected using the control condition, pH 5.45. It might be due to the strong bonds of entrapped starch in the secondary cell wall consisting of hemicellulose, lignin, and cellulose, which are extremely hard to release without any external treatment [75]. The purpose of alkaline with abundant [OH-] was to break the alkyl aryl linkages that can be disrupted under pH 10 or above [18]. Without breaking bonds, only a small amount of starch would be available. The low concentration of available starch and other saccharides could create insufficient concentration for methanogens. It has been reported that commonly pH pretreatment was used to release the hydrogen bonds in the polysaccharides to the monomers to ease the microorganisms and proceeded to generate methane [75].
The addition of ZVI was found to increase the cumulative methane production in the optimized value of alkaline pretreatment. After using pH 10 for alkaline pretreatment, adding 50 g ZVI kg TVS−1 of ZVI in the anaerobic digestion showed consistent methane production throughout the experiment (Figure 4b). The effect of ZVI was shown to elongate the biogas production process instead of bursting the methane generation at the same production time. This result was similar to Abdelsalam et al.’s [76] result, which emphasized the difference in the cumulative biogas production with the slight increase in daily biogas production. The performance of ZVI was related to the enhancement of biological processes in the substrate and the cells of microorganisms [62]. Thus, the main effect detected would be the continuous conversion of substrate to methane.
On the other hand, other concentrations of ZVI did not positively affect the methane production compared with the control (non-ZVI substrate with optimum alkaline pretreatment). Cumulative biogas production in the control condition was only lower than 50–60 g ZVI kg TVS−1, while the higher concentrations tended to have detrimental effects on methane production. High concentrations of ZVI were reported to have an inhibitory activity on the methanogens in the system. In contrast, the lower concentration possessed subtle effects on the cell lysis and the activity of methanogens [6].
Heat pretreatment is often used to enhance the effectiveness of alkaline pretreatment. It is common to have these two pretreatments combined (Table S1). Increasing the pH pretreatment was reported to positively correlate with the heat to produce higher methane production with the heat as the complementation in this concept [9]. The result revealed that 30 min of heat at 100 °C treatment in the cassava wastewater with the condition of pH 10 showed significantly higher biogas production (significance using DMRT with α = 0.05) up to more than 3-fold higher than the substrate with no heat pretreatment (Figure 4c), followed by 20 min of heat pretreatment and the rest of treatment. It was higher than most of the alkaline–heat combination pretreatments (22–97% increase) [17]. The duration of the heat application becomes crucial to the suitability of the substrate to the methanogenesis process. Conversely, a highly long period of heat exposure, longer than 30 min heat pretreatment, can initiate the formation of inhibitors due to continuous harsh conditions [77].
It is also important to note that the wastewater was not suitable for any methane production at the rate only with ZVI addition with no alkaline and heat pretreatment. It is similar to the control condition in the alkaline pretreatment, proving that the unavailable substrate for digestion can cause failure in methane production even though the microbial factors were already enhanced by adding ZVI [77,78].
3.4. ZVI Characterization
The XRD pattern of the irons was analyzed after the production. Most ZVI ions were oxidized to iron oxides in the form of Fe3+ and Fe2+ which consisted of siderite (FeCO3) and FeO, which are essential factors in rusting iron; therefore, it can react with carbonate as in Equations (2)–(4).
3Fe2O3 + CO → 2Fe3O4 + CO2
Fe3O4 + CO → 3FeO + CO2
The formation of carbon dioxide from the process can cause the abundance of the increased level of CO2 from which the significant factor of CH4 precursor can be obtained. In the optimum condition, where most of the ZVI ions were employed in the production of CH4, iron oxides would be found mainly in the form of Fe3+ as an indication that the oxidation process took place to enhance the biogas production [79]. Moreover, excess available Fe2+ could act as the SCODs removal agent and thus limit biogas production [80]. It has also been reported that even in the natural environment, excess Fe3+ can activate iron-reduction microorganisms, which further inhibit methanogens [81].
It is found that ZVI with the concentration of 50 g ZVI kg TVS−1 had an Fe2+ percentage of 5.67% (Figure 5) as the highest percentage of oxidized Fe+3, indicating the successful process of enhancing methane production. In the usual production of methane from VFA, bicarbonate was often formed as the typical intermediate product before producing methane from hydrogen and carbon dioxide (Equations (5)–(9)):
CH3CH2CH2COO− + 2H2O → 2CH3COO− + H+ + 2H2
4H2 + CO2 → CH4 + 2H2O
4H2 + 2CO2 → CH3COO− + H+ + 2H2O
CH3COO− + H+ → CH4 + CO2
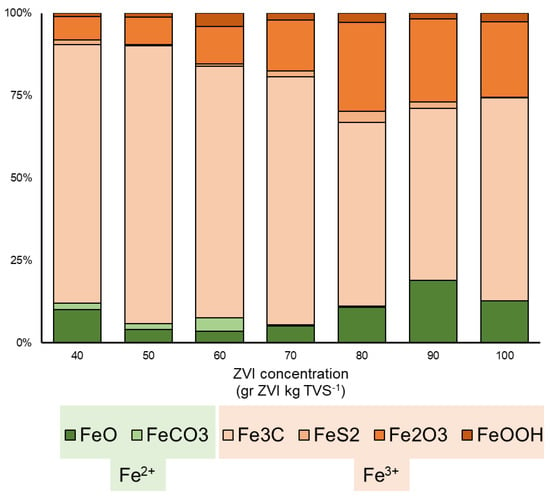
Figure 5.
Forms of iron in the different initial concentrations of ZVI.
Apart from the usual reactions of methane production, ZVI also acted in several intermediate products such as hydrogen ions, carbon dioxide, and fatty acids (10–12).
