Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids
Abstract
1. Introduction
2. Arrested Anaerobic Digestion
2.1. Understanding Methanogenesis and Its Inhibition
- the hydrogenotrophic pathway where CO2 is reduced to CH4, with H2 acting as the electron donor.
- In the aceticlastic pathway, CH4 is produced from acetate.
- In the methylotropic pathway, methylated compounds are reduced to CH4.
2.2. Parameters Affecting Arrested Methanogenesis
2.2.1. pH
2.2.2. HRT
2.3. New Emerging Technologies
2.3.1. Bioaugmentation
2.3.2. Electro-Fermentation
2.3.3. Re-Wiring Hydrogen Fermentation for VFA
3. Extraction and Purification of VFA
3.1. Adsorption
3.2. Membrane-Based Technologies
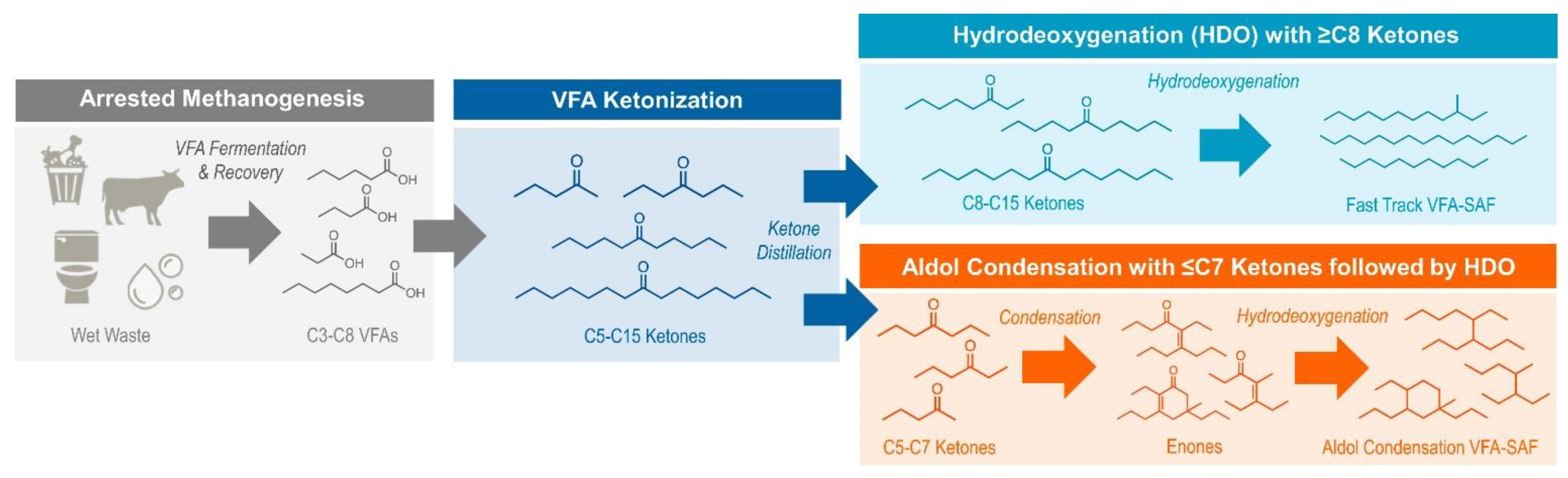
4. Role of VFA in Producing Sustainable Aviation Fuel
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perez-Zabaleta, M.; Khatami, K.; Cetecioglu, Z. From waste to bioplastics: Bio-based conversion of volatile fatty acids to polyhydroxyalkanoates. Access Microbiol. 2020, 2, 1030. [Google Scholar] [CrossRef]
- Agnihotri, S.; Yin, D.-M.; Mahboubi, A.; Sapmaz, T.; Varjani, S.; Qiao, W.; Koseoglu-Imer, D.Y.; Taherzadeh, M.J. A Glimpse of the World of Volatile Fatty Acids Production and Application: A review. Bioengineered 2022, 13, 1249–1275. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Yang, Y.-H. Microbial production of volatile fatty acids: Current status and future perspectives. Rev. Environ. Sci. Bio/Technol. 2017, 16, 327–345. [Google Scholar] [CrossRef]
- Kim, N.-J.; Lim, S.-J.; Chang, H.N. Volatile Fatty Acid Platform: Concept and Application. In Emerging Areas in Bioengineering; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2018; pp. 173–190. [Google Scholar]
- Veluswamy, G.K.; Shah, K.; Ball, A.S.; Guwy, A.J.; Dinsdale, R.M. A techno-economic case for volatile fatty acid production for increased sustainability in the wastewater treatment industry. Environ. Sci. Water Res. Technol. 2021, 7, 927–941. [Google Scholar] [CrossRef]
- Wang, W.-C.; Tao, L. Bio-jet fuel conversion technologies. Renew. Sustain. Energy Rev. 2016, 53, 801–822. [Google Scholar] [CrossRef]
- Fernandes, L.R.; Gomes, A.C.; Lopes, A.; Albuquerque, A.; Simões, R.M. Sugar and volatile fatty acids dynamic during anaerobic treatment of olive mill wastewater. Environ. Technol. 2016, 37, 997–1007. [Google Scholar] [CrossRef]
- Chang, H.N.; Kim, N.-J.; Kang, J.; Jeong, C.M. Biomass-derived volatile fatty acid platform for fuels and chemicals. Biotechnol. Bioprocess Eng. 2010, 15, 1–10. [Google Scholar] [CrossRef]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; Cardayre, S.B.d. Microbial Biosynthesis of Alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef]
- Consortium, N.A.B. Fermentation of Lignocellulosic Sugars Process Strategy. Retrieved March 2012, 6, 2013. [Google Scholar]
- Yang, J.; Xin, Z.; He, Q.; Corscadden, K.; Niu, H. An overview on performance characteristics of bio-jet fuels. Fuel 2019, 237, 916–936. [Google Scholar] [CrossRef]
- Krishnan, M.S.; Ho, N.W.; Tsao, G.T. Fermentation kinetics of ethanol production from glucose and xylose by recombinant Saccharomyces 1400(pLNH33). Appl. Biochem. Biotechnol. 1999, 77–79, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J.; Rova, U.; Christakopoulos, P.; Matsakas, L. Volatile Fatty Acids (VFAs) Generated by Anaerobic Digestion Serve as Feedstock for Freshwater and Marine Oleaginous Microorganisms to Produce Biodiesel and Added-Value Compounds. Front. Microbiol. 2021, 12, 614612. [Google Scholar] [CrossRef] [PubMed]
- Murali, N.; Srinivas, K.; Ahring, B.K. Biochemical Production and Separation of Carboxylic Acids for Biorefinery Applications. Fermentation 2017, 3, 22. [Google Scholar] [CrossRef]
- National Overview: Facts and Figures on Materials, Wastes and Recycling. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/national-overview-facts-and-figures-materials#NationalPicture (accessed on 2 October 2022).
