Cobrançosa Table Olive Fermentation as per the Portuguese Traditional Method, Using Potentially Probiotic Lactiplantibacillus pentosus i106 upon Alternative Inoculation Strategies
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Table Olives Samples
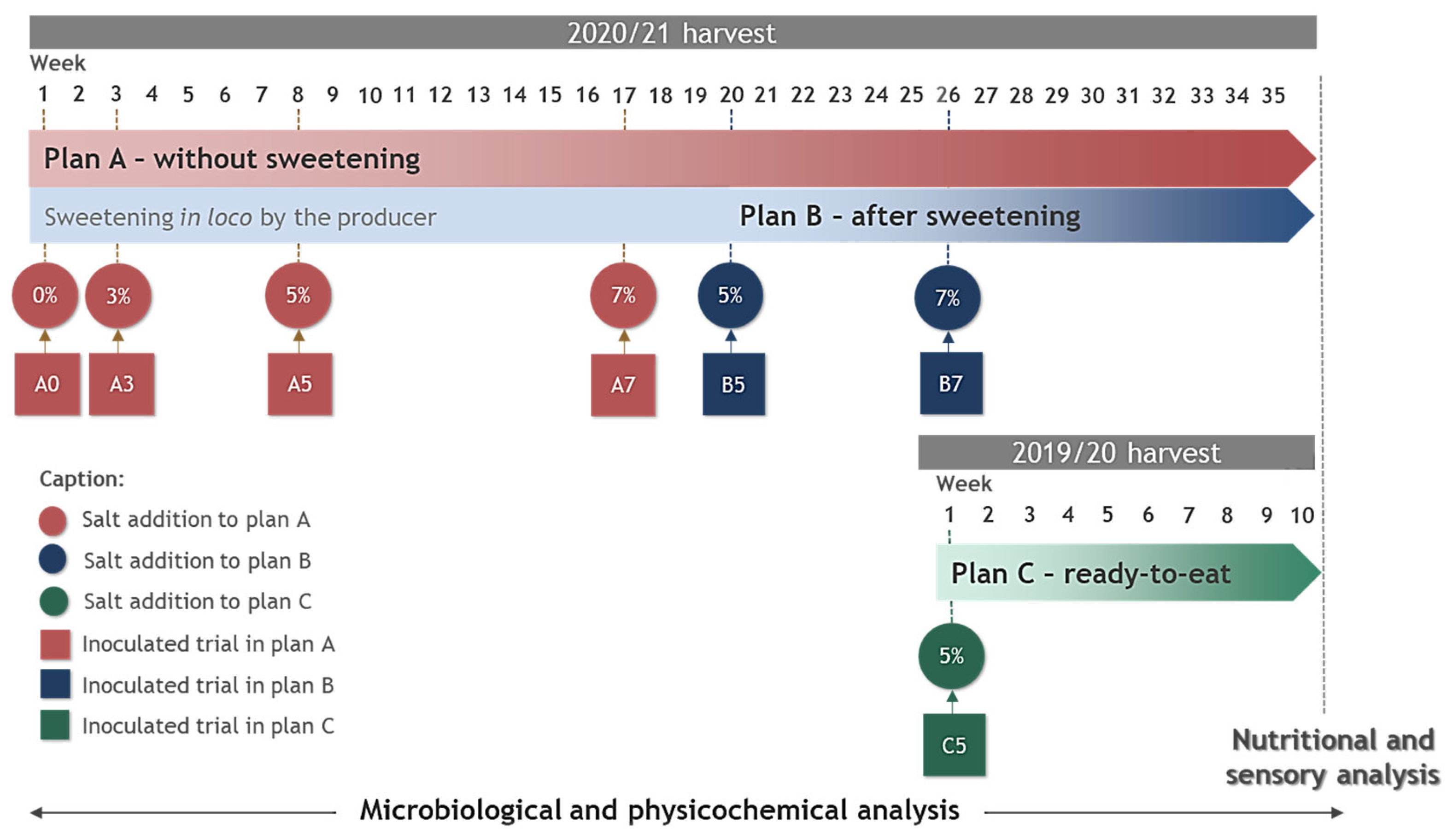
2.3. Experimental Design
2.4. Microbiological Analysis
2.5. Physicochemical Analysis
2.6. Nutritional Analysis
2.7. Sensory Analysis
2.8. Statistical Analysis
3. Results
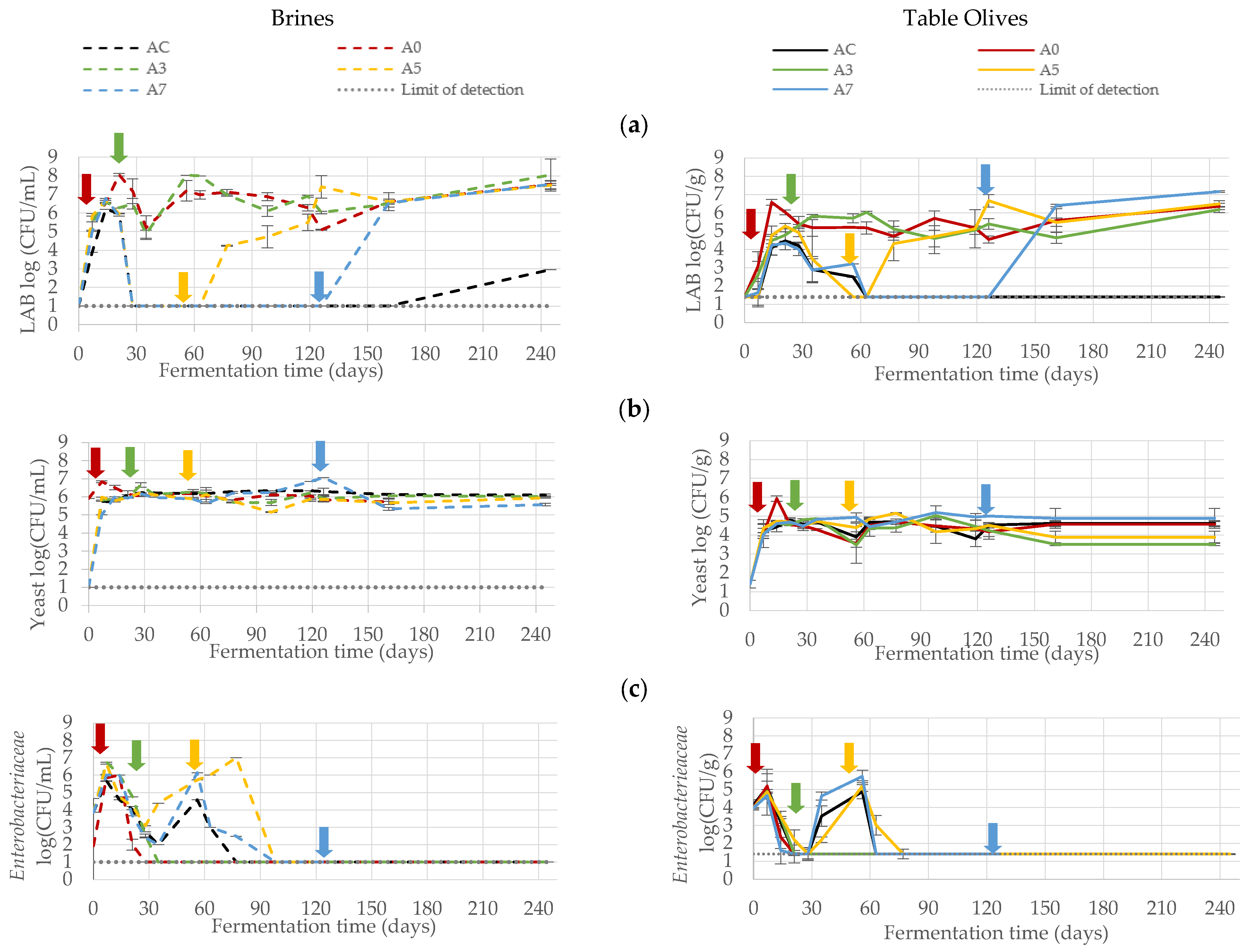
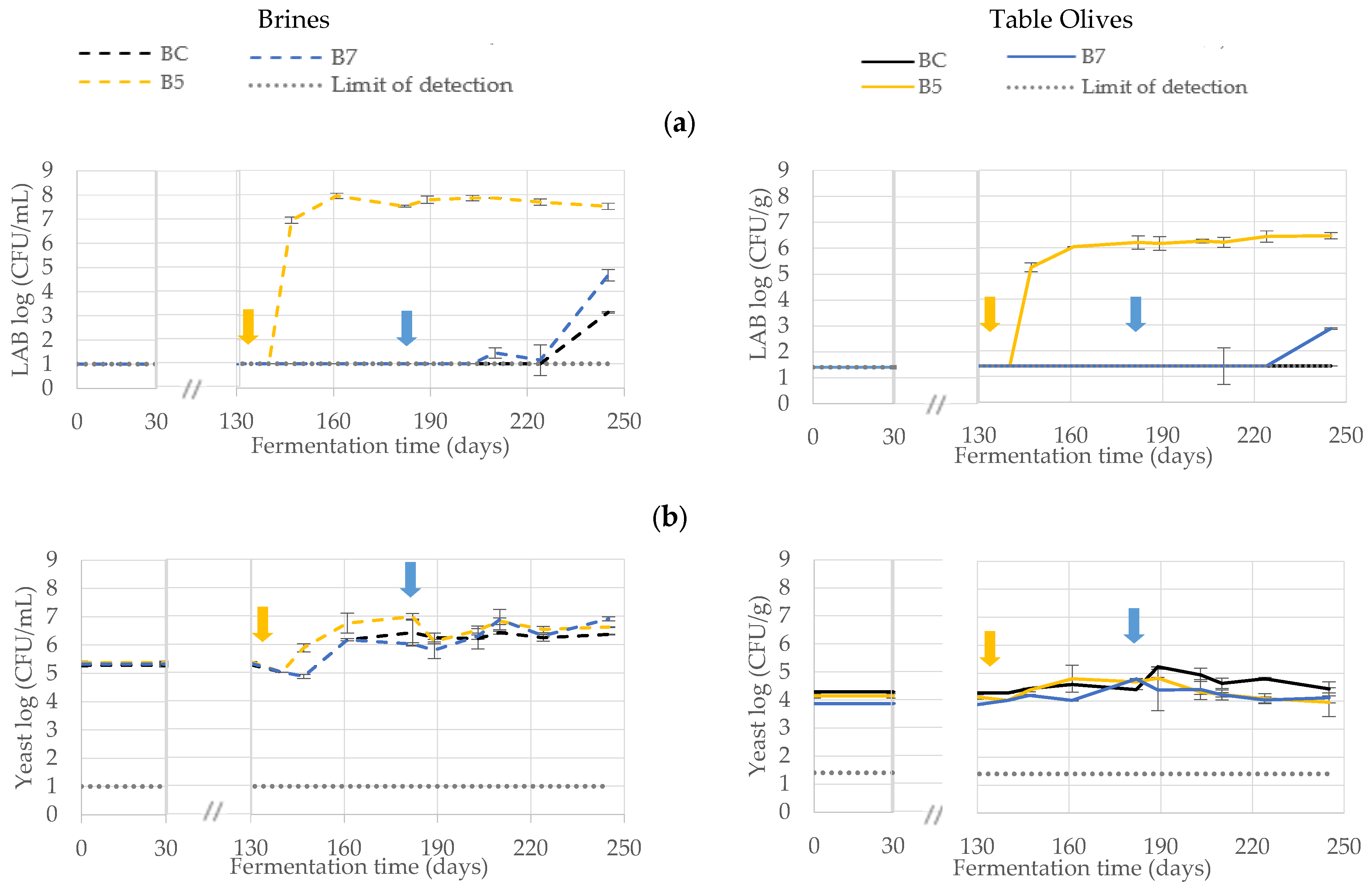
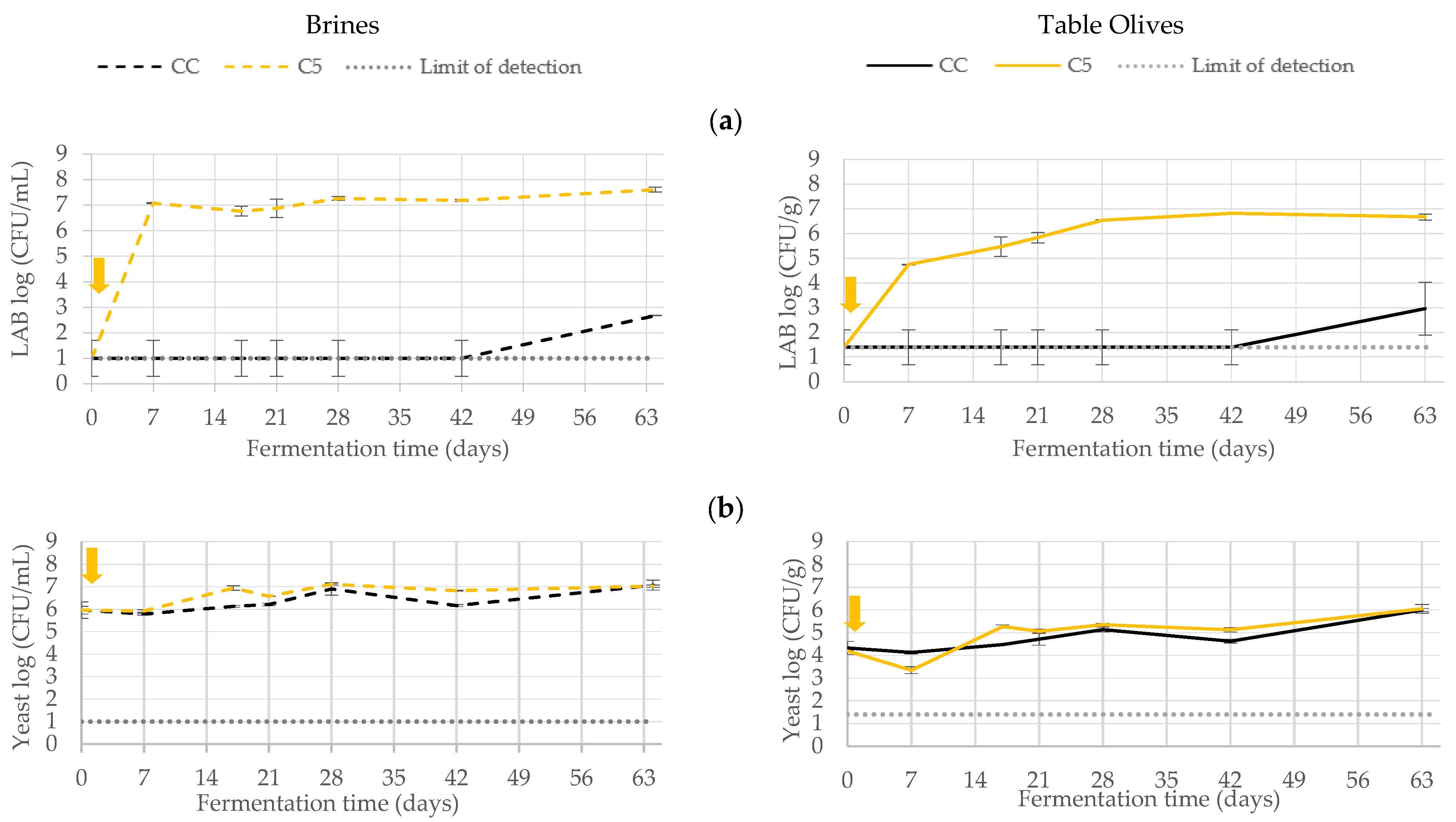
3.1. Microbiological Monitoring
3.2. Physicochemical Monitoring
3.2.1. Salt Content, pH, and Total Titratable Acidity
3.2.2. Phenolic Composition
3.3. Nutritional Composition
3.4. Sensory Assessment
4. Discussion
5. Conclusions
6. Future Research
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perpetuini, G.; Prete, R.; Garcia-Gonzalez, N.; Khairul Alam, M.; Corsetti, A. Table olives more than a fermented food. Foods 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, T.G.; Costa, H.S.; Oliveira, M.B.P.P. An overview of Portuguese olive oils and table olives with Protected Designation of Origin. Eur. J. Lipid Sci. Technol. 2019, 121, 1800129. [Google Scholar] [CrossRef]
- Benitez-Cabello, A.; Calero-Delgado, B.; Rodriguez-Gomez, F.; Bautista-Gallego, J.; Garrido-Fernandez, A.; Jimenez-Diaz, R.; Arroyo-Lopez, F.N. The use of multifunctional yeast-lactobacilli starter cultures improves fermentation performance of Spanish-style green table olives. Food Microbiol. 2020, 91, 103497. [Google Scholar] [CrossRef] [PubMed]
- Campus, M.; Degirmencioglu, N.; Comunian, R. Technologies and trends to improve table olive quality and safety. Front. Microbiol. 2018, 9, 617. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Macedo, A.C.; Malcata, F.X. A review on adventitious lactic acid bacteria from table olives. Foods 2020, 9, 948. [Google Scholar] [CrossRef]
- Saúde, C.; Barros, T.; Mateus, T.; Quintas, C.; Pires-Cabral, P. Effect of chloride salts on the sensory and nutritional properties of cracked table olives of the Maçanilha Algarvia cultivar. Food Biosci. 2017, 19, 73–79. [Google Scholar] [CrossRef]