8H+ + 4Fe0 + CO2 → CH4 + 4Fe2+ + 2H2O
Fe0 + VFA → CH4 + 4Fe2+ + CO2
Different from the direct interaction of ZVI with the substrate to enhance the formation of CH4 [63], several indirect enhancements through the biological process were also involved. Methanogens often depend on metal ions such as Fe for their enzymatic system [78]. Theoretically, methanogens are also under the advantageous condition when ZVI is available and abundant in the system, more than the acetogen group [82,83]. On the other hand, the excess accumulation of Fe in the form of ZVI, Fe2+, or Fe3+ potentially reached the toxic level for anaerobic activities in the system [84]. The excess amount of these ions can also cause the switch from methanogenesis activity to iron reduction agent in some microorganisms [85]. This iron reduction process could also explain the low concentration of Fe3+ in 100 g ZVI kg TVS−1 than in the 90 g ZVI kg TVS−1 (Figure 5).
Observation of the microstructure in the different concentrations of ZVI added to the substrate was conducted to clarify the results in biogas and ionic compounds of oxidized ZVI. It was found that the structure of ZVI was different under different initial concentrations, as is shown in Figure 6. The microstructure of ZVI was found to be smooth in 40 g, 70 g, 80 g, 90 g, and 100 g ZVI in g of ZVI kg of TVS−1. Pore-like structures were only evident in 50 g and 60 g ZVI in g of ZVI kg of TVS−1. Xi et al. [86] explained that the pore-like form indicated the reaction product of oxidized ZVI. In line with that, Wang et al. [87] also reported that the assimilated and oxidized ZVI is often characterized by the change from a smooth structure to a smaller granule-like structure after successful digestion in the AD system. Liu, Tong, Li, Wang, Liu, and Zhang [79] also explained the transition from the ball-like forms, which consists of transitioning from a smooth surface structure to a coarse-like structure after precipitation, which can indicate the unsuccessful change of ZVI through oxidation and other reaction processes [88].
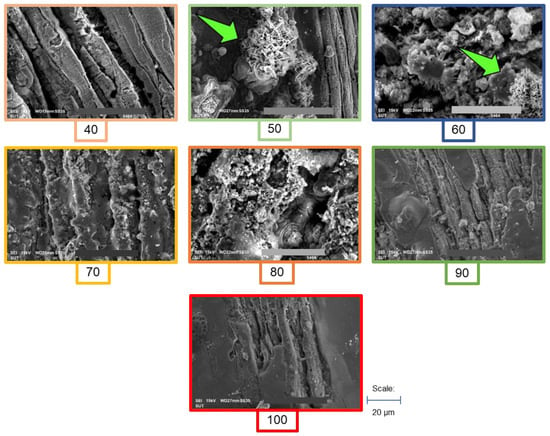
Figure 6.
SEM images of different concentrations of ZVI in cassava wastewater. The number below indicates the concentration of g ZVI in g of ZVI kg of TVS−1. Green arrows indicate the pore-like structure.
3.5. Continuous System
Using the optimized condition of alkaline and heat pretreatment with ZVI addition in the system, a continuous-stirred tank reactor (CSTR) consisting of 6 reaction tubes was operated using two different HRTs (20 and 60 days). It is important to note that the ZVI may exhibit different effects under semi- and continuous systems [89]. Thus, the switch of the system to continuous was conducted. The wastewater was digested under two conditions to evaluate the effect of the pH and heat pretreatments with ZVI on the system. It has been shown that there were two different patterns of the impact of ZVI in the pretreatment under continuous reactors. In the 20-day HRT, the significance of the ZVI addition only showed in the first 20-day period, while, in the 60-day HRT, the noticeable increase could be observed for up to 30 days. The rise of methane production also varied from a slight increase on almost all days of the continuous system to an increase up to five times higher than the treatment with only pH adjustment (alkalinity) and the control (without any additional treatment). This pattern has been previously reported by Dong, Kyung Choi, and Woo Lee [89] and Calabrò, Fazzino, Folino, Scibetta, and Sidari [24]. It was explained as the effect of ZVI accumulation that can be an inhibition factor for the methanogens [62]. However, this result differed from Calabrò et al. (2019), who displayed methane production stability after adding ZVI. The instability of the biogas production after the 20- and 30-day period in HRT of 20 and 60 days, respectively, may also be resulted from the accumulation of Fe2+ and Fe3+ ions in the system. Iron ions could not be removed after oxidizing in a continuous AD system, potentially inhibiting more methanogenesis than homoacetogenesis [90,91].
Significant decreases in methane in the presence of ZVI were also found in the early stage of the continuous system with the 20-day HRT (Figure 7a). Similarly, methane production fluctuated before it went down to a similar product with the CTRL and pH treatments on day 20 in the HRT of 60 days. These patterns were previously reported in several studies [92,93]. The dramatical decrease in methane production at the early stage was found in the previous research caused by the different compositions of organic matter loaded into the digestion with different pretreatments that possibly reduced the methane production capacity in the continuous system [93]. Lower HRT was also reported to result in an early decrease in methane production compared with the higher HRT. The high flowrate in low HRT possibly caused the dramatic change in the microbial community and reduced its capacity to produce optimum methane even under the optimized treatment/pretreatment condition [94]. A severe decrease in methane production between day 20 and day 25 was observed in the 60-day HRT (Figure 7b). The direct decrease may be explained as the accumulation of ZVI that directly inhibits methane generation. Methanogens have been reported to be very sensitive to excess ZVI. The increase in ZVI accumulation eventually reached a sufficient concentration for the methanogens to significantly decrease methane production [95].
