Ergothioneine Production by Submerged Fermentation of a Medicinal Mushroom Panus conchatus
Abstract
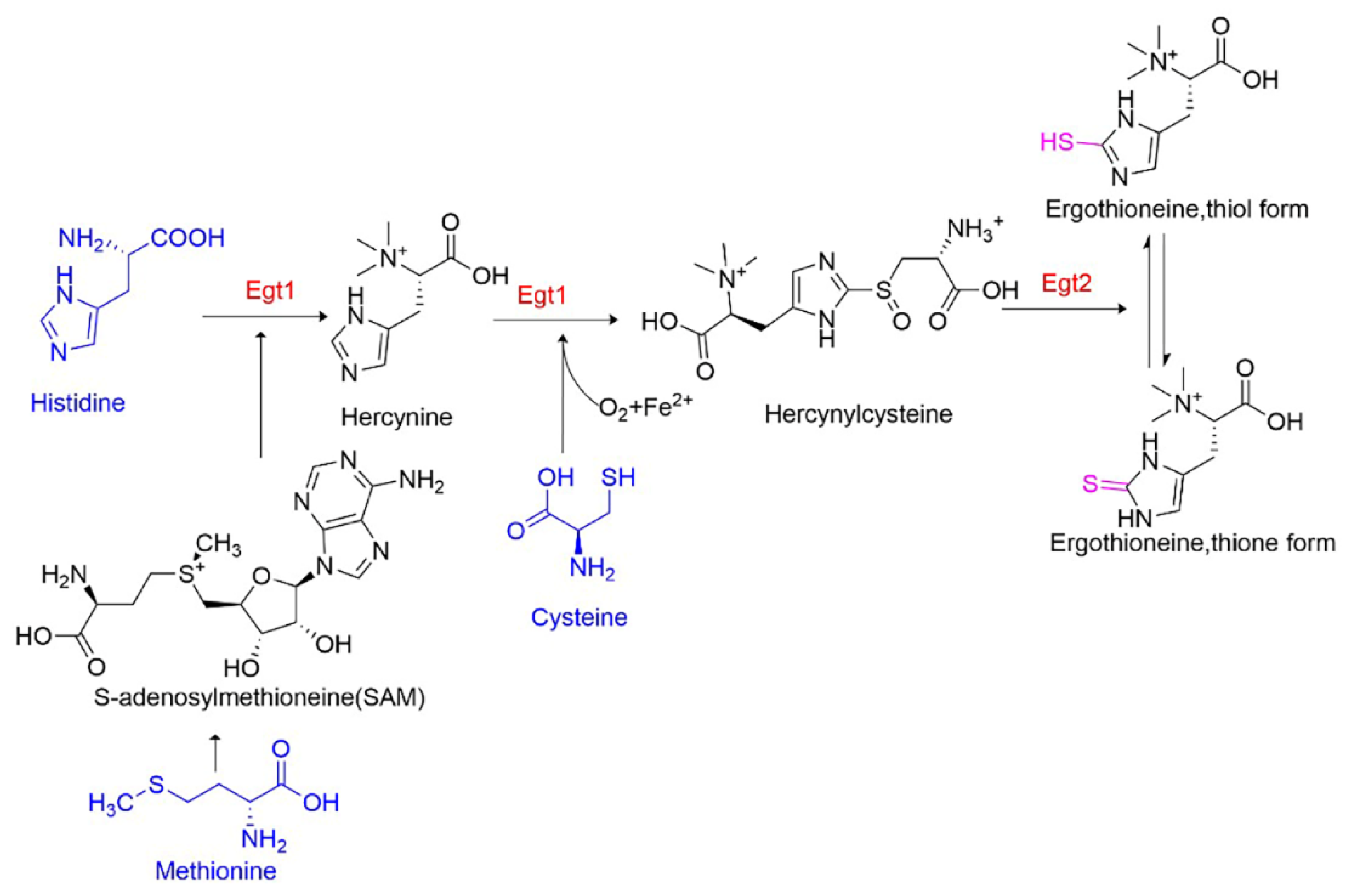
:1. Introduction
2. Materials and Methods
2.1. Strains and Growth Conditions
2.2. Determination of Dry Cell Weight and Substrate Consumption
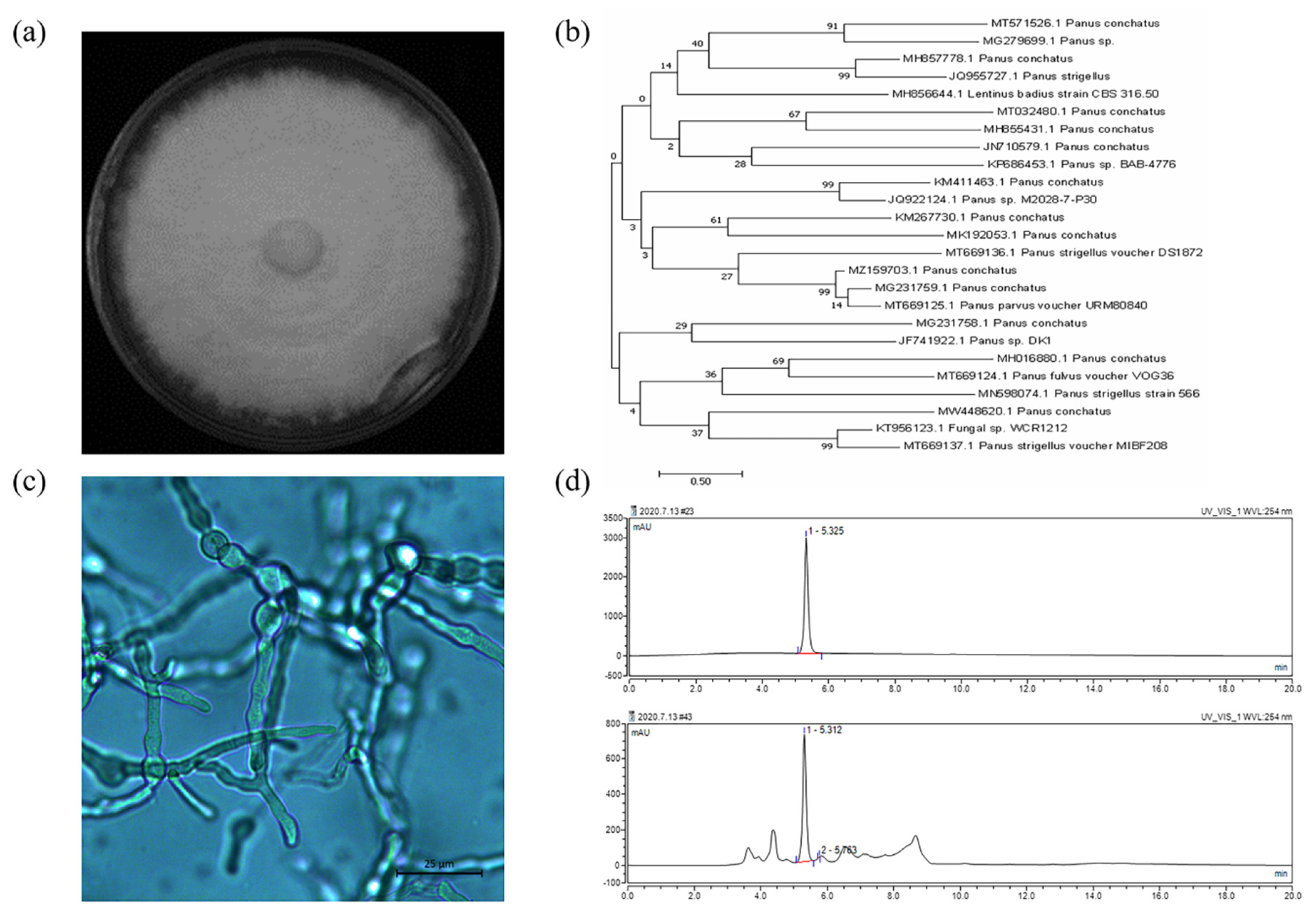
2.3. Genotypic Identification and Phenotypic Identification of Panus conchatus
2.4. Optimization of Cultivation Medium and Environmental Conditions
2.5. EGT Extraction and Analysis
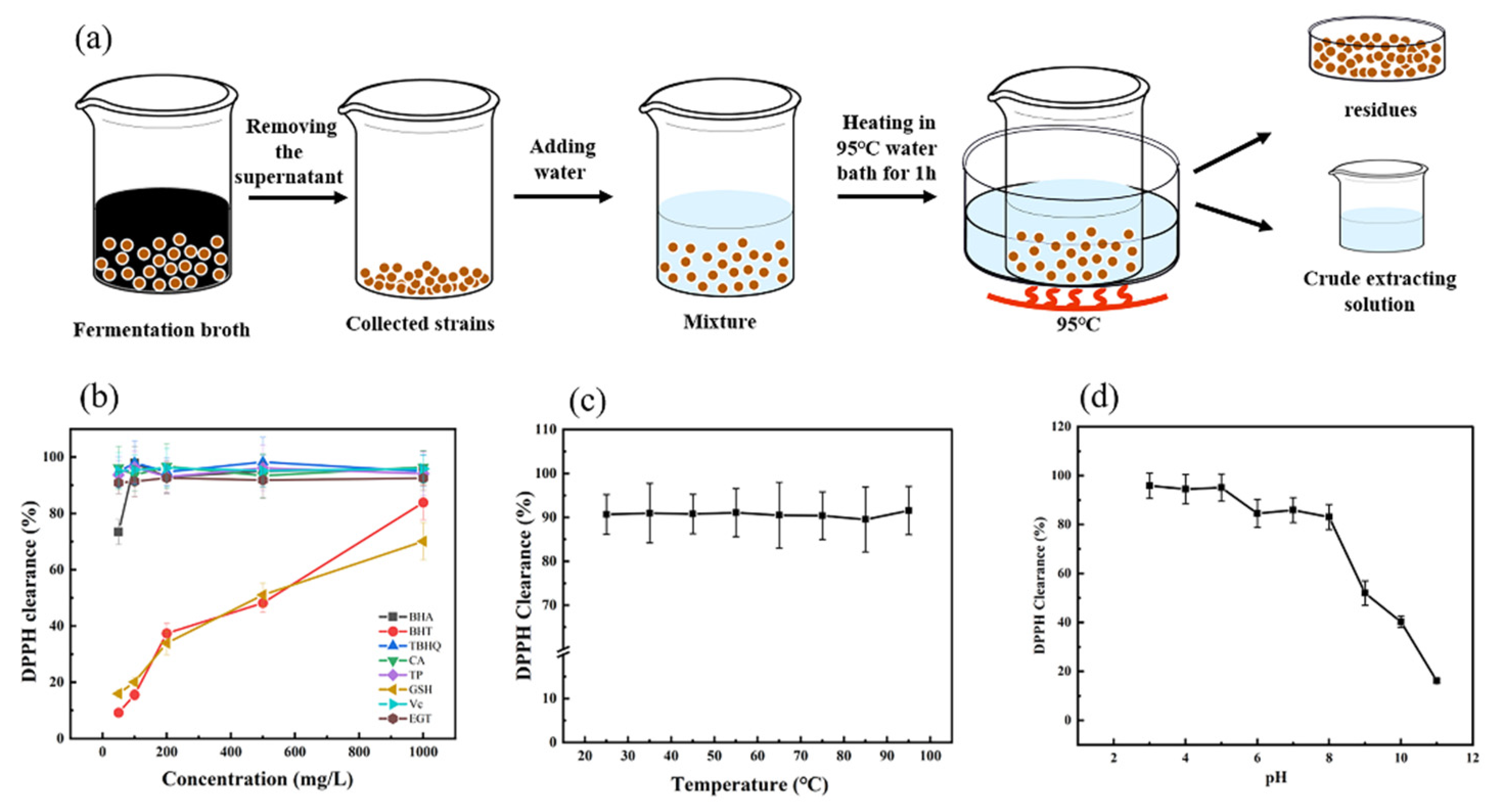
2.6. Evaluation and Stability of Ergothioneine Radical Scavenging Ability by 2,2-Di-Phenyl-1-Picrylhydrazyl
2.7. Statistical Analysis
3. Results and Discussion
3.1. Genotypic Identification and Phenotypic Identification of Panus conchatus
3.2. Choosing the Optimal Nitrogen Sources
3.3. Choosing the Optimal Carbon Sources
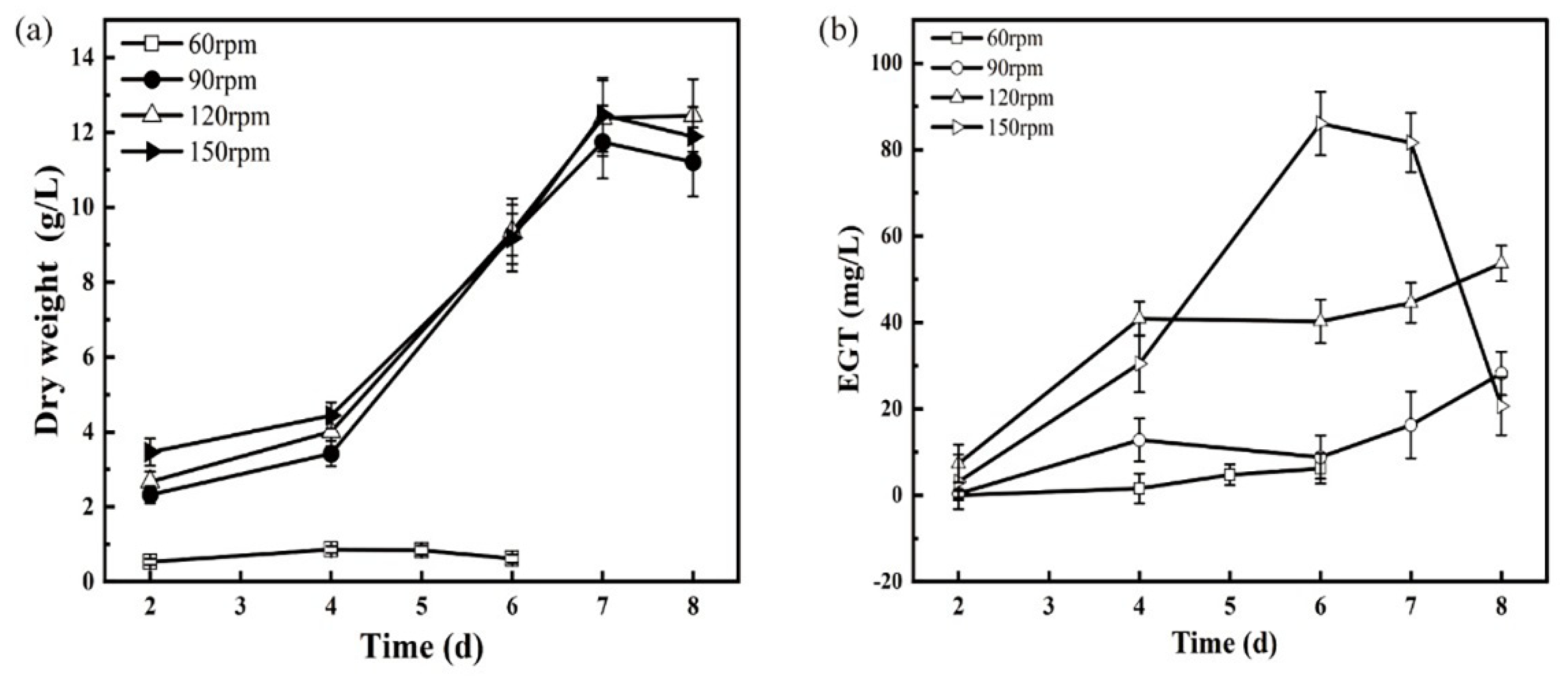
3.4. Effect of Oxygen Supply Conditions
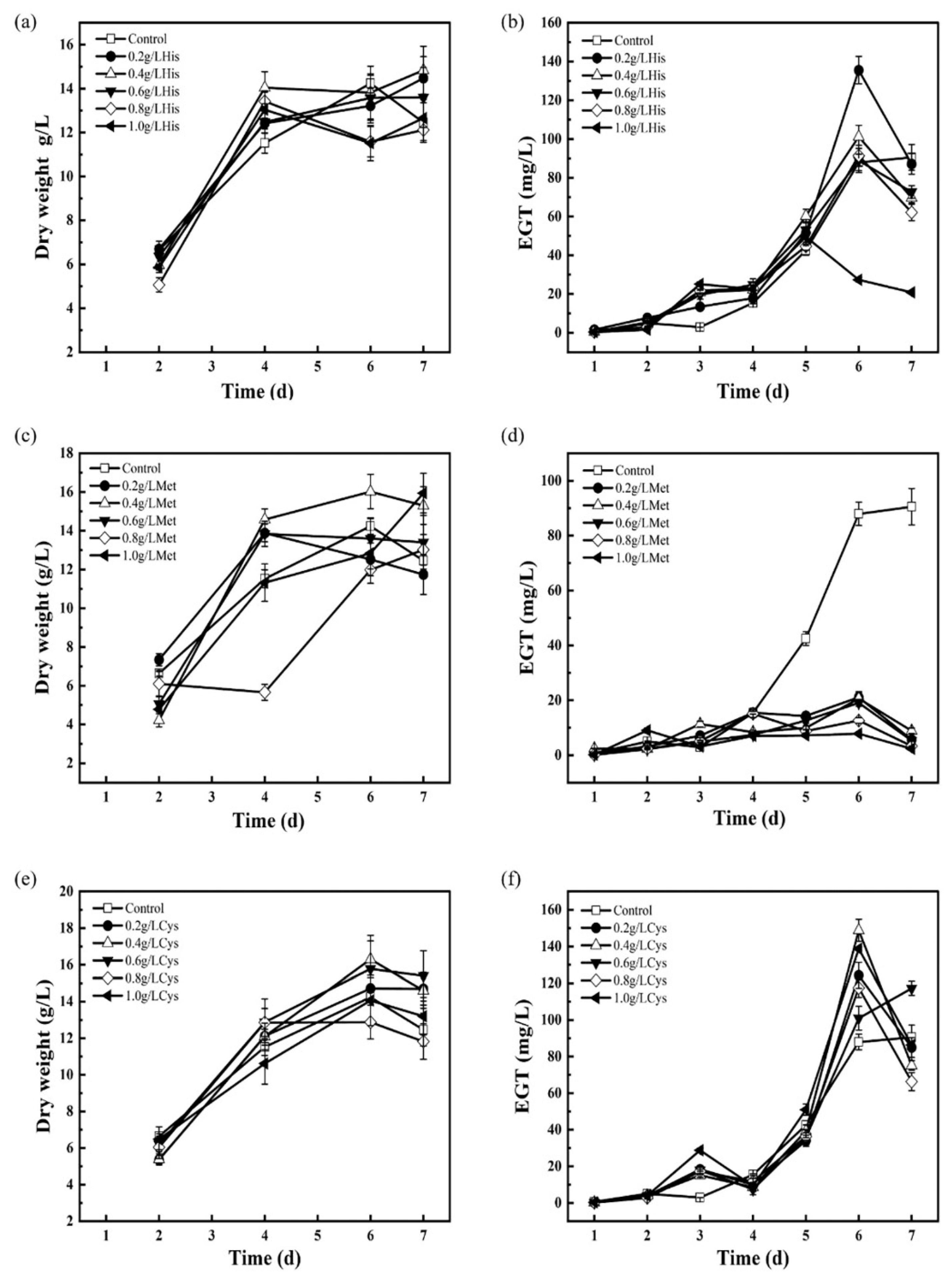
3.5. Adding Precursors to Enhance EGT Biosynthesis
3.6. Evaluation of Radical Scavenging Ability and Stability of Crude Ergothioneine Extract
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hartman, P.E. Erogothioneine as antioxidant. Method Enzymol. 1990, 186, 310–318. [Google Scholar]
- Chaves, N.A.; Pires Alegria, T.G.; Dantas, L.S.; Soares Netto, L.E.; Miyamoto, S.; Bonini Domingos, C.R.; da Silva, D.G.H. Impaired antioxidant capacity causes a disruption of metabolic homeostasis in sickle erythrocytes. Free Radic. Biol. Med. 2019, 141, 34–46. [Google Scholar] [PubMed]
