Abstract
The cost of fermentable sugars added as a substrate is one major problem for economic lactic acid (LA) production. Old oil palm trunks (OPT) squeezed sap, the agricultural wastes on replanting and pruning of oil palm (Elaeis guineensis), contained mainly glucose and fructose as a potential feedstock to use as a vast carbon source for LA production. To improve the LA yield and productivity, various fermentation modes were performed by Lactobacillus rhamnosus ATCC 10863 using OPT sap as a basal medium. A modified constant feed mode of fed-batch and repeated fed-batch fermentation using undiluted OPT sap feed medium can achieve a high average LA concentration of 95.94 g/L, yield of 1.04 g/g, and productivity of 6.40 g/L/h) at 11 h cultivation time. It can also provide open and open repeated batch fermentation with an average LA concentration of 91.30 g/L, yield of 0.87 g/g, and productivity of 3.88 g/L/h at 21 h fermentation time.
1. Introduction
Lactic acid (LA) is widely used in food and related industries as a preservative, natural additive, solvent, curing agent, flavoring agent, and buffering agent, with a global market size of USD 2.9 billion in 2021 expected to expand at a compound annual growth rate of 8.0% from 2022 to 2030 [1].
The use of LA as a starter for the production of polylactic (PLA), a biodegradable and biocompatible biopolymer, drives up demand in the LA market [2]. Approximately 90% of LA is produced industrially worldwide through microbial fermentation as opposed to chemical synthesis [3]. When compared to the chemical method, fermentation has a number of benefits, including the ability to produce optically pure LA from agricultural wastes, low energy consumption, and low environmental impact [4].
Carbon sources are the key factor affecting LA production costs. Most often, a plentiful, affordable raw material, such as agricultural residues and industrial by-products like molasses, acid whey, green alfalfa juice, food wastes, etc., was employed as a substrate for LA fermentation [5,6,7,8,9,10]. Other fermentation feedstock, such as starchy and cellulosic materials, i.e., tapioca starch, sweet sorghum juice, potato, wheat, rye-barley, malt, rice, xylan-derived sugars, galactan, and lignin were studied for cost-effective LA production processes [3,8,11,12,13].
Oil palm trunks (OPT) are the agricultural wastes of oil palm (Elaeis guineensis) generated from replanting and pruning in the plantation sites. OPT contains fibrous materials which can be used in many industries, such as plywood, laminated veneer lumber, wood-cement composites, gypsum board, pulp and paper making, animal feeds, and other value-added products [14,15]. The squeezed sap from old OPT contained mainly glucose, fructose, sucrose, and small amounts of galactose, xylose, and rhamnose, whose total amounts varied between 16.97–140 g/L [16,17,18]. Additionally, oil palm sap contains minerals, vitamins, organic acids, and amino acids [15,19].
Numerous microorganisms have been studied for LA production, such as Lactobacillus sp. [6], Lactobacillus casei CCDM 198 [20], Lactobacillus plantarum [3], L. rhamnosus ATCC 10863 [9]. L. rhamnosus LA-04-1 [21], Bacillus coagulans A534 [8], Enterococcus mundtii QU 25 [22], and Lactococcus lactis subsp. lactis NBRC 12007 [23].
The most frequent methods for LA production are batch, fed-batch, repeated batch, and continuous fermentation. Regarding the nature and variety of substrate, microbial activity, and viscosity of the fermentation broth, many fermentation procedures can be used [5]. Batch mode produces high yields by fully utilizing the substrate when the cost of raw materials is low [9]. However, the substrate and final product inhibition of the traditional batch process resulted in decreased productivity [24]. Different feeding strategies were created to prevent these issues. By inoculating a portion or all of the cells from the previous batch into the next, repeated batch operations entailed several cycles of fermentation. In Rhizopus oryzae, repeated batch culture combines the advantages of batch and continuous cultures, such as the feasibility of high product content due to the complete bioconversion of substrate. The one and two-reactor system was also conducted [4]. Additionally, fed-batch fermentation may offer a variety of operating modes depending on the substrate, end products, and microorganisms. The external substrate concentration can be changed with the right feed rate [19]. To feed a suitable medium, repeated fed-batch or cyclic fed-batch can be overcome by process design, and numerous cycles offer without limiting substrate or product. LA production could be carried out via non-sterile fermentation because it minimized the need for equipment, reduced energy usage, simplified the fermentation process, and saved labor [4,25]. Zhao et al. [4] developed open repeated fermentation to synthesize optically pure LA.
To increase LA yield and productivity using OPT sap as a base substrate, the feasibility of open batch, repeat open batch, fed-batch, and repeated fed-batch fermentation was examined in this study. To lessen the product inhibition, the fed-batch and repeated the fed-batch fermentation’s constant feed mode modification to dilute product concentration can be used.
