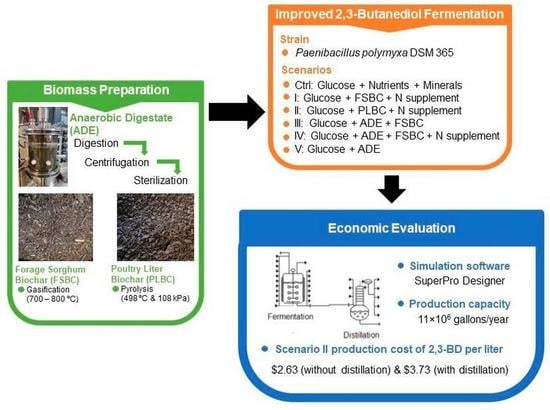
From Agricultural Wastes to Fermentation Nutrients: A Case Study of 2,3-Butanediol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. 2,3-Butanediol Fermentation with Anaerobic Digestate Supplementation
2.3. 2,3-Butanediol Fermentation in Media Containing Biochar
2.4. 2,3-Butanediol Fermentation in Media with Combination of ADE and Biochar
2.5. 2,3-Butanediol Fermentation in Media with Both ADE and Biochar and Organic Nitrogen Supplementation
2.6. Analytical Methods and Calculations
2.7. Process Design and Economic Evaluation
2.8. Statistical Analysis
3. Results and Discussion