- Ahring, B.K.; Ibrahim, A.A.; Mladenovska, Z. Effect of temperature increase from 55 to 65 °C on performance and microbial population dynamics of an anaerobic reactor treating cattle manure. Water Res. 2001, 35, 2446–2452. [Google Scholar] [CrossRef]
- Malik, S.N.; Madhu, K.; Mhaisalkar, V.A.; Vaidya, A.N.; Mudliar, S.N. Pretreatment of yard waste using advanced oxidation processes for enhanced biogas production. Biomass Bioenergy 2020, 142, 105780. [Google Scholar] [CrossRef]
- Lee, J.T.E.; Khan, M.U.; Dai, Y.; Tong, Y.W.; Ahring, B.K. Influence of wet oxidation pretreatment with hydrogen peroxide and addition of clarified manure on anaerobic digestion of oil palm empty fruit bunches. Bioresour. Technol. 2021, 332, 125033. [Google Scholar] [CrossRef]
- Khan, M.U.; Ahring, B.K. Anaerobic Digestion of Digested Manure Fibers: Influence of Thermal and Alkaline Thermal Pretreatment on the Biogas Yield. BioEnergy Res. 2021, 14, 891–900. [Google Scholar] [CrossRef]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef]
- Kuruti, K.; Nakkasunchi, S.; Begum, S.; Juntupally, S.; Arelli, V.; Anupoju, G.R. Rapid generation of volatile fatty acids (VFA) through anaerobic acidification of livestock organic waste at low hydraulic residence time (HRT). Bioresour. Technol. 2017, 238, 188–193. [Google Scholar] [CrossRef]
- Cheah, Y.-K.; Vidal-Antich, C.; Dosta, J.; Mata-Álvarez, J. Volatile fatty acid production from mesophilic acidogenic fermentation of organic fraction of municipal solid waste and food waste under acidic and alkaline pH. Environ. Sci. Pollut. Res. 2019, 26, 35509–35522. [Google Scholar] [CrossRef]
- Kuruti, K.; Gangagni Rao, A.; Gandu, B.; Kiran, G.; Mohammad, S.; Sailaja, S.; Swamy, Y.V. Generation of bioethanol and VFA through anaerobic acidogenic fermentation route with press mud obtained from sugar mill as a feedstock. Bio Technol. 2015, 192, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Demirel, B.; Yenigun, O. Anaerobic acidogenesis of dairy wastewater: The effects of variations in hydraulic retention time with no pH control. J. Chem. Technol. Biotechnol. 2004, 79, 755–760. [Google Scholar] [CrossRef]
- Murali, N.; Fernandez, S.; Ahring, B.K. Fermentation of wet-exploded corn stover for the production of volatile fatty acids. Bioresour. Technol. 2017, 227, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Murali, N.; Srinivas, K.; Ahring, B.K. Increasing the Production of Volatile Fatty Acids from Corn Stover Using Bioaugmentation of a Mixed Rumen Culture with Homoacetogenic Bacteria. Microorganisms 2021, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- Van, D.P.; Fujiwara, T.; Leu Tho, B.; Song Toan, P.P.; Hoang Minh, G. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef]
- Wainaina, S.; Lukitawesa; Kumar Awasthi, M.; Taherzadeh, M.J. Bioengineering of anaerobic digestion for volatile fatty acids, hydrogen or methane production: A critical review. Bioengineered 2019, 10, 437–458. [Google Scholar] [CrossRef]
- Ramos-Suarez, M.; Zhang, Y.; Outram, V. Current perspectives on acidogenic fermentation to produce volatile fatty acids from waste. Rev. Environ. Sci. Bio/Technol. 2021, 20, 439–478. [Google Scholar] [CrossRef]
- Braga Nan, L.; Trably, E.; Santa-Catalina, G.; Bernet, N.; Delgenès, J.-P.; Escudié, R. Biomethanation processes: New insights on the effect of a high H2 partial pressure on microbial communities. Biotechnol. Biofuels 2020, 13, 141. [Google Scholar] [CrossRef]
- Holtzapple, M.T.; Davison, R.R.; Ross, M.K.; Albrett-Lee, S.; Nagwani, M.; Lee, C.M.; Lee, C.; Adelson, S.; Kaar, W.; Gaskin, D.; et al. Biomass conversion to mixed alcohol fuels using the MixAlco process. Appl. Biochem. Biotechnol. 1999, 77–79, 609–631. [Google Scholar] [CrossRef]
- Taco Vasquez, S.; Dunkleman, J.; Chaudhuri, S.K.; Bond, A.; Holtzapple, M.T. Biomass conversion to hydrocarbon fuels using the MixAlco™ process at a pilot-plant scale. Biomass Bioenergy 2014, 62, 138–148. [Google Scholar] [CrossRef]
- Reyes, J.L.R. Rewiring Anaerobic Digestion: Production of Biofuel Intermediates and High-Value Chemicals from Cellulosic Wastes; Colarado State University: Fort Collins, CO, USA, 2019. [Google Scholar]
- Xu, Y.; He, Z. Enhanced volatile fatty acids accumulation in anaerobic digestion through arresting methanogenesis by using hydrogen peroxide. Water Environ. Res. 2021, 93, 2051–2059. [Google Scholar] [CrossRef] [PubMed]
- Glass, J.; Orphan, V. Trace Metal Requirements for Microbial Enzymes Involved in the Production and Consumption of Methane and Nitrous Oxide. Front. Microbiol. 2012, 3, 61. [Google Scholar] [CrossRef] [PubMed]
- Czatzkowska, M.; Harnisz, M.; Korzeniewska, E.; Koniuszewska, I. Inhibitors of the methane fermentation process with particular emphasis on the microbiological aspect: A review. Energy Sci. Eng. 2020, 8, 1880–1897. [Google Scholar] [CrossRef]
- Ziemiński, K.; Frac, M. Methane fermentation process as anaerobic digestion of biomass: Transformations, stages and microorganisms. Afr. J. Biotechnol. 2012, 11, 4127–4139. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. Bio/Technol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Chen, S.; He, Q. Persistence of Methanosaeta populations in anaerobic digestion during process instability. J. Ind. Microbiol. Biotechnol. 2015, 42, 1129–1137. [Google Scholar] [CrossRef]
- Di Maria, F. Chapter 3—The Recovery of Energy and Materials From Food Waste by Codigestion with Sludge: Internal Environment of Digester and Methanogenic Pathway. In Food Bioconversion; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2017; pp. 95–125. [Google Scholar]