- Pahila, J.; Kaneda, H.; Nagasaka, R.; Koyama, T.; Ohshima, T. Effects of ergothioneine-rich mushroom extracts on lipid oxidation and discoloration in salmon muscle stored at low temperatures. Food Chem. 2017, 233, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Shime, H.; Matsumoto, M.; Kasahara, M.; Seya, T. Anti-oxidative amino acid L-ergothioneine modulates the tumor microenvironment to facilitate adjuvant vaccine immunotherapy. Front. Immunol. 2019, 10, 671. [Google Scholar] [PubMed]
- Cheah, I.K.; Tang, R.M.Y.; Yew, T.S.Z.; Lim, K.H.C.; Halliwell, B. Administration of pure ergothioneine to healthy human subjects: Uptake, metabolism, and effects on biomarkers of oxidative damage and inflammation. Antioxid. Redox Signal. 2017, 26, 193–206. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Cao, L.; Zhang, W.; Lu, R.; Bian, J.S.; Nie, X. Therapeutic potential of sulfur-containing natural products in inflammatory diseases. Pharmacol. Ther. 2020, 216, 107687. [Google Scholar]
- Nakamichi, N.; Nakayama, K.; Ishimoto, T.; Masuo, Y.; Wakayama, T.; Sekiguchi, H.; Sutoh, K.; Usumi, K.; Iseki, S.; Kato, Y. Food-derived hydrophilic antioxidant ergothioneine is distributed to the brain and exerts antidepressant effect in mice. Brain Behav. 2016, 6, e00477. [Google Scholar] [CrossRef]
- Nishida, K.; Takeuchi, K.; Hosoda, A.; Sugano, S.; Morisaki, E.; Ohishi, A.; Nagasawa, K. Ergothioneine ameliorates oxaliplatin-induced peripheral neuropathy in rats. Life Sci. 2018, 207, 516–524. [Google Scholar]
