Evaluation of Gas Production, Fermentation Parameters, and Nutrient Degradability in Different Proportions of Sorghum Straw and Ammoniated Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experiment Material
2.2. Experiment Design
2.3. Experiment Method
2.3.1. In Situ Nylon Bag Test
2.3.2. In Vitro Test
2.4. In Vitro Degradability and Fermentation Parameter Test
2.5. Parameter Simulation and Statistical Analysis
2.5.1. Determination of Rumen Degradability and Calculation Method of Degradation Parameters in In Situ Nylon Bag Method
2.5.2. In Vitro Gas Production and Gas Production Parameters
2.5.3. Calculation Method of In Vitro Degradability
2.6. Data Processing and Analysis
3. Results
3.1. In Situ Nylon Bag Test Results
3.2. In Vitro Degradability Test Results
3.3. In Vitro Gas Production Test Results
3.4. In Vitro Fermentation Parameter Test Results
4. Discussion
4.1. Evaluation of Mixed Ratio of Sorghum Straw and Ammoniated Wheat Straw by In Situ Nylon Bag Method
4.2. Evaluation of Degradability In Vitro
4.3. Effect of In Vitro Gas Production Method Evaluation
4.4. Evaluation of Fermentation Parameters In Vitro
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| S | Sorghum straw |
| AWS | Ammoniated wheat straw |
| IVDMD | In vitro dry matter degradability |
| IVOMD | In vitro organic matter degradability |
| DM | Dry matter |
| OM | Organic matter |
| CP | Crude protein |
| ED | Effective degradability |
References
- Kaur, R.; Garcia, S.C.; Fulkerson, W.J.; Barchia, I.M. Degradation kinetics of leaves, petioles and stems of forage rape (Brassica napus) as affected by maturity. Anim. Feed Sci. Technol. 2011, 168, 165–178. [Google Scholar] [CrossRef]
- Getachew, G.; DePeters, E.J.; Robinson, P.H.; Fadel, J.G. Use of an in vitro rumen gas production technique to evaluate microbial fermentation of ruminant feeds and its impact on fermentation products. Anim. Feed Sci. Technol. 2005, 123, 547–559. [Google Scholar] [CrossRef]
- Li, S.L.; Shi, H.T.; Cao, Z.J.; Wang, Y.J. Scientific utilization and evaluation technology of forage. Chin. J. Anim. Nutr. 2014, 26, 3149–3158. [Google Scholar]
- Ørskov, E. Recent advances in evaluation of roughages as feeds for ruminants. In Advances in Animal Nutrition; Farell, D.J., Ed.; University of New England Printery: Armidale, Australia, 1989; pp. 102–108. [Google Scholar]