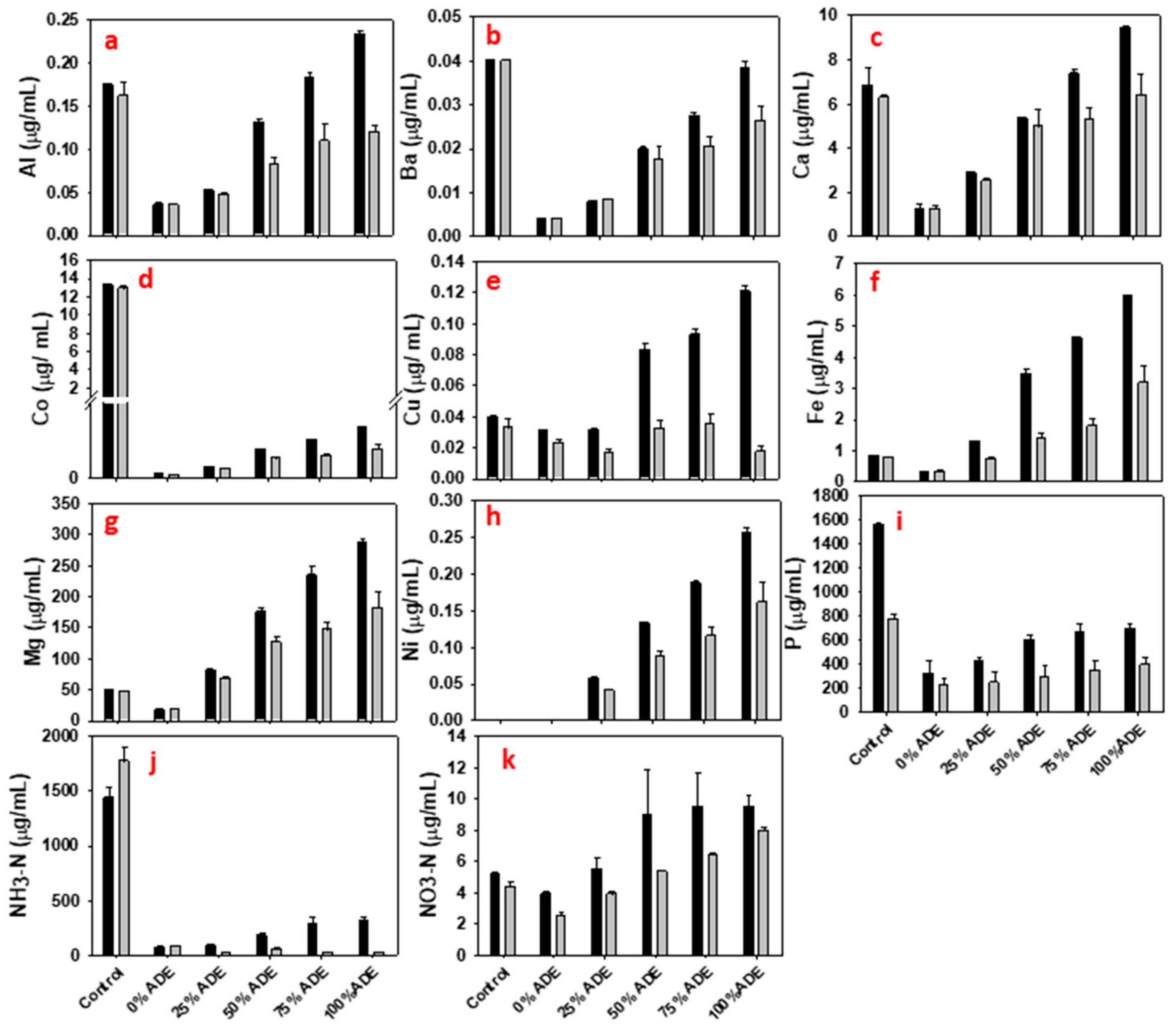
3.1. 2,3-Butanediol Fermentation in Media with Anaerobic Digestion Effluent
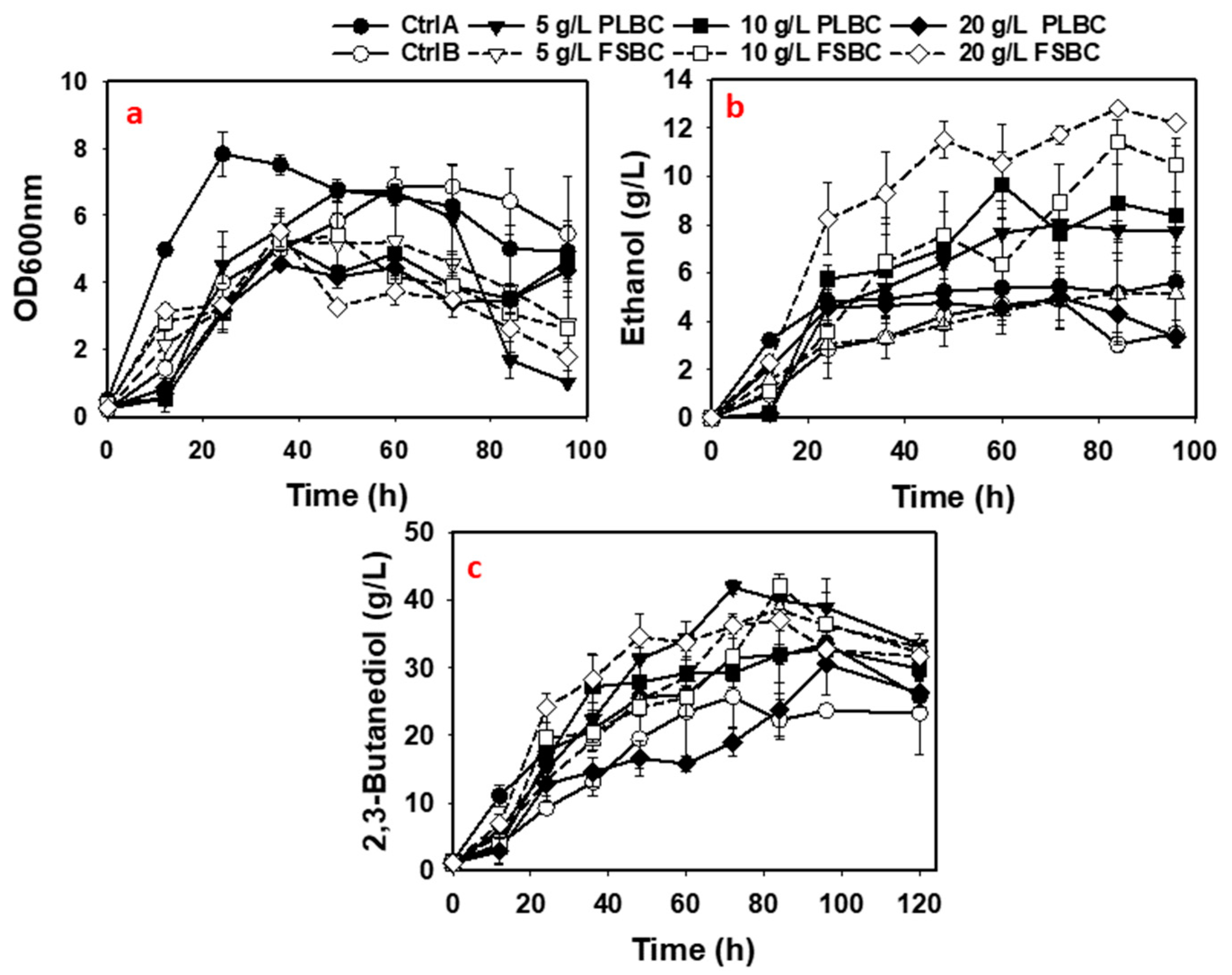
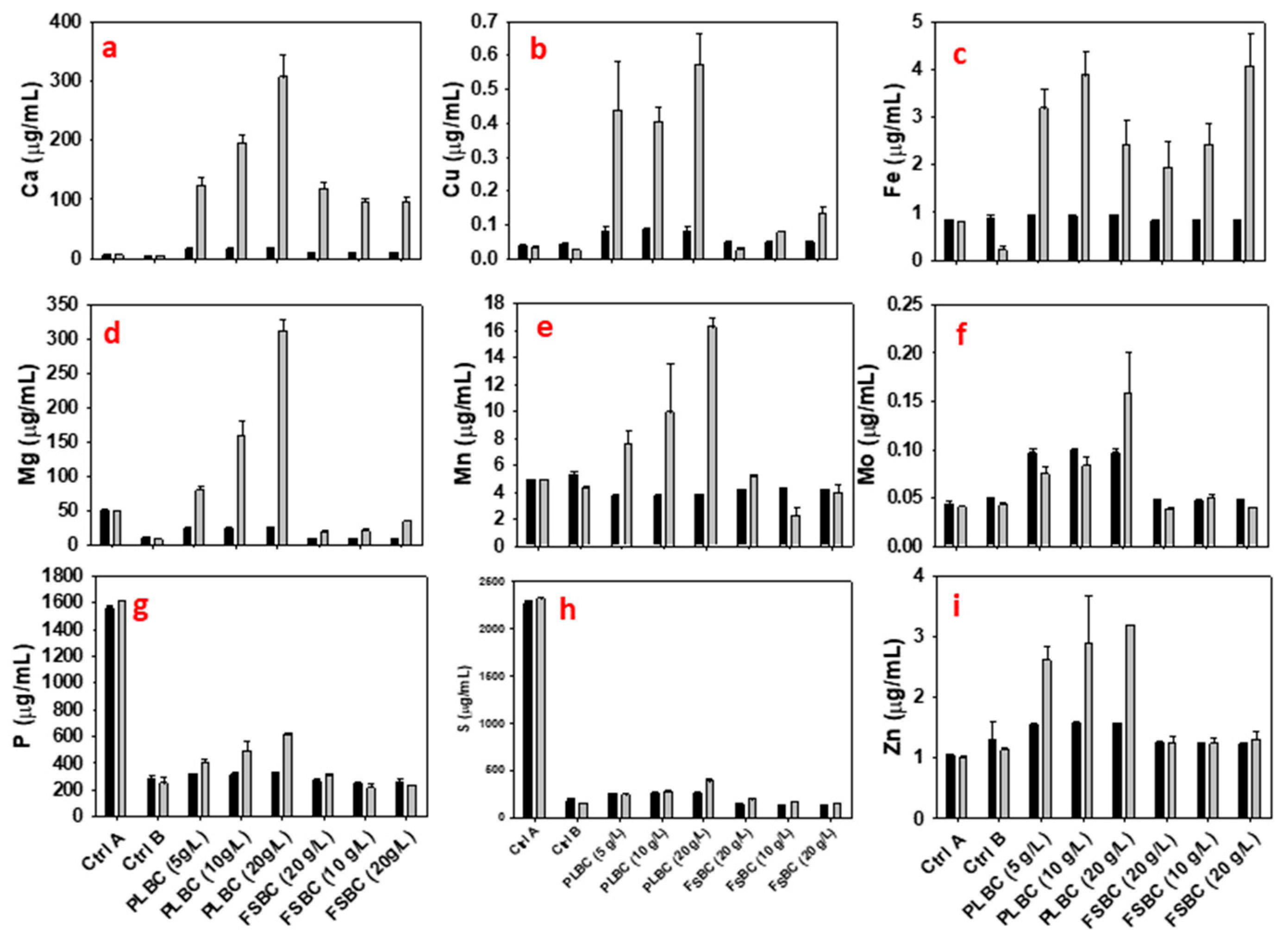
3.2. 2,3-Butanediol Fermentation in Media Containing Biochar
3.3. 2,3-Butanediol Fermentation in Media with Combination of ADE and Biochar
3.4. 2,3-Butanediol Fermentation in Media with Both ADE and Biochar and Organic Nitrogen Supplementation
3.5. Cost Analysis and Economic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Natural Resources Defense Council Report. Available online: http://www.nrdc.org/food/wasted-food.asp (accessed on 19 August 2022).