- Tyler, E.F.; Misra, S.L.; McGhee, C.N.J.; Zhang, J. Corneal nerve plexus changes induced by Oxaliplatin chemotherapy and Ergothioneine antioxidant supplementation. Clin. Exp. Ophthalmol. 2020, 48, 264–266. [Google Scholar] [CrossRef]
- Han, Y.W.; Tang, X.Y.; Zhang, Y.T.; Hu, X.C.; Ren, L.J. The current status of biotechnological production and the application of a novel antioxidant ergothioneine. Crit. Rev. Biotechnol. 2021, 41, 580–593. [Google Scholar] [CrossRef]
- Ramirez-Martinez, A.; Wesolek, N.; Yadan, J.C.; Moutet, M.; Roudot, A.C. Intake assessment of L-ergothioneine in some European countries and in the United States. Hum. Ecol. Risk Assess. 2016, 22, 667–677. [Google Scholar] [CrossRef]
- Kitsanayanyong, L.; Ohshima, T. Ergothioneine: A potential antioxidative and antimelanosis agent for food quality preservation. FEBS Lett. 2022, 596, 1330–1347. [Google Scholar] [CrossRef] [PubMed]
- Hseu, Y.C.; Gowrisankar, Y.V.; Chen, X.Z.; Yang, Y.C.; Yang, H.L. The antiaging activity of ergothioneine in UVA-irradiated human dermal fibroblasts via the inhibition of the AP-1 pathway and the activation of Nrf2-mediated antioxidant genes. Oxidative Med. Cell. Longev. 2020, 2020, 2576823. [Google Scholar] [CrossRef]
- Tsay, G.J.; Lin, S.Y.; Li, C.Y.; Mau, J.L.; Tsai, S.Y. Comparison of single and combined use of ergothioneine, ferulic acid, and glutathione as antioxidants for the prevention of ultraviolet B radiation-induced photoaging damage in human skin fibroblasts. Processes 2021, 9, 1204. [Google Scholar] [CrossRef]