- Ahring, B.; Murali, N.; Srinivas, K. Fermentation of Cellulose with a Mixed Microbial Rumen Culture with and without Methanogenesis. Ferment. Technol. 2018, 7. [Google Scholar] [CrossRef]
- Patra, A.; Park, T.; Kim, M.; Yu, Z. Rumen methanogens and mitigation of methane emission by anti-methanogenic compounds and substances. J. Anim. Sci. Biotechnol. 2017, 8, 13. [Google Scholar] [CrossRef]
- Jankowska, E.; Chwiałkowska, J.; Stodolny, M.; Oleskowicz-Popiel, P. Effect of pH and retention time on volatile fatty acids production during mixed culture fermentation. Bioresour. Technol. 2015, 190, 274–280. [Google Scholar] [CrossRef]
- Wu, H.; Dalke, R.; Mai, J.; Holtzapple, M.; Urgun-Demirtas, M. Arrested methanogenesis digestion of high-strength cheese whey and brewery wastewater with carboxylic acid production. Bioresour. Technol. 2021, 332, 125044. [Google Scholar] [CrossRef]
- Yin, J.; Wang, K.; Yang, Y.; Shen, D.; Wang, M.; Mo, H. Improving production of volatile fatty acids from food waste fermentation by hydrothermal pretreatment. Bioresour. Technol. 2014, 171, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, S.; Parchami, M.; Mahboubi, A.; Horváth, I.S.; Taherzadeh, M.J. Food waste-derived volatile fatty acids platform using an immersed membrane bioreactor. Bioresour. Technol. 2019, 274, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, M.; Eyice, O.; Cetecioglu, Z. A comprehensive study of volatile fatty acids production from batch reactor to anaerobic sequencing batch reactor by using cheese processing wastewater. Bioresour. Technol. 2020, 311, 123529. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.J.; Massanet-Nicolau, J.; Mulder, M.J.J.; Premier, G.; Dinsdale, R.; Guwy, A. Increased biohydrogen yields, volatile fatty acid production and substrate utilisation rates via the electrodialysis of a continually fed sucrose fermenter. Bioresour. Technol. 2017, 229, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Eryildiz, B.; Lukitawesa; Taherzadeh, M.J. Effect of pH, substrate loading, oxygen, and methanogens inhibitors on volatile fatty acid (VFA) production from citrus waste by anaerobic digestion. Bioresour. Technol. 2020, 302, 122800. [Google Scholar] [CrossRef] [PubMed]
- Cheah, Y.-K.; Dosta, J.; Mata-Álvarez, J. Enhancement of Volatile Fatty Acids Production from Food Waste by Mature Compost Addition. Molecules 2019, 24, 2986. [Google Scholar] [CrossRef]
- Dahiya, S.; Sarkar, O.; Swamy, Y.V.; Venkata Mohan, S. Acidogenic fermentation of food waste for volatile fatty acid production with co-generation of biohydrogen. Bioresour. Technol. 2015, 182, 103–113. [Google Scholar] [CrossRef]
- Yarimtepe, C.C.; Oz, N.A.; Ince, O. Volatile fatty acid production dynamics during the acidification of pretreated olive mill wastewater. Bioresource Technol. 2017, 241, 936–944. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, Y.; Zhou, J.; Xu, C.; Zhou, Q. Effects of pH on the hydrolysis of lignocellulosic wastes and volatile fatty acids accumulation: The contribution of biotic and abiotic factors. Bioresour. Technol. 2012, 110, 321–329. [Google Scholar] [CrossRef]
- Lukitawesa; Patinvoh, R.J.; Millati, R.; Sárvári-Horváth, I.; Taherzadeh, M.J. Factors influencing volatile fatty acids production from food wastes via anaerobic digestion. Bioengineered 2020, 11, 39–52. [Google Scholar] [CrossRef]
- Yang, X.; Liu, X.; Chen, S.; Liu, G.; Wu, S.; Wan, C. Volatile Fatty Acids Production from Codigestion of Food Waste and Sewage Sludge Based on <i>β</i>-Cyclodextrins and Alkaline Treatments. Archaea 2016, 2016, 1698163. [Google Scholar] [CrossRef] [PubMed]
- Magdalena, J.A.; Greses, S.; González-Fernández, C. Impact of Organic Loading Rate in Volatile Fatty Acids Production and Population Dynamics Using Microalgae Biomass as Substrate. Sci. Rep. 2019, 9, 18374. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.X.; Zhou, Y.; Vadivelu, V.M. Enhanced volatile fatty acid production and microbial population analysis in anaerobic treatment of high strength wastewater. J. Water Process Eng. 2020, 33, 101058. [Google Scholar] [CrossRef]
- Huang, X.; Shen, C.; Liu, J.; Lu, L. Improved volatile fatty acid production during waste activated sludge anaerobic fermentation by different bio-surfactants. Chem. Eng. J. 2015, 264, 280–290. [Google Scholar] [CrossRef]
- Wan, J.; Fang, W.; Zhang, T.; Wen, G. Enhancement of fermentative volatile fatty acids production from waste activated sludge by combining sodium dodecylbenzene sulfonate and low-thermal pretreatment. Bioresour. Technol. 2020, 308, 123291. [Google Scholar] [CrossRef]
- Ma, H.; Liu, H.; Zhang, L.; Yang, M.; Fu, B.; Liu, H. Novel insight into the relationship between organic substrate composition and volatile fatty acids distribution in acidogenic co-fermentation. Biotechnol. Biofuels 2017, 10, 137. [Google Scholar] [CrossRef]
- Yin, D.M.; Uwineza, C.; Sapmaz, T.; Mahboubi, A.; De Wever, H.; Qiao, W.; Taherzadeh, M.J. Volatile Fatty Acids (VFA) Production and Recovery from Chicken Manure Using a High-Solid Anaerobic Membrane Bioreactor (AnMBR). Membranes 2022, 12, 1133. [Google Scholar] [CrossRef]
- Koster, I.W.; Cramer, A. Inhibition of methanogenesis from acetate in granular sludge by long-chain Fatty acids. Appl. Environ. Microbiol. 1987, 53, 403–409. [Google Scholar] [CrossRef]