- Havlik, P.; Valin, H.; Herrero, M.; Obersteiner, M.; Schmid, E.; Rufino, M.C.; Mosnier, A.; Thornton, P.K.; Böttcher, H.; Conant, R.T.; et al. Climate change mitigation through livestock system transitions. Proc. Natl. Acad. Sci. USA 2014, 111, 3709–3714. [Google Scholar] [CrossRef] [PubMed]
- Task Force on National Greenhouse Gas Inventories. Available online: https://www.ipcc-nggip.iges.or.jp/index.html (accessed on 19 August 2022).
- Okonkwo, C.C.; Ujor, V.; Cornish, K.; Ezeji, T.C. Inactivation of the levansucrase gene in Paenibacillus polymyxa DSM 365 diminishes exopolysaccharide biosynthesis during 2,3-butanediol fermentation. Appl. Environ. Microbiol. 2020, 86, e00196-20. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Production of 2,3-butanediol from non-detoxified wheat straw hydrolysate: Impact of microbial inhibitors on Paenibacillus polymyxa DSM 365. Ind. Crops Prod. 2021, 159, 113047. [Google Scholar] [CrossRef]
- Celinska, E.; Grajek, W. Biotechnological production of 2,3-butanediol—Current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Kopke, M.; Mihalcea, C.; Liew, F.M.; Tizard, J.H.; Ali, M.S.; Connolly, J.J.; Al-Sinawi, B.; Simpson, S.D. 2,3-Butanediol production by acetogenic bacteria, an alternative route to chemical synthesis, using industrial waste gas. Appl. Environ. Microbiol. 2011, 77, 5467–5475. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.C.; Ujor, V.; Ezeji, T.C. Investigation of relationship between 2,3-butanediol toxicity and production during growth of Paenibacillus polymyxa. New Biotechnol. 2017, 34, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Transparency Market Research. 2,3-Butanediol Market (Application: Intermediate Chemicals, Plastics, Food Additives, Cosmetics, and Others [Pesticides and Anti-Freeze Agents])-Industry Analysis, Size, Share, Growth, Trends, and Forecasts, 2020–2030. 2020. Available online: www.transparencymarketresearch.com/2,3-butanediol-market.html (accessed on 22 July 2021).
- Tinoco, D.; Pateraki, C.; Koutinas, A.A.; Freire, D.M.G. Bioprocess development for 2,3-butanediol production by Paenibacillus strains. ChemBioEng Rev. 2021, 8, 44–62. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Yepez, B.; Kopsahelis, N.; Freire, D.M.G.; Machado de Castro, A.; Papanikolaou, S.; Kookos, I.K. Techno-economic evaluation of a complete bioprocess for 2,3-butanediol production from renewable resources. Bioresour. Technol. 2016, 204, 55–64. [Google Scholar] [CrossRef]
- Ujor, V.C.; Okonkwo, C.C.; Rush, B.B.; McCrea, G.E.; Ezeji, T.C. Harnessing the residual nutrients in anaerobic digestate for ethanol fermentation and digestate remediation using Saccharomyces cerevisiae. Fermentation 2020, 6, 52. [Google Scholar] [CrossRef]
- Campos, J.L.; Crutchnik, D.; Franchi, O.; Pavissich, J.P.; Belmonte, M.; Pedrouso, A.; Mosquera-Corral, A.; Val del Rio, A. Nitrogen, and phosphorus recovery from anaerobically pretreated agro-wastes: A review. Front. Sustain. Food Syst. 2019, 2, 91. [Google Scholar] [CrossRef]
- Kumar, S.; Posmanik, R.; Spatari, S.; Ujor, V.C. Repurposing anaerobic digestate for economical biomanufacturing and water recovery. Appl. Microbiol. Biotechnol. 2022, 106, 1419–1434. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Kumar, A.; Zhang, H. Enhanced ethanol production by Clostridium ragsdalei from syngas by incorporating biochar in the fermentation medium. Bioresour. Technol. 2018, 247, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Adesanya, Y.; Okonkwo, C.; Zhang, H.; Huhnke, R.L.; Ezeji, T. Feasibility of using biochar and mineral nutrients replacement for acetone-butanol-ethanol production from non-detoxified switchgrass hydrolysate. Bioresour. Technol. 2020, 298, 122569. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Patil, K.; Bellmer, D.; Wang, D.; Yuan, W.; Huhnke, R. Effects of biomass feedstocks and gasification conditions on the physicochemical properties of char. Energies 2013, 6, 3972. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Ujor, V.; Mishra, P.K.; Ezeji, T.C. Process development for enhanced 2,3-butanediol production by Paenibacillus polymyxa DSM 365. Fermentation 2017, 3, 18. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and selective method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Okonkwo, C.C.; Azam, M.M.; Ezeji, T.C.; Qureshi, N. Enhancing ethanol production from cellulosic sugars using Scheffersomyces (Pichia) stipitis. Bioprocess Biosyst. Eng. 2016, 39, 1023–1032. [Google Scholar] [CrossRef]
- Adesanya, Y.; Atiyeh, H.K.; Olorunsogbon, T.; Asmita, K.; Okonkwo, C.C.; Ujor, V.C.; Shah, A.; Ezeji, T.C. Viable strategies for enhancing acetone-butanol-ethanol production from non-detoxified switchgrass hydrolysates. Bioresour. Technol. 2022, 344, 126167. [Google Scholar] [CrossRef]
- Zang, G.; Shah, A.; Wan, C. Techno-economic analysis of co-production of 2,3-butanediol, furfural, and technical lignin via biomass processing based on deep eutectic solvent pretreatment. Biofuels Bioprod. Biorefining 2020, 14, 326–343. [Google Scholar] [CrossRef]