- Fujitani, Y.; Alamgir, K.M.; Tani, A. Ergothioneine production using methylobacterium species, yeast, and fungi. J. Biosci. Bioeng. 2018, 126, 715–722. [Google Scholar] [CrossRef]
- Pfeiffer, C.; Bauer, T.; Surek, B.; Schoemig, E.; Gruendemann, D. Cyanobacteria produce high levels of ergothioneine. Food Chem. 2011, 129, 1766–1769. [Google Scholar] [CrossRef]
- Kalaras, M.D.; Richie, J.P.; Calcagnotto, A.; Beelman, R.B. Mushrooms: A rich source of the antioxidants ergothioneine and glutathione. Food Chem. 2017, 233, 429–433. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ho, K.J.; Liang, C.H.; Tsai, C.H.; Huang, L.Y.; Mau, J.L. Preparation of culinary-medicinal king oyster mushroom Pleurotus eryngii fermented products with high ergothioneine content and their taste quality. Int. J. Med. Mushrooms 2012, 14, 85–93. [Google Scholar] [CrossRef]
- Tepwong, P.; Giri, A.; Sasaki, F.; Fukui, R.; Ohshima, T. Mycobial enhancement of ergothioneine by submerged cultivation of edible mushroom mycelia and its application as an antioxidative compound. Food Chem. 2012, 131, 247–258. [Google Scholar] [CrossRef]
- Chen, S.Y.; Ho, K.J.; Hsieh, Y.J.; Wang, L.T.; Mau, J.L. Contents of lovastatin, gamma-aminobutyric acid and ergothioneine in mushroom fruiting bodies and mycelia. LWT-Food Sci. Technol. 2012, 47, 274–278. [Google Scholar] [CrossRef]
- Hu, W.; Song, H.; Her, A.S.; Bak, D.W.; Naowarojna, N.; Elliott, S.J.; Qin, L.; Chen, X.; Liu, P. Bioinformatic and biochemical characterizations of C-S bond formation and cleavage enzymes in the fungus Neurospora crassa ergothioneine biosynthetic pathway. Org. Lett. 2014, 16, 5382–5385. [Google Scholar] [CrossRef] [PubMed]