- Chiu, P.C.; Lee, M. 2-Bromoethanesulfonate affects bacteria in a trichloroethene-dechlorinating culture. Appl. Environ. Microbiol. 2001, 67, 2371–2374. [Google Scholar] [CrossRef][Green Version]
- Sarioglu, M.; Akkoyun, S.; Bisgin, T. Inhibition effects of heavy metals (copper, nickel, zinc, lead) on anaerobic sludge. Desalination Water Treat. 2010, 23, 55–60. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef]
- Koniuszewska, I.; Czatzkowska, M.; Harnisz, M.; Korzeniewska, E. The Impact of Antimicrobial Substances on the Methanogenic Community during Methane Fermentation of Sewage Sludge and Cattle Slurry. Appl. Sci. 2021, 11, 369. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Lichtfouse, E.; Li, Z.; Kumar, P.S.; Liu, J.; Feng, D.; Yang, Q.; Liu, F. Effect of Antibiotics on the Microbial Efficiency of Anaerobic Digestion of Wastewater: A Review. Front. Microbiol. 2021, 11, 611613. [Google Scholar] [CrossRef] [PubMed]
- Tice, R.C.; Kim, Y. Methanogenesis control by electrolytic oxygen production in microbial electrolysis cells. Int. J. Hydrog. Energy 2014, 39, 3079–3086. [Google Scholar] [CrossRef]
- Coban, H.; Ertekin, E.; Ince, O.; Turker, G.; Akyol, Ç.; Ince, B. Degradation of oxytetracycline and its impacts on biogas-producing microbial community structure. Bioprocess Biosyst. Eng. 2016, 39, 1051–1060. [Google Scholar] [CrossRef]
- Aydin, S.; Ince, B.; Ince, O. Application of real-time PCR to determination of combined effect of antibiotics on Bacteria, Methanogenic Archaea, Archaea in anaerobic sequencing batch reactors. Water Res. 2015, 76, 88–98. [Google Scholar] [CrossRef]
- Rusanowska, P.; Zieliński, M.; Dębowski, M.; Harnisz, M.; Korzeniewska, E.; Amenda, E. Inhibition of Methane Fermentation by Antibiotics Introduced to Municipal Anaerobic Sludge. Proceedings 2018, 2, 1274. [Google Scholar]
- Ettwig, K. Degradation of 2-Bromo-Ethane Sulfonate (BES) and 2-Mercapto-Ethane Sulfonate (Coenzyme M) by Anaerobic Enrichment Cultures; Marine Biological Laboratry, The University of Chicago: Woods Hole, MA, USA, 2006. [Google Scholar]
- Park, S.-G.; Rhee, C.; Shin, S.G.; Shin, J.; Mohamed, H.O.; Choi, Y.-J.; Chae, K.-J. Methanogenesis stimulation and inhibition for the production of different target electrobiofuels in microbial electrolysis cells through an on-demand control strategy using the coenzyme M and 2-bromoethanesulfonate. Environ. Int. 2019, 131, 105006. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, L.; Loh, K.-C. Review and perspectives of enhanced volatile fatty acids production from acidogenic fermentation of lignocellulosic biomass wastes. Bioresour. Bioprocess. 2021, 8, 68. [Google Scholar] [CrossRef]
- Taconi, K.A.; Zappi, M.E.; Todd French, W.; Brown, L.R. Methanogenesis under acidic pH conditions in a semi-continuous reactor system. Bioresour. Technol. 2008, 99, 8075–8081. [Google Scholar] [CrossRef] [PubMed]
- Horn, M.A.; Matthies, C.; Küsel, K.; Schramm, A.; Drake, H.L. Hydrogenotrophic methanogenesis by moderately acid-tolerant methanogens of a methane-emitting acidic peat. Appl. Environ. Microbiol. 2003, 69, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Staley, B.F.; Reyes, F.L.d.l.; Barlaz, M.A. Effect of Spatial Differences in Microbial Activity, pH, and Substrate Levels on Methanogenesis Initiation in Refuse. Appl. Environ. Microbiol. 2011, 77, 2381–2391. [Google Scholar] [CrossRef] [PubMed]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile Fatty Acid Production from Organic Waste with the Emphasis on Membrane-Based Recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Lv, N.; Cai, G.; Pan, X.; Li, Y.; Wang, R.; Li, J.; Li, C.; Zhu, G. pH and hydraulic retention time regulation for anaerobic fermentation: Focus on volatile fatty acids production/distribution, microbial community succession and interactive correlation. Bioresour. Technol. 2022, 347, 126310. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. The effects of pH on the production of volatile fatty acids and microbial dynamics in long-term reactor operation. J. Environ. Manag. 2022, 319, 115700. [Google Scholar] [CrossRef]
- Jie, W.; Peng, Y.; Ren, N.; Li, B. Volatile fatty acids (VFAs) accumulation and microbial community structure of excess sludge (ES) at different pHs. Bioresour. Technol. 2014, 152, 124–129. [Google Scholar] [CrossRef]
- Khatami, K.; Atasoy, M.; Ludtke, M.; Baresel, C.; Eyice, Ö.; Cetecioglu, Z. Bioconversion of food waste to volatile fatty acids: Impact of microbial community, pH and retention time. Chemosphere 2021, 275, 129981. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, Y.; Zhang, H.; Jiang, S.; Zhou, Q.; Gu, G. Improved Bioproduction of Short-Chain Fatty Acids (SCFAs) from Excess Sludge under Alkaline Conditions. Environ. Sci. Technol. 2006, 40, 2025–2029. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, D.-B.; Li, X.-M.; Yang, Q.; Chen, H.; Zhong, Y.; Zeng, G. Free Nitrous Acid Serving as a Pretreatment Method for Alkaline Fermentation to Enhance Short-Chain Fatty Acid Production from Waste Activated Sludge. Water Res. 2015, 78, 111–120. [Google Scholar] [CrossRef]
- Atasoy, M.; Eyice, O.; Schnürer, A.; Cetecioglu, Z. Volatile fatty acids production via mixed culture fermentation: Revealing the link between pH, inoculum type and bacterial composition. Bioresour. Technol. 2019, 292, 121889. [Google Scholar] [CrossRef] [PubMed]