- Portilha-Cunha, M.F.; Malcata, F.X.; Reis, P.J.M.; Macedo, A.C. Towards a starter culture of Lactobacillus plantarum AFS13: Assessment of more relevant effects for in vitro production and preservation thereof, via fractional factorial design methodology. LWT 2020, 133, 110119. [Google Scholar] [CrossRef]
- Reis, P.J.M.; Tavares, T.G.; Rocha, J.M.; Malcata, F.X.; Macedo, A.C. Cobrançosa table olives: Characterization of processing method and lactic acid bacteria profile throughout spontaneous fermentation. Appl. Sci. 2022, 12, 9738. [Google Scholar] [CrossRef]
- Cosmai, L.; Campanella, D.; De Angelis, M.; Summo, C.; Paradiso, V.M.; Pasqualone, A.; Caponio, F. Use of starter cultures for table olives fermentation as possibility to improve the quality of thermally stabilized olive-based paste. LWT—Food Sci. Technol. 2018, 90, 381–388. [Google Scholar] [CrossRef]
- Bonatsou, S.; Tassou, C.C.; Panagou, E.Z.; Nychas, G.E. Table olive fermentation using starter cultures with multifunctional potential. Microorganisms 2017, 5, 30. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Tsaltas, D. Current status, recent advances, and main challenges on table olive fermentation: The present meets the future. Front. Microbiol. 2022, 12, 797295. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Fernandez, A.; Benitez-Cabello, A.; Rodriguez-Gomez, F.; Jimenez-Diaz, R.; Arroyo-Lopez, F.N.; Morales, M.L. Relating starter cultures to volatile profile and potential markers in green Spanish-style table olives by compositional data analysis. Food Microbiol. 2021, 94, 103659. [Google Scholar] [CrossRef] [PubMed]
- Lanza, B.; Zago, M.; Di Marco, S.; Di Loreto, G.; Cellini, M.; Tidona, F.; Bonvini, B.; Bacceli, M.; Simone, N. Single and multiple inoculum of Lactiplantibacillus plantarum strains in table olive lab-scale fermentations. Fermentation 2020, 6, 126. [Google Scholar] [CrossRef]
- Pino, A.; De Angelis, M.; Todaro, A.; Van Hoorde, K.; Randazzo, C.L.; Caggia, C. Fermentation of Nocellara Etnea table olives by functional starter cultures at different low salt concentrations. Front. Microbiol. 2018, 9, 1125. [Google Scholar] [CrossRef]
- Coimbra-Gomes, J.; Reis, P.J.M.; Tavares, T.G.; Malcata, F.X.; Macedo, A.C. Study of lactic acid bacteria biodiversity in fermented Cobrançosa table olives to determine their probiotic potential. Foods 2022, 11, 3050. [Google Scholar] [CrossRef]