- Kwan, T.H.; Ong, K.L.; Haque, M.A.; Kulkarni, S.; Lin, C.S.K. Biorefinery of food and beverage waste valorisation for sugar syrups production: Techno-economic assessment. Process Saf. Environ. Prot. 2019, 121, 194–208. [Google Scholar] [CrossRef]
- LPELC. Impact of Fluctuating Fertilizer Prices on Poultry Manure Nutrient Value—Livestock and Poultry Environmental Learning Community. 2019. Available online: https://lpelc.org/impact-of-fluctuating-fertilizer-prices-on-poultry-manure-nutrient-value/ (accessed on 20 November 2021).
- Superpro Database. Superpro Designer®; v12; Intelligen Inc.: Scotch Plains, NJ, USA, 2021. [Google Scholar]
- Echemi.com. Available online: https://www.echemi.com/produce/pr1703241191-ammonium-acetate.html/ (accessed on 16 July 2021).
- Eisen-Golden. Available online: https://www.eisengolden.com/storefront/index.php?route=product/product&product_id=2236&search=Potassium+phosphate (accessed on 16 July 2021).
- Cardoso, V.M.; Campani, G.; Sanos, M.P.; Silva, G.G.; Pires, M.C.; Goncalves, V.M.; Giordano, R.C.; Sargo, C.R.; Horta, A.C.L.; Zangirolami, T.C. Cost analysis based on bioreactor cultivation conditions: Production of a soluble recombinant protein using Escherichia coli BL21(DE3). Biotechnol. Rep. 2020, 26, e00441. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Saha, B.; Cotta, M.; Singh, V. An economic evaluation of biological conversion of wheat straw to butanol: A biofuel. Energy Convers. Manag. 2013, 65, 456–462. [Google Scholar] [CrossRef]
- Harvianto, G.R.; Haider, J.; Hong, J.; van Duc Long, N.; Shim, J.J.; Cho, M.H.; Kim, W.K.; Lee, M. Purification of 2,3-butanediol from fermentation broth: Process development and techno-economic analysis. Biotechnol. Biofuels 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-C.; Bian, Y.-Q.; Han, R.-Z.; Dong, J.-J.; Ni, Y. Cloning, expression, and Characterization of budC gene encoding meso-2,3-butanediol dehydrogenase from Bacillus licheniformis. Appl. Biochem. Biotechnol. 2016, 178, 604–617. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Q.; Zhan, S.; Li, Y.; Lin, H.; Sun, S.; Sha, L.; Hu, K.; Guan, X.; Shan, Y. A new NAD(H)-dependent meso-2,3-butanediol dehydrogenase from an industrially potential strain Serratia marcescens H30. Appl. Microbiol. Biotechnol. 2014, 98, 1175–1184. [Google Scholar] [CrossRef]
- Colak, F.; Olgun, A.; Atar, N.; Yazicioglu, D. Heavy metal resistances and biosorptive behaviors of Paenibacillus polymyxa: Batch and column studies. J. Ind. Eng. Chem. 2013, 19, 863–869. [Google Scholar] [CrossRef]
- Yegorenkova, I.V.; Tregubova, K.V.; Matora, L.Y.; Burygin, G.L.; Ignatov, V.V. Biofilm formation by Paenibacillus polymyxa strains differing in the production and rheological properties of their exopolysaccharides. Curr. Microbiol. 2011, 62, 1554–1559. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Huang, J.-X.; Zhou, W.-W. Isolation, characterization and cytoprotective effects against UV radiation of exopolysaccharide produced from Paenibacillus polymyxa PYQ1. J. Biosci. Bioeng. 2020, 130, 283–289. [Google Scholar] [CrossRef]
- Grinev, V.S.; Tregubova, K.V.; Anis’kov, A.A.; Sigida, E.N.; Shirokov, A.A.; Fedonenko, Y.P.; Yegorenkova, I.V. Isolation, structure, and potential biotechnological applications of the exopolysaccharide from Paenibacillus polymyxa 92. Carbohydr. Polym. 2020, 232, 115780. [Google Scholar] [CrossRef]
- Acosta, M.P.; Valdman, E.; Leite, S.; Battaglini, F.; Ruzal, S. Biosorption of copper by Paenibacillus polymyxa cells and their exopolysaccharide. World J. Microbiol. Biotechnol. 2005, 21, 1157–1163. [Google Scholar] [CrossRef]
- Puri, A.; Padda, K.P.; Chanway, C.P. Evidence of nitrogen fixation and growth promotion in canola (Brassica napus L.) by an endophytic diazotroph Paenibacillus polymyxa P2b-2R. Biol. Fertil. Soils 2016, 52, 119–125. [Google Scholar] [CrossRef]
- Jeong, H.; Choi, S.-K.; Ryu, C.-M.; Park, S.-H. Chronicle of a soil bacterium: Paenibacillus polymyxa E681 as a tiny guardian of plant and human health. Front. Microbiol. 2019, 10, 467. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ujor, V.C. Complete Genome Sequence of Paenibacillus polymyxa DSM 365, a soil bacterium of agricultural and industrial importance. Microbiol. Resour. Announc. 2022, 11, e0032922. [Google Scholar] [CrossRef]
- Pasari, N.; Gupta, M.; Eqbal, D.; Yazdani, S.S. Genome analysis of Paenibacillus polymyxa A18 gives insights into the features associated with its adaptation to the termite gut environment. Sci. Rep. 2019, 9, 6091. [Google Scholar] [CrossRef]
- Wang, X.-X.; Hu, H.-Y.; Liu, D.-H.; Song, Y.-Q. The implementation of high fermentative 2,3-butanediol production from xylose by simultaneous additions of yeast extract, Na2EDTA, and acetic acid. New Biotechnol. 2016, 33, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Okonkwo, C.C. Process Development and Metabolic Engineering to Enhance 2,3-Butanediol Production by Paenibacillus polymyxa DSM 365. PhD Dissertation, The Ohio State University, Wooster, OH, USA, 2017. Available online: https://www.proquest.com/docview/2458764038/fulltextPDF/DC07C1D7989342BEPQ/1?accountid=8120 (accessed on 1 December 2022).