- Lund, P.A.; De Biase, D.; Liran, O.; Scheler, O.; Mira, N.P.; Cetecioglu, Z.; Fernández, E.N.; Bover-Cid, S.; Hall, R.; Sauer, M.; et al. Understanding How Microorganisms Respond to Acid pH Is Central to Their Control and Successful Exploitation. Front. Microbiol. 2020, 11, 556140. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Das, D. Chapter 3—Potential of Hydrogen Production From Biomass. In Science and Engineering of Hydrogen-Based Energy Technologies; de Miranda, P.E.V., Ed.; Academic Press: London, UK, 2019; pp. 123–164. [Google Scholar]
- Khan, M.A.; Ngo, H.H.; Guo, W.; Liu, Y.; Nghiem, L.D.; Chang, S.W.; Nguyen, D.D.; Zhang, S.; Luo, G.; Jia, H. Optimization of hydraulic retention time and organic loading rate for volatile fatty acid production from low strength wastewater in an anaerobic membrane bioreactor. Bioresour. Technol. 2019, 271, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Swiatkiewicz, J.; Slezak, R.; Krzystek, L.; Ledakowicz, S. Production of Volatile Fatty Acids in a Semi-Continuous Dark Fermentation of Kitchen Waste: Impact of Organic Loading Rate and Hydraulic Retention Time. Energies 2021, 14, 2993. [Google Scholar] [CrossRef]
- Fitamo, T.; Boldrin, A.; Boe, K.; Angelidaki, I.; Scheutz, C. Co-digestion of food and garden waste with mixed sludge from wastewater treatment in continuously stirred tank reactors. Bioresour. Technol. 2016, 206, 245–254. [Google Scholar] [CrossRef]
- Bruni, C.; Foglia, A.; Eusebi, A.L.; Frison, N.; Akyol, Ç.; Fatone, F. Targeted Bio-Based Volatile Fatty Acid Production from Waste Streams through Anaerobic Fermentation: Link between Process Parameters and Operating Scale. ACS Sustain. Chem. Eng. 2021, 9, 9970–9987. [Google Scholar] [CrossRef]
- Volker, A.R.; Gogerty, D.S.; Bartholomay, C.; Hennen-Bierwagen, T.; Zhu, H.; Bobik, T.A. Fermentative production of short-chain fatty acids in Escherichia coli. Microbiology 2014, 160, 1513–1522. [Google Scholar] [CrossRef][Green Version]
- Ehsanipour, M.; Suko, A.V.; Bura, R. Fermentation of lignocellulosic sugars to acetic acid by Moorella thermoacetica. J. Ind. Microbiol. Biotechnol. 2016, 43, 807–816. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. Butyric acid dominant volatile fatty acids production: Bio-augmentation of mixed culture fermentation by Clostridium butyricum. J. Environ. Chem. Eng. 2020, 8, 104496. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. Bioaugmented Mixed Culture by Clostridium aceticum to Manipulate Volatile Fatty Acids Composition From the Fermentation of Cheese Production Wastewater. Front. Microbiol. 2021, 12, 658494. [Google Scholar] [CrossRef]
- Atasoy, M.; Cetecioglu, Z. Bioaugmentation as a strategy for tailor-made volatile fatty acid production. J. Environ. Manag. 2021, 295, 113093. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.H.; Ren, Z.J.; Tao, L. Value Proposition of Untapped Wet Wastes: Carboxylic Acid Production through Anaerobic Digestion. iScience 2020, 23, 101221. [Google Scholar] [CrossRef]
- Fu, B.; Jin, X.; Conrad, R.; Liu, H.; Liu, H. Competition Between Chemolithotrophic Acetogenesis and Hydrogenotrophic Methanogenesis for Exogenous H2/CO2 in Anaerobically Digested Sludge: Impact of Temperature. Front. Microbiol. 2019, 10, 2418. [Google Scholar] [CrossRef] [PubMed]
- Ren, N.Q.; Chua, H.; Chan, S.Y.; Tsang, Y.F.; Wang, Y.J.; Sin, N. Assessing optimal fermentation type for bio-hydrogen production in continuous-flow acidogenic reactors. Bioresour. Technol. 2007, 98, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Sravan, J.S.; Butti, S.K.; Sarkar, O.; Mohan, S.V. Chapter 5.1—Electrofermentation: Chemicals and Fuels. In Microbial Electrochemical Technology; Mohan, S.V., Varjani, S., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 723–737. [Google Scholar]
- Tanvir, R.U.; Ahmed, M.; Lim, T.T.; Li, Y.; Hu, Z. Chapter One—Arrested methanogenesis: Principles, practices, and perspectives. In Advances in Bioenergy; Li, Y., Zhou, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 7, pp. 1–66. [Google Scholar]
- Bhagchandanii, D.D.; Babu, R.P.; Sonawane, J.M.; Khanna, N.; Pandit, S.; Jadhav, D.A.; Khilari, S.; Prasad, R. A Comprehensive Understanding of Electro-Fermentation. Fermentation 2020, 6, 92. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of Wastes into Bioelectricity and Chemicals by Using Microbial Electrochemical Technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef]
- Hirano, S.; Matsumoto, N.; Morita, M.; Sasaki, K.; Ohmura, N. Electrochemical control of redox potential affects methanogenesis of the hydrogenotrophic methanogen Methanothermobacter thermautotrophicus. Lett. Appl. Microbiol. 2013, 56, 315–321. [Google Scholar] [CrossRef]
- Sasaki, D.; Morita, M.; Sasaki, K.; Watanabe, A.; Ohmura, N. Increased growth of a hydrogenotrophic methanogen in co-culture with a cellulolytic bacterium under cathodic electrochemical regulation. Biosci. Biotechnol. Biochem. 2013, 77, 1096–1099. [Google Scholar] [CrossRef][Green Version]
- Jiang, Y.; Lu, L.; Wang, H.; Shen, R.; Ge, Z.; Hou, D.; Chen, X.; Liang, P.; Huang, X.; Ren, Z.J. Electrochemical Control of Redox Potential Arrests Methanogenesis and Regulates Products in Mixed Culture Electro-Fermentation. ACS Sustain. Chem. Eng. 2018, 6, 8650–8658. [Google Scholar] [CrossRef]
- Yang, G.; Fang, H.; Wang, J.; Jia, H.; Zhang, H. Enhanced anaerobic digestion of up-flow anaerobic sludge blanket (UASB) by blast furnace dust (BFD): Feasibility and mechanism. Int. J. Hydrog. Energy 2019, 44, 17709–17719. [Google Scholar] [CrossRef]