- Coimbra-Gomes, J.; Tavares, T.G.; Reis, P.J.M.; Faria, M.A.; Malcata, F.X.; Macedo, A.C. Evaluating the probiotic potential of lactic acid bacteria implicated in natural fermentation of table olives, cv. Cobrançosa. Food Biosci. 2022, submitted.
- Blana, Β.; Stamatiou, A.; Nychas, G.-J.N.; Panagou, Ε.Ζ. Implementation of a probiotic culture of Lactobacillus pentosus in large scale fermentation of cv. Halikidiki table olives. In Proceedings of the 4th Panhellenic Conference “Biotechnology and Food Technology”, Athens, Greece, 11–13 October 2013. [Google Scholar]
- Panagou, E.Z.; Schillinger, U.; Franz, C.M.; Nychas, G.J. Microbiological and biochemical profile of cv. Conservolea naturally black olives during controlled fermentation with selected strains of lactic acid bacteria. Food Microbiol. 2008, 25, 348–358. [Google Scholar] [CrossRef]
- Abriouel, H.; Benomar, N.; Lucas, R.; Gálvez, A. Culture-independent study of the diversity of microbial populations in brines during fermentation of naturally-fermented Aloreña green table olives. Int. J. Food Microbiol. 2011, 144, 487–496. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis, 17th ed.; AOAC, Ed.; The Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000; Volume II. [Google Scholar]
- Fernandez-Diez, M.J.; Castro, R.; Garrido, A.; Gonzales, F.C.; Gonçales, F.P.; Millan, A.C.G. Biotecnologia de la Aceituna de Mesa; Instituto de la Grasa y sus Derivados: Seville, Spain, 1985. [Google Scholar]
- Rodrigues, N.; Oliveira, C.; Casal, S.; Pereira, J.A.; Ramalhosa, E. “Table olive flours”: An ingredient rich in bioactive compounds? Appl. Sci. 2022, 12, 1661. [Google Scholar] [CrossRef]
- Anagnostopoulos, D.A.; Goulas, V.; Xenofontos, E.; Vouras, C.; Nikoloudakis, N.; Tsaltas, D. Benefits of the use of lactic acid bacteria starter in green cracked Cypriot table olives fermentation. Foods 2020, 9, 17. [Google Scholar] [CrossRef]
- IOC. Standards, Methods and Guides; International Olive Council: Madrid, Spain, 2021; Available online: https://www.internationaloliveoil.org/wp-content/uploads/2021/07/DEC3-4-_113-Table-olives-ENG.pdf (accessed on 2 July 2022).