- Psaki, O.; Maina, S.; Vlysidis, A.; Papanikolaou, S.; Machado de Castro, A.; Freire, D.M.G.; Dheskali, E.; Kookos, I.; Koutinas, A. Optimization of 2,3-butanediol production by Enterobacter ludwigii using sugarcane molasses. Biochem. Eng. J. 2019, 152, 107370. [Google Scholar] [CrossRef]



| Components/Stock (g/L) a | YE (g/L) | Tryp (g/L) | KH2PO4 (g/L) | K2HPO4 (g/L) | (NH4)2SO4 (g/L) | CH3CO NH4 | MgSO4 (g/L) | CoCl2 (g/L) | MOPS (g/L) | Trace Element | BC (g/L) | ADE (% Vol.) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatments b | (g/L) | (mL/L) | ||||||||||
| A: glucose media containing ADE | ||||||||||||
| Ctrl | 5.0 | 3.5 | 3.5 | 2.75 | 3.0 | 4.0 | 0.2 | 0.05 | 10.0 | 3.0 | -- | |
| 0-ADE | - | - | - | - | - | - | 0.2 | - | - | 3.0 | -- | |
| 25-ADE | - | - | - | - | - | - | 0.2 | - | - | 3.0 | 25.0 | |
| 50-ADE | - | - | - | - | - | - | 0.2 | - | - | 3.0 | 50.0 | |
| 75-ADE | - | - | - | - | - | - | 0.2 | - | - | 3.0 | 75.0 | |
| 100-ADE | - | - | - | - | - | - | 0.2 | - | - | 3.0 | 100.0 | |
| B: glucose media containing biochar | ||||||||||||
| Ctrl A | 5.0 | 3.5 | 3.5 | 2.75 | 3.0 | 4.0 | 0.2 | 0.05 | 10.0 | 3.0 | - | - |
| Ctrl B | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | - | - |
| 5-PLBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 5.0 | - |
| 10-PLBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 10.0 | - |
| 20-PLBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 20.0 | - |
| 5-FSBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 5.0 | - |
| 10-FSBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 10.0 | - |
| 20-FSBC | 5.0 | 3.5 | - | - | - | - | - | - | - | 3.0 | 20.0 | - |
| C: glucose media containing ADE and biochar | ||||||||||||
| Ctrl A | 5.0 | 3.5 | 3.5 | 2.75 | 3.0 | 4.0 | 0.2 | 0.05 | 10.0 | 3.0 | - | - |
| Ctrl B | - | - | - | - | - | - | - | - | - | 3.0 | - | - |
| 75-ADE + 5-PLBC | - | - | - | - | - | - | - | - | - | 3.0 | 5.0 | 75.0 |
| 75-ADE + 10-FSBC | - | - | - | - | - | - | - | - | - | 3.0 | 10.0 | 75.0 |
| 5-PLBC | 3.0 | 5.0 | - | |||||||||
| 10-FSBC | 3.0 | 10.0 | - | |||||||||
| D: glucose media containing ADE, biochar, and organic nitrogen supplementation | ||||||||||||
| Ctrl A | 5.0 | 3.5 | 3.5 | 2.75 | 3.0 | 4.0 | 0.2 | 0.05 | 10.0 | 3.0 | - | - |
| Ctrl B | - | - | - | - | - | - | - | - | - | 3.0 | - | - |
| 75-ADE + 5-PLBC + 12.5% N | 0.63 | 0.44 | - | - | - | - | - | - | - | 3.0 | 5.0 | 75.0 |
| 75-ADE + 5-PLBC + 25% N | 1.25 | 0.88 | - | - | - | - | - | - | - | 3.0 | 5.0 | 75.0 |
| 75-ADE + 5-PLBC + 50% N | 2.5 | 1.75 | - | - | - | - | - | - | - | 3.0 | 5.0 | 75.0 |
| 75-ADE + 10-FSBC + 12.5% N | 0.63 | 0.44 | - | - | - | - | - | - | - | 3.0 | 10.0 | 75.0 |
| 75-ADE + 10-FSBC + 25% N | 1.25 | 0.88 | - | - | - | - | - | - | - | 3.0 | 10.0 | 75.0 |
| 75-ADE + 10-FSBC + 50% N | 2.5 | 1.75 | - | - | - | - | - | - | - | 3.0 | 10.0 | 75.0 |
| Production Scenario | Media Composition |
|---|---|
| Control | Mineral/elemental nutrients |
| Scenario I | 5 g/L FSBC + organic N |
| Scenario II | 5 g/L PLBC + organic N |
| Scenario III | 75% ADE + 10 g/L FSBC |
| Scenario IV | 75% ADE + 10 g/L FSBC + 12.5% organic N |