- Wei, C.Z.; Zhang, M.M.; Sun, Y.R.; Jiang, M.Y.; Xie, X.L. Research progress on nutritional value evaluation of roughage for meat sheep. In Proceedings of the National Sheep Production and Academic Seminar, Bengbu, China, 16 August 2018; p. 150. [Google Scholar]
- Li, Y.L.; Ding, J.; Lu, L.; Cao, Y.C. Ruminant feed nutritional value evaluation and the model application. China Herbiv. Sci. 2014, 34, 45–50. [Google Scholar]
- France, J.; Dijkstra, J.; Dhanoa, M.S.; Lopez, S.; Bannink, A. Estimating the extent of degradation of ruminant feeds from a description of their gas production profiles observed in vitro: Derivation of models and other mathematical considerations. Br. J. Nutr. 2000, 83, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grander, D.; Singh, N.P.; Dhiman, K.R. Nutritional evaluation of cultivated fodder crops grown under agro-climate of Tripura. Indian J. Anim. Sci. 2009, 79, 1143–1148. [Google Scholar]
- Liu, B.; Qin, T. Application of wheat straw used in animal production. Feed Rev. 2015, 11, 12–14. [Google Scholar]
- Kang, J.; Kuang, Y.B.; Sheng, J. Analysis of nutritive value of 10 forages straw. Prata Sci. 2014, 31, 1951–1956. [Google Scholar]
- Muzaffar, M.H. Simplification of Urea Treatment Method to Improve the Nutritive Value of Cereal Straw; AGRIS: Rome, Italy, 2005; Volume 22, pp. 46–54. [Google Scholar]
- Menke, K.H. Estimation of the energetic feed value obtained from chemical analysis and in vitro gas production using rumen fluid. Anim. Res. Dev. 1988, 28, 7–55. [Google Scholar]
- Akinfemi, A.; Adua, M.M.; Adu, O.A. Evaluation of nutritive values of tropical feed sources and by-products using in vitro gas production technique in ruminant animals. Emir. J. Food Agric. 2013, 24, 348–353. [Google Scholar]
- Lutakome, P.; Kabi, F.; Tibayungwa, F.; Laswaic, G.H.; Kimambo, A.; Ebong, C. Rumen liquor from slaughtered cattle as inoculum for feed evaluation. Anim. Nutr. 2017, 3, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Broderick, G.A.; Kang, J.H. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 1980, 63, 64–75. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef] [Green Version]
- Dijkstra, J.; Neal, H.D.S.C.; Beever, D.E.; France, J. Simulation of nutrient digestion, absorption and outflow in the rumen: Model description. J. Nutr. 1992, 122, 2239–2256. [Google Scholar] [CrossRef]