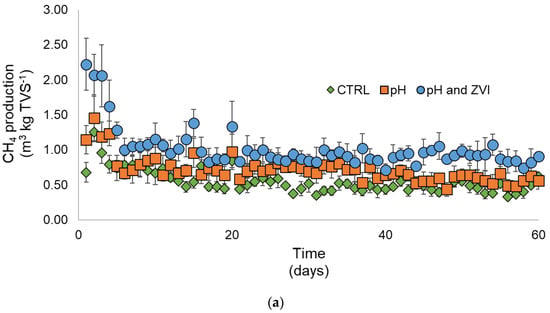
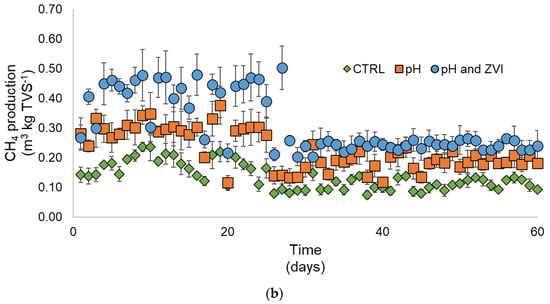
Figure 7.
Methane production from continuous reactors using a combination of alkaline and heat pretreatment with additional ZVI. (a) HRT 20 and (b) HRT 60.
In the two HRTs, cumulative methane production from the wastewater showed a significant increase after alkaline and heat pretreatment with and without the addition of ZVI (Table 2). It showed that overall removal of COD and VFAs were affected by the alkaline and heat pretreatments; meanwhile, the addition of ZVI did not raise the cumulative methane production. It proved that the ZVI mainly involved the late methanogenesis stage instead of the hydrolysis and acidogenesis phases. It is in line with the finding of Puyol et al. [96] that even though the iron ions can enhance the hydrolysis of substrates, methanogens enhancement is the most affected part in the addition of ZVI. The higher methane production from similar substrate removal (VFAs and COD) can also indicate an increase in the methanogenesis rate to balance the hydrolysis and acidogenesis rates [97].

Table 2.
Methane production and substrate removal efficiencies of cassava wastewater by two different HRTs.
Overall, each pretreatment and addition of ZVI showed better methane production than in the literature using similar pretreatments and ZVI applications (Table S2). This study also highlighted the critical finding of ZVI and alkaline–heat pretreatments. It has been found that ZVI addition in the pretreated wastewater enhanced the acetogenesis–methanogenesis more than the hydrolysis–acidogenesis. Here, the results indicate that pretreatments entirely focused on the hydrolysis process. On the other hand, ZVI, which was reported to have versatile advantages in hydrolysis–methanogenesis, mainly affected the methanogens in the physiological function after the heat and alkaline pretreatments.
This enhancement showed the promising application of ZVI with alkaline and heat pretreatments. However, several limitations shall be highlighted for further experiments.
- Removal of nutrients in the cassava pulp and cassava wastewater was still low, especially in N and P elements. Although the complete removal of these nutrients is infrequent by anaerobic digestion [98], these contaminants are still essential to consider as they can be significant causes for eutrophication if the effluents are discharged [99].
- The specific concentration of ZVI addition (50–60 g of ZVI kg of TVS−1) showed the application’s vulnerability to enhancing methane production. If there is a slight change in TVs of the substrate, the result may be lower than the absence of ZVI [100].
- Accumulating the oxidized products of ZVI can be inefficient in the process, and later it can negatively affect the total costs [101]. It is essential to control and change the system periodically with the new inocula for the continuous advantage of the ZVI.
- Combining ZVI addition with alkaline and heat pretreatments is a promising method for increasing methane production. However, an exact calculation for a larger scale must be conducted to ensure the capital and operational costs are still lower than the benefit from the combination of the pretreatments and ZVI addition [102].
- The continuous system successfully confirmed the positive effect of ZVI addition with heat and alkaline pretreatments using two different HRTs based on the reactor in the field. It is important to note that the performance of several HRT experiments was not conducted in this study. However, it may be necessary to develop this combination of pretreatment with optimization of HRTs to ensure a broad application of substrate and robust confirmation of the effect in the continuous system [103].
- Although ZVI is considered a non-toxic substance, contamination in the effluent can negatively impact the label of green energy from biogas. Thus, the control of ZVI and its derivatives must be considered [104].
4. Conclusions
The combination of alkaline and heat pretreatments increased methane production in the batch and semicontinuous systems. Alkaline pretreatment at pH 10 with 30 min of heat exposure at 100 °C showed the highest methane production from the substrate. During the anaerobic digestion process, after the optimum pretreatment, an additional 50 g of ZVI kg of TVS−1 resulted in the most increased methane production up to 4.18 m3 CH4 kg TVS−1. According to the removal results, only COD, including the VFAs that were greatly removed from the substrate, consisted of cassava wastewater and cassava pulp. Thus, the alkaline pretreatment and addition of ZVI yielded promising results. In light of the results, the pretreatments play essential roles in degrading complex and robust bonds of saccharides that are important as primary biogas substrates. Meanwhile, the addition of ZVI at a sufficient concentration can enhance the methanogenesis of the degraded substrates.