| Scenario V | 75% ADE only |
| Parameters | Price ($/Kg) | References |
|---|---|---|
| Yeast Extract | 2.30 | [25] a |
| Tryptone | 1.65 | [22] |
| Ammonium Sulfate | 0.08 | [25] a |
| Ammonium Acetate | 1.25 | [26] b |
| Potassium Phosphate Monobasic | 1.82 | [22] |
| Potassium Phosphate Dibasic | 74.77 | [27] c |
| Glucose | 0.77 | [28] |
| Cobalt Chloride | 14.00 | [28] |
| MOPS Sodium Salt | 0.18 | [22] |
| NaOH | 0.33 | [23] |
| Magnesium sulfate | 0.35 | [25] a |
| Trace Elements | 0.22 | [16] |
| Biochar | 0.10 | [16] |
| Anaerobic Digestion Effluent (ADE) | 0.11 | [24] |
| Water | 0.0003 | [29] |
| Inoculum | 0.005 | [21] |
| Ethanol credit | 0.84 | [21] |
| Medium A * | Ctrl | 0% ADE | 25% ADE | 50% ADE | 75% ADE | 100% ADE |
|---|---|---|---|---|---|---|
| Max. OD | 7.8 ± 0.3 a | 2.2 ± 0.1 b | 1.8 ± 0.1 b | 2.1 ± 0.1 b | 3.0 ± 0.4 b | 2.5 ± 0.1 b |
| Ethanol (g/L) | 7.6 ± 0.5 a | 0.6 ± 0.1 b | 1.3 ± 0.3 b | 2.0 ± 0.7 b | 2.0 ± 0.3 b | 2.0 ± 0.7 b |
| Acetoin (g/L) | 1.0 ± 0.2 a | 0.0 ± 0.0 b | 1.4 ± 0.1 a | 1.7 ± 0.2 a | 2.2 ± 0.4 b | 1.7 ± 0.1 a |
| EPS (g/L) | 2.3 ± 0.1 a | 0.9 ± 0.4 a | 3.7 ± 1.7 a | 4.6 ± 1.1 b | 7.3 ± 1.6 b | 4.8 ± 0.5 b |
| Glucose 0 h (g/L) | 114.4 ± 2.2 a | 115.4 ± 2.6 a | 106.5 ± 5.9 b | 113.3 ± 5.4 a | 110.7 ± 8.6 a | 99.6 ± 1.4 b |
| Glucose U (g/L) | 105.5 ± 5.0 a | 18.3 ± 4.7 b | 27.9 ± 3.2 b | 42.1 ± 7.7 b | 57.6 ± 6.2 b | 59.5 ± 2.7 b |
| 2,3-BD (g/L) | 32.5 ± 1.5 a | 4.2 ± 0.2 b | 6.6 ± 0.8 b | 10.4 ± 1.2 b | 16.7 ± 1.0 b | 14.2 ± 0.7 b |
| 2,3-BD yield (g/g) | 0.31 ± 0.01 a | 0.20 ± 0.00 b | 0.25 ± 0.00 b | 0.22 ± 0.02 b | 0.27 ± 0.01 b | 0.24 ± 0.00 b |
| 2,3-BD PT (g/L/h) | 0.30 ± 0.01 a | 0.05 ± 0.00 b | 0.10 ± 0.01 b | 0.10 ± 0.01 b | 0.23 ± 0.01 b | 0.13 ± 0.01 b |
| * Medium | Ctrl A | Ctrl B | 5-PLBC | 10-PLBC | 20-PLBC | 5-FSBC | 10-FSBC | 20-FSBC |
|---|---|---|---|---|---|---|---|---|
| Max. OD | 7.8 ± 0.7 a | 4.7 ± 0.1 bc | 6.7 ± 0.3 ab | 5.2 ± 0.3 bc | 4.6 ± 0.1 c | 5.3 ± 0.8 bc | 5.4 ± 0.2 bc | 5.5 ± 0.5 bc |
| Ethanol (g/L) | 5.6 ± 0.4 a | 4.5 ± 1.4 a | 8.0 ± 1.2 ab | 9.7 ± 1.4 abc | 5.0 ± 1.3 a | 5.1 ± 1.7 ab | 11.4 ± 0.9 bc | 12.8 ± 0.2 c |
| Acetoin (g/L) | 4.0 ± 0.0 ab | 1.0 ± 0.4 b | 5.2 ± 2.5 ab | 1.2 ± 0.0 b | 2.8 ± 1.1 ab | 4.6 ± 1.1 ab | 6.8 ± 0.7 a | 6.2 ± 0.5 a |
| EPS (g/L) | 1.8 ± 0.3 a | 1.7 ± 0.7 a | 1.6 ± 0.6 a | 2.0 ± 0.8 a | 4.0 ± 1.2 ab | 6.1 ± 0.8 b | 2.6 ± 0.2 a | 3.3 ± 0.2 a |
| Glucose 0 h (g/L) | 115.5 ± 3.7 a | 122.0 ± 5.5 a | 115.6 ± 2.6 a | 100.2 ± 1.6 b | 120.7 ± 4.5 a | 119.4 ± 9.2 a | 118.9 ± 5.4 a | 117.0 ± 1.1 a |
| Glucose utilized (g/L) | 108.8 ± 9.0 a | 76.3 ± 9.7 b | 110.6 ± 3.5 a | 94.6 ± 2.2 ab | 86.0 ± 1.9 b | 103.1 ± 3.7 a | 113.1 ± 0.7 a | 115.6 ± 2.6 a |
| 2,3-BD (g/L) | 33.6 ± 1.3 ab | 25.9 ± 9.0 b | 41.9 ± 1.0 a | 32.8 ± 1.7 ab | 30.6 ± 4.6 ab | 38.7 ± 1.6 a | 42.1 ± 1.9 a | 37.1 ± 1.5 a |
| 2,3-BD yield (g/g) | 0.33 ± 0.01 a | 0.34 ± 0.10 a | 0.38 ± 0.01 a | 0.34 ± 0.01 a | 0.36 ± 0.05 a | 0.38 ± 0.02 a | 0.37 ± 0.02 a | 0.32 ± 0.01 a |
| 2,3-BD PT (g/L/h) | 0.37 ± 0.04 ab | 0.31 ± 0.08 ab | 0.58 ± 0.01 c | 0.38 ± 0.02 ab | 0.32 ± 0.05 abc | 0.46 ± 0.02 ac | 0.50 ± 0.02 abc | 0.44 ± 0.02 b |