- Marcondes, M.I.; Valadares Filho, S.C.; Detmann, E.; Valadares, R.F.D.; Silva, L.F.C.; Fonseca, M.A. Rumen degradation and intestinal digestibility of crud the estimation of protein degradability in the rumen from incubation measurements weighted according to rate of passage. e protein in feeds for cattle. Rev. Bras. Zootec. 2009, 38, 2247–2257. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Zhang, X.M.; Wang, Z.S.; Peng, Q.H.; Zou, H.W. Ruminal degradation characteristics and small intestinal digestibility of rumen undegraded protein of six kinds of commonly used roughages for steers. Chin. J. Anim. Nutr. 2014, 26, 2145–2154. [Google Scholar]
- Hao, S.H.; Ding, N.; Zhao, J.X.; Ren, Y.S.; Zhang, W.J.; Xiang, B.W.; Zhang, J.X. Ruminal degradability characteristics of different roughages for sheep. China Herbiv. Sci. 2017, 37, 16–21. [Google Scholar]
- Van Zijderveld, S.M.; Fonken, B.; Dijkstra, J.; Gerrits, W.J.J.; Perdok, H.B.; Fokkink, W.; Newbold, J.R. Effects of a combination of feed additives on methane production, diet digestibility, and animal performance in lactating dairy cows. J. Dairy Sci. 2011, 94, 1445–1454. [Google Scholar] [CrossRef] [Green Version]
- Li, H.G.; Diao, X.G.; Zhao, J.X.; Ren, Y.S.; Zhang, C.X. Ruminal degradability characteristics of four unconventional roughages in mutton sheep. China Herbiv. Sci. 2017, 37, 18–22. [Google Scholar]
- Liu, Y.F.; Ma, J.; Wang, Y.; Cao, Z.; Li, S.; Yu, X. Degradation characteristics of common roughage and roughage forage in the rumen of dairy cows. Chin. J. Anim. Nutr. 2018, 30, 1592–1602. [Google Scholar]
- Yu, S.C.; Cao, S.Q.; Ren, Y.S.; Zhang, C.X.; Zhang, W.J.; Xiang, B.W.; Zhang, J.X. Nutritional value and rumen degradability characteristics of common crop straws for sheep. Chin. J. Anim. Sci. 2017, 53, 69–74. [Google Scholar]
- Lin, F.D.; Knabe, D.A.; Tanksley, T.D., Jr. Apparent digestibility of amino acids, gross energy and starch in corn, sorghum, wheat, barley, oat groats and wheat middlings for growing pigs. J. Anim. Sci. 1987, 64, 1655. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.A.; Cushnahan, A.; Mayne, C.S.; Unsworth, E.F. The effect of partial replacement of grass silage with fresh grass on rumen fermentation characteristics and rumen outflow rates in cattle. Grass Forage Sci. 1996, 51, 32–41. [Google Scholar] [CrossRef]
- Polyorach, S.; Poungchompu, O.; Wanapat, M.; Kang, S.; Cherdthong, A. Optimal cultivation time for yeast and lactic acid bacteria in fermented milk and effects of fermented soybean meal on rumen degradability using nylon bag technique. Asian-Australas. J. Anim. Sci. 2016, 29, 1273–1279. [Google Scholar] [CrossRef] [Green Version]
