Abstract
Antimicrobial resistance demands the development of therapeutic alternatives such as prebiotics, probiotics, and nutraceuticals. The aim of this study was to assess the antimicrobial proprieties of the nutraceutical fermented defatted “alperujo”, derived from olive oil production, in a laying hen farm (n = 122,250) endemic with avian intestinal spirochetosis (Brachyspira spp.). Part of the batch (n = 1440) was divided into six groups of 240 hens each that included 80 or 108-week-old laying hens, supplemented with 0%, 2%, or 6% fermented defatted ‘alperujo’ for a month. At the end of the experiment, eight hens from each group were autopsied and cecal content was subjected to (i) Brachyspira culture and species identification by PCRs, and (ii) direct DNA extraction and Brachyspira qPCR. Furthermore, the ceca were processed for histopathology. Microbiological isolation revealed B. pilosicoli and B. hyodysenteriae co-infection in all groups. The 80-week-old hen group 2% supplemented showed a reduction in the cecal Brachyspira content (qPCR) compared with non-supplemented hens. Cecal histopathology showed a diffuse mild infiltration of lymphocytes, plasma cells, and heterophils; and hyperplasia of the gut-associated lymphoid tissue hyperplasia which decreased in severity in 80-week-old supplemented hens. The reduction in Brachyspira colonization and the severity of the lesions observed in supplemented hens highlights a potential protective function against avian intestinal spirochetosis.
1. Introduction
Avian intestinal spirochetosis is a worldwide neglected disease of domestic avian species and waterfowl caused by Brachyspira spp. [1]. Some species are regarded as pathogens of poultry: B. intermedia, B. pilosicoli, and B. alvinipulli [2,3,4]. Furthermore, B. pilosicoli has zoonotic potential leading to food-borne infection in immunocompromised people [5,6]. Brachyspira infection of laying hens causes chronic diarrhea, discomfort, and mortality [4,7]. Avian intestinal spirochetosis productive performance impairment and economic importance is due to fecal staining of eggshells, delayed onset of lay in pullets, reduced egg production, poor chick quality from infected breeders, and reduction of feed conversion ratio [4,7,8]. The cost of avian intestinal spirochetosis was estimated at 14 million pounds (over 16 million euro) in the United Kingdom in 2006 [7]. Antimicrobial treatment (ampicillin, tiamulin, lincomycin, zinc bacitracin) is the preferred tool to control Brachyspira infections in laying hen farms [2,7,8,9]. However, relapse after treatment and emerging antimicrobial resistance have been reported for avian spirochetes, demanding re-orientation of avian intestinal spirochetosis management [3,8,10].
Probiotic, prebiotic, and nutraceutical products are compounds with antimicrobial proprieties as feed additives or supplements in poultry [11,12,13]. Specifically, nutraceuticals are compounds derived from natural sources with benefits other than nutrition [14]. The olive oil two-phase extraction system produces the semisolid by-product ‘alperujo’ [15] that can be adapted to animal feed through bacterial fermentation, fat hydrolysis, desiccation, and grinding [16]. The nutraceutical obtained, fermented defatted ‘alperujo’, has been tested in laying hens and broilers showing an improvement of the intestinal health by means of higher bacterial diversity and enhancement of mucosal histological parameters [16,17]. Improvement of some productive parameters has been also reported in laying hens (reduced broken eggs) [16] and broilers (body weight) [17]. In addition, the co-administration of biscuit flour and fermented defatted ‘alperujo’ has also proved beneficial for intestinal health and production performance in laying hens [18].
Olives contain phenolic compounds with beneficial properties (antioxidant, anti-inflammatory, antithrombotic, antimicrobial, and anti-coccidian) that are retained in olive oil by-products [19,20,21]. The antimicrobial properties of fermented defatted ‘alperujo’ have been previously investigated in broilers experimentally challenged with Salmonella Typhimurium [22]. The results of these experiments showed that dietary supplementation delayed and reduced Salmonella Typhimurium colonization in the cecum of challenged broilers, contributing to prophylactic and therapeutic measures to reduce salmonellosis prevalence in poultry farms [22]. However, the effectiveness of fermented defatted ‘alperujo’ to reduce colonization by other enteric bacterial pathogens aside from Salmonella is unexplored. Therefore, in this study, the potential benefits of fermented defatted ‘alperujo’ were assessed to control avian intestinal spirochetosis in a laying hen farm.