- Piepho, H.P. Letters in mean comparisons: What they do and don’t mean. J. Agron. 2018, 110, 431–434. [Google Scholar] [CrossRef]
- Rocha, J.; Borges, N.; Pinho, O. Table olives and health: A review. J. Nutr. Sci. 2020, 9, e57. [Google Scholar] [CrossRef] [PubMed]
- Pires-Cabral, P.; Nunes, P.; Barros, T.; Quintas, C. Physicochemical, nutritional and microbiological characteristics of traditional table olives from Southern Portugal. Emir. J. Food Agric. 2018, 30, 611–620. [Google Scholar] [CrossRef]
- Malheiro, R.; Casal, S.; Sousa, A.; de Pinho, P.G.; Peres, A.M.; Dias, L.G.; Bento, A.; Pereira, J.A. Effect of cultivar on sensory characteristics, chemical composition, and nutritional value of stoned green table olives. Food Bioproc. Technol. 2012, 5, 1733–1742. [Google Scholar] [CrossRef]
- Galanakis, C.M. Olive fruit dietary fiber: Components, recovery and applications. Trends Food Sci. Technol. 2011, 22, 175–184. [Google Scholar] [CrossRef]
- Emmanouilidou, M.G.; Koukourikou-Petridou, M.; Gerasopoulos, D.; Kyriacou, M.C. Oxidative stability, fatty-acid and phenolic composition of Cypriot monovarietal virgin olive oils with progressive fruit maturity. J. Food Compos. Anal. 2021, 104, 104191. [Google Scholar] [CrossRef]
- Reboredo-Rodriguez, P.; Olmo-Garcia, L.; Figueiredo-Gonzalez, M.; Gonzalez-Barreiro, C.; Carrasco-Pancorbo, A.; Cancho-Grande, B. Effect of olive ripening degree on the antidiabetic potential of biophenols-rich extracts of Brava Gallega virgin olive oils. Food Res. Int. 2020, 137, 109427. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.; Figueiredo, C.; Santos, C.; Silva, A.M.S. Phenolic and lipophilic metabolite adjustments in Olea europaea (olive) trees during drought stress and recovery. Phytochemistry 2021, 185, 112695. [Google Scholar] [CrossRef]
- Valente, S.; Machado, B.; Pinto, D.; Santos, C.; Silva, A.M.S.; Dias, M.C. Modulation of phenolic and lipophilic compounds of olive fruits in response to combined drought and heat. Food Chem. 2020, 329, 127191. [Google Scholar] [CrossRef]
- Hurtado, A.; Reguant, C.; Bordons, A.; Rozes, N. Influence of fruit ripeness and salt concentration on the microbial processing of Arbequina table olives. Food Microbiol. 2009, 26, 827–833. [Google Scholar] [CrossRef]
- Campus, M.; Cauli, E.; Scano, E.; Piras, F.; Comunian, R.; Paba, A.; Daga, E.; Di Salvo, R.; Sedda, P.; Angioni, A.; et al. Towards controlled fermentation of table olives: LAB starter driven process in an automatic pilot processing plant. Food Bioproc. Technol. 2017, 10, 1063–1073. [Google Scholar] [CrossRef]
- De Bellis, P.; Sisto, A.; Lavermicocca, P. Probiotic bacteria and plant-based matrices: An association with improved health-promoting features. J. Funct. Foods 2021, 87, 104821. [Google Scholar] [CrossRef]
- Penland, M.; Pawtowski, A.; Pioli, A.; Maillard, M.B.; Debaets, S.; Deutsch, S.M.; Falentin, H.; Mounier, J.; Coton, M. Brine salt concentration reduction and inoculation with autochthonous consortia: Impact on Protected Designation of Origin Nyons black table olive fermentations. Food Res. Int. 2022, 155, 111069. [Google Scholar] [CrossRef] [PubMed]
- Tassou, C.C.; Panagou, E.Z.; Katsaboxakis, K.Z. Microbiological and physicochemical changes of naturally black olives fermented at different temperatures and NaCl levels in the brines. Food Microbiol. 2002, 19, 605–615. [Google Scholar] [CrossRef]