2. Materials and Methods
2.1. Chemicals and Culture Media
Yeast extract, peptone, De Man-Rogosa-Sharpe (MRS) agar, and MRS broth were purchased from HimediaTM (Mumbai, India). A standard 33-element TraceCERT was purchased from Sigma-Aldrich (St. Louis, MO, USA). All other reagents and standards were of analytical grade.
2.2. Oil Palm Trunk (OPT) Sap Preparation
Old oil palm trees (approximately 20 years old) left for replanting, located at Nakhon Si Thammarat, Thailand, were felled down, cut into small logs, and sliced into small pieces around 10 × 1.5–2.0 × 150–200 cm3. The palm disks were immediately squeezed, passed through a sugar cane pressing machine to get oil palm trunk sap (OPT sap). The OPT sap was centrifuged at 4000 rpm for 40 min at 4 °C and collected at −18 °C for the experiment (Figure 1). The sugar and mineral compositions of OPT sap were determined using the standard methods described by Saelee [26].

Figure 1.
Oil palm tree (a), shedding and logging (b), slicing to disks (c), squeezing (d), and oil palm squeezed sap (OPT sap) (e).
2.3. Microorganism and Seed Preparation
A homofermentative LA bacterium, Lactobacillus rhamnosus ATCC 10863, was used in this investigation. The microorganism was maintained in MRS broth containing 30% glycerol at −20 °C. For seed preparation, double propagation was performed on MRS broth. First, the 250 µL of L. rhamnosus ATCC 10863 stock culture was transferred to 5 mL MRS broth, and static culture was performed by incubation at 40 °C, 48 h. Then, one percentage (v/v) was transferred to the MRS seed medium composed of 20 g/L glucose, 10 g/L bacto-tryptone, 10 g/L yeast extract, 2 g/L K2HPO4, 5 g/L CH3COONa∙3H2O, 0.2 g/L MgSO4∙7H2O, and 5 g/L MnSO4∙4H2O [15]. The pH was brought to 7.0 using 6 N NaOH or conc. HCl. The medium sterilization was autoclaved at 121 °C for 15 min. Before being utilized as a seed inoculum, fermentation was carried out in a shake-flask at 150 rpm, 40 °C for 18 h.
2.4. Open Batch and Open Repeated Batch Fermentation
Bath fermentation was conducted in a 2 L stirred tank fermentor (Bioflo, New Brunswick Scientific Co., Picataway, NJ, USA) containing a 1.2 L OPT sap base medium. The addition of 10 g/L yeast extract, 5 g/L peptone and trace salts (1.5 g/L CH3COONa∙3H2O, 1.5 g/L KH2PO4, 1.5 g/L K2HPO4, 0.2 g/L MgSO4∙7H2O, and 0.05 g/L MnSO4∙4H2O) were performed [27]. Non-sterile OPT sap and trace salts were used as a basal medium. Fermentation was operated at 40 °C, with an agitation speed of 200 rpm without aeration. The pH was controlled at 6.5 by using 6N Ca(OH)2. Nitrogen gas was flushed to the OPT sap-based medium before inoculation and after sampling to maintain an anaerobic condition. Prior to the 10% v/v inoculation, the OD620 nm of the pre-seed was adjusted to a constant value of 4. Samples were aseptically withdrawn every 3 h intervals from the fermenter to measure the viable cells and biomass growth at OD620 nm. The culture broth was heated (70 °C, 30 min), centrifuged (10,000× g, 10 min) at room temperature (26–29 °C), and filtered through a 0.45 µm filter membrane. The sugars and LA concentration were examined in the clear supernatant, as detailed below.
When the maximum OD620 nm was reached close to the stationary phase, open repeated batch fermentation was carried out by removing the fermentation broth and replacing it with a nonsterile OPT sap medium. Open repeated batch fermentation was performed in the following cycles using the same non-sterile open conditions. The fermentation broth was taken off until there was only 0.2 L left. The following cycle began with the operation of three cycles of fermentation after filling 1.2 L of fresh OPT sap with a nutrient supplement without sterilization.
2.5. Fed Batch and Repeated Fed Batch Fermentation
For fed-batch fermentation, undiluted OPT sap was used as the main substrate. A 10% seed formulation was inoculated into a 2 L fermenter (BioFlo, New Brunswick Scientific Co., USA). The medium was sterilized at 110 °C for 10 min. Without aeration, fermentation was conducted with temperature, agitation, and pH automatically controlled at 40 °C, 200 rpm, and 6.5 (6 N Ca(OH)2), respectively. The medium was flushed with nitrogen gas before inoculation and intermittently applied after sample collection. Fed-batch fermentation started with batch fermentation mode in a 2 L fermenter containing 0.4 L OPT sap-based medium. Ten percent of pre-seed was aseptically added, and fermentation was performed using a modified constant rate fed-batch fermentation. The feeding was initiated at 12 h of cultures and fed-batch performed by a pulse feed of 0.2 L feed medium at the beginning of the fed-batch mode followed by a constant feed of 0.6 L of an OPT sap fermentation medium at a volumetric feed rate, resulting in the final volume of 1.2 L. The feed was stopped after 9 h.