2. Materials and Methods
2.1. Laying Hens and Rearing Conditions
This study was performed in a commercial laying hen farm (Hy-Line 2015, n = 122,250), from which part (n = 1440) was divided into six groups of 240 hens each (720 80-week-old laying hens and 720 108-week-old laying hens, see below) and monitored for a month. Hens were kept in an intensive housing system under homogeneous environmental conditions (24–32 °C, 50–70% humidity). Feed and water were supplied ad libitum.
2.2. Diet, Groups, and Sampling
For the dietary inclusion, fermented defatted ‘alperujo’ was prepared as previously detailed [16]. ‘Alperujo’ first underwent a controlled anaerobic bacterial fermentation, was then defatted with chemical solvents (fat hydrolysis), followed by desiccation at 80 °C in a low oxygen content atmosphere, and finally ground. Fermented defatted ‘alperujo’ composition is detailed in Table 1.

Table 1.
Fermented defatted ‘alperujo’ composition [16].
Fermented defatted ‘alperujo’ was added to the commercial feed (Table 2) in a 2% or 6% respect to the feed weight by employing a mixing machine

Table 2.
Ingredient composition of the commercial feed.
All laying hens were fed the same commercial formulation as used in the farm, but some hens were supplemented with 2% or 6% fermented defatted ‘alperujo’ depending on the group: (1) 80-week-old hens infected with Brachyspira spp., (2) 80-week-old hens infected with Brachyspira spp. and dietary supplemented with 2% fermented defatted ‘alperujo’, (3) 80-week-old hens infected with Brachyspira spp. and dietary supplemented with 6% fermented defatted ‘alperujo’, (4) 108-week-old hens infected with Brachyspira spp., (5) 108-week-old hens infected with Brachyspira spp. and dietary supplemented with 2% fermented defatted ‘alperujo’, and (6) 108-week-old hens infected with Brachyspira spp. and dietary supplemented with 6% fermented defatted ‘alperujo’.
One month after dietary supplementation, eight hens from each group (n = 48 in total) were euthanized. During autopsies, cecal content was sampled in parallel and subjected to (i) Brachyspira culture, DNA extraction, and identification by polymerase chain reaction (PCR), and (ii) direct DNA extraction from cecal content and Brachyspira quantification by real time PCR (qPCR) [23]. Additionally, ceca samples were collected for histopathological studies.
2.3. Brachyspira Culture and Confirmation by PCR
For Brachyspira culture and confirmation, cecal samples were cultured onto MacConkey agar, MacConkey agar supplemented with cefotaxime (CTX, 1 mg/L), and trypto-casein soy agar (TSA) plates. Plates were incubated at 37 °C in anaerobic conditions (80% N2, 10% H2, and 10% CO2). Plates were inspected after 72 h and, if negative, daily for 10 days. Morphologically compatible cultures were sub-cultured until pure, and DNA was extracted for further identification by polymerase chain reaction (PCR). Brachyspira isolates were identified using previously described PCR protocols for B. intermedia, B. pilosicoli, and B. hyodysenteriae. Briefly, a PCR was performed based on the amplification of the NADH oxidase (nox) gene of B. intermedia (567 base pair region) and B. hyodysenteriae (1268 base pair region) [24], and the 16S rRNA gene (823 base pair region) of B. pilosicoli [25].
2.4. Quantitative Real-Time PCR for Brachyspira
Direct DNA extraction from cecal samples was carried out using a commercial kit (FASTI001-1 FavorPrep Stool DNA Isolation Mini Kit, Favorgen-Europe, Vienna, Austria), following the manufacturer’s specifications (elution volume of 200 µL), coupled with a specific qPCR assay for quantitative detection of Brachyspira spp., as described previously [23]. Briefly, a multiplex qPCR assay was performed based on the amplification of a 198 base pair portion of the NADH oxidase gene, using TaqMan probes for detecting and quantifying B. hyodysenteriae, B. pilosicoli, and B. intermedia. The detection limit for all three targeted species was 1–10 viable cells and 10 fg DNA per reaction [23]. Genome equivalent/g was calculated performing a standard curve based on 10-fold serial dilutions of known amounts of positive control DNA.