- Servili, M.; Settanni, L.; Veneziani, G.; Esposto, S.; Massitti, O.; Taticchi, A.; Urbani, S.; Montedoro, G.F.; Corsetti, A. The use of Lactobacillus pentosus 1MO to shorten the debittering process time of black table olives (cv. Itrana and Leccino): A pilot-scale application. J. Agric. Food Chem. 2006, 54, 3869–3875. [Google Scholar] [CrossRef] [PubMed]
- Anagnostopoulos, D.A.; Kamilari, E.; Tsaltas, D. Evolution of bacterial communities, physicochemical changes and sensorial attributes of natural whole and cracked Picual table olives during spontaneous and inoculated fermentation. Front. Microbiol. 2020, 11, 1128. [Google Scholar] [CrossRef] [PubMed]
- Mateus, T.; Santo, D.; Saude, C.; Pires-Cabral, P.; Quintas, C. The effect of NaCl reduction in the microbiological quality of cracked green table olives of the Maçanilha Algarvia cultivar. Int. J. Food Microbiol. 2016, 218, 57–65. [Google Scholar] [CrossRef]
- Aponte, M.; Ventorino, V.; Blaiotta, G.; Volpe, G.; Farina, V.; Avellone, G.; Lanza, C.M.; Moschetti, G. Study of green Sicilian table olive fermentations through microbiological, chemical and sensory analyses. Food Microbiol. 2010, 27, 162–170. [Google Scholar] [CrossRef]
- Panagou, E.Z.; Tassou, C.C.; Katsaboxakis, C.Z. Induced lactic acid fermentation of untreated green olives of the Conservolea cultivar by Lactobacillus pentosus. J. Sci. Food Agric. 2003, 83, 667–674. [Google Scholar] [CrossRef]
- Papadelli, M.; Zoumpopoulou, G.; Georgalaki, M.; Anastasiou, R.; Manolopoulou, E.; Lytra, I.; Papadimitriou, K.; Tsakalidou, E. Evaluation of two lactic acid bacteria starter cultures for the fermentation of natural black table olives (Olea europaea L. cv Kalamon). Pol. J. Microbiol. 2015, 64, 265–271. [Google Scholar] [CrossRef]
- Sousa, A.; Malheiro, R.; Casal, S.; Bento, A.; Pereira, J.A. Antioxidant activity and phenolic composition of cv. Cobrançosa olives affected through the maturation process. J. Funct. Foods 2014, 11, 20–29. [Google Scholar] [CrossRef]
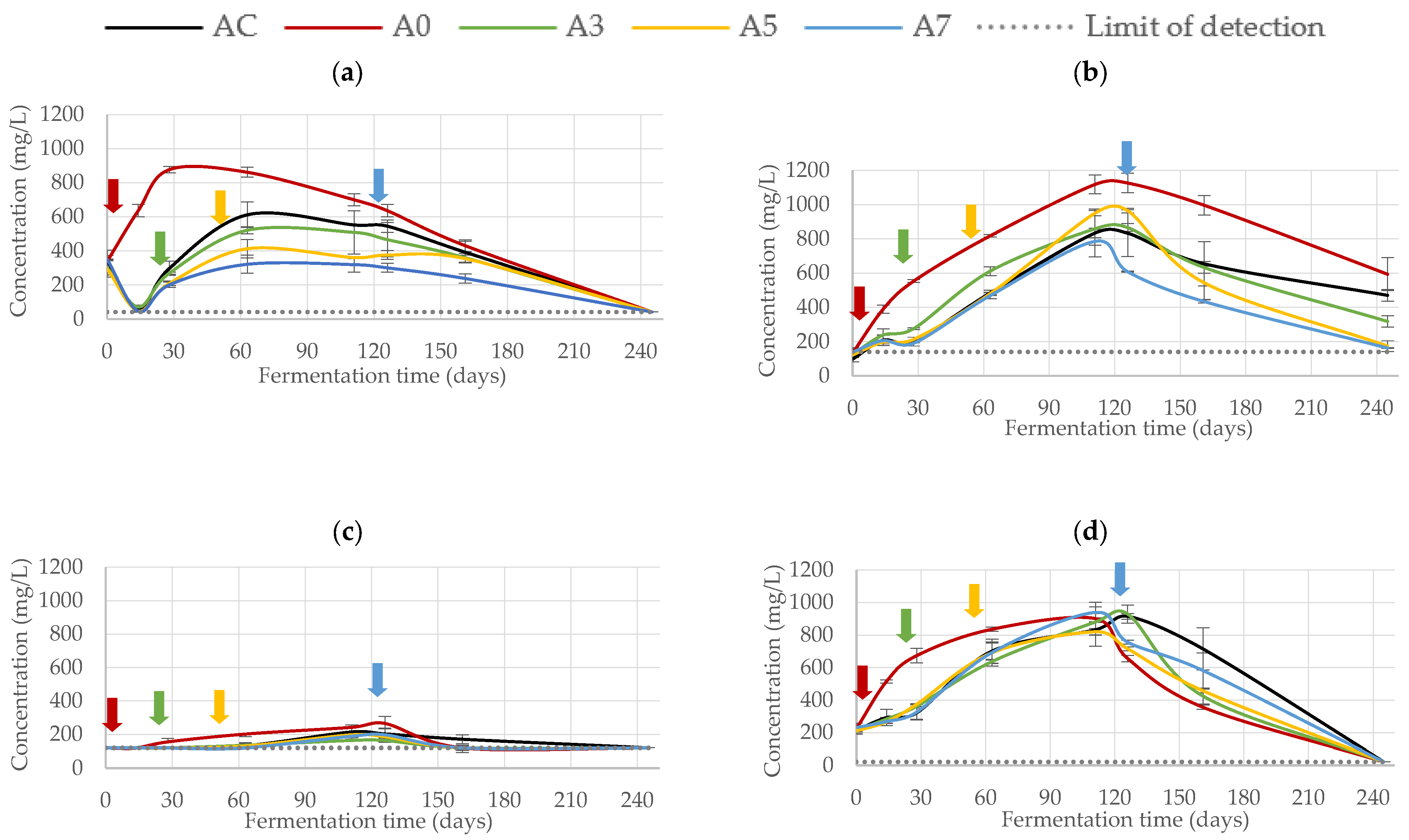
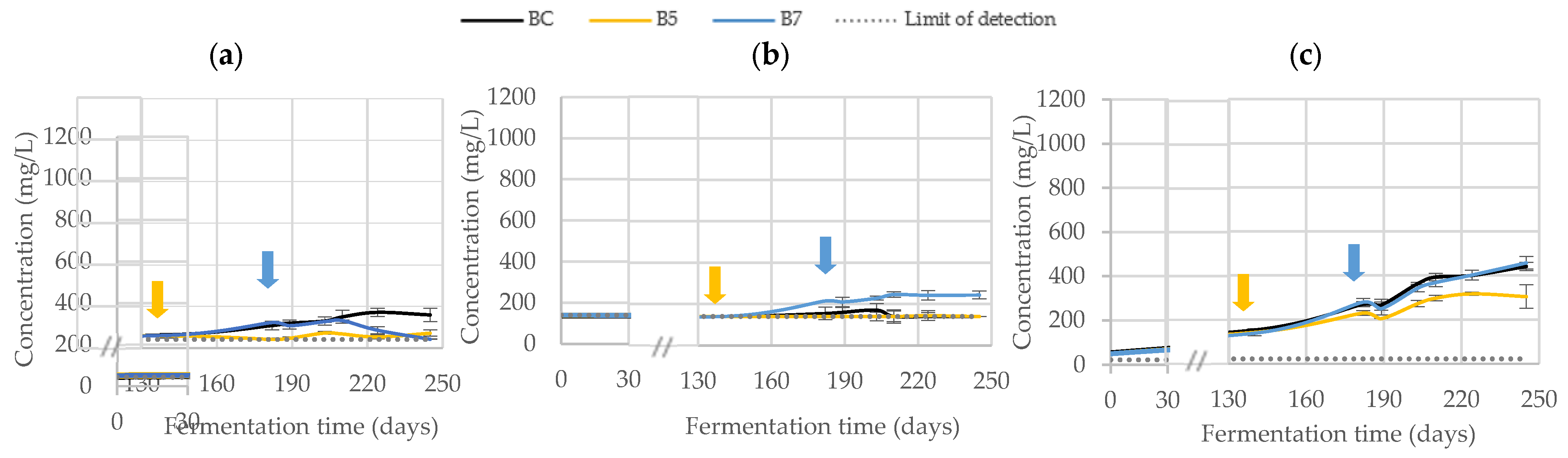
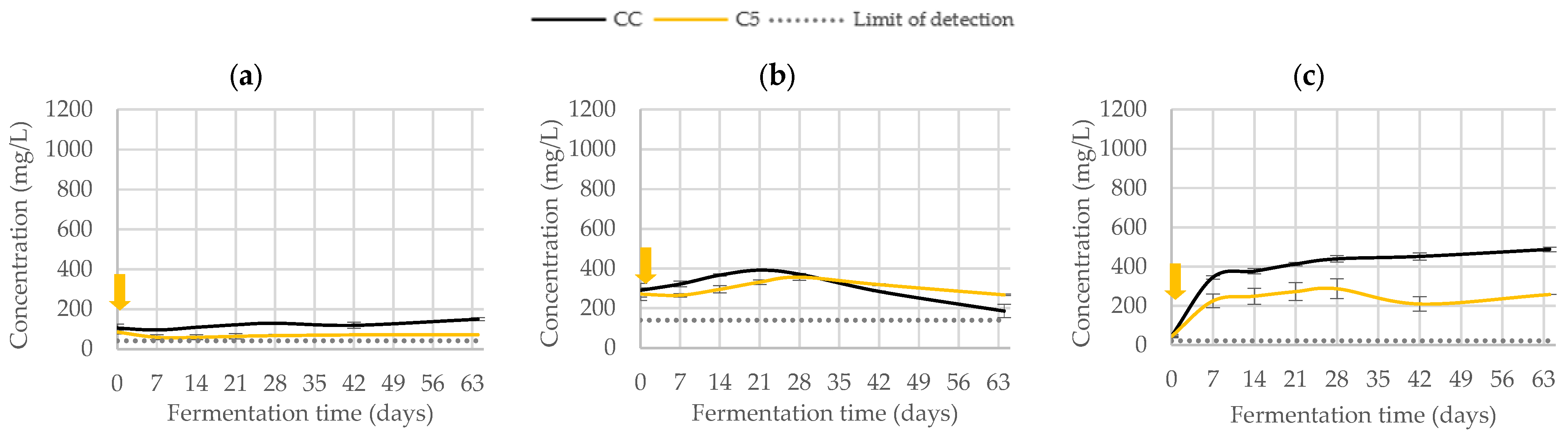
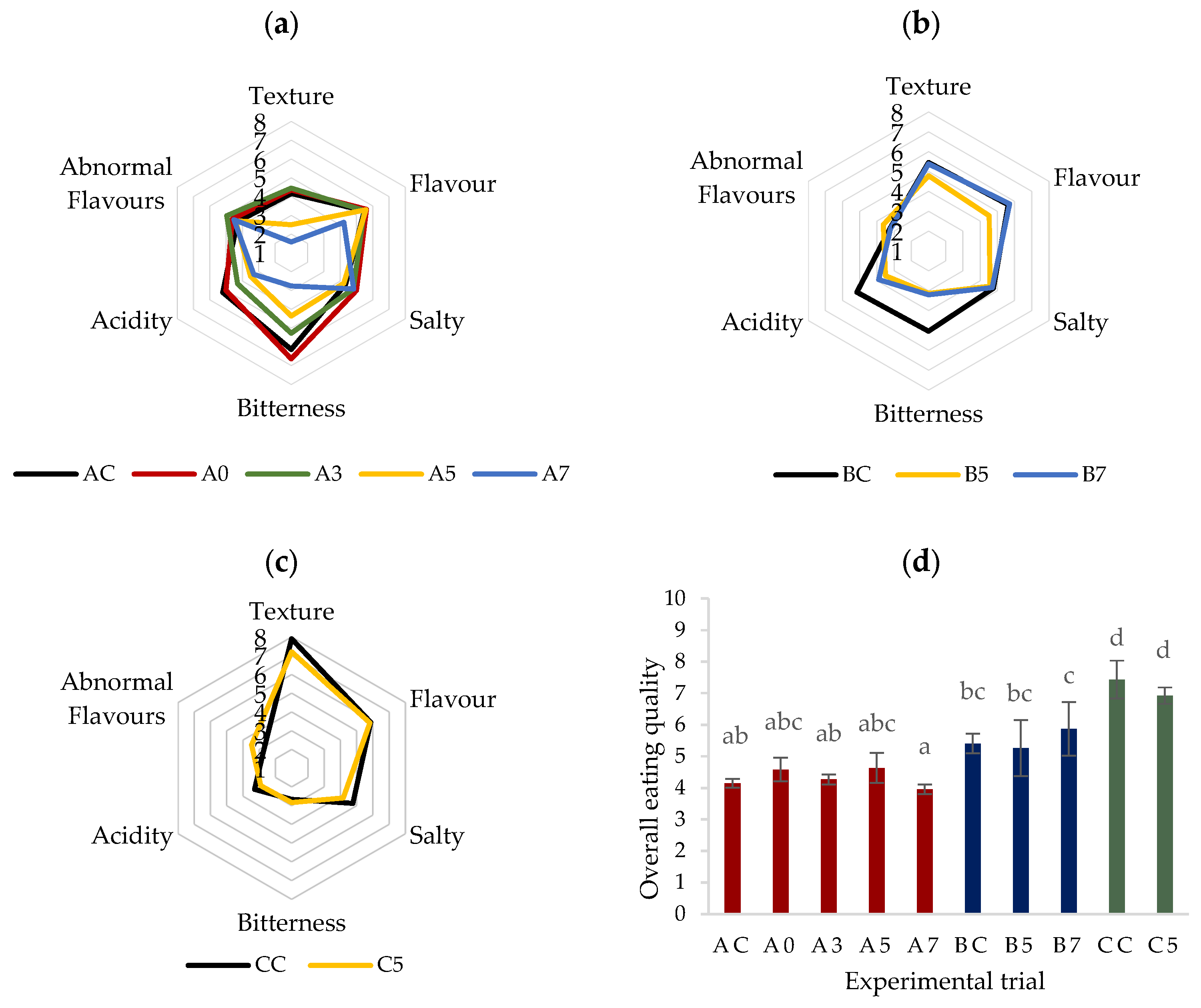
| Trial | Fermentation (Days) | |||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 14 | 28 | 63 | 126 | 245 | p-Value | ||
| Salt (%) | AC | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 2.90 ± 0.14 aB | 5.00 ± 0.00 C | 6.70 ± 0.28 D | 5.50 ± 0.00 aC | <0.001 |
| A0 | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 3.80 ± 0.28 bB | 5.00 ± 0.00 C | 6.75 ± 0.35 E | 5.80 ± 0.00 abD | <0.001 | |
| A3 | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 3.00 ± 0.00 aB | 5.00 ± 0.00 C | 6.25 ± 0.35 E | 5.65 ± 0.07 abD | <0.001 | |
| A5 | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 2.85 ± 0.07 aB | 5.00 ± 0.00 C | 6.00 ± 0.00 D | 5.95 ± 0.21 bD | <0.001 | |
| A7 | 0.00 ± 0.00 A | 0.00 ± 0.00 A | 3.00 ± 0.00 aB | 4.90 ± 0.14 C | 6.80 ± 0.28 E | 5.50 ± 0.00 aD | <0.001 | |
| p-value | 1.000 | 1.000 | 0.006 | 0.486 | 0.124 | 0.023 | ||
| pH | AC | 5.20 ± 0.00 | 4.56 ± 0.01 | 4.40 ± 0.03 b | 4.44 ± 0.03 | 4.20 ± 0.07 ab | 4.56 ± 0.06 a | 0.082 |