Nevertheless, nutrient removal shall be considered to be complete in the other process apart from the biogas generation as the pulp contains a sufficient amount of N and P to trigger detrimental effects to the environment.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation9020108/s1, Figure S1. Characterization of synthesized ZVI and its comparison with previous studies’ results.; Table S1: Comparison of Heat and pH pretreatments’ effect on biogas production from several anaerobic digestions; Table S2: Comparison of ZVI pretreatments’ effect on biogas production from several anaerobic digestions. References [105,106,107,108] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, N.B. and T.P.; methodology, N.B. and T.P.; software, M.P.; validation, N.B., C.P. and M.P.; formal analysis, C.P. and M.P.; investigation, T.P. and C.P.; resources, N.B.; data curation, M.P.; writing—original draft preparation, M.P.; writing—review and editing, N.B.; visualization, M.P.; supervision, N.B.; project administration, C.P.; funding acquisition, N.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Suranaree University of Technology, grant project One Research One Graduate (OROG) Academic Year 1/2018. The APC was funded by the Suranaree University of Technology.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank all the members of the School of Environmental Engineering, Suranaree University of Technology, Thailand, for technical support and the apparatus during the experiment.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matsuo, Y.; Yanagisawa, A.; Yamashita, Y. A global energy outlook to 2035 with strategic considerations for Asia and Middle East energy supply and demand interdependencies. Energy Strategy Rev. 2013, 2, 79–91. [Google Scholar] [CrossRef]
- Chien, F.; Kamran, H.W.; Albashar, G.; Iqbal, W. Dynamic planning, conversion, and management strategy of different renewable energy sources: A Sustainable Solution for Severe Energy Crises in Emerging Economies. Int. J. Hydrogen Energy 2021, 46, 7745–7758. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Lomwongsopon, P.; Aramrueang, N. Mild chemical pretreatment of cassava pulp for enhancing high-load anaerobic digestion. Bioresour. Technol. Rep. 2022, 17, 100896. [Google Scholar] [CrossRef]
- Jia, T.; Wang, Z.; Shan, H.; Liu, Y.; Gong, L. Effect of nanoscale zero-valent iron on sludge anaerobic digestion. Resour. Conserv. Recycl. 2017, 127, 190–195. [Google Scholar] [CrossRef]
- Atelge, M.R.; Atabani, A.E.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.Ş.; Unalan, S.; et al. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Dos Santos, A.L.M.; Castro, A.L.S.; Salomon, K.R.; De Souza, T.S.O.; Vich, D.V. Global research trends on anaerobic digestion and biogas production from cassava wastewater: A bibliometric analysis. J. Chem. Technol. Biotechnol. 2022, 97, 1379–1389. [Google Scholar] [CrossRef]
- Pedersen, M.; Johansen, K.S.; Meyer, A.S. Low temperature lignocellulose pretreatment: Effects and interactions of pretreatment pH are critical for maximizing enzymatic monosaccharide yields from wheat straw. Biotechnol. Biofuels 2011, 4, 11. [Google Scholar] [CrossRef]
- Mussatto, S.I.; Fernandes, M.; Milagres, A.M.F.; Roberto, I.C. Effect of hemicellulose and lignin on enzymatic hydrolysis of cellulose from brewer’s spent grain. Enzym. Microb. Technol. 2008, 43, 124–129. [Google Scholar] [CrossRef]
- Triolo, J.M.; Sommer, S.G.; Møller, H.B.; Weisbjerg, M.R.; Jiang, X.Y. A new algorithm to characterize biodegradability of biomass during anaerobic digestion: Influence of lignin concentration on methane production potential. Bioresour. Technol. 2011, 102, 9395–9402. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Duan, X.; Jiang, Y.; Sun, J.; Yang, S.; Yang, B.; He, S.; Liang, H.; Luo, Y. Modification of hemicellulose polysaccharides during ripening of postharvest banana fruit. Food Chem. 2009, 115, 43–47. [Google Scholar] [CrossRef]
- Binder, J.B.; Raines, R.T. Fermentable sugars by chemical hydrolysis of biomass. Proc. Natl. Acad. Sci. USA 2010, 107, 4516–4521. [Google Scholar] [CrossRef]
- Gaewchingduang, S.; Pengthemkeerati, P. Enhancing efficiency for reducing sugar from cassava bagasse by pretreatment. Int. J. Environ. Ecol. Eng. 2010, 4, 477–480. [Google Scholar]
- Appels, L.; Baeyens, J.; Degrève, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. Sci. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Kim, D.-H.; Jeong, E.; Oh, S.-E.; Shin, H.-S. Combined (alkaline+ultrasonic) pretreatment effect on sewage sludge disintegration. Water Res. 2010, 44, 3093–3100. [Google Scholar] [CrossRef]
- Toutian, V.; Barjenbruch, M.; Loderer, C.; Remy, C. Impact of process parameters of thermal alkaline pretreatment on biogas yield and dewaterability of waste activated sludge. Water Res. 2021, 202, 117465. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Sukwanitch, K. Comparison of Biogas Production Improvement from Cassava Pulp between Acid and Alkaline Hydrolysis Processes. Master’s Thesis, Chiang Mai University, Chiangmai, Thailand, 2011. [Google Scholar]
- Varongchayakul, S.; Songkasiri, W.; Chaiprasert, P. Optimization of Cassava Pulp Pretreatment by Liquid Hot Water for Biomethane Production. BioEnergy Res. 2021, 14, 1312–1327. [Google Scholar] [CrossRef]