2.5. Histological Processing
Ceca samples obtained during the autopsies were fixed in 10% buffered formalin, automatically dehydrated in ethanol series and xylene substitute, and embedded in synthetic paraffin (Citadel 2000 Tissue Processor, Thermo Fisher Scientific, Waltham, MA, USA). Paraffin blocks (Histo Star Embedding Workstation, Thermo Fisher Scientific) were cut at 4 µm sections (Finesse ME+ Microtome, Thermo Fisher Scientific) and stained with hematoxylin-eosin (Gemini AS Automated Slide Stainer, Thermo Fisher Scientific), mounted (CTM6 Coverslipper, Thermo Fisher Scientific), and examined under light microscopy (DM2000 LED, Leica, Wetzlar, Germany).
2.6. Statistical Analysis
The qPCR results (genome equivalent/g) were compared according to the percentage of supplementation using a Kruskal–Wallis test, followed by a Holm–Bonferroni post hoc test. In addition, this comparison was performed for each age group.
3. Results
3.1. Brachyspira Culture and Confirmation on Cecal Content
Regardless of the group, Brachyspira was cultured from all cecal contents as detailed in Table 3. Species identification revealed B. pilosicoli and B. hyodysenteriae co-infection in all the groups (Table 3). B. intermedia was not detected in any sample.

Table 3.
Brachyspira detection in cecal content according to the groups.
3.2. Quantitative Real-Time PCR for Brachyspira
In 80-week-old laying hens, dietary supplementation with fermented defatted ‘alperujo’ showed a reduction in the cecal Brachyspira content (Figure 1) in the 2% group (median: 1.59 × 106) compared with non-supplemented hens (median: 3.30 × 106). No statistical differences were observed in the 2% and in 6% group (median: 3.65 × 106) compared with controls (p > 0.05).
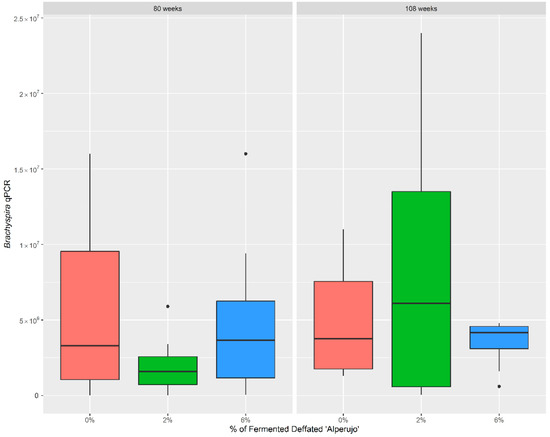
Figure 1.
Distribution of the Brachyspira spp. qPCR (genome equivalent/g) observed among groups. Boxes indicate the interquartile range, middle highlighted bars inside boxes indicate median values, and top and bottom of the box indicate the 75th and 25th percentile. Whiskers denote 97.5th and 2.5th percentile. Dots represent outliers. Dietary supplementation with fermented defatted ‘alperujo’ showed a reduction in the cecal Brachyspira content in 80-week-old laying hens.
In 108-week-old laying hens, dietary supplementation with fermented defatted ‘alperujo’ did not show any reduction in the cecal Brachyspira content (Figure 1) in 2% (median: 6.10 × 106) or 6% (median: 4.15 × 106) groups compared with non-supplemented hens (median: 3.75 × 106). No statistically significant differences were found among groups (p > 0.05).
3.3. Histopathology
Histopathological study of the cecum in non-supplemented laying hens regardless of the age group showed a moderate chronic diffuse lymphoplasmacytic and heterophilic typhlitis, displaying also mild to moderate epithelial hyperplasia and moderate gut-associated lymphoid tissue (GALT) hyperplasia (Figure 2a).
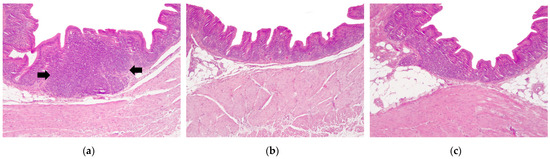
Figure 2.
Histopathological study of the cecum of 80 and 108-week-old laying hens infected with Brachyspira and supplemented with fermented defatted ‘alperujo’. (a) Non-supplemented groups. Moderate chronic diffuse lymphoplasmacytic and heterophilic typhlitis and marked GALT hyperplasia (arrows). (b) Two percent supplemented groups. There is a marked reduction in the lesion severity compared with non-supplemented hens. (c) Six percent supplemented groups. There is a reduction in the inflammation compared with non-supplemented hens. Hematoxylin-eosin, 100×, scale bar: 200 µm.