| A0 | 5.20 ± 0.00 E | 4.93 ± 0.01 D | 3.83 ± 0.01 aA | 3.95 ± 0.04 A | 4.22 ± 0.06 abB | 4.65 ± 0.13 abC | <0.001 | |
| A3 | 5.20 ± 0.00 D | 4.62 ± 0.01 C | 3.87 ± 0.11 aAB | 3.61 ± 0.03 A | 4.09 ± 0.09 aB | 4.83 ± 0.09 abcC | <0.001 | |
| A5 | 5.20 ± 0.00 C | 4.60 ± 0.05 B | 4.40 ± 0.03 bB | 4.54 ± 0.03 B | 4.08 ± 0.02 aA | 5.08 ± 0.16 bcC | <0.001 | |
| A7 | 5.20 ± 0.00 B | 4.60 ± 0.01 A | 4.42 ± 0.02 bA | 4.60 ± 0.01 B | 4.39 ± 0.08 bA | 5.19 ± 0.14 cB | <0.001 | |
| p-value | 1.000 | 0.584 | <0.001 | 0.532 | 0.030 | 0.010 | ||
| TTA * | AC | 0.15 ± 0.04 A | 0.18 ± 0.01 bA | 0.10 ± 0.02 bA | 0.23 ± 0.03 aA | 0.40 ± 0.06 abB | 0.23 ± 0.01 A | 0.006 |
| A0 | 0.16 ± 0.01 A | 0.40 ± 0.00 dB | 0.56 ± 0.01 cC | 0.54 ± 0.03 bBC | 0.46 ± 0.04 bBC | 0.27 ± 0.04 BC | <0.001 | |
| A3 | 0.17 ± 0.02 A | 0.18 ± 0.00 bA | 0.19 ± 0.01 bA | 0.59 ± 0.01 bD | 0.42 ± 0.04 abC | 0.19 ± 0.03 B | <0.001 | |
| A5 | 0.15 ± 0.01 AB | 0.16 ± 0.01 aAB | 0.11 ± 0.00 aA | 0.20 ± 0.02 aAB | 0.51 ± 0.01 bC | 0.17 ± 0.03 B | <0.001 | |
| A7 | 0.18 ± 0.01 AB | 0.20 ± 0.00 cB | 0.09 ± 0.00 aA | 0.18 ± 0.03 aAB | 0.27 ± 0.03 aB | 0.14 ± 0.01 B | 0.004 | |
| p-value | 0.665 | <0.001 | <0.001 | <0.001 | 0.015 | 0.090 | ||
| Trial | Fermentation Time (Days) | |||||
|---|---|---|---|---|---|---|
| 0 | 140 | 182 | 245 | p-Value | ||
| Salt (%) | BC | 4.35 ± 0.21 B | 3.75 ± 0.07 aA | 6.50 ± 0.00 D | 5.90 ± 0.14 C | <0.001 |
| B5 | 4.35 ± 0.21 A | 4.25 ± 0.07 bA | 6.20 ± 0.28 B | 5.55 ± 0.07 B | 0.001 | |
| B7 | 4.35 ± 0.21 B | 3.75 ± 0.07 aA | 6.50 ± 0.00 D | 5.60 ± 0.00 C | <0.001 | |
| p-value | 1.000 | 0.009 | 0.253 | 0.057 | ||
| pH | BC | 4.17 ± 0.05 B | 3.98 ± 0.02 bA | 4.01 ± 0.01 aA | 4.17 ± 0.04 aB | 0.009 |
| B5 | 4.19 ± 0.01 B | 3.76 ± 0.02 aA | 4.24 ± 0.02 bB | 4.83 ± 0.08 bC | <0.001 | |
| B7 | 4.18 ± 0.04 C | 3.98 ± 0.02 bA | 4.00 ± 0.05 aAB | 4.71 ± 0.04 bD | <0.001 | |
| p-value | 0.859 | 0.003 | 0.019 | 0.003 | ||
| TTA * | BC | 0.05 ± 0.00 A | 0.21 ± 0.01 C | 0.12 ± 0.01 abB | 0.11 ± 0.01 bB | <0.001 |
| B5 | 0.05 ± 0.00 A | 0.16 ± 0.02 B | 0.15 ± 0.01 bB | 0.07 ± 0.01 aA | 0.003 | |
| B7 | 0.05 ± 0.00 A | 0.14 ± 0.02 B | 0.07 ± 0.02 aAB | 0.07 ± 0.01 aAB | 0.038 | |
| p-value | 1.000 | 0.130 | 0.017 | 0.032 | ||
| Trial | Fermentation Time (Days) | ||||
|---|---|---|---|---|---|
| 0 | 7 | 64 | p-Value | ||
| Salt (%) | CC | 5.10 ± 0.14 | 5.75 ± 0.35 | 5.75 ± 0.21 | 0.104 |
| C5 | 5.00 ± 0.00 A | 6.00 ± 0.00 C | 5.75 ± 0.07 B | <0.001 | |
| p-value | 0.423 | 0.423 | 1.000 | ||
| pH | CC | 4.03 ± 0.04 A | 4.04 ± 0.01 A | 4.40 ± 0.08 B | 0.011 |
| C5 | 4.10 ± 0.03 B | 3.58 ± 0.01 A | 4.46 ± 0.09 C | 0.001 | |
| p-value | 0.192 | < 0.001 | 0.598 | ||
| TTA * | CC | 0.14 ± 0.03 | 0.14 ± 0.02 | 0.10 ± 0.03 | 0.228 |
| C5 | 0.14 ± 0.01 B | 0.29 ± 0.01 C | 0.07 ± 0.01 A | <0.001 | |
| p-value | 0.771 | 0.005 | 0.312 | ||
| Trial | Content per 100 g of Edible Part of Table Olives | Energetic Value (Kcal/100 g) | ||||||
|---|---|---|---|---|---|---|---|---|
| Humidity | Ashes | Salt | Proteins | Fats | Fiber | Carbohydrates | ||
| AC | 66.97 ± 0.42 abc | 3.22 ± 0.04 a | 2.60 ± 0.15 a | 0.92 ± 0.13 ab | 22.21 ± 0.49 c | 3.78 ± 0.11 ab | 1.90 ± 0.06 a | 218.73 ± 4.50 bc |
| A0 | 66.28 ± 0.81 a | 3.29 ± 0.05 ab | 2.88 ± 0.01 ab | 1.03 ± 0.05 abc | 22.82 ± 0.06 c | 4.46 ± 0.51 ab | 2.13 ± 0.25 a | 226.92 ± 2.36 c |