For repeated fed-batch fermentation, after the 1st batch, 800 mL of fermentation broth was drawn off until the 0.4 L volume remained. The next cycle was started by adding feed medium until the total volume was 1.2 L. Then, 3 cycles of fed-batch fermentation were performed. The feeding medium contained the same composition as the fermentation medium. Five mL samples were taken every 3 h for analysis.
2.6. Kinetic Parameters Calculation
LA concentration (g/L) was calculated from the volumetric fermentation broth and the total LA produced at the end of fermentation. The total sugars (g/L) or residual sugars (g/L) were expressed by the sum of glucose, fructose, and sucrose in the volumetric fermentation broth at test time. The total sugar consumption (g/L) was calculated as the initial sugars (g/L) minus the residual sugars (g/L). In the batch mode, the LA yield, YLA, was calculated based on the total LA produced per gram of total sugar consumed (g/g) as Equation (1), with representing the LA concentration at the end of fermentation, denoting the LA concentration at the beginning of fermentation, denoting the total sugars concentration detected at the beginning of fermentation (g/L), and denoting the total sugar concentration detected at the end of fermentation (g/L). The LA productivity (QLA, g/L/h) was calculated using the ratio of LA concentration (g/L) to the entire fermentation time (h) as Equation (2), with t denoting the fermentation time (h).
In repeated modes, LA yield (YLA) (g/g) was calculated as Equation (3). LA productivity (QLA) (g/L/h) was calculated as Equation (4), where VF, VFE, and VI were the total final volume, total feed volume, and total initial volume (L) of each cycle, respectively. PF and PI represented the final and initial concentrations of total LA in the fermentation broth (g/L) of each cycle, respectively. SF, SFE, and SI represented the final, feed, and initial concentrations of total sugars in the fermentation broth (g/L), respectively, and t represented the fermentation time (h).
2.7. Analytical Methods
Sugar composition in fresh OPT sap was analyzed by a High Performance Liquid Chromatography (HPLC) system (Waters, Milford, MA, USA), 300 × 7.8 mm Animex HPX-87P column (Biorad, Hercules, CA, USA). Deionized water (DI) as a mobile phase was isocratically performed at 0.6 mL/min, and the column oven was set at 80 °C. The Refractive index (RI) detector was set at 50 °C. Before a 10 µL injection, samples were diluted with DI to the proper dilution and filtered using a 0.45 µm filter. The external standards of 1%, D(-)-fructose, D-(+)-glucose anhydrous, D(+) galactose, sucrose, D-(+)- maltose monohydrate, L-rhamnose monohydrate, and arabinose were used for quantification. Unknown concentration was calculated by a linear relationship plot between the peak area and known concentrations of standards [26].
LA and sugars concentrations of fermentation broth were determined using HPLC (Waters, USA), with Animex HPX 87H column (300 × 7.8 mm) (Biorad, USA) with RI detector (Waters 2410, Waters, USA). The eluent 0.005 M H2SO4 (0.6 mL/min), detector, and column were set at 50 °C. Before a 10 µL injection, samples were diluted with DI to the proper dilution and filtered using a 0.45 μm filter. Different concentrations of 1% L (+) lactic acid, ethanol, D-(+)-glucose anhydrous, D(-)-fructose, and sucrose were used as a calibration standard. To determine the correlation coefficient (r2), slope (a), and y-intercept values, a linear regression of standards was plotted.
Using an inductively coupled plasma optical emission spectrometer (ICP-OES, Varian 730-ES, Belrose, NSW, Australia), mineral contents were examined. Nitric acid (2%) was used to dilute 10 mL of OPT sap, which was then left overnight. Each sample underwent centrifugation, filtration, and dilution in DI to the proper concentration. TraceCERT, 33 elements (Sigma-Aldrich, St. Louis, MO, USA) of standards, aluminum (Al), boron (B), calcium (Ca), copper (Cu), iron (Fe), magnesium (Mg), manganese (Mn), nickel (Ni), phosphorous (P), potassium (K), sodium (Na), sulfur (S), and zinc (Zn) were used.
The absorbance at 620 nm (OD620 nm) (spectrophotometer UV-1601, Shimadzu Co., Tokyo, Japan) was measured to estimate bacterial growth. Viable cells (cfu/mL) were examined using the spread plate technique on MRS agar with anaerobic incubation at 40 °C in an aerobic jar.