- Zhang, Q.; He, J.; Tian, M.; Mao, Z.; Tang, L.; Zhang, J.; Zhang, H. Enhancement of methane production from cassava residues by biological pretreatment using a constructed microbial consortium. Bioresour. Technol. 2011, 102, 8899–8906. [Google Scholar] [CrossRef]
- Lerdlattaporn, R.; Phalakornkule, C.; Trakulvichean, S.; Songkasiri, W. Implementing circular economy concept by converting cassava pulp and wastewater to biogas for sustainable production in starch industry. Sustain. Environ. Res. 2021, 31, 20. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Quan, X.; Chen, S. Enhanced anaerobic digestion of waste activated sludge digestion by the addition of zero valent iron. Water Res. 2014, 52, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Calabrò, P.S.; Fazzino, F.; Folino, A.; Scibetta, S.; Sidari, R. Improvement of semi-continuous anaerobic digestion of pre-treated orange peel waste by the combined use of zero valent iron and granular activated carbon. Biomass Bioenergy 2019, 129, 105337. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Quan, X. Zero-valent iron enhanced methanogenic activity in anaerobic digestion of waste activated sludge after heat and alkali pretreatment. Waste Manag. 2015, 38, 297–302. [Google Scholar] [CrossRef]
- Noubactep, C. The suitability of metallic iron for environmental remediation. Environ. Prog. Sustain. Energy 2010, 29, 286–291. [Google Scholar] [CrossRef]
- Karri, S.; Sierra-Alvarez, R.; Field, J.A. Zero valent iron as an electron-donor for methanogenesis and sulfate reduction in anaerobic sludge. Biotechnol. Bioeng. 2005, 92, 810–819. [Google Scholar] [CrossRef]
- Dieter, D.; Angelika, S. Biogas from Waste and Renewable Resources: An Introduction; Wiley-VCH: Weiheim, Germany, 2008. [Google Scholar]
- Tchbanoglous, G.; Burton, F.L.; Stensel, H.D. Wastewater Engineering: Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Andronikou, M.; Lytras, N.; Chrysanthou, G.; Koutsokeras, L.; Constantinides, G.; Stylianou, M.; Agapiou, A.; Vyrides, I. Biogas upgrading to methane and removal of volatile organic compounds in a system of zero-valent iron and anaerobic granular sludge. Environ. Sci. Pollut. Res. 2022, 29, 87245–87256. [Google Scholar] [CrossRef]
- Zou, Y.; Zhang, S.; Huo, L.; Sun, G.; Lu, X.; Jiang, M.; Yu, X. Wetland saturation with introduced Fe(III) reduces total carbon emissions and promotes the sequestration of DOC. Geoderma 2018, 325, 141–151. [Google Scholar] [CrossRef]
- Yingchon, U. The Increase in Efficiency of Biogas Production from Treated Cassava Pulp by Zero Valent Iron (Fe0). Master’s Thesis, Suranaree University of Technology, Nakhon Ratchasima, Thailand, 2017. [Google Scholar]
- American Public Health Association. APHA Standard Methods for the Examination of Water and Wastewater; American Public Health Association (APHA): Washington, DC, USA, 2005. [Google Scholar]
- Chen, R.; Nie, Y.; Ji, J.; Utashiro, T.; Li, Q.; Komori, D.; Li, Y.-Y. Submerged anaerobic membrane bioreactor (SAnMBR) performance on sewage treatment: Removal efficiencies, biogas production and membrane fouling. Water Sci. Technol. 2017, 76, 1308–1317. [Google Scholar] [CrossRef]
- Intanoo, P.; Rangsanvigit, P.; Malakul, P.; Chavadej, S. Optimization of separate hydrogen and methane production from cassava wastewater using two-stage upflow anaerobic sludge blanket reactor (UASB) system under thermophilic operation. Bioresour. Technol. 2014, 173, 256–265. [Google Scholar] [CrossRef]
- Lavine, B.K.; Auslander, G.; Ritter, J. Polarographic studies of zero valent iron as a reductant for remediation of nitroaromatics in the environment. Microchem. J. 2001, 70, 69–83. [Google Scholar] [CrossRef]
- Bang, S.; Johnson, M.D.; Korfiatis, G.P.; Meng, X. Chemical reactions between arsenic and zero-valent iron in water. Water Res. 2005, 39, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Chandel, M.K. Enhancement of biogas production from organic fraction of municipal solid waste using alkali pretreatment. J. Mater. Cycles Waste Manag. 2020, 22, 757–767. [Google Scholar] [CrossRef]
- Xi, Y.; Mallavarapu, M.; Naidu, R. Reduction and adsorption of Pb2+ in aqueous solution by nano-zero-valent iron—A SEM, TEM and XPS study. Mater. Res. Bull. 2010, 45, 1361–1367. [Google Scholar] [CrossRef]
- Moshi, A.P.; Temu, S.G.; Nges, I.A.; Malmo, G.; Hosea, K.M.M.; Elisante, E.; Mattiasson, B. Combined production of bioethanol and biogas from peels of wild cassava Manihot glaziovii. Chem. Eng. J. 2015, 279, 297–306. [Google Scholar] [CrossRef]
- Mañunga, T.; Barrios-Pérez, J.D.; Zaiat, M.; Rodríguez-Victoria, J.A. Evaluation of pretreatment methods and initial pH on mixed inoculum for fermentative hydrogen production from cassava wastewater. Biofuels 2022, 13, 301–308. [Google Scholar] [CrossRef]
- Tg, I.; Haq, I.; Kalamdhad, A.S. 14-Factors affecting anaerobic digestion for biogas production: A review. In Advanced Organic Waste Management; Hussain, C., Hait, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 223–233. [Google Scholar] [CrossRef]
- Kayhanian, M.; Hardy, S. The impact of four design parameters on the performance of a high-solids anaerobic digestion of municipal solid waste for fuel gas production. Environ. Technol. 1994, 15, 557–567. [Google Scholar] [CrossRef]