- Meng, M.J.; Tu, Y.L.; Bai, Y.F.; Yan, S.; Gao, L.; Liu, P.; Liu, J. Combination effects on wheat straw and non-conventional feeds. Chin. J. Anim. Nutr. 2016, 28, 3005–3014. [Google Scholar]
- Estrada, J.I.C.; Delagarde, R.; Faverdin, P.; Peyraud, J.L. Dry matter intake and eating rate of grass by dairy cows is restricted by internal, but not external water. Anim. Feed Sci. Technol. 2004, 114, 59–74. [Google Scholar] [CrossRef]
- Li, W.J.; Diao, Q.Y. Research progress on dry matter intake of mutton sheep and related influencing factors. Chin. J. Anim. Sci. 2016, 52, 95–99. [Google Scholar]
- Luis, E.S.; Sullivan, T.W.; Nelson, L.A. Nutritional value of proso millets, sorghum grains, and corn in turkey starter diets. Poult. Sci. 1982, 61, 321–326. [Google Scholar] [CrossRef]
- Xie, C.Y.; Yang, H.J.; Yao, X.B.; Yue, Q.; Wang, J.Q. Comparison of in situ nylon bag technique and in vitro gas production for evaluating the feedstuff of ruminant. Chin. J. Anim. Sci. 2007, 43, 39–42. [Google Scholar]
- Fan, Q.S.; Diao, Q.Y.; Bi, Y.L.; Wang, B.; Cheng, S.R.; Fu, T.; Tu, Y. Effects of three kinds of plant cakes on in vitro gas production, rumen fermentation and degradation characteristics in beef cattle. Feed Ind. 2018, 39, 9–17. [Google Scholar]
- Yang, H.J.; Li, D.H.; Xie, C.Y.; Yue, Q. Effects of ferulic acid esterase supplementation on rumen fermentation characteristics of Chinese wildrye, corn stalk, rice straw and wheat straw by in vitro batch culture. Chin. J. Anim. Nutr. 2010, 22, 207–211. [Google Scholar]
- Metzler-Zebeli, B.U.; Scherr, C.; Sallaku, E.; Drochner, W.; Zebeli, Q. Evaluation of associative effects of total mixed ration for dairy cattle using in vitro gas production and different rumen inocula. J. Sci. Food Agric. 2012, 92, 2479–2485. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.H.; Getachew, G.; Cone, J.W. Evaluation of the extent of associative effects of two groups of four feeds using an in vitro gas production procedure. Anim. Feed Sci. Technol. 2009, 150, 9–17. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, S.; Jiang, H.; Tan, Z. In vitro fermentation characteristics of crop straws and their combined utilization. Chin. J. Appl. Ecol. 2005, 16, 1862–1867. [Google Scholar]