3. Results and Discussion
3.1. Some Chemical Compositions of OPT Sap
Some chemical compositions of OPT sap are shown in Table 1. In this investigation, the predominant sugars were glucose and fructose, which were 55.69 ± 1.42 and 40.10 ± 2.35 g/L, respectively. Additionally, trace amounts of cellobiose (2.037 ± 0.09 g/L) and sucrose (3.89 ± 0.44 g/L) were discovered in OPT sap. The high total free sugar content value of 101.71 ± 3.31 g/L was higher than that reported by other studies [15,28,29]. However, Noparat et al. [29] observed that the top of OPT had 18.06 ± 1.6 g/L of total sugars. Juice from oil palm fronds (OPF) was found to have a high average amount of sugars of 76.09 ± 2.85 g/L [30]. Similar main sugar component values were found, with glucose having the largest content [28,29]. Yamada et al. [31] also reported on the composition of the glucose, fructose, and sucrose found in OPT juice. In contrast to this study, sucrose initially dominates the sugars in OPT sap but rapidly declines at the beginning of storage. Because sucrose was decomposed into glucose and fructose, the amount of these two sugars increased while sucrose was reduced in the OPT sap. The composition of OPT juice varies with the variety, soil, climate, locality, processed method, and storage time [18]. OPT sap also contained some minerals, such as P (0.257 ± 0.009 g/L), Na (0.257 ± 0.080 g/L), K (1.676 ± 0.417 g/L), Mg (0.503 ± 0.145 g/L), Ca (0.488 ± 0.150 g/L), and S (0.694 ± 0.218 g/L) (Table 1).

Table 1.
Chemical characteristics of OPT sap.
3.2. Open Batch Fermentation and Open Repeated Batch Fermentation
To examine the economics of LA production, open batch LA fermentation of OPT sap by L. rhamnosus ATCC 10863 was studied (Figure 2). The maximum LA concentration was 93.99 ± 2.91 g/L at 15 h of cultivation, which resulted in a yield of 0.95 g/g and an LA productivity of 6.27 g/L/h. This was greater than Ouyang et al.’s study [32], which produced LA with an average productivity of 1.04 g/L/h and a yield of 74.5% using open fermentation of lignocellulosic hydrolyzates by Bacillus sp. strain NL01. Since L. rhamnosus ATCC 10863 requires a temperature of 40 °C for optimal growth, this may not be appropriate for other bacteria. Additionally, a non-sterilized or open fermentation technique could delay the Millard reaction and the development of furfural compounds, which generally proceed during sterilization [4]. According to Abdel-Rahman et al. [33], E. mundtii QU 25 produced a maximum LA concentration of 132 g/L at a yield of 0.853 g/g and productivity of 6.99 g/L/h. under non-sterile conditions. Open batch fermentation of OPT sap by L. rhamnosus ATCC 10863 rapidly devoured sugar during 15 h of incubation, the remaining sugars less than 4.72 ± 0.73 g/L, and completely utilized sugar at 21 h of cultivation. LA concentration, yield, and production efficiency were decreased to 91.30 g/L, 0.88 g/g, and 4.35 g/L/h at 21 h cultivation. A high cell concentration of 9.15 × 1011 cfu/mL was attained using the open batch mode, which was in accordance with a high cell growth rate as measured by the OD620nm at 32.60 ± 2.79. This suggested that L. rhamnosus had no trouble consuming the fermentable sugars present in OPT sap, such as glucose and fructose. Glucose (57.43 g/L initially) and fructose (44.70 g/L initially) were consumed concurrently, but fructose was more rapidly and completely utilized at 9 h (Figure 2). According to Senedese et al. [9], L. rhamnosus ATCC 10863 most frequently used glucose as a substrate, with a maximum LA of 16.5 g/L at 14 h batch fermentation. Additionally, adopting thermophile fermentation decreases the cost of industrial LA manufacturing [34].
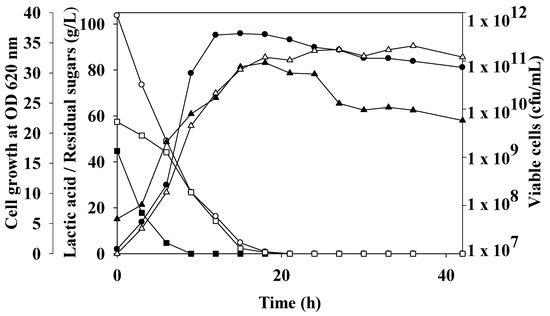
Figure 2.
Open batch fermentation of oil palm trunk sap by L. rhamnosus ATCC 10863. Culture was performed in a 2 L fermenter containing 1.2 L working volume at a temperature of 40 °C, agitation speed of 200 rpm, and pH 6.5. Lactic acid (●), residual sugars (○), glucose (□), fructose (■), viable cells (▲), and cell growth at OD620nm (∆) was indicated.
LA generation was impacted by inoculums and LA buildup. Batch fermentation was carried out repeatedly to increase LA productivity over batch culture. Because certain cells from the final culture broth are frequently reused, repeated batch culture offers several advantages over batch culture. These benefits include reducing the time needed to clean and sterilize the fermenter, skipping the time needed to prepare the seeds, and reducing the time needed for the primary culture by increasing the initial inoculation volume [4].