- Takusagawa, S.; Satoh, Y.; Ohtsu, I.; Dairi, T. Ergothioneine production with Aspergillus oryzae. Biosci. Biotechnol. Biochem. 2019, 83, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Estrada, A.E.R.; Lee, H.J.; Beelman, R.B.; Jimenez-Gasco, M.d.M.; Royse, D.J. Enhancement of the antioxidants ergothioneine and selenium in Pleurotus eryngii var. eryngii basidiomata through cultural practices. World J. Microbiol. Biotechnol. 2009, 25, 1597–1607. [Google Scholar] [CrossRef]
- Liang, C.H.; Huang, L.Y.; Ho, K.J.; Lin, S.Y.; Mau, J.L. Submerged cultivation of mycelium with high ergothioneine content from the culinary-medicinal king oyster Mushroom Pleurotus eryngii (higher basidiomycetes) and its composition. Int. J. Med. Mushrooms 2013, 15, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Chien, S.C.; Wang, S.Y.; Mau, J.L. Submerged cultivation of mycelium with high ergothioneine content from the culinary-medicinal golden oyster mushroom, Pleurotus citrinopileatus (higher basidiomycetes). Int. J. Med. Mushrooms 2015, 17, 749–761. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Li, Z.H.; Feng, T.; Liu, J.K. A new cadinane sesquiterpenoid from cultures of the basidiomycete Panus conchatus. Nat. Prod. Res. 2018, 32, 2333–2337. [Google Scholar] [CrossRef]