Supplemented laying hens showed a marked reduction in the intestinal lesions, displaying a reduction in the severity of the lymphoplasmacytic and heterophilic inflammation in the cecal lamina propria and less/absence of GALT hyperplasia. Histopathological changes were milder in 2% supplemented group (Figure 2b) compared with 6% supplemented group (Figure 2c).
4. Discussion
Avian intestinal spirochetosis is an emerging disease which includes the genus Brachyspira, which groups different species recognized as poultry pathogens. Here, before investigating the effectiveness of the nutraceutical tested against Brachyspira, we confirmed the natural infection and revealed two major species infecting (co-infecting) the laying hens of the flock: B. pilosicoli and B. hyodysenteriae. Studies performed in the United States of America (USA) also revealed B. pilosicoli, but in a reduced number of flocks (23.8%) and with a low within-flock prevalence (10.7%) compared with other Brachyspira spp. [26]. Although B. intermedia seem to be more prevalent in the USA, we did not find B. intermedia in any cecal sample [26]. On the other hand, B. hyodysenteriae has been previously reported in poultry [27]. This suggests that different Brachyspira spp. may be involved in avian intestinal spirochetosis, depending on the geographic region. In addition, studies focusing on the diversity of Brachyspira spp. in laying hen flocks also found the presence of different Brachyspira spp. in fecal samples as reported here [25].
Antimicrobials are the main control strategy for avian intestinal spirochetosis in poultry [2,7,8,9]. Despite the current antibiotic use, antimicrobial resistance and subsequent use restriction in the European Union demands development of therapeutic alternatives in animal health, such as prebiotics, probiotics, and nutraceuticals. Dietary composition has been studied in the context of Brachyspira infection in laying hens. For example, the use of different diets based on wheat, barley, or barley and sorghum showed that laying hens fed wheat had significantly more colonization by B. intermedia than the other hens fed with other cereals [28]. Furthermore, the wheat variety also seems to influence B. intermedia intestinal colonization in laying hens [29]. Few natural compounds have been tested as antimicrobial alternatives in poultry models of Brachyspira infection. Essential oils have been tested in vitro and in vivo in a study [30]. Of them, cinnamaldehyde was proven inhibitory for B. intermedia in vitro and was shown to reduce B. intermedia in the cecum of pullets in vivo [30]. However, the underlying mechanisms were not clearly defined, with microbiota and mucosal structure changes considered to be the main mechanisms involved in B. intermedia reduction [30]. The use of the probiotic Lactobacillus reuteri LM1 in drinking water was demonstrated useful to reduce B. pilosicoli clinical signs, histological intestinal lesions, and improve performance in laying hens, possibly through bacterial competition in the gut lumen and/or intestinal microenvironment acidification [31].
Here, the nutraceutical fermented defatted ‘alperujo’, derived from the olive oil production, was assessed to control Brachyspira infection in a laying hen farm endemic with avian intestinal spirochetosis. Our results showed a reduction in Brachyspira spp. cecal content in 80-week-old laying hens supplemented at 2%. Similar to our results, the employment of this nutraceutical at 2% has proven valuable to control cecal Salmonella Typhimurium colonization in broilers under experimental conditions [22]. The bacteriostatic capacity of this nutraceutical has been attributed to the inhibition of bacterial division via reduction of the ATP intracellular concentration through the synergistic action of phenols and polyphenols, particularly hydroxytyrosol [22].
The effects on the intestinal health of the nutraceutical tested here also comprise microbiota modulation and morphologic changes in the intestine [16,17,18,22]. The evaluation of the intestinal mucosa provides valuable information on health and disease in laying hens. Herein, the histopathological results in Brachyspira-infected non-supplemented laying hens revealed evident cecal inflammation. While some authors did not report histological lesions in Brachyspira-infected laying hens [9], others describe the attachment of Brachyspira to the intestinal epithelium before invading the mucosa and inducing a variable lymphoplasmacytic typhlocolitis [8,27,31]. Here, the supplementation with fermented defatted ‘alperujo’ markedly reduced intestinal inflammation regardless of the age range, especially at 2% supplementation. These results coincide with those obtained in broilers infected with Salmonella Typhimurium and supplemented with fermented defatted ‘alperujo’, which display a reduction in the intestinal inflammation compared with non-supplemented Salmonella-infected controls [22]. Our results also coincide with those obtained in laying hens 21 days after B. pilosicoli challenge and supplemented with the probiotic Lactobacillus reuteri LM1, which showed a marked reduction in the severity of histopathological lesions [31]. This highlights the need to monitor histopathological changes during efficacy trials testing antimicrobial compounds.