Figure 3 depicts the results of four batches of open repeated batch fermentation of OPT sap by L. rhamnosus ATCC 10863. Compared to the batch cycle, the first cycle’s fermentation period was cut by 18 h. According to the aforementioned non-sterile batch fermentation, each fermentation cycle took place over 21 h. The highest LA concentrations were 94.15, 99.08, 84.80, and 85.60 g/L for the first batch through the third repeated cycle, respectively (Table 2). This is due to the amount of LA at the beginning of fermentation, which was 103.19–112.81 g/L. The maximum yield and productivity for the LA were achieved by the first open batch, which had values of 0.91 g/g and 4.31 g/L/h, respectively. The LA productivity and yield were 4.65, 3.26, and 3.28 g/L/h, and 0.82, 0.77, and 0.95 g/g, respectively, in the first, second, and third repeated cycles. Unfermented sugars remained after fermentation until the medium was refilled with fresh medium. This might lead to an accumulation at the start of fermentation for each cycle, equating to a decreased yield and productivity. In this investigation, the four batches, respective average LA, yield, and productivity values were 91.30 g/L, 0.87 ± 0.08 g/g, and 3.88 ± 0.71 g/L/h. Compared to the experiment by Abdel-Rahman et al. [33], which was conducted for 11 runs with the same yield (0.761–0.832 g/g glucose consumed). Zhao et al. [4] suggested that high cell density did not always result in an improvement in LA production since the number of viable cells declined while the number of non-viable cells increased in the latter cycles of open repeated batch fermentation.
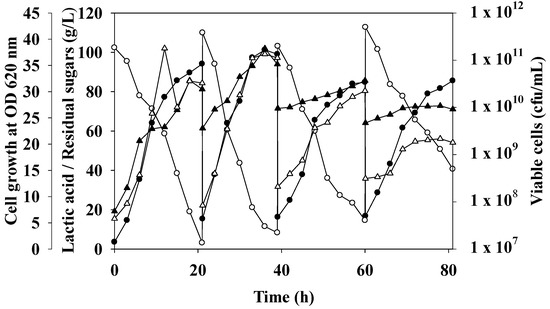
Figure 3.
Open repeated batch fermentation of oil palm trunk sap by L. rhamnosus ATCC 10863. Culture was performed in a 2 L fermenter containing 1.2 L working volume at a temperature of 40 °C, agitation speed of 200 rpm, pH 6.5. Lactic acid (●), residual sugars (○), viable cells (▲), and cell growth at OD620nm (∆) was indicated.

Table 2.
Kinetics of open repeated batch fermentation of oil palm trunk sap by L. rhamnosus ATCC 10863.
3.3. Fed Batch and Repeated Fed Batch Fermentation of OPT Sap
Fed-batch fermentation patterns of LA production, sugars consumption, and biomass growth of L. rhamnosus ATCC 10863 using concentrated OPT sap with nutrient supplementation are shown in Figure 4. It was started with 0.4 L OPT sap-based medium in batch fermentation and changed to fed-batch after the 12 h cultivation. Then, a modified constant feed mode was performed. Herein, 0.2 L of feed medium was pulse-fed at the start of the batch feeding process, and 0.6 L of OPT sap fermentation medium was delivered continuously at a rate of 67.7 mL/h to create a 1.2 L working volume. The feed was stopped after 9 h. For 9 h of fermentation, this feeding strategy may sustain total sugar levels between 6.49 and 8.57 g/L with high cell concentrations between 4.29 × 109 and 1.69 × 1010 cfu/mL. When the fed-batch mode was finished, product efficiency increased to 7.19 g/L/h, which was greater than batch mode (6.05 g/L/h). Compared to batch fermentation, the yield in the fed-batch mode was marginally lower. However, L. rhamnosus B103 with pH-stat fed-batch fermentation produced 143.7 g/L of LA from dairy waste, with a productivity of 1.17 g/L/h [24]. Repeated fed-batch fermentation is currently a good tactic for improving LA fermentation as it lowers operating costs, because no seed purchase improved LA fermentation, which inhibited the higher LA production rate in fed-batch mode [6,34].
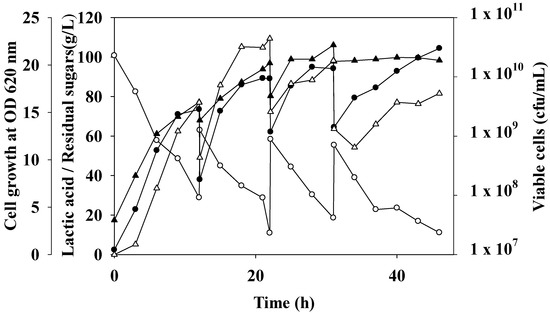
Figure 4.
Fed-batch and repeated fed-batch fermentation for lactic acid production by L. rhamnosus ATCC 10863 using undiluted oil palm trunk sap with nutrient supplementation. Cells cultivated in 2 L fermenter containing 1.2 L working volume with 0.8 L OPT sap feed medium. A 0.2 L of feed medium was added at the beginning of feeding; the other was constant fed at the rate of 67.7 mL/h. Fermentation was performed at a temperature of 40 °C, agitation speed of 200 rpm, pH 6.5. Lactic acid (●), residual sugars (○), viable cells (▲), and cell growth at OD 620 nm (∆) was indicated.