- Tellez-Tellez, M.; Fernandez, F.J.; Montiel-Gonzalez, A.M.; Sanchez, C.; Diaz-Godinez, G. Growth and laccase production by Pleurotus ostreatus in submerged and solid-state fermentation. Appl. Microbiol. Biotechnol. 2008, 81, 675–679. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nagasaka, R.; Ohshima, T. Effects of extraction solvents, cooking procedures and storage conditions on the contents of ergothioneine and phenolic compounds and antioxidative capacity of the cultivated mushroom Flammulina velutipes. Int. J. Food Sci. Technol. 2012, 47, 1193–1205. [Google Scholar] [CrossRef]
- Szabo, M.R.; Iditoiu, C.; Chambre, D.; Lupea, A.X. Improved DPPH determination for antioxidant activity spectrophotometric assay. Chem. Pap. 2007, 61, 214–216. [Google Scholar] [CrossRef]
- Wu, C.Y.; Mau, J.L.; Liang, Z.C. The influence of cultivation conditions on mycelial growth and exopolysaccharide production of culinary-medicinal mushroom, Pleurotus citrinopileatus Singer (Agaricomycetideae). Int. J. Med. Mushrooms 2008, 10, 279–292. [Google Scholar] [CrossRef]
- Kim, H.M.; Paik, S.Y.; Ra, K.S.; Koo, K.B.; Yun, J.W.; Choi, J.W. Enhanced production of exopolysaccharides by fed-batch culture of Ganoderma resinaceum DG-6556. J. Microbiol. 2006, 44, 233–242. [Google Scholar] [PubMed]
- Riley, G.L.; Tucker, K.G.; Paul, G.C.; Thomas, C.R. Effect of biomass concentration and mycelial morphology on fermentation broth rheology. Biotechnol. Bioeng. 2000, 68, 160–172. [Google Scholar] [CrossRef]
- Tepwong, P.; Giri, A.; Ohshima, T. Effect of mycelial morphology on ergothioneine production during liquid fermentation of Lentinula edodes. Mycoscience 2012, 53, 102–112. [Google Scholar] [CrossRef]