- Cao, J.-Y.; Kong, Z.-Y.; Ye, M.-W.; Ling, T.; Chen, K.; Xu, J.-L.; Zhou, C.-X.; Liao, K.; Zhang, L.; Yan, X.-J. Comprehensive comparable study of metabolomic and transcriptomic profiling of Isochrysis galbana exposed to high temperature, an important diet microalgal species. Aquaculture 2020, 521, 735034. [Google Scholar] [CrossRef]
- Costa, R.C.; Ramos, M.D.N.; Fleck, L.; Gomes, S.D.; Aguiar, A. Critical analysis and predictive models using the physicochemical characteristics of cassava processing wastewater generated in Brazil. J. Water Process Eng. 2022, 47, 102629. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sumardiono, S. Predicting kinetic model of biogas production and biodegradability organic materials: Biogas production from vinasse at variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Budiyono, B.; Syaichurrozi, I.; Sumardiono, S. Biogas production from bioethanol waste: The effect of pH and urea addition to biogas production rate. Waste Technol. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Peres, S.; Monteiro, M.R.; Ferreira, M.L.; do Nascimento Junior, A.F.; de Los Angeles, M.P.F.P. Anaerobic Digestion Process for the Production of Biogas from Cassava and Sewage Treatment Plant Sludge in Brazil. BioEnergy Res. 2019, 12, 150–157. [Google Scholar] [CrossRef]
- Chantawan, N.; Moungprayoon, A.; Lunprom, S.; Reungsang, A.; Salakkam, A. High-solid dark fermentation of cassava pulp and cassava processing wastewater for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 40672–40682. [Google Scholar] [CrossRef]
- Panichnumsin, P.; Nopharatana, A.; Ahring, B.; Chaiprasert, P. Production of methane by co-digestion of cassava pulp with various concentrations of pig manure. Biomass Bioenergy 2010, 34, 1117–1124. [Google Scholar] [CrossRef]
- Achi, C.G.; Hassanein, A.; Lansing, S. Enhanced Biogas Production of Cassava Wastewater Using Zeolite and Biochar Additives and Manure Co-Digestion. Energies 2020, 13, 491. [Google Scholar] [CrossRef]
- Dongsheng, Z.; Wenqiang, G.; Guozhang, C.; Shuai, L.; Weizhou, J.; Youzhi, L. Removal of heavy metal lead(II) using nanoscale zero-valent iron with different preservation methods. Adv. Powder Technol. 2019, 30, 581–589. [Google Scholar] [CrossRef]
- Mossmann, A.; Dotto, G.L.; Hotza, D.; Jahn, S.L.; Foletto, E.L. Preparation of polyethylene–supported zero–valent iron buoyant catalyst and its performance for Ponceau 4R decolorization by photo–Fenton process. J. Environ. Chem. Eng. 2019, 7, 102963. [Google Scholar] [CrossRef]
- Lü, Y.; Li, J.; Li, Y.; Liang, L.; Dong, H.; Chen, K.; Yao, C.; Li, Z.; Li, J.; Guan, X. The roles of pyrite for enhancing reductive removal of nitrobenzene by zero-valent iron. Appl. Catal. B Environ. 2019, 242, 9–18. [Google Scholar] [CrossRef]
- Shao, Y.; Zhao, P.; Yue, Q.; Wu, Y.; Gao, B.; Kong, W. Preparation of wheat straw-supported Nanoscale Zero-Valent Iron and its removal performance on ciprofloxacin. Ecotoxicol. Environ. Saf. 2018, 158, 100–107. [Google Scholar] [CrossRef]
- Singhal, R.K.; Gangadhar, B.; Basu, H.; Manisha, V.; Naidu, G.R.K.; Reddy, A.V.R. Remediation of Malathion Contaminated Soil Using Zero Valent Iron Nano-Particles. Am. J. Anal. Chem. 2012, 3, 7. [Google Scholar] [CrossRef]
- Bochmann, G.; Montgomery, L.F.R. 4-Storage and pre-treatment of substrates for biogas production. In The Biogas Handbook; Wellinger, A., Murphy, J., Baxter, D., Eds.; Woodhead Publishing: Cambridge, UK, 2013; pp. 85–103. [Google Scholar] [CrossRef]
- Thys, R.C.S.; Westfahl, H.; Noreña, C.P.Z.; Marczak, L.D.F.; Silveira, N.P.; Cardoso, M.B. Effect of the Alkaline Treatment on the Ultrastructure of C-Type Starch Granules. Biomacromolecules 2008, 9, 1894–1901. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, P.; Vyrides, I. In situ biogas upgrading and enhancement of anaerobic digestion of cheese whey by addition of scrap or powder zero-valent iron (ZVI). J. Environ. Manag. 2021, 280, 111651. [Google Scholar] [CrossRef] [PubMed]
- Meissner, Y.; Lamprecht, A. Alternative Drug Delivery Approaches for the Therapy of Inflammatory Bowel Disease. J. Pharm. Sci. 2008, 97, 2878–2891. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wei, W.; Wu, S.-L.; Ni, B.-J. Zerovalent iron effectively enhances medium-chain fatty acids production from waste activated sludge through improving sludge biodegradability and electron transfer efficiency. Environ. Sci. Technol. 2020, 54, 10904–10915. [Google Scholar] [CrossRef]
- Domrongpokkaphan, V.; Phalakornkule, C.; Khemkhao, M. In-situ methane enrichment of biogas from anaerobic digestion of palm oil mill effluent by addition of zero valent iron (ZVI). Int. J. Hydrogen Energy 2021, 46, 30976–30987. [Google Scholar] [CrossRef]
- Hu, Y.; Hao, X.; Zhao, D.; Fu, K. Enhancing the CH4 yield of anaerobic digestion via endogenous CO2 fixation by exogenous H2. Chemosphere 2015, 140, 34–39. [Google Scholar] [CrossRef]
- Thongchul, N.; Navankasattusas, S.; Yang, S.-T. Production of lactic acid and ethanol by Rhizopus oryzae integrated with cassava pulp hydrolysis. Bioprocess Biosyst. Eng. 2010, 33, 407–416. [Google Scholar] [CrossRef]
- Barua, V.B.; Rathore, V.; Kalamdhad, A.S. Anaerobic co-digestion of water hyacinth and banana peels with and without thermal pretreatment. Renew. Energy 2019, 134, 103–112. [Google Scholar] [CrossRef]