In the following cycle of fed-batch fermentation, a modified repeated fed-batch fermentation was carried out for 9 h. For the first batch, first fed-batch, second fed-batch, and third fed-batch cycles, respectively, the maximal LA production values were 73.49, 89.25, 94.16, and 104.42 g/L. In the first batch, the first fed-batch, the second fed-batch, and the third fed-batch cycles, respectively, there were 28.80, 28.76, 18.56, and 0 g/L of total sugars left at the end of the cycle. The highest cell density was 1.34 × 1010, 3.44 × 1010, and 2.11 × 1010 cfu/mL for the first fed-batch, second fed-batch, and third fed-batch cycle, respectively. The sugar levels were fairly low (10.90 to 18.56 g/L) at the end of each fed-batch fermentation medium. During the first 9 h of cultivation, the sugar concentration was rapidly reduced, and after 15 h of cultivation, the last fed-batch cycle, it was totally consumed.
Kinetic parameters of three cycles of repeated fed-batch fermentation for LA production of OPT sap by L. rhamnosus ATCC 10863 were investigated (Table 3). The maximum volumetric LA productivities for the first fed-batch, second fed-batch, and third fed-batch cycles were 7.19, 7.16, and 4.86 g/L/h, respectively. These first and second fed batches had a greater fermentation rate than the initial batch (6.05 g/L/h). In the first cycle, the highest LA productivity was attained. This study showed that adding feed medium at the beginning of fermentation helps to prevent dilution of the culture broth, which could alter the growth rate and by-product accumulation in the culture broth [35]. According to Boontawan et al. [36], the fed-batch fermentation had a slightly lower glucose consumption rate than the previous fermentation. In addition, inadequate glucose utilization dramatically decreased fermentation performance. LA is the cause of this inhibition. Both bacterial development and LA production would greatly benefit from removing lactate ions from the fermentation broth. Nolasco-Hipolito et al. [37] emphasized that increasing the feed amount of glucose free-solution eliminated the end-product inhibition by LA. This inherent feature makes it easier to maintain LA concentrations between 35 and 40 g/L, which batch fermentation does not impede. As a result, it is feasible to boost the formation of LA by increasing the glucose concentration in the feed medium. In this investigation, the repeated fed-batch procedures combined nutrient supplementation with low concentrations of undiluted OPT sap to lengthen the period of productive batch fermentation and prevent substrate and catabolite suppression. This also suggested that initial sugar and LA concentrations should be taken into account to increase the productivity and yield of LA.

Table 3.
Kinetics of fed-batch and repeated fed-batch fermentation of oil palm trunk sap by L. rhamnosus ATCC 10863.
4. Conclusions
To increase the LA yield and productivity of undiluted OPT sap by L. rhamnosus ATCC 10863, various fermentation procedures were investigated. With 11 h of fermentation time for the production of LA as opposed to 21 h for repeated open batch fermentation, a modified constant feed mode of fed-batch and repeated fed-batch fermentation might increase yield, productivity, and short time. Additionally, when employing OPT sap as a cheap, abundant carbon source, all these techniques, including open batch and open repeated batch fermentation, could aid in extending the LA fermentation. However, utilizing cheap nitrogen sources as supplements would be worth considering.
Funding
This research was funded by the new strategic research project (P2P), Walailak University, Thailand.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The author would like to thank Manat Chaijan for editing support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lactic Acid Market Size, Share & Trends Analysis Report by Raw Material (Sugarcane, Corn, Cassava), by Application (PLA, Food & Beverages, Personal Care, Pharmaceuticals), by Region, and Segment Forecasts. 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/lactic-acid-and-poly-lactic-acid-market (accessed on 2 June 2022).