- Koutinas, A.A.; Wang, R.; Webb, C. Evaluation of wheat as generic feedstock for chemical production. Ind. Crops Prod. 2004, 20, 75–88. [Google Scholar] [CrossRef]
- Quinzii, C.M.; Lopez, L.C.; Gilkerson, R.W.; Dorado, B.; Coku, J.; Naini, A.B.; Lagier-Tourenne, C.; Schuelke, M.; Salviati, L.; Carrozzo, R.; et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ(10) deficiency. Faseb J. 2010, 24, 3733–3743. [Google Scholar] [CrossRef]
- Tanaka, N.; Kawano, Y.; Satoh, Y.; Dairi, T.; Ohtsu, I. Gram-scale fermentative production of ergothioneine driven by overproduction of cysteine in Escherichia coli. Sci. Rep. 2019, 9, 1895. [Google Scholar]
- Hidese, R.; Mihara, H.; Esaki, N. Bacterial cysteine desulfurases: Versatile key players in biosynthetic pathways of sulfur-containing biofactors. Appl. Microbiol. Biotechnol. 2011, 91, 47–61. [Google Scholar]
- Uprety, M.C.; Revis, B. Elevated temperature studies on stability of ascorbic acid in certain fruit juice and aqueous vehicles. J. Pharm. Sci. 1964, 53, 1248–1251. [Google Scholar] [CrossRef]
| Nitrogen Source | Glycerol Consumption Rate g/L/h | Dry Cell Weight g/L | EGT Yield mg/L | Fermentation Time, h | EGT Productivity mg/L/h |
|---|---|---|---|---|---|
| Peptone | 0.22 ± 0.016 a | 21.66 ± 2.72 a | 20.22 ± 1.76 a | 192 | 0.11 |
| Soy peptone | 0.38 ± 0.011 c | 16.08 ± 1.13 b | 40.13 ± 3.89 c | 144 | 0.28 |
| Casein peptone | 0.23 ± 0.019 a | 10.16 ± 1.26 c | 33.41 ± 2.23 b | 240 | 0.14 |
| Soybean meal powder | 0.27 ± 0.027 b | - | 19.90 ± 1.76 a | 240 | 0.08 |
| Beef extract | 0.37 ± 0.019 c | 16.74 ± 1.50 b | 21.33 ± 1.17 a | 96 | 0.22 |