| * Medium | Ctrl A | Ctrl B | 75-ADE+ 5-PLBC | 75-ADE+ 10-FSBC | 5-PLBC | 10-FSBC |
|---|---|---|---|---|---|---|
| Max. OD | 7.9 ± 0.9 a | 1.6 ± 0.1 b | 3.1 ± 0.2 c | 3.3 ± 0.8 c | 1.4 ± 0.1 b | 1.6 ± 0.2 b |
| Ethanol (g/L) | 6.3 ± 0.6 a | 0.2 ± 0.0 b | 2.0 ± 0.4 c | 1.9 ± 1.4 cd | 0.7 ± 0.1 de | 1.2 ± 0.2 be |
| Acetoin (g/L) | 3.2 ± 0.0 a | 0.1 ± 0.0 b | 5.7 ± 1.2 c | 4.0 ± 0.4 ac | 2.3 ± 0.0 ab | 2.7 ± 0.8 a |
| EPS (g/L) | 1.9 ± 0.2 a | 0.7 ± 0.2 b | 6.4 ± 0.3 c | 8.1 ± 0.1 d | 1.5 ± 0.1 e | 2.1 ± 0.3 a |
| Glucose 0 h (g/L) | 103.3 ± 2.2 a | 97.4 ± 4.9 ab | 115.4 ± 3.1 ac | 106.0 ± 6.0 a | 104.1 ± 7.0 a | 100.1 ± 2.0 |
| Glucose utilized (g/L) | 93.2 ± 1.1 a | 10.3 ± 1.5 b | 52.2 ± 0.1 c | 61.8 ± 1.9 d | 25.1 ± 5.4 e | 32.1 ± 5.2 ce |
| 2,3-BD (g/L) | 32.2 ± 6.3 a | 2.5 ± 0.1 b | 18.1 ± 1.5 cd | 22.4 ± 1.0 c | 7.7 ± 1.5 be | 11.3 ± 1.6 de |
| 2,3-BD yield (g/g) | 0.35 ± 0.07 a | 0.25 ± 0.03 a | 0.39 ± 0.00 a | 0.35 ± 0.02 a | 0.31 ± 0.01 a | 0.35 ± 0.03 a |
| 2,3-BD PT (g/L/h) | 0.38 ± 0.07 a | 0.11 ± 0.00 b | 0.21 ± 0.02 cd | 0.26 ± 0.02 c | 0.09 ± 0.02 b | 0.13 ± 0.02 bd |
| * Medium | Ctrl A | Ctrl B | 75-ADE+ 5-PLBC+ | 75-ADE+ 5-PLBC+ | 75-ADE+ 5-PLBC+ | 75-ADE+ 10-FSBC+ | 75-ADE+ 10-FSBC+ | 75-ADE+ 10-FSBC+ |
|---|---|---|---|---|---|---|---|---|
| +12.5% N | +25% N | +50% N | +12.5% N | +25% N | +50% N | |||
| Max. OD | 7.9 ± 0.9 a | 1.6 ± 0.1 b | 4.8 ± 0.3 c | 8.9 ± 0.6 a | 7.8 ± 0.5 ac | 5.7 ± 0.3 c | 8.8 ± 1.9 a | 8.5 ± 0.1 a |
| Ethanol (g/L) | 6.3 ± 0.6 a | 0.2 ± 0.0 b | 3.6 ± 0.1 c | 4.2 ± 0.6 c | 6.9 ± 1.5 a | 2.0 ± 0.5 bc | 3.4 ± 0.7 c | 7.8 ± 1.6 a |
| Acetoin (g/L) | 3.2 ± 0.0 a | 0.1 ± 0.0 b | 1.5 ± 0.4 c | 6.8 ± 0.4 d | 6.0 ± 1.4 d | 5.3 ± 0.4 d | 6.7 ± 0.2 d | 9.7 ± 2.6 e |
| EPS (g/L) | 2.1 ± 0.1 a | 1.0 ± 0.1 b | 5.3 ± 0.4 c | 6.1 ± 0.3 c | 7.0 ± 0.1 cd | 5.8 ± 0.1 c | 5.4 ± 0.3 c | 6.5 ± 0.2 cd |
| Glucose (g/L) | 103.3 ± 2.2 a | 97.4 ± 4.9 a | 112.0 ± 1.7 a | 112.1 ± 5.0 a | 107.5 ± 11.9 a | 113.4 ± 0.8 ab | 108.1 ± 7.5 a | 111.4 ± 3.6 a |
| Glucose utilized (g/L) | 93.2 ± 1.1 a | 10.3 ± 1.5 b | 76.6 ± 6.9 c | 96.4 ± 1.5 a | 101.7 ± 2.7 a | 99.1 ± 3.6 a | 106.0 ± 9.6 a | 99.0 ± 0.5 a |
| 2,3-BD (g/L) | 32.2 ± 6.3 ab | 2.5 ± 0.1 c | 22.9 ± 1.6 d | 31.4 ± 3.1 b | 31.9 ± 2.5 ab | 37.1 ± 1.5 a | 36.0 ± 0.9 ab | 34.3 ± 1.2 ab |
| 2,3-BD yield (g/g) | 0.35 ± 0.07 abc | 0.25 ± 0.03 c | 0.30 ± 0.04 bc | 0.33 ± 0.03 ab | 0.30 ± 0.02 bc | 0.37 ± 0.00 a | 0.34 ± 0.02 ab | 0.35 ± 0.01 ab |
| 2,3-BD PT (g/L/h) | 0.38 ± 0.07 a | 0.11 ± 0.00 b | 0.27 ± 0.02 a | 0.52 ± 0.05 c | 0.52 ± 0.04 c | 0.62 ± 0.03 d | 0.60 ± 0.01 cd | 0.57 ± 0.02 cd |
| Production Scenario | Conc. of 2,3-BD after Fermentation (g/L) | Cost without Distillation | Cost with Distillation | Distillation Cost ($/L) b | ||
|---|---|---|---|---|---|---|
| $/L | Fold ↓ in Cost a | $/L | Fold ↓ in Cost a | |||
| Control | 32.5 | 5.96 | - | 8.38 | - | 2.42 |
| Scenario I | 38.7 | 2.91 | 2.05 | 4.14 | 2.03 | 1.23 |
| Scenario II | 41.9 | 2.63 | 2.27 | 3.73 | 2.24 | 1.11 |
| Scenario III | 22.4 | 2.70 | 2.21 | 4.63 | 1.81 | 1.94 |