- Macedo, J.V.C.; Ranke, F.F.D.B.; Escaramboni, B.; Campioni, T.S.; Núñez, E.G.F.; Neto, P.D.O. Cost-effective lactic acid production by fermentation of agro-industrial residues. Biocatal. Agric. Biotechnol. 2020, 27, 101706. [Google Scholar] [CrossRef]
- Derabli, B.; Nancib, A.; Nancib, N.; Aníbal, J.; Raposo, S.; Rodrigues, B.; Boudrant, J. Opuntia ficus indica waste as a cost effective carbon source for lactic acid production by Lactobacillus plantarum. Food Chem. 2022, 370, 131005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Li, F.; Hua, D.; Ma, C.; Ma, Y.; Xu, P. Kinetics of d-lactic acid production by Sporolactobacillus sp. strain CASD using repeated batch fermentation. Bioresour. Technol. 2010, 101, 6499–6505. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Hassan, S.E.-D.; Alrefaey, H.M.A.; El-Belely, E.F.; Elsakhawy, T.; Fouda, A.; Desouky, S.G.; Khattab, S.M.R. Subsequent improvement of lactic acid production from beet molasses by Enterococcus hirae ds10 using different fermentation strategies. Bioresour. Technol. Rep. 2021, 13, 100617. [Google Scholar] [CrossRef]
- Chenebault, C.; Moscoviz, R.; Trably, E.; Escudié, R.; Percheron, B. Lactic acid production from food waste using a microbial consortium: Focus on key parameters for process upscaling and fermentation residues valorization. Bioresour. Technol. 2022, 354, 127230. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Li, H.; Deng, Z.; Liu, J. Lactic acid production from mesophilic and thermophilic fermentation of food waste at different pH. J. Environ. Manag. 2022, 304, 114312. [Google Scholar] [CrossRef]
- Olszewska-Widdrat, A.; Alexandri, M.; López-Gómez, J.P.; Schneider, R.; Venus, J. Batch and continuous lactic acid fermentation based on a multi-substrate approach. Microorganisms 2020, 8, 1084. [Google Scholar] [CrossRef]
- Senedese, A.L.C.; Filho, R.M.; Maciel, M.R.W. L-lactic acid production by Lactobacillus rhamnosus ATCC 10863. Sci. World J. 2015, 2015, 6. [Google Scholar] [CrossRef]
- Xu, K.; Xu, P. Efficient production of L-lactic using co-feeding strategy based on cane molasses/glucose carbon sources. Bioresour. Technol. 2014, 153, 23–25. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A. Enterococcus faecium s6 Enabled efficient homofermentative lactic acid production from xylan-derived sugars. Fermentation 2022, 8, 134. [Google Scholar] [CrossRef]
- Sharma, A.; Pranaw, K.; Singh, S.; Khare, S.K.; Chandel, A.K.; Nain, P.K.S.; Nain, L. Efficient two-step lactic acid production from cassava biomass using thermostable enzyme cocktail and lactic acid bacteria: Insights from hydrolysis optimization and proteomics analysis. 3 Biotech. 2020, 10, 409. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, B.; Liu, B.; Yang, C.; Yu, B.; Li, Q.; Ma, C.; Xu, P.; Ma, Y. Efficient production of L-lactic acid from cassava powder by Lactobacillus rhamnosus. Bioresour. Technol. 2010, 101, 7895–7901. [Google Scholar] [CrossRef]
- Dirkes, R.; Neubauer, P.R.; Rabenhorst, J. Pressed sap from oil palm (Elaeis guineensis) trunks: A revolutionary growth medium for the biotechnological industry? Biofuels Bioprod. Bioref. 2021, 15, 931–944. [Google Scholar] [CrossRef]
- Kosugi, A.; Tanaka, R.; Magara, K.; Murata, Y.; Arai, T.; Sulaiman, O.; Hashim, R.; Hamid, Z.A.; Yahya, M.K.; Yusof, M.N.; et al. Ethanol and lactic acid production using sap squeezed from old oil palm trunks felled for replanting. J. Biosci. Bioeng. 2010, 110, 322–325. [Google Scholar] [CrossRef]
- Hau, E.H.; The, S.S.; Yeo1, S.K.; Chua, B.L.; Mah, S.H. Transformation of oil palm biomass into value-added components. Rev. Agric. Sci. 2022, 10, 36–55. [Google Scholar]
- Wong, L.J.; H’ng, P.S.; Abdullah, L.C.; Paridah, M.T.; Chin, K.L. Effect of chemical steeping on yields of glucose and xylose from dilute acid hydrolysis of extract from oil palm trunk. BioResources 2022, 17, 207–222. [Google Scholar] [CrossRef]
- Ezzatzadegan, L.; Yusof, R.; Morad, N.A.; Shabanzadeh, P.; Muda, N.S.; Borhani, T.N. Experimental and artificial intelligence modelling study of oil palm trunk sap fermentation. Energies 2021, 14, 2137. [Google Scholar] [CrossRef]
- Liu, B.; Yang, M.; Qi, B.; Chen, X.; Su, Z.; Wan, Y. Optimizing l-(+)-lactic acid production by thermophile Lactobacillus plantarum As.1.3 using alternative nitrogen sources with response surface method. Biochem. Eng. J. 2010, 52, 212–219. [Google Scholar] [CrossRef]