| A5 | 67.90 ± 0.29 abc | 3.46 ± 0.01 bc | 2.95 ± 0.08 b | 1.16 ± 0.15 abc | 20.69 ± 0.48 bc | 4.66 ± 0.44 ab | 2.14 ± 0.21 a | 208.68 ± 2.08 b |
| A7 | 66.36 ± 0.35 a | 3.49 ± 0.09 c | 2.94 ± 0.02 b | 0.89 ± 0.07 a | 19.81 ± 1.30 b | 5.84 ± 1.15 ab | 3.20 ± 0.67 abc | 207.70 ± 7.02 b |
| BC | 66.60 ± 1.22 ab | 3.97 ± 0.03 de | 3.70 ± 0.09 c | 1.18 ± 0.17 abc | 21.08 ± 0.32 bc | 4.80 ± 0.50 ab | 2.38 ± 0.25 ab | 213.54 ± 1.56 bc |
| B5 | 67.86 ± 0.23 ab | 3.81 ± 0.02 d | 3.64 ± 0.02 c | 1.04 ± 0.12 abc | 21.34 ± 0.02 bc | 3.64 ± 0.25 a | 2.32 ± 0.15 ab | 212.72 ± 1.74 bc |
| B7 | 67.12 ± 1.39 ab | 3.86 ± 0.06 d | 3.64 ± 0.08 c | 1.13 ± 0.02 abc | 20.73 ± 0.09 b | 4.43 ± 0.86 ab | 2.73 ± 0.52 abc | 210.91 ± 4.55 b |
| CC | 70.22 ± 0.02 c | 4.07 ± 0.02 e | 3.61 ± 0.06 c | 1.34 ± 0.13 c | 15.34 ± 0.44 a | 5.98 ± 0.34 b | 3.47 ± 0.19 b | 167.89 ± 2.99 a |
| C5 | 70.02 ± 0.82 c | 4.12 ± 0.02 e | 4.02 ± 0.06 d | 1.47 ± 0.08 c | 14.64 ± 0.38 ª | 9.41 ± 0.37 c | 3.78 ± 0.15 c | 171.58 ± 5.14 a |
| p-value | 0.003 | <0.001 | <0.001 | 0.009 | <0.001 | <0.001 | 0.002 | <0.001 |
| Fatty Acid (%Relative) | Trial | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| AC | A3 | A5 | A7 | BC | B5 | B7 | CC | C5 | p-Value | |
| Dodecanoic acid (C12:0) | nd | nd | nd | nd | nd | nd | nd | 0.02 ± 0.04 | nd | - |
| Myristic acid (C14:0) | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.474 |
| Pentadecylic acid (C15:0) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.106 |
| Palmitic Acid (C16:0) | 12.53 ± 0.43 a | 12.41 ± 0.27 a | 12.73 ± 0.28 a | 12.72 ± 0.11 a | 12.75 ± 0.48 a | 12.75 ± 0.38 a | 12.64 ± 0.37 a | 14.10 ± 0.04 b | 13.62 ± 0.43 b | <0.001 |
| Heptadecylic acid (C17:0) | 0.15 ± 0.02 | 0.15 ± 0.02 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.185 |
| Stearic acid (C18:0) | 4.20 ± 0.35 ab | 3.87 ± 0.17 ab | 3.80 ± 0.42 ab | 4.25 ± 0.32 ab | 4.44 ± 1.08 b | 3.63 ± 0.65 ab | 3.38 ± 0.39 ab | 3.15 ± 0.2 a | 3.85 ± 0.25 ab | 0.024 |
| Arachidic acid (C20:0) | 0.44 ± 0.04 bc | 0.43 ± 0.02 bc | 0.45 ± 0.02 c | 0.41 ± 0.03 abc | 0.41 ± 0.05 abc | 0.37 ± 0.04 ab | 0.39 ± 0.02 abc | 0.35 ± 0.01 a | 0.41 ± 0.02 abc | 0.002 |
| Heneicosylic acid (C21:0) | 0.04 ± 0.00 ab | 0.05 ± 0.02 ab | 0.04 ± 0.00 ab | 0.04 ± 0.00 ab | 0.03 ± 0.00 a | 0.04 ± 0.01 ab | 0.04 ± 0.01 ab | 0.06 ± 0.01 b | 0.06 ± 0.01 b | 0.006 |
| Behenic acid (C22:0) | 0.09 ± 0.01 ab | 0.10 ± 0.01 ab | 0.10 ± 0.00 b | 0.09 ± 0.01 ab | 0.08 ± 0.00 ab | 0.08 ± 0.01 a | 0.08 ± 0.00 ab | 0.08 ± 0.00 ab | 0.09 ± 0.00 ab | 0.005 |
| Lignoceric acid (C24:0) | 0.06 ± 0.01 ab | 0.07 ± 0.00 ab | 0.07 ± 0.00 ab | 0.06 ± 0.00 ab | 0.06 ± 0.00 ab | 0.06 ± 0.00 ab | 0.05 ± 0.01 a | 0.06 ± 0.00 ab | 0.07 ± 0.00 b | 0.021 |
| ∑ SFA | 17.49 ± 0.50 abc | 17.09 ± 0.39 ab | 17.31 ± 0.32 abc | 17.69 ± 0.34 bc | 17.90 ± 0.66 bc | 17.06 ± 0.43 ab | 16.70 ± 0.17 a | 17.93 ± 0.23 bc | 18.19 ± 0.39 c | <0.001 |
| Palmitoleic acid (cis-C16:1) | 0.93 ± 0.18 a | 0.98 ± 0.05 a | 0.95 ± 0.02 a | 1.05 ± 0.04 ab | 0.94 ± 0.12 a | 1.09 ± 0.21 ab | 1.05 ± 0.04 ab | 1.37 ± 0.09 bc | 1.27 ± 0.02 c | <0.001 |
| Ginkgolic acid (C17:1) | 0.26 ± 0.04 | 0.25 ± 0.02 | 0.24 ± 0.02 | 0.24 ± 0.03 | 0.22 ± 0.03 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.22 ± 0.01 | 0.336 |