- Schuchmann, K.; Müller, V. Autotrophy at the thermodynamic limit of life: A model for energy conservation in acetogenic bacteria. Nat. Rev. Microbiol. 2014, 12, 809–821. [Google Scholar] [CrossRef]
- Koster, I.W.; Koomen, E. Ammonia inhibition of the maximum growth rate (μm) of hydrogenotrophic methanogens at various pH-levels and temperatures. Appl. Microbiol. Biotechnol. 1988, 28, 500–505. [Google Scholar] [CrossRef]
- Hunik, J.H.; Hamelers, H.V.M.; Koster, I.W. Growth-rate inhibition of acetoclastic methanogens by ammonia and pH in poultry manure digestion. Biol. Wastes 1990, 32, 285–297. [Google Scholar] [CrossRef]
- Song, J.; Wang, Y.; Zhang, S.; Song, Y.; Xue, S.; Liu, L.; Lvy, X.; Wang, X.; Yang, G. Coupling biochar with anaerobic digestion in a circular economy perspective: A promising way to promote sustainable energy, environment and agriculture development in China. Renew. Sustain. Energy Rev. 2021, 144, 110973. [Google Scholar] [CrossRef]
- Wadchasit, P.; Rakmak, N.; O-Thong, S.; Rattanasak, U.; Imai, T.; Jitpinit, S.; Nuithitikul, K. Improvement of biogas production and quality by addition of struvite precipitates derived from liquid anaerobic digestion effluents of palm oil wastes. J. Environ. Chem. Eng. 2023, 11, 109081. [Google Scholar] [CrossRef]
- Quan, X.; Ye, C.; Xiong, Y.; Xiang, J.; Wang, F. Simultaneous removal of ammonia, P and COD from anaerobically digested piggery wastewater using an integrated process of chemical precipitation and air stripping. J. Hazard. Mater. 2010, 178, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Lanet, P.; Deluchat, V.; Baudu, M. Relevant design parameters for a reactor used in P removal with ZVI-based materials. J. Ind. Eng. Chem. 2021, 104, 8–21. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, Y.; Qi, Q.; Zhang, P.; Zhang, Y.; Tong, Y.W.; He, Y. The bio-chemical cycle of iron and the function induced by ZVI addition in anaerobic digestion: A review. Water Res. 2020, 186, 116405. [Google Scholar] [CrossRef]
- Jia, L.; Sun, H.; Zhou, Q.; Dai, R.; Wu, W. Integrated evaluation for advanced removal of nitrate and phosphorus in novel PHBV/ZVI-based biofilters: Insight into functional genes and key enzymes. J. Clean. Prod. 2022, 349, 131199. [Google Scholar] [CrossRef]
- Cheawchanlertfa, P.; Tongsuk, P.; Sutheeworapong, S.; Waeonukul, R.; Pason, P.; Poomputsa, K.; Ratanakhanokchai, K.; Kosugi, A.; Tachaapaikoon, C. A novel amylolytic/xylanolytic/cellulolytic multienzyme complex from Clostridium manihotivorum that hydrolyzes polysaccharides in cassava pulp. Appl. Microbiol. Biotechnol. 2021, 105, 6719–6733. [Google Scholar] [CrossRef]
- Abdelsalam, E.; Samer, M.; Attia, Y.A.; Abdel-Hadi, M.A.; Hassan, H.E.; Badr, Y. Influence of zero valent iron nanoparticles and magnetic iron oxide nanoparticles on biogas and methane production from anaerobic digestion of manure. Energy 2017, 120, 842–853. [Google Scholar] [CrossRef]
- Mirmohamadsadeghi, S.; Karimi, K.; Azarbaijani, R.; Parsa Yeganeh, L.; Angelidaki, I.; Nizami, A.-S.; Bhat, R.; Dashora, K.; Vijay, V.K.; Aghbashlo, M.; et al. Pretreatment of lignocelluloses for enhanced biogas production: A review on influencing mechanisms and the importance of microbial diversity. Renew. Sustain. Energy Rev. 2021, 135, 110173. [Google Scholar] [CrossRef]
- Glass, J.; Orphan, V. Trace Metal Requirements for Microbial Enzymes Involved in the Production and Consumption of Methane and Nitrous Oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Tong, Q.; Li, Y.; Wang, N.; Liu, B.; Zhang, X. Biogas production and metal passivation analysis during anaerobic digestion of pig manure: Effects of a magnetic Fe3O4/FA composite supplement. RSC Adv. 2019, 9, 4488–4498. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yuan, R.; Liu, C.; Zhou, B. Effect of Fe2+ adding period on the biogas production and microbial community distribution during the dry anaerobic digestion process. Process Saf. Environ. Prot. 2020, 136, 234–241. [Google Scholar] [CrossRef]
- Yamamoto, S.; Morii, H. Suppression of Methane Production via the Promotion of Fe2+ Oxidation in Paddy Fields. Commun. Soil Sci. Plant Anal. 2020, 51, 1114–1122. [Google Scholar] [CrossRef]
- Palacios, P.A.; Francis, W.R.; Rotaru, A.-E. A Win–Loss Interaction on Fe0 Between Methanogens and Acetogens from a Climate Lake. Front. Microbiol. 2021, 12, 919. [Google Scholar] [CrossRef]
- Paulo, L.M.; Stams, A.J.M.; Sousa, D.Z. Methanogens, sulphate and heavy metals: A complex system. Rev. Environ. Sci. Bio/Technol. 2015, 14, 537–553. [Google Scholar] [CrossRef]
- Hu, J.; Wu, H.; Sun, Z.; Peng, Q.-A.; Zhao, J.; Hu, R. Ferrous Iron Addition Decreases Methane Emissions Induced by Rice Straw in Flooded Paddy Soils. ACS Earth Space Chem. 2020, 4, 843–853. [Google Scholar] [CrossRef]
- Sivan, O.; Shusta, S.S.; Valentine, D.L. Methanogens rapidly transition from methane production to iron reduction. Geobiology 2016, 14, 190–203. [Google Scholar] [CrossRef]
- Xi, Y.; Luo, Y.; Zou, J.; Li, J.; Liao, T.; Zhang, L.; Wang, C.; Li, X.; Lin, G. Kinetics of Arsenic Removal in Waste Acid by the Combination of CuSO4 and Zero-Valent Iron. Processes 2019, 7, 401. [Google Scholar] [CrossRef]