- Cone, J.W.; Van Gelder, A.H. Influence of protein fermentation on gas production profiles. Anim. Feed Sci. Technol. 1999, 76, 251–264. [Google Scholar] [CrossRef]
- Hai, C.X. Study on Nutritive Value of Assessing Native Grass on Qinghai Plateau by Gas Production Method In Vitro. Master’s Thesis, Qing Hai University, Xining, China, 2007. [Google Scholar]
- Chen, L.; Yu, C.; Zhao, L.; Zhou, C.; Li, B. Study on in vitro fermentation characteristics of different crop straws in Tibet region. Chin. J. Anim. Nutr. 2017, 29, 719–728. [Google Scholar]
- Wang, F.; Xu, Y.J.; Niu, J.L.; Zhao, M.; Zhang, Y.D.; Zhang, K.Z. Nutrient value evaluation of different type feeds by in vitro gas production method. China Anim. Husb. Vet. Med. 2016, 43, 76–83. [Google Scholar]
- Krause, K.M.; Oetzel, G.R. Understanding and preventing subacute ruminal acidosis in dairy herds: A review. Anim. Feed Sci. Technol. 2006, 126, 215–236. [Google Scholar] [CrossRef]
- Hristov, A.N.; Ropp, J.K.; Hunt, C.W. Effect of barley and its amylopectin content on ruminal fermentation and bacterial utilization of ammonia-N in vitro. Anim. Feed Sci. Technol. 2002, 99, 25–36. [Google Scholar] [CrossRef]
- Han, Z.K.; Chen, J. The Digestion and Metabolism of Ruminant Rumen; Science Press: Beijing, China, 1988. [Google Scholar]
- Liu, D.C.; Lu, D.X.; Hou, X.Z.; Gao, M.; Sun, H.Z. Effect of dietary forage of different qualities on rumen fermentation and predominant fibrolytic bacterial populations. Sci. Agric. Sin. 2008, 41, 1199–1206. [Google Scholar]
- Ørskov, E.R. Starch digestion and utilization in ruminants. J. Anim. Sci. 1986, 63, 1624–1633. [Google Scholar] [CrossRef] [PubMed]
| Item | Sorghum Straw | Wheat Straw | Ammoniated Wheat Straw |
|---|---|---|---|
| DM (%) | 89.3 | 91.60 | 92.35 |
| CP (%) | 6.36 | 2.80 | 6.03 |
| Crude fiber (%) | 34.63 | 40.63 | 34.40 |
| Ether extract (%) | 1.82 | 1.20 | 1.87 |
| Ca (%) | 0.77 | 0.26 | |
| P (%) | 0.20 | 0.03 |
| Item | Concentration | Nutrition Level | Concentration |
|---|---|---|---|
| Corn | 29.29 | DM (%) | 90.27 |
| Soybean meal | 9.00 | DE (MJ/kg) | 10.32 |
| Cottonseed meal | 1.50 | CP (%) | 11.03 |
| Alfalfa hay | 26.00 | DE/CP (MJ/g) | 0.094 |
| Oat grass | 33.00 | Ca (%) | 0.53 |
| Salt | 0.70 | P (%) | 0.23 |
| Additive premix 1 | 0.51 | ||
| Total | 100.00 |
| A Sorghum Straw:Ammoniated Wheat Straw | B Time (h) | Ruminal Degradability | |||
|---|---|---|---|---|---|
| DM% | OM% | CP% | Ash% | ||
| A1 (2:8) | B1 (6 h) | 11.54 | 16.22 | 14.86 | 18.35 |