The age of the animal is known to influence epidemiology and pathogenesis in avian intestinal spirochetosis, as initial infection of pullets leads to amplification and transmission within the farm [5,25,32]. Therefore, two age groups (80- and 108-week-old laying hens) were independently studied to assess age differences. The results showed no positive effects in 108-week-old hens. This may be explained by the increased rate of colonization of Brachyspira in older animals [32]. Concordantly, some authors have set that intestinal spirochetes significantly increase in chickens older than 40 weeks of age [5]. The expected increased Brachyspira content in the ceca of older hens may explain the decreased effectiveness of the tested nutraceutical in this age range.
5. Conclusions
This study showed that dietary supplementation with fermented defatted ‘alperujo’ at 2%, but not 6%, promotes the cecal reduction of Brachyspira (B. pilosicoli and B. hyodysenteriae) and diminished cecal inflammation in 80-week-old laying hens. This nutraceutical can contribute to the control of avian intestinal spirochetosis, thereby reducing the use of antimicrobials in poultry.
Author Contributions
Conceptualization, L.D. and A.R.-B.; methodology, A.R.-M., M.U.-R., A.G.-B., C.B., and N.G.; software, A.R.-M. and A.G.-B.; validation, M.U.-R., C.B., N.G., L.D., and A.R.-B.; formal analysis, A.R.-M. and A.G.-B.; investigation, A.R.-M., M.U.-R., A.G.-B., C.B., N.G., L.D., and A.R.-B.; resources, L.D. and A.R.-B.; data curation, A.R.-M. and A.G.-B.; writing—original draft preparation, A.R.-M.; writing—review and editing, M.U.-R., A.G.-B., C.B., N.G., L.D., and A.R.-B.; visualization, A.R.-M., L.D., and A.R.-B.; supervision, A.R.-B.; project administration, L.D. and A.R.-B.; funding acquisition, L.D. and A.R.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Spanish Ministry of Economy, Industry, and Competitiveness, grant number RTC-2015-3621-2; and the Autonomous Community of Madrid, grant number S2013/ABI-2747. A.R.-M. is a recipient of a Spanish Government-funded Ph.D. contract for Research Staff Training (FPI) granted by the Spanish Ministry of Science and Innovation and the Spanish Ministry of Universities (RTI2018-098658-B-C22; PRE2019-087439).
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Ethics Committee of the Complutense University of Madrid and the Community of Madrid (PROEX 152/19).
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank S. Cruz, G. Torre, I. Martínez, Á. Fernández-Manzano, S. Gómez, M. García, E. Rivero, N. Maasoumi, and E. Martínez (VISAVET Health Surveillance Centre, Complutense University of Madrid) for their technical support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Swayne, D.E.; Eaton, K.A.; Stoutenburg, J.; Trott, D.J.; Hampson, D.J.; Jensen, N.S. Identification of a new intestinal spirochete with pathogenicity for chickens. Infect. Immun. 1995, 63, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Hampson, D.J.; Oxberry, S.L.; Stephens, C.P. Influence of in-feed zinc bacitracin and tiamulin treatment on experimental avian intestinal spirochaetosis caused by Brachyspira intermedia. Avian Pathol. 2002, 31, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Herbst, W.; Willems, H.; Heuser, J.; Ewers, C. Isolation and antimicrobial susceptibility of Brachyspira species from feces of layer chickens in Germany. Tierarztl. Prax. Ausg. G Grosstiere Nutztiere 2018, 46, 29–34. [Google Scholar]
- Stephens, C.P.; Hampson, D.J. Experimental infection of broiler breeder hens with the intestinal spirochaete Brachyspira (Serpulina) pilosicoli causes reduced egg production. Avian Pathol. 2002, 31, 169–175. [Google Scholar] [CrossRef]