| Oleic acid (cis-C18:1) | 65.52 ± 0.82 ab | 67.10 ± 1.66 bc | 66.72 ± 0.84 abc | 64.80 ± 1.68 ab | 64.94 ± 1.66 ab | 67.06 ± 0.94 bc | 68.51 ± 0.52 c | 65.79 ± 0.15 ab | 64.19 ± 0.82 a | <0.001 |
| Eicosaenoic acid (C20:1) | 0.22 ± 0.02 b | 0.21 ± 0.01 ab | 0.22 ± 0.00 b | 0.21 ± 0.01 ab | 0.21 ± 0.01 ab | 0.21 ± 0.01 ab | 0.21 ± 0.01 ab | 0.18 ± 0.01 a | 0.20 ± 0.02 ab | 0.009 |
| Docosaenoic acid (C22:1) | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.00 | 0.01 ± 0.01 | 0.02 ± 0.02 | 0.02 ± 0.00 | 0.729 |
| ∑ MUFA | 66.95 ± 0.83 a | 68.56 ± 1.63 ab | 68.15 ± 0.83 ab | 66.32 ± 1.73 a | 66.32 ± 1.78 a | 68.62 ± 1.11 ab | 70.02 ± 0.51 b | 67.62 ± 0.09 a | 65.90 ± 0.84 a | <0.001 |
| Linoleic acid (C18:2) | 10.17 ± 0.88 bc | 8.24 ± 0.66 a | 9.12 ± 0.72 ab | 10.19 ± 0.56 bc | 10.72 ± 0.87 c | 9.20 ± 0.82 abc | 7.93 ± 0.27 a | 8.79 ± 0.34 ab | 10.12 ± 0.50 bc | <0.001 |
| α-Linolenic acid (C18:3) | 0.86 ± 0.06 ab | 0.81 ± 0.02 a | 0.87 ± 0.03 ab | 0.85 ± 0.10 ab | 0.76 ± 0.07 a | 0.74 ± 0.06 a | 0.84 ± 0.03 ab | 0.84 ± 0.02 ab | 0.95 ± 0.05 b | <0.001 |
| ∑ PUFA | 11.03 ± 0.91 bc | 9.04 ± 0.66 a | 9.99 ± 0.72 abc | 11.04 ± 0.57 bc | 11.49 ± 0.93 c | 9.94 ± 0.83 abc | 8.77 ± 0.29 a | 9.63 ± 0.34 ab | 11.07 ± 0.54 bc | <0.001 |
| Palmitelaidic acid (trans-C16:1) | nd | nd | nd | nd | nd | nd | nd | nd | nd | - |
| Elaidic acid (trans-C18:1) | nd | 0.01 ± 0.01 | 0.01 ± 0.00 | 0.01 ± 0.01 | 0.01 ± 0.01 | nd | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.957 |
| Linoelaidic acid (trans-C18:2) | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.01 | 0.01 ± 0.00 | 0.02 ± 0.02 | 0.01 ± 0.00 | 0.382 |
| ∑ trans | 0.02 ± 0.00 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.02 | 0.02 ± 0.01 | 0.779 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coimbra-Gomes, J.; Reis, P.J.M.; Tavares, T.G.; Silva, A.A.; Mendes, E.; Casal, S.; Malcata, F.X.; Macedo, A.C. Cobrançosa Table Olive Fermentation as per the Portuguese Traditional Method, Using Potentially Probiotic Lactiplantibacillus pentosus i106 upon Alternative Inoculation Strategies. Fermentation 2023, 9, 12. https://doi.org/10.3390/fermentation9010012
Coimbra-Gomes J, Reis PJM, Tavares TG, Silva AA, Mendes E, Casal S, Malcata FX, Macedo AC. Cobrançosa Table Olive Fermentation as per the Portuguese Traditional Method, Using Potentially Probiotic Lactiplantibacillus pentosus i106 upon Alternative Inoculation Strategies. Fermentation. 2023; 9(1):12. https://doi.org/10.3390/fermentation9010012
Chicago/Turabian StyleCoimbra-Gomes, Joana, Patrícia J. M. Reis, Tânia G. Tavares, Afonso A. Silva, Eulália Mendes, Susana Casal, Francisco Xavier Malcata, and Angela C. Macedo. 2023. "Cobrançosa Table Olive Fermentation as per the Portuguese Traditional Method, Using Potentially Probiotic Lactiplantibacillus pentosus i106 upon Alternative Inoculation Strategies" Fermentation 9, no. 1: 12. https://doi.org/10.3390/fermentation9010012
APA StyleCoimbra-Gomes, J., Reis, P. J. M., Tavares, T. G., Silva, A. A., Mendes, E., Casal, S., Malcata, F. X., & Macedo, A. C. (2023). Cobrançosa Table Olive Fermentation as per the Portuguese Traditional Method, Using Potentially Probiotic Lactiplantibacillus pentosus i106 upon Alternative Inoculation Strategies. Fermentation, 9(1), 12. https://doi.org/10.3390/fermentation9010012