| B2 (12 h) | 14.74 | 18.92 | 16.97 | 24.38 | |
| B3 (24 h) | 22.90 | 22.66 | 19.56 | 27.90 | |
| B4 (48 h) | 28.60 | 30.06 | 21.60 | 32.00 | |
| A2 (3:7) | B1 (6 h) | 20.11 | 17.69 | 15.26 | 22.20 |
| B2 (12 h) | 25.02 | 21.16 | 19.15 | 26.35 | |
| B3 (24 h) | 29.63 | 26.46 | 22.49 | 30.12 | |
| B4 (48 h) | 34.20 | 32.77 | 24.02 | 35.74 | |
| A3 (4:6) | B1 (6 h) | 19.98 | 17.31 | 16.26 | 24.15 |
| B2 (12 h) | 22.61 | 20.11 | 20.52 | 28.51 | |
| B3 (24 h) | 24.31 | 23.58 | 23.13 | 34.61 | |
| B4 (48 h) | 31.25 | 31.49 | 26.06 | 37.42 | |
| A4 (5:5) | B1 (6 h) | 19.50 | 18.88 | 18.40 | 25.38 |
| B2 (12 h) | 23.78 | 24.56 | 22.00 | 33.30 | |
| B3 (24 h) | 26.36 | 27.47 | 24.20 | 36.62 | |
| B4 (48 h) | 29.05 | 31.05 | 28.40 | 38.42 | |
| A5 (6:4) | B1 (6 h) | 18.47 | 19.29 | 19.20 | 27.14 |
| B2 (12 h) | 21.51 | 24.95 | 21.62 | 35.05 | |
| B3 (24 h) | 25.11 | 27.39 | 23.49 | 39.21 | |
| B4 (48 h) | 29.87 | 33.71 | 26.79 | 41.50 | |
| A6 (7:3) | B1 (6 h) | 21.79 | 22.55 | 20.40 | 28.24 |
| B2 (12 h) | 26.66 | 26.98 | 23.35 | 34.58 | |
| B3 (24 h) | 32.29 | 31.55 | 25.50 | 37.00 | |
| B4 (48 h) | 39.54 | 33.49 | 28.57 | 42.70 | |
| A7 (8:2) | B1 (6 h) | 22.83 | 22.52 | 21.30 | 31.43 |
| B2 (12 h) | 27.65 | 26.23 | 24.33 | 33.78 | |
| B3 (24 h) | 32.91 | 32.36 | 27.50 | 39.65 | |
| B4 (48 h) | 40.51 | 38.70 | 31.40 | 42.32 | |
| A | A1 (2:8) | 19.45 d | 21.97 e | 18.25 f | 25.66 e |
| A2 (3:7) | 24.67 bc | 24.52 d | 20.23 e | 28.60 d | |
| A3 (4:6) | 24.54 c | 23.12 e | 21.49 ed | 31.17 c | |
| A4 (5:5) | 23.74 c | 25.49 cd | 23.25 bc | 33.43 b | |
| A5 (6:4) | 27.24 b | 26.34 c | 22.77 cd | 35.72 a | |
| A6 (7:3) | 30.07 a | 28.64 b | 24.45 b | 35.63 a | |
| A7 (8:2) | 30.97 a | 29.95 a | 26.13 a | 36.80 a | |
| B | B1 (6 h) | 19.17 d | 19.21 d | 17.95 d | 25.27 d |
| B2 (12 h) | 23.14 c | 23.27 c | 21.13 c | 30.85 c | |
| B3 (24 h) | 27.64 b | 27.35 b | 23.69 b | 35.01 b | |
| B4 (48 h) | 33.29 a | 33.04 a | 26.69 a | 38.58 a | |
| SEM | 0.65 | 0.56 | 0.40 | 0.61 | |
| p-value | A | <0.001 | <0.001 | <0.001 | <0.001 |
| B | <0.001 | <0.001 | <0.001 | <0.001 | |
| A × B | 0.011 | <0.001 | 0.329 | 0.012 | |
| A Sorghum Straw:Ammoniated Wheat Straw | DM | CP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a (%) | b (%) | c (%/h) | a + b | ED (%) | a (%) | b (%) | c (%/h) | a + b | ED (%) | |
| A1 (2:8) | 5.19 b | 27.95 | 0.04 ab | 33.14 ab | 20.77 d | 11.56 ab | 10.91 | 0.06 | 22.48 c | 18.58 d |