- Bano, L.; Merialdi, G.; Bonilauri, P.; Dall’Anese, G.; Capello, K.; Comin, D.; Cattoli, G.; Sanguinetti, V.; Hampson, D.J.; Agnoletti, F. Prevalence, disease associations and risk factors for colonization with intestinal spirochaetes (Brachyspira spp.) in flocks of laying hens in north-eastern Italy. Avian Pathol. 2008, 37, 281–286. [Google Scholar] [CrossRef]
- Verlinden, M.; Pasmans, F.; Garmyn, A.; De Zutter, L.; Haesebrouck, F.; Martel, A. Occurrence of viable Brachyspira spp. on carcasses of spent laying hens from supermarkets. Food Microbiol. 2012, 32, 321–324. [Google Scholar] [CrossRef]
- Burch, D.G.; Harding, C.; Alvarez, R.; Valks, M. Treatment of a field case of avian intestinal spirochaetosis caused by Brachyspira pilosicoli with tiamulin. Avian Pathol. 2006, 35, 211–216. [Google Scholar] [CrossRef]
- Woodward, M.J.; Mappley, L.; Le Roy, C.; Claus, S.P.; Davies, P.; Thompson, G.; La Ragione, R.M. Drinking water application of Denagard® Tiamulin for control of Brachyspira pilosicoli infection of laying poultry. Res. Vet. Sci. 2015, 103, 87–95. [Google Scholar] [CrossRef]
- Hampson, D.J.; Phillips, N.D.; Pluske, J.R. Dietary enzyme and zinc bacitracin reduce colonisation of layer hens by the intestinal spirochaete Brachyspira intermedia. Vet. Microbiol. 2002, 86, 351–360. [Google Scholar] [CrossRef]
- Hampson, D.J.; Stephens, C.P.; Oxberry, S.L. Antimicrobial susceptibility testing of Brachyspira intermedia and Brachyspira pilosicoli isolates from Australian chickens. Avian Pathol. 2006, 35, 12–16. [Google Scholar] [CrossRef]
- Abudabos, A.M.; Hussein, E.O.S.; Ali, M.H.; Al-Ghadi, M.Q. The effect of some natural alternative to antibiotics on growth and changes in intestinal histology in broiler exposed to Salmonella challenge. Poult. Sci. 2018, 98, 1441–1446. [Google Scholar] [CrossRef] [PubMed]
- Fasina, Y.O.; Bowers, J.B.; Hess, J.B.; McKee, S.R. Effect of dietary glutamine supplementation on Salmonella colonization in the ceca of young broiler chicks. Poult. Sci. 2010, 89, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Gong, S.; Wang, Y.; Sun, Q.; Duo, K.; Fei, P. Antibacterial activity of olive oil polyphenol extract against Salmonella Typhimurium and Staphylococcus aureus: Possible Mechanisms. Foodborne Pathog. Dis. 2019, 17, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Nasri, H.; Baradaran, A.; Shirzad, H.; Rafieian-Kopaei, M. New concepts in nutraceuticals as alternative for pharmaceuticals. Int. J. Prev. Med. 2014, 5, 1487–1499. [Google Scholar]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Rebollada-Merino, A.; Bárcena, C.; Ugarte-Ruiz, M.; Porras, N.; Mayoral-Alegre, F.J.; Tomé-Sánchez, I.; Domínguez, L.; Rodríguez-Bertos, A. Effects on intestinal mucosal morphology, productive parameters and microbiota composition after supplementation with fermented defatted alperujo (FDA) in laying hens. Antibiotics 2019, 8, 215. [Google Scholar] [CrossRef]
- Rebollada-Merino, A.; Ugarte-Ruiz, M.; Hernández, M.; Miguela-Villoldo, P.; Abad, D.; Cuesta-Álvaro, P.; Rodríguez-Lázaro, D.; de Juan, L.; Domínguez, L.; Rodríguez-Bertos, A. Dietary supplementation with fermented defatted “alperujo” induces modifications of the intestinal mucosa and cecal microbiota of broiler chickens. Poult. Sci. 2020, 99, 5308–5315. [Google Scholar] [CrossRef]
- Porras, N.; Rebollada-Merino, A.; Bárcena, C.; Mayoral-Alegre, F.J.; Lomillos, J.M.; Domínguez, L.; Rodríguez-Bertos, A. Effect of biscuit flour and fermented defatted “alperujo” co-administration on intestinal mucosa morphology and productive performance in laying hens. Animals 2021, 11, 1075. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Alli, I.; Ereifej, K.; Alhamad, M.; Rahman Al-Tawaha, A.; Rababah, T. Optimization, characterization and quantification of phenolic compounds in olive cake. Food Chem. 2010, 123, 117–122. [Google Scholar] [CrossRef]