- Paulova, L.; Chmelik, J.; Branska, B.; Patakova, P.; Drahokoupil, M.; Melzoch, K. Comparison of lactic acid production by L. casei in batch, fed-batch and continuous cultivation, testing the use of feather hydrolysate as a complex nitrogen source. Braz. Arch. Biol. Technol. 2020, 63, e20190151. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, C.; Cai, D.; Wang, Z.; Qin, P.; Tan, T. The optimization of l-lactic acid production from sweet sorghum juice by mixed fermentation of Bacillus coagulans and Lactobacillus rhamnosus under unsterile conditions. Bioresour. Technol. 2016, 218, 1098–1105. [Google Scholar] [CrossRef]
- Wang, Y.; Chan, K.L.; Abdel-Rahman, M.A.; Sonomoto, K.; Leu, S.-Y. Dynamic simulation of continuous mixed sugar fermentation with increasing cell retention time for lactic acid production using Enterococcus mundtii QU 25. Biotechnol. Biofuels. 2020, 13, 2–16. [Google Scholar]
- Kawai, M.; Harada, R.; Yoda, N.; Yamasaki-Yashiki, S.; Fukusaki, E.; Katakura, Y. Suppression of lactate production by using sucrose as a carbon source in lactic acid bacteria. J. Biosci. Bioeng. 2020, 129, 47–51. [Google Scholar] [CrossRef]
- Bernardo, M.P.; Coelho, L.F.; Sass, D.C.; Contiero, J. l-(+)-Lactic acid production by Lactobacillus rhamnosus B103 from dairy industry waste. Braz. J. Microbiol. 2016, 47, 640–646. [Google Scholar] [CrossRef]
- Akao, S.; Tsuno, H.; Horie, T.; Mori, S. Effects of pH and temperature on products and bacterial community in l-lactate batch fermentation of garbage under unsterile condition. Water Res. 2007, 41, 2636–2642. [Google Scholar] [CrossRef]
- Saelee, N. Effects of soil salinity on nutritional compositions of fresh Jak (Nypa fruticans) sap. J. Food Composit Anal. 2022, 114, 104767. [Google Scholar] [CrossRef]
- Saelee, N.; Sriroth, K. Nitrogen and salt supplementation of oil palm trunk juice and Its optimization conditions to enhance lactic acid production by Lactobacillus rhamnosus TISTR 108. Walailak J. Sci. Tech. 2015, 12, 279–289. [Google Scholar]
- Komonkiat, I.; Cheirsilp, B. Felled oil palm trunk as a renewable source for biobutanol production by Clostridium spp. Bioresour. Technol. 2013, 146, 200–207. [Google Scholar] [CrossRef]
- Noparat, P.; Prasertsan, P.; O-Thong, S. Isolation and characterization of high hydrogen-producing strain Clostridium beijerinckii PS-3 from fermented oil palm sap. Int. J. Hydrogen Energy 2011, 36, 14086–14092. [Google Scholar] [CrossRef]
- Zahari, M.A.K.M.; Zakaria, M.R.; Ariffin, H.; Mokhtar, M.N.; Salihon, J.; Shirai, Y.; Hassan, M.A. Renewable sugars from oil palm frond juice as an alternative novel fermentation feedstock for value-added products. Bioresour. Technol. 2012, 110, 566–571. [Google Scholar] [CrossRef]
- Yamada, H.; Tanaka, R.; Sulaiman, O.; Hashim, R.; Hamid, Z.A.A.; Yahya, M.K.A.; Kosugi, A.; Arai, T.; Murata, Y.; Nirasawa, S.; et al. Old oil palm trunk: A promising source of sugars for bioethanol production. Biomass Bioenergy 2010, 34, 1608–1613. [Google Scholar] [CrossRef]
- Ouyang, J.; Ma, R.; Zheng, Z.; Cai, C.; Zhang, M.; Jiang, T. Open fermentative production of L-lactic acid by Bacillus sp. strain NL01 using lignocellulosic hydrolyzates as low-cost raw material. Bioresour Technol. 2013, 135, 380–475. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.A.; Tashiro, Y.; Zendoa, T.; Sonomoto, K. Improved lactic acid productivity by an open repeated batch fermentation system using Enterococcus mundtii QU 25. RSC Adv. 2013, 3, 8437–8445. [Google Scholar] [CrossRef]
- Michelson, T.; Kask, K.; Jogi, E.; Talpsep, E.; Suitso, I.; Nurk, A. L(+)-lactic acid producer Bacillus coagulans SIM-7 DSM 14043 and its comparison with Lactobacillus delbrueckii spp. lactis DSM 20073. Enzyme Microb. Technol. 2006, 19, 861–867. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Steinbüchel, A. High-cell-density cyclic fed-batch fermentation of a poly(3-hydroxybutyrate)-accumulating thermophile, Chelatococcus sp. strain MW10. Appl. Environ. Microbiol. 2010, 76, 7890–7895. [Google Scholar] [CrossRef]
- Boontawan, P.; Kanchanathawee, S.; Boontawan, A. Extractive fermentation of l-(+)-lactic acid by Pediococcus pentosaceus using electrodeionization (EDI) technique. Biochem. Eng. J. 2011, 54, 192–199. [Google Scholar] [CrossRef]
- Nolasco-Hipolito, C.; Carvajal-Zarrabal, O.; Kelvin, E.; Tan, Y.H.; Kohei, M.; Nyoel, S.A.; Shoji, E.; Dieng, H.; Bujang, K. Scaling up of lactic acid fermentation using Enterococcus faecalis. IOP Conf. Ser. Mater. Sci. Eng. 2019, 495, 012049. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).