- Wang, D.; Ma, W.; Han, H.; Li, K.; Xu, H.; Fang, F.; Hou, B.; Jia, S. Enhanced anaerobic degradation of Fischer-Tropsch wastewater by integrated UASB system with Fe-C micro-electrolysis assisted. Chemosphere 2016, 164, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Youcai, Z.; Ran, W. Chapter 2 - Bioproduction of volatile fatty acids from vegetable waste. In Biomethane Production from Vegetable and Water Hyacinth Waste; Youcai, Z., Ran, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 63–170. [Google Scholar]
- Rittmann, S.; Herwig, C. A comprehensive and quantitative review of dark fermentative biohydrogen production. Microb. Cell Factories 2012, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, K.; Mohd Soom, M.A. Trends in bio-hydrogen generation—A review. Environ. Sci. 2006, 3, 255–271. [Google Scholar] [CrossRef]
- Wang, A.-J.; Cao, G.-L.; Liu, W.-Z. Biohydrogen Production from Anaerobic Fermentation. In Biotechnology in China III: Biofuels and Bioenergy; Bai, F.-W., Liu, C.-G., Huang, H., Tsao, G.T., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 143–163. [Google Scholar]
- Patel, S.K.S.; Kalia, V.C. Integrative biological hydrogen production: An overview. Ind. J. Microbiol. 2013, 53, 3–10. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Fermentative hydrogen production using pretreated microalgal biomass as feedstock. Microb. Cell Factories 2018, 17, 22. [Google Scholar] [CrossRef] [PubMed]
- Osman, A.I.; Deka, T.J.; Baruah, D.C.; Rooney, D.W. Critical challenges in biohydrogen production processes from the organic feedstocks. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
- Ergal, İ.; Gräf, O.; Hasibar, B.; Steiner, M.; Vukotić, S.; Bochmann, G.; Fuchs, W.; Rittmann, S.K.M.R. Biohydrogen production beyond the Thauer limit by precision design of artificial microbial consortia. Commun. Biol. 2020, 3, 443. [Google Scholar] [CrossRef]
- Gavala, H.N.; Skiadas, I.V.; Ahring, B.K. Biological hydrogen production in suspended and attached growth anaerobic reactor systems. Int. J. Hydrog. Energy 2006, 31, 1164–1175. [Google Scholar] [CrossRef]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. The Future Perspectives of Dark Fermentation: Moving from Only Biohydrogen to Biochemicals. In Biorefinery: Integrated Sustainable Processes for Biomass Conversion to Biomaterials, Biofuels, and Fertilizers; Bastidas-Oyanedel, J.-R., Schmidt, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 375–412. [Google Scholar]
- Bastidas-Oyanedel, J.-R.; Bonk, F.; Thomsen, M.H.; Schmidt, J.E. Dark fermentation biorefinery in the present and future (bio)chemical industry. Rev. Environ. Sci. Bio/Technol. 2015, 14, 473–498. [Google Scholar] [CrossRef]
- Karekar, S.C.; Srinivas, K.; Ahring, B.K. Continuous in-situ extraction of acetic acid produced by Acetobacterium woodii during fermentation of hydrogen and carbon dioxide using Amberlite FPA53 ion exchange resins. Bioresour. Technol. Rep. 2020, 12, 100568. [Google Scholar] [CrossRef]
- Darwish, A.S.; Warrag, S.E.E.; Lemaoui, T.; Alseiari, M.K.; Hatab, F.A.; Rafay, R.; Alnashef, I.; Rodríguez, J.; Alamoodi, N. Green Extraction of Volatile Fatty Acids from Fermented Wastewater Using Hydrophobic Deep Eutectic Solvents. Fermentation 2021, 7, 226. [Google Scholar] [CrossRef]
- López-Garzón, C.S.; Straathof, A.J.J. Recovery of carboxylic acids produced by fermentation. Biotechnol. Adv. 2014, 32, 873–904. [Google Scholar] [CrossRef] [PubMed]
- Reyhanitash, E.; Zaalberg, B.; Ijmker, H.M.; Kersten, S.R.A.; Schuur, B. CO2-enhanced extraction of acetic acid from fermented wastewater. Green Chem. 2015, 17, 4393–4400. [Google Scholar] [CrossRef]
- Saboe, P.O.; Manker, L.P.; Michener, W.E.; Peterson, D.J.; Brandner, D.G.; Deutch, S.P.; Kumar, M.; Cywar, R.M.; Beckham, G.T.; Karp, E.M. In situ recovery of bio-based carboxylic acids. Green Chem. 2018, 20, 1791–1804. [Google Scholar] [CrossRef]
- Rocha, M.A.A.; Raeissi, S.; Hage, P.; Weggemans, W.M.A.; van Spronsen, J.; Peters, C.J.; Kroon, M.C. Recovery of volatile fatty acids from water using medium-chain fatty acids and a cosolvent. Chem. Eng. Sci. 2017, 165, 74–80. [Google Scholar] [CrossRef]
- Petersen, A.M.; Franco, T.; Görgens, J.F. Comparison of recovery of volatile fatty acids and mixed ketones as alternative downstream processes for acetogenisis fermentation. Biofuels Bioprod. Biorefining 2018, 12, 882–898. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Fufachev, E.; van Munster, K.D.; van Beek, M.B.M.; Sprakel, L.M.J.; Edelijn, C.N.; Weckhuysen, B.M.; Kersten, S.R.A.; Bruijnincx, P.C.A.; Schuur, B. Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater. Green Chem. 2019, 21, 2023–2034. [Google Scholar] [CrossRef]
- Fayad, N.; Yehya, T.; Audonnet, F.; Vial, C. Preliminary purification of volatile fatty acids in a digestate from acidogenic fermentation by electrocoagulation. Sep. Purif. Technol. 2017, 184, 220–230. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; Premier, G.C.; Dinsdale, R.M.; Reilly, M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015, 189, 279–284. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Zhang, X.; Feng, H.; Xu, T. In-situ combination of fermentation and electrodialysis with bipolar membranes for the production of lactic acid: Continuous operation. Bioresour. Technol. 2013, 147, 442–448. [Google Scholar] [CrossRef]
- Eregowda, T.; Rene, E.R.; Rintala, J.; Lens, P.N.L. Volatile fatty acid adsorption on anion exchange resins: Kinetics and selective recovery of acetic acid. Sep. Sci. Technol. 2020, 55, 1449–1461. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Kersten, S.R.A.; Schuur, B. Recovery of Volatile Fatty Acids from Fermented Wastewater by Adsorption. ACS Sustain. Chem. Eng. 2017, 5, 9176–9184. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Razali, Y.S.; Ismail, N.; Rafatullah, M.; Azan Tajarudin, H. Selective adsorption and recovery of volatile fatty acids from fermented landfill leachate by activated carbon process. Sci. Total Environ. 2020, 707, 134533. [Google Scholar] [CrossRef] [PubMed]