- Gerasopoulos, K.; Stagos, D.; Kokkas, S.; Petrotos, K.; Kantas, D.; Goulas, P.; Kouretas, D. Feed supplemented with byproducts from olive oil mill wastewater processing increases antioxidant capacity in broiler chickens. Food Chem. Toxicol. 2015, 82, 42–49. [Google Scholar] [CrossRef]
- Nasopoulou, C.; Lytoudi, K.; Zabetakis, I. Evaluation of olive pomace in the production of novel broilers with enhanced in vitro antithrombotic properties. Eur. J. Lipid Sci. Technol. 2018, 120, 1700290. [Google Scholar] [CrossRef]
- Rebollada-Merino, A.; Ugarte-Ruiz, M.; Hernández, M.; Miguela-Villoldo, P.; Abad, D.; Rodríguez-Lázaro, D.; de Juan, L.; Domínguez, L.; Rodríguez-Bertos, A. Reduction of Salmonella Typhimurium cecal colonisation and improvement of intestinal health in broilers supplemented with fermented defatted ‘alperujo’, an olive oil by-product. Animals 2020, 10, 1931. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Hampson, D.J. Development of a multiplex qPCR for detection and quantitation of pathogenic intestinal spirochaetes in the faeces of pigs and chickens. Vet. Microbiol. 2009, 137, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, R.F.; Stanton, T.B.; Jensen, N.S.; Suriyaarachichi, D.S.; Hampson, D.J. Differentiation of Serpulina species by NADH oxidase gene (nox) sequence comparisons and nox-based polymerase chain reaction tests. Vet. Microbiol. 1999, 67, 47–60. [Google Scholar] [CrossRef]
- Phillips, N.D.; La, T.; Hampson, D.J. A cross-sectional study to investigate the occurrence and distribution of intestinal spirochaetes (Brachyspira spp.) in three flocks of laying hens. Vet. Microbiol. 2005, 105, 189–198. [Google Scholar] [CrossRef]
- Myers, S.E.; Dunn, P.A.; Phillips, N.D.; La, T.; Hampson, D.J. Brachyspira intermedia and Brachyspira pilosicoli are commonly found in older laying flocks in Pennsylvania. Avian Dis. 2009, 53, 533–537. [Google Scholar] [CrossRef]
- Harms, M.; Schmidt, V.; Heydel, T.; Hauptmann, J.; Ahlers, C.; Bergmann, R.; Baums, C.G. Differentiation of Brachyspira spp. isolated from laying hens using PCR-based methods and matrix-assisted laser desorption/ionization time-of-flight mass spectrometry. J. Vet. Diagn. Investig. 2018, 30, 545–553. [Google Scholar] [CrossRef]
- Phillips, N.D.; La, T.; Pluske, J.R.; Hampson, D.J. A wheat-based diet enhances colonization with the intestinal spirochaete Brachyspira intermedia in experimentally infected laying hens. Avian Pathol. 2004, 33, 451–457. [Google Scholar] [CrossRef]
- Phillips, N.D.; La, T.; Pluske, J.R.; Hampson, D.J. The wheat variety used in the diet of laying hens influences colonization with the intestinal spirochaete Brachyspira intermedia. Avian Pathol. 2004, 33, 586–590. [Google Scholar] [CrossRef]
- Verlinden, M.; Pasmans, F.; Mahu, M.; Vande Maele, L.; De Pauw, N.; Yang, Z.; Haesebrouck, F.; Martel, A. In vitro sensitivity of poultry Brachyspira intermedia isolates to essential oil components and in vivo reduction of Brachyspira intermedia in rearing pullets with cinnamaldehyde feed supplementation. Poult. Sci. 2013, 92, 1202–1207. [Google Scholar] [CrossRef]
- Mappley, L.J.; Tchórzewska, M.A.; Nunez, A.; Woodward, M.J.; Bramley, P.M.; La Ragione, R.M. Oral treatment of chickens with Lactobacillus reuteri LM1 reduces Brachyspira pilosicoli-induced pathology. J. Med. Microbiol. 2013, 62, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Stephens, C.P.; Hampson, D.J. Evaluation of tiamulin and lincomycin for the treatment of broiler breeders experimentally infected with the intestinal spirochaete Brachyspira pilosicoli. Avian Pathol. 2002, 31, 299–304. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).