| Scenario IV | 37.1 | 3.26 | 1.83 | 4.98 | 1.68 | 1.73 |
| Scenario V | 16.7 | 3.42 | 1.74 | 5.95 | 1.41 | 2.53 |
| Percentage of Total Operating Cost | Control | Scenario I | Scenario II | Scenario III | Scenario IV | Scenario V |
|---|---|---|---|---|---|---|
| Raw Materials (%) | 56.6 | 67.2 | 67.2 | 71.7 | 58.6 | 70.8 |
| Labor-dependent (%) | 0.4 | 0.8 | 0.8 | 0.8 | 0.7 | 0.7 |
| Facility-dependent (%) | 31.9 | 23.3 | 22.8 | 20.8 | 30.2 | 21.3 |
| Laboratory/QC/QA (%) | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Utilities (%) | 11.1 | 8.6 | 9.0 | 6.5 | 10.4 | 7.1 |
| Total operating cost (%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Total operating cost (×106 $) | 247.6 | 122.9 | 113.7 | 112.9 | 134.2 | 143.2 |
| Percentage of Total Operating Cost | Control | Scenario I | Scenario II | Scenario III | Scenario IV | Scenario V |
|---|---|---|---|---|---|---|
| Raw Materials (%) | 37.5 | 47.6 | 47.9 | 42.5 | 38.4 | 41.7 |
| Labor-dependent (%) | 0.3 | 0.6 | 0.6 | 0.5 | 0.5 | 0.4 |
| Facility-dependent (%) | 22.8 | 16.6 | 16.3 | 12.2 | 19.8 | 12.3 |
| * Facility-dependent (distillation) (%) | 30.3 | 26.9 | 28.0 | 35.3 | 34.1 | 35.8 |
| Laboratory/QC/QA (%) | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Utilities (%) | 7.9 | 6.1 | 6.4 | 3.8 | 6.8 | 4.1 |
| Utilities (distillation) (%) | 1.2 | 2.1 | 0.6 | 5.5 | 0.4 | 5.6 |
| Total operating cost (%) | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Total operating cost (×106 $) | 346.1 | 173.0 | 159.1 | 192.3 | 205.0 | 247.5 |
| Control | Scenario I | Scenario II | Scenario III | Scenario IV | Scenario V | |
|---|---|---|---|---|---|---|
| Total Glucose consumed (MT/yr) | 124,727 | 107,443 | 107,443 | 118,555 | 112,301 | 148,655 |
| Total 2,3-BD produced (MT/yr) | 41,000 | 41,000 | 41,000 | 41,000 | 41,000 | 41,000 |
| Yield (MT 2,3-BD/MT glucose) | 0.33 | 0.38 | 0.38 | 0.35 | 0.37 | 0.28 |
| Glucose utilization (MT glucose/MT 2,3-BD) | 3.03 | 2.63 | 2.63 | 2.86 | 2.70 | 3.57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonkwo, C.C.; Duduyemi, A.; Ujor, V.C.; Atiyeh, H.K.; Iloba, I.; Qureshi, N.; Ezeji, T.C. From Agricultural Wastes to Fermentation Nutrients: A Case Study of 2,3-Butanediol Production. Fermentation 2023, 9, 36. https://doi.org/10.3390/fermentation9010036
Okonkwo CC, Duduyemi A, Ujor VC, Atiyeh HK, Iloba I, Qureshi N, Ezeji TC. From Agricultural Wastes to Fermentation Nutrients: A Case Study of 2,3-Butanediol Production. Fermentation. 2023; 9(1):36. https://doi.org/10.3390/fermentation9010036
Chicago/Turabian StyleOkonkwo, Christopher Chukwudi, Ademola Duduyemi, Victor Chinomso Ujor, Hasan K. Atiyeh, Ifeanyi Iloba, Nasib Qureshi, and Thaddeus Chukwuemeka Ezeji. 2023. "From Agricultural Wastes to Fermentation Nutrients: A Case Study of 2,3-Butanediol Production" Fermentation 9, no. 1: 36. https://doi.org/10.3390/fermentation9010036
APA StyleOkonkwo, C. C., Duduyemi, A., Ujor, V. C., Atiyeh, H. K., Iloba, I., Qureshi, N., & Ezeji, T. C. (2023). From Agricultural Wastes to Fermentation Nutrients: A Case Study of 2,3-Butanediol Production. Fermentation, 9(1), 36. https://doi.org/10.3390/fermentation9010036