- Demiral, H.; Yildirim, M.E. Recovery of acetic acid from waste streams by extractive distillation. Water Sci. Technol. 2003, 47, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Tugtas, A.E. Recovery of volatile fatty acids via membrane contactor using flat membranes: Experimental and theoretical analysis. Waste Manag. 2014, 34, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Aghapour Aktij, S.; Zirehpour, A.; Mollahosseini, A.; Taherzadeh, M.J.; Tiraferri, A.; Rahimpour, A. Feasibility of membrane processes for the recovery and purification of bio-based volatile fatty acids: A comprehensive review. J. Ind. Eng. Chem. 2020, 81, 24–40. [Google Scholar] [CrossRef]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of mixed volatile fatty acids from anaerobically fermented organic wastes by vapor permeation membrane contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Struyf, A. Extraction and purification of volatile fatty acids. In Master [120]: Bioingénieur en Chimie et Bioindustries; Université Catholique de Louvain (UCL): Louvain, Belgium, 2018. [Google Scholar]
- Rebecchi, S.; Pinelli, D.; Bertin, L.; Zama, F.; Fava, F.; Frascari, D. Volatile fatty acids recovery from the effluent of an acidogenic digestion process fed with grape pomace by adsorption on ion exchange resins. Chem. Eng. J. 2016, 306, 629–639. [Google Scholar] [CrossRef]
- Yousuf, A.; Bonk, F.; Bastidas-Oyanedel, J.-R.; Schmidt, J.E. Recovery of carboxylic acids produced during dark fermentation of food waste by adsorption on Amberlite IRA-67 and activated carbon. Bioresour. Technol. 2016, 217, 137–140. [Google Scholar] [CrossRef]
- Garrett, B.; Srinivas, K.; Ahring, B. Performance and Stability of Amberlite™ IRA-67 Ion Exchange Resin for Product Extraction and pH Control during Homolactic Fermentation of Corn Stover Sugars. Biochem. Eng. J. 2014, 94, 1–8. [Google Scholar] [CrossRef]
- Outram, V.; Zhang, Y. Solvent-free membrane extraction of volatile fatty acids from acidogenic fermentation. Bioresour. Technol. 2018, 270, 400–408. [Google Scholar] [CrossRef]
- Chalmers Brown, R.; Tuffou, R.; Massanet Nicolau, J.; Dinsdale, R.; Guwy, A. Overcoming nutrient loss during volatile fatty acid recovery from fermentation media by addition of electrodialysis to a polytetrafluoroethylene membrane stack. Bioresour. Technol. 2020, 301, 122543. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, H.; Dessì, P.; Trudu, S.; Asunis, F.; Lens, P.N.L. Silicone membrane contactor for selective volatile fatty acid and alcohol separation. Process Saf. Environ. Prot. 2021, 148, 125–136. [Google Scholar] [CrossRef]
- Woo, H.C.; Kim, Y.H. Eco-efficient recovery of bio-based volatile C2–6 fatty acids. Biotechnol. Biofuels 2019, 12, 92. [Google Scholar] [CrossRef] [PubMed]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of Volatile Fatty Acids from Model Anaerobic Effluents Using Various Membrane Technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Pervez, M.N.; Mahboubi, A.; Uwineza, C.; Zarra, T.; Belgiorno, V.; Naddeo, V.; Taherzadeh, M.J. Factors influencing pressure-driven membrane-assisted volatile fatty acids recovery and purification-A review. Sci. Total Environ. 2022, 817, 152993. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Leininger, A.; Jassby, D.; Tsesmetzis, N.; Ren, Z.J. Will Membranes Break Barriers on Volatile Fatty Acid Recovery from Anaerobic Digestion? ACS EST Eng. 2021, 1, 141–153. [Google Scholar] [CrossRef]
- Huq, N.A.; Hafenstine, G.R.; Huo, X.; Nguyen, H.; Tifft, S.M.; Conklin, D.R.; Stück, D.; Stunkel, J.; Yang, Z.; Heyne, J.S.; et al. Toward net-zero sustainable aviation fuel with wet waste–derived volatile fatty acids. Proc. Natl. Acad. Sci. USA 2021, 118, e2023008118. [Google Scholar] [CrossRef] [PubMed]
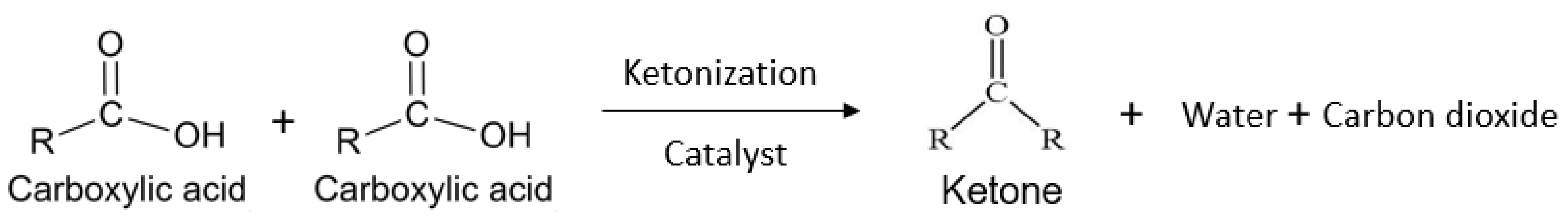
- Pham, T.N.; Sooknoi, T.; Crossley, S.P.; Resasco, D.E. Ketonization of Carboxylic Acids: Mechanisms, Catalysts, and Implications for Biomass Conversion. ACS Catal. 2013, 3, 2456–2473. [Google Scholar] [CrossRef]
- Boekaerts, B.; Sels, B.F. Catalytic advancements in carboxylic acid ketonization and its perspectives on biomass valorisation. Appl. Catal. B Environ. 2021, 283, 119607. [Google Scholar] [CrossRef]
- Gliński, M.; Kijeński, J.; Jakubowski, A. Ketones from monocarboxylic acids: Catalytic ketonization over oxide systems. Appl. Catal. A Gen. 1995, 128, 209–217. [Google Scholar] [CrossRef]
- Pacchioni, G. Ketonization of Carboxylic Acids in Biomass Conversion over TiO2 and ZrO2 Surfaces: A DFT Perspective. ACS Catal. 2014, 4, 2874–2888. [Google Scholar] [CrossRef]
- Whyte, A.; Yoon, T.P. Selective Cross-Ketonization of Carboxylic Acids Enabled by Metallaphotoredox Catalysis. Angew. Chem. Int. Ed. 2022, 134, e202213739. [Google Scholar] [CrossRef]
- Renz, M. Ketonization of Carboxylic Acids by Decarboxylation: Mechanism and Scope. Eur. J. Org. Chem. 2005, 2005, 979–988. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, X.; Zhang, Q.; Liang, Y.; Zhao, X.; Wang, C.; Ma, L. Synthesis of branched alkane fuel from biomass-derived methyl-ketones via self-aldol condensation and hydrodeoxygenation. Fuel 2021, 299, 120889. [Google Scholar] [CrossRef]