| A2 (3:7) | 14.31 a | 21.71 | 0.06 ab | 36.03 ab | 27.98 b | 8.37 b | 16.01 | 0.09 | 24.38 bc | 20.31 c |
| A3 (4:6) | 18.45 a | 29.86 | 0.01 b | 48.31 a | 27.11 b | 10.36 b | 16.48 | 0.08 | 26.84 abc | 21.77 c |
| A4 (5:5) | 16.22 a | 17.41 | 0.08 a | 29.67 b | 24.81 c | 14.99 ab | 16.81 | 0.05 | 31.80 ab | 23.99 b |
| A5 (6:4) | 15.20 a | 21.76 | 0.03 ab | 36.96 ab | 24.82 c | 17.09 a | 15.10 | 0.03 | 32.19 ab | 23.47 b |
| A6 (7:3) | 16.69 a | 33.87 | 0.03 ab | 50.56 a | 31.73 a | 17.43 a | 12.42 | 0.05 | 29.85 abc | 24.93 b |
| A7 (8:2) | 18.15 a | 29.32 | 0.03 ab | 47.46 a | 32.58 a | 18.03 a | 15.94 | 0.04 | 33.97 a | 26.88 a |
| SEM | 1.02 | 1.65 | 0.01 | 2.07 | 0.86 | 0.91 | 0.64 | 0.01 | 1.04 | 0.59 |
| p-value | <0.001 | 0.066 | 0.057 | 0.005 | <0.001 | 0.001 | 0.064 | 0.183 | 0.002 | <0.001 |
| A Sorghum Straw:Ammoniated Wheat Straw | IVDMD% | IVOMD% |
|---|---|---|
| A1 (2:8) | 42.76 c | 45.21 cd |
| A2 (3:7) | 44.90 bc | 47.91 cd |
| A3 (4:6) | 45.89 bc | 49.25 bcd |
| A4 (5:5) | 48.59 ab | 46.88 cd |
| A5 (6:4) | 49.00 ab | 52.26 abc |
| A6 (7:3) | 48.56 a | 54.69 ab |
| A7 (8:2) | 52.01 a | 56.78 a |
| SEM | 2.84 | 3.95 |
| p-value | 0.011 | 0.001 |
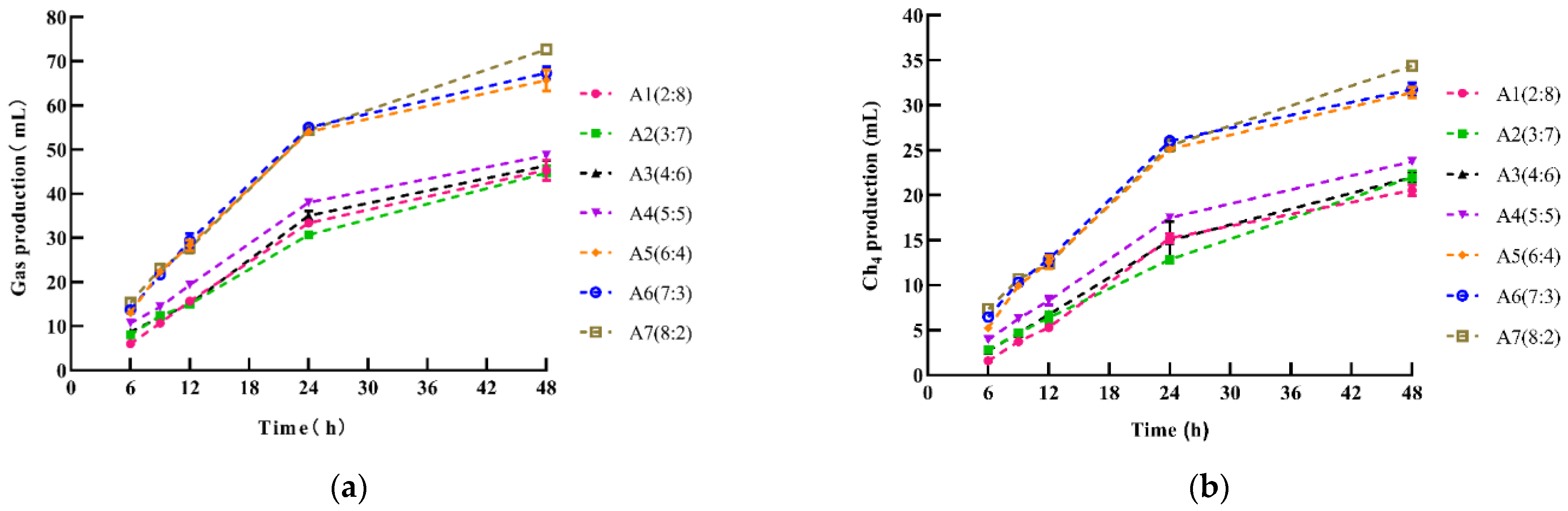
| A Sorghum Straw:Ammoniated Wheat Straw | B Time (h) | Gas Production (mL) | CH4 Production (mL) |
|---|---|---|---|
| A1 (2:8) | B1 (6 h) | 6.00 | 1.61 |
| B2 (9 h) | 10.67 | 3.74 | |
| B3 (12 h) | 15.67 | 5.30 | |
| B4 (24 h) | 33.33 | 15.23 | |
| B5 (48 h) | 45.33 | 20.54 | |
| A2 (3:7) | B1 (6 h) | 8.33 | 2.79 |
| B2 (9 h) | 12.33 | 4.68 | |
| B3 (12 h) | 15.00 | 6.39 | |
| B4 (24 h) | 30.67 | 12.84 | |
| B5 (48 h) | 44.67 | 22.00 | |
| A3 (4:6) | B1 (6 h) | 10.00 | 2.72 |
| B2 (9 h) | 12.00 | 4.64 | |
| B3 (12 h) | 16.00 | 6.73 | |
| B4 (24 h) | 35.00 | 14.96 | |
| B5 (48 h) | 46.33 | 21.98 | |
| A4 (5:5) | B1 (6 h) | 11.00 | 3.92 |
| B2 (9 h) | 14.33 | 6.26 | |
| B3 (12 h) | 19.33 | 8.30 | |
| B4 (24 h) | 38.00 | 17.49 | |
| B5 (48 h) | 48.67 | 23.74 | |
| A5 (6:4) | B1 (6 h) | 13.00 | 5.22 |
| B2 (9 h) | 22.33 | 9.95 | |
| B3 (12 h) | 28.33 | 12.54 | |
| B4 (24 h) | 54.00 | 25.12 | |
| B5 (48 h) | 65.67 | 31.41 | |
| A6 (7:3) | B1 (6 h) | 14.50 | 6.45 |
| B2 (9 h) | 21.67 | 10.33 | |
| B3 (12 h) | 29.33 | 12.89 | |
| B4 (24 h) | 55.00 | 26.05 | |
| B5 (48 h) | 67.33 | 31.76 | |
| A7 (8:2) | B1 (6 h) | 16.67 | 7.34 |
| B2 (9 h) | 23.00 | 10.68 | |
| B3 (12 h) | 27.67 | 12.39 | |