- Wang, T.; Qin, Y.; Cao, Y.; Han, B.; Ren, J. Simultaneous addition of zero-valent iron and activated carbon on enhanced mesophilic anaerobic digestion of waste-activated sludge. Environ. Sci. Pollut. Res. 2017, 24, 22371–22381. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, B.; Zhou, T.; Wu, X.; Mao, J. An insight in magnetic field enhanced zero-valent iron/H2O2 Fenton-like systems: Critical role and evolution of the pristine iron oxides layer. Sci. Rep. 2016, 6, 24094. [Google Scholar] [CrossRef] [PubMed]
- Dong, D.; Kyung Choi, O.; Woo Lee, J. Influence of the continuous addition of zero valent iron (ZVI) and nano-scaled zero valent iron (nZVI) on the anaerobic biomethanation of carbon dioxide. Chem. Eng. J. 2022, 430, 132233. [Google Scholar] [CrossRef]
- Kato, S. Microbial extracellular electron transfer and its relevance to iron corrosion. Microb. Biotechnol. 2016, 9, 141–148. [Google Scholar] [CrossRef]
- Keller, A.A.; Garner, K.; Miller, R.J.; Lenihan, H.S. Toxicity of Nano-Zero Valent Iron to Freshwater and Marine Organisms. PLoS ONE 2012, 7, e43983. [Google Scholar] [CrossRef]
- Gao, M.; Zhang, S.; Ma, X.; Guan, W.; Song, N.; Wang, Q.; Wu, C. Effect of yeast addition on the biogas production performance of a food waste anaerobic digestion system. R. Soc. Open Sci. 2020, 7, 200443. [Google Scholar] [CrossRef] [PubMed]
- Han, W.-B.; Zhao, Y.-Z.; Chen, H. Study on Biogas Production of Joint Anaerobic Digestion with Excess Sludge and Kitchen Waste. Procedia Environ. Sci. 2016, 35, 756–762. [Google Scholar] [CrossRef]
- Sivagurunathan, P.; Anburajan, P.; Kumar, G.; Kim, S.-H. Effect of hydraulic retention time (HRT) on biohydrogen production from galactose in an up-flow anaerobic sludge blanket reactor. Int. J. Hydrogen Energy 2016, 41, 21670–21677. [Google Scholar] [CrossRef]
- Tian, W.; Li, J.; Zhu, L.; Li, W.; He, L.; Gu, L.; Deng, R.; Shi, D.; Chai, H.; Gao, M. Insights of enhancing methane production under high-solid anaerobic digestion of wheat straw by calcium peroxide pretreatment and zero valent iron addition. Renew. Energy 2021, 177, 1321–1332. [Google Scholar] [CrossRef]
- Puyol, D.; Flores-Alsina, X.; Segura, Y.; Molina, R.; Padrino, B.; Fierro, J.L.G.; Gernaey, K.V.; Melero, J.A.; Martinez, F. Exploring the effects of ZVI addition on resource recovery in the anaerobic digestion process. Chem. Eng. J. 2018, 335, 703–711. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, L.; Wang, T.; Ren, J.; Cao, Y.; Zhou, S. Impacts of ferric chloride, ferrous chloride and solid retention time on the methane-producing and physicochemical characterization in high-solids sludge anaerobic digestion. Renew. Energy 2019, 139, 1290–1298. [Google Scholar] [CrossRef]
- Chernicharo, C.A.L. Post-Treatment Options for the Anaerobic Treatment of Domestic Wastewater. Rev. Environ. Sci. Bio/Technol. 2006, 5, 73–92. [Google Scholar] [CrossRef]
- Yoshida, H.; Ten Hoeve, M.; Christensen, T.H.; Bruun, S.; Jensen, L.S.; Scheutz, C. Life cycle assessment of sewage sludge management options including long-term impacts after land application. J. Clean. Prod. 2018, 174, 538–547. [Google Scholar] [CrossRef]
- Tang, J.; Liu, Z.; Zhao, M.; Miao, H.; Shi, W.; Huang, Z.; Xie, L.; Ruan, W. Enhanced biogas biological upgrading from kitchen wastewater by in-situ hydrogen supply through nano zero-valent iron corrosion. J. Environ. Manag. 2022, 310, 114774. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Cerrillo, M.; Burgos, L.; Ruiz, B.; Barrena, R.; Moral-Vico, J.; Font, X.; Sánchez, A.; Bonmatí, A. In-situ methane enrichment in continuous anaerobic digestion of pig slurry by zero-valent iron nanoparticles addition under mesophilic and thermophilic conditions. Renew. Energy 2021, 180, 372–382. [Google Scholar] [CrossRef]
- Santos, F.S.; Ricci, B.C.; França Neta, L.S.; Amaral, M.C.S. Sugarcane vinasse treatment by two-stage anaerobic membrane bioreactor: Effect of hydraulic retention time on changes in efficiency, biogas production and membrane fouling. Bioresour. Technol. 2017, 245, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Lu, J.; Ye, J.; Zhou, Y. The effects and mechanisms of zero-valent iron on anaerobic digestion of solid waste: A mini-review. J. Clean. Prod. 2021, 278, 123567. [Google Scholar] [CrossRef]
- Su, L.; Shi, X.; Guo, G.; Zhao, A.; Zhao, Y. Stabilization of sewage sludge in the presence of nanoscale zero-valent iron (nZVI): Abatement of odor and improvement of biogas production. J. Mater. Cycles Waste Manag. 2013, 15, 461–468. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Huang, W.; Huang, W.; Li, F.; Lei, Z.; Zhang, Z. Enhanced anaerobic digestion of ammonia-rich swine manure by zero-valent iron: With special focus on the enhancement effect on hydrogenotrophic methanogenesis activity. Bioresour. Technol. 2018, 270, 172–179. [Google Scholar] [CrossRef]
- Yangin-Gomec, C.; Olmez-Hanci, T.; Arslan-Alaton, I.; Khoei, S.; Fakhri, H. Iopamidol degradation with ZVI- and ZVA-activated chemical oxidation: Investigation of toxicity, anaerobic inhibition and microbial communities. J. Environ. Chem. Eng. 2018, 6, 7318–7326. [Google Scholar] [CrossRef]
- Zhang, Y.; An, X.; Quan, X. Enhancement of sludge granulation in a zero valence iron packed anaerobic reactor with a hydraulic circulation. Process Biochem. 2011, 46, 471–476. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).