- Bayahia, H. Gas-phase ketonization of acetic acid over Co–Mo and its supported catalysts. J. Taibah Univ. Sci. 2018, 12, 191–196. [Google Scholar] [CrossRef]

| Substrate | Inhibition of Methanogenesis | VFA Yield | VFA Type | HRT | Temperature (◦C) | Mode | Reference |
|---|---|---|---|---|---|---|---|
| High-strength cheese whey and brewery wastewater | Acid shock & heat treatment of inoculum | 78 g/L | Total | - | 40 | Batch | [44] |
| 30 g/L | 4 | 40 | Fed-Batch | ||||
| Livestock organic waste (Cattle manure–poultry litter) | Low pH-5.5 | 3.5 g/L | Ac, Pr, Bu | 4 | 35 | Fed-batch | [21] |
| Primary sewage sludge–organic wastes | Low pH-5.5 | 17.242 g COD/L | Total | 7 | 35 | Fed-batch | [20] |
| Glucose | H2O2 | 1.233 g/L | Total | - | 35 | Batch | [34] |
| Wet exploded corn stover | BES | 49.31 g/L | Ac, Pr, Bu | 6 | 37 | Fed-batch | [26] |
| Wet exploded corn stover | Rumen culture as inoculum | 40.8 g/L | Ac, Pr, Bu | 6 | 37 | Fed-batch | [25] |
| Food waste | Low pH-6 | 34.05 g/L | Ac, Pr, Bu, Va | - | 30 | Batch | [45] |
| Food waste | Low HRT, high OLR | 7.5 g/L | Total | 6.67 | 37 | Fed-batch | [46] |
| Cheese production WW | - | 0.97 g COD/g SCOD | Total | - | 35 | Batch | [47] |
| Sucrose | Heat inactivation of methanogens in inoculum | 37 g/L | Ac, Bu | 2 | 35 | Continuous | [48] |
| Citrus waste | Low pH-6, O2 | 0.793 g VFA/g VS | Total | - | 37.5 | Batch | [49] |
| Food waste–mature compost | Low pH-6, acidogenic reactor effluent as inoculum | 20 g COD/L | Total | 5 | 37 | Fed-batch | [50] |
| Organic MSW–food waste | Low pH-6, acidogenic reactor effluent as inoculum | 11.73 g /L | Total | 3.5 | 37 | Fed-batch | [22] |
| Food waste | High OLR, pH 10 | 6.3 g/L | Ac, Pr, Bu | - | 28 | Batch | [51] |
| Olive mill WW | Low pH-5, high OLR | 27 g/L | Total | 2 | - | Batch | [52] |
| Wetland plant litter | High pH-12, | 0.127 g/g dry matter | Total | 25 | 25 | Batch | [53] |
| Food waste | Low pH-6, O2 | 0.8 g VFA/g VS | Total | - | 37 | Batch | [54] |
| Food waste–sewage sludge | High pH -10 | 8.631 g/ L | Total | - | 35 | Batch | [55] |
| Microalgae | High OLR | 36.8 g/ L | Total | 8 | 25 | Fed-batch | [56] |
| Palm oil mill effluent | Low HRT | 10.5 g/L | Total | 5 | 29 | Fed-batch | [57] |
| Waste activated sludge | Bio-surfactants-surfactin, rhamnolipid, saponin | 3.3 g COD/L | Total | - | 30 | Batch | [58] |
| Waste activated sludge | Low thermal pretreatment, sodium dodecylbenzene sulfonate | 0.32 g COD/g VS | Total | - | 37 | Batch | [59] |
| Food waste–waste activated sludge | High pH-10, BES | 0.343 g COD/g VS | Ac, Pr, Bu, Va | - | 35 | Batch | [60] |
| Chicken manure | Thermal shock | 0.9 g VFA/g VS | Ac, Pr, Bu | 10 | 37 | Fed-batch | [61] |
| Bioreactor | Acetic Acid (g/L) | Propionic Acid (g/L) | Butyric Acid (g/L) | Total VFA in Acetic Acid Equivalents (g/L) | Total VFA Yield in Acetic Acid Equivalents (g/g VS) |
|---|---|---|---|---|---|
| Control; With Methanogenesis [25] | 12.26 | 10.08 | 2.42 | 31.09 | 1.25 g/gVS |
| Control; (BES-added) Without Methanogenesis | 9.29 | 5.63 | 1.23 | 21.41 | 0.95 g/gVS |
| Bioaugmentation with A. ruminis after BES addition | 16.99 | 6.88 | 2.98 | 32.33 | 1.34 g/gVS |
| Bioaugmentation with A. woodii after BES addition | 30.8 | 7.91 | 3.89 | 49.31 | 2.19 g/gVS |
| Materials Used | VFA Recovered | Acid Recovery Efficiency (%) | Regeneration Method | References |
|---|---|---|---|---|
| Purolite A103S Plus | Ac, Bu | 66.16 | Not reported | [139] |
| Amberlyst A21 | Total VFA | Up to 80 | Not reported | [140] |
| Amberlite IRA-67 | Ac, Bu, La | 75 | Thermal | [141] |
| Amberlite IRA-67, Dowex optipore L-493 | Ac, Pr | Up to 85 | Alkali wash | [132] |
| Amberlite FPA53 | Ac | 42.36 | Strong alkali wash | [121] |
| Non-functionalized polystyrene-divinylbenzene-based resin | Total VFA | 75.5 | N2 stripping | [133] |
| Activated Carbon | Ac, Bu | Up to 80 | Not reported | [134] |
| Amberlite IRA-67 | La | Not reported | Alkali wash | [142] |
| Operation Technique | VFA Recovered | Recovery Efficiency | Fouling/ Regeneration | Membrane Details | References |
|---|---|---|---|---|---|
| Vapor permeation | Total VFA | Up to 95% | Not reported | Trioctylamine-filled PTFE membrane; area—19.25 cm2 | [138] |
| Membrane extraction | Total VFA | Not reported | Water rinsing | Silicone membrane; area—24.3 m2/Lferm | [143] |
| Membrane extraction coupled with electrodialysis | Total VFA | Up to 98% | Alkali wash | PTFE membrane; membrane configuration—1,3 and 5 membranes stacked. Total active area of 64 cm2, 192 cm2 and 320 cm2 respectively. | [144] |
| Membrane extraction | Total VFA | Up to 21.5% | Not reported | Silicone membrane; area—125 cm2 | [145] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giduthuri, A.T.; Ahring, B.K. Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids. Fermentation 2023, 9, 13. https://doi.org/10.3390/fermentation9010013
Giduthuri AT, Ahring BK. Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids. Fermentation. 2023; 9(1):13. https://doi.org/10.3390/fermentation9010013
Chicago/Turabian StyleGiduthuri, Anthony T., and Birgitte K. Ahring. 2023. "Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids" Fermentation 9, no. 1: 13. https://doi.org/10.3390/fermentation9010013
APA StyleGiduthuri, A. T., & Ahring, B. K. (2023). Current Status and Prospects of Valorizing Organic Waste via Arrested Anaerobic Digestion: Production and Separation of Volatile Fatty Acids. Fermentation, 9(1), 13. https://doi.org/10.3390/fermentation9010013