| Yeast powder | 0.24 ± 0.020 b | 17.50 ± 2.26 b | 36.64 ± 0.98 b | 240 | 0.15 |
| Carbon Source | Dry Cell Weight g/L | EGT Yield mg/L | Fermentation Time, h | EGT Productivity mg/L/h |
|---|---|---|---|---|
| Molasses | 9.70 ± 0.54 a | 81.44 ± 5.01 g | 96 | 0.85 |
| Fructose | 7.93 ± 0.55 a | 22.69 ± 1.99 f | 144 | 0.16 |
| Sucrose | 8.65 ± 0.70 a | 11.75 ± 0.57 c | 192 | 0.06 |
| Maltose | 11.37 ± 0.91 a | 14.74 ± 1.11 d | 192 | 0.08 |
| Glucose | 13.12 ± 0.76 b | 18.78 ± 1.67 e | 96 | 0.20 |
| Glycerol | 9.74 ± 0.41 a | 33.35 ± 1.12 a | 96 | 0.35 |
| Dextrin | - | 7.20 ± 0.88 b | 192 | 0.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Han, Y.; Hu, X.; Gong, C.; Ren, L. Ergothioneine Production by Submerged Fermentation of a Medicinal Mushroom Panus conchatus. Fermentation 2022, 8, 431. https://doi.org/10.3390/fermentation8090431
Zhu M, Han Y, Hu X, Gong C, Ren L. Ergothioneine Production by Submerged Fermentation of a Medicinal Mushroom Panus conchatus. Fermentation. 2022; 8(9):431. https://doi.org/10.3390/fermentation8090431
Chicago/Turabian StyleZhu, Min, Yiwen Han, Xuechao Hu, Changbin Gong, and Lujing Ren. 2022. "Ergothioneine Production by Submerged Fermentation of a Medicinal Mushroom Panus conchatus" Fermentation 8, no. 9: 431. https://doi.org/10.3390/fermentation8090431
APA StyleZhu, M., Han, Y., Hu, X., Gong, C., & Ren, L. (2022). Ergothioneine Production by Submerged Fermentation of a Medicinal Mushroom Panus conchatus. Fermentation, 8(9), 431. https://doi.org/10.3390/fermentation8090431