| B4 (24 h) | 54.33 | 25.52 | |
| B5 (48 h) | 72.67 | 34.39 | |
| A | A1 (2:8) | 22.20 d | 9.28 d |
| A2 (3:7) | 22.13 d | 9.74 d | |
| A3 (4:6) | 23.40 d | 10.21 d | |
| A4 (5:5) | 26.20 c | 11.94 c | |
| A5 (6:4) | 36.67 b | 16.85 b | |
| A6 (7:3) | 37.40 ab | 17.50 ab | |
| A7 (8:2) | 38.60 a | 18.06 a | |
| B | B1 (6 h) | 10.76 e | 4.29 e |
| B2 (9 h) | 16.62 d | 7.18 d | |
| B3 (12 h) | 21.48 c | 9.22 c | |
| B4 (24 h) | 42.91 b | 19.60 b | |
| B5 (48 h) | 55.81 a | 26.54 a | |
| SEM | 0.17 | 0.09 | |
| p-value | A | <0.001 | <0.001 |
| B | <0.001 | <0.001 | |
| A × B | <0.001 | <0.001 | |
| A Sorghum Straw:Ammoniated Wheat Straw | B Time (h) | pH | NH3-N (mg/dL) | Total Volatile Fatty Acid (mM) | Acetate (%) | Propionate (%) | Butyrate (%) | Acetate/ Propionate |
|---|---|---|---|---|---|---|---|---|
| A1 (2:8) | B1 (6 h) | 6.79 | 25.37 | 44.40 | 70.79 | 11.55 | 10.96 | 6.22 |
| B2 (9 h) | 6.78 | 28.75 | 50.85 | 70.52 | 11.67 | 10.88 | 6.04 | |
| B3 (12 h) | 6.73 | 16.07 | 50.53 | 71.69 | 12.02 | 10.27 | 5.98 | |
| B4 (24 h) | 6.66 | 14.33 | 57.46 | 82.2 | 15.32 | 7.84 | 5.39 | |
| B5 (48 h) | 6.57 | 17.08 | 98.79 | 71.04 | 14.32 | 7.89 | 5.10 | |
| A2 (3:7) | B1 (6 h) | 6.85 | 23.16 | 36.41 | 70.58 | 12.01 | 10.85 | 5.97 |
| B2 (9 h) | 6.84 | 26.21 | 39.87 | 70.17 | 12.54 | 10.68 | 5.60 | |
| B3 (12 h) | 6.74 | 31.53 | 54.55 | 70.46 | 12.43 | 10.79 | 5.67 | |
| B4 (24 h) | 6.68 | 15.54 | 82.59 | 69.8 | 15.04 | 9.18 | 4.64 | |
| B5 (48 h) | 6.68 | 18.25 | 97.42 | 70.82 | 15.32 | 8.17 | 4.62 | |
| A3 (4:6) | B1 (6 h) | 6.91 | 23.74 | 39.83 | 70.67 | 12.58 | 10.27 | 5.67 |
| B2 (9 h) | 6.87 | 16.28 | 45.73 | 70.52 | 12.46 | 10.58 | 5.66 | |
| B3 (12 h) | 6.88 | 13.29 | 48.73 | 72.12 | 12.42 | 9.86 | 5.82 | |
| B4 (24 h) | 6.56 | 19.63 | 93.02 | 71.28 | 13.89 | 8.72 | 5.13 | |
| B5 (48 h) | 6.58 | 23.86 | 107.88 | 70.22 | 15.5 | 8.36 | 4.53 | |
| A4 (5:5) | B1 (6 h) | 6.91 | 11.18 | 39.17 | 71.37 | 12.8 | 9.95 | 5.54 |
| B2 (9 h) | 6.82 | 20.32 | 43.65 | 70.41 | 12.95 | 10.45 | 5.44 | |
| B3 (12 h) | 6.87 | 22.34 | 46.6 | 73.42 | 12.33 | 9.11 | 5.96 | |
| B4 (24 h) | 6.59 | 33.26 | 84.48 | 71.88 | 14.05 | 8.59 | 5.12 | |
| B5 (48 h) | 6.62 | 40.55 | 109.37 | 71.23 | 15.01 | 8.09 | 4.76 | |
| A5 (6:4) | B1 (6 h) | 6.92 | 16.51 | 29.01 | 73.32 | 13.09 | 8.83 | 5.51 |
| B2 (9 h) | 6.94 | 19.63 | 40.15 | 73.88 | 13.14 | 8.46 | 5.62 | |
| B3 (12 h) | 6.89 | 38.87 | 50.97 | 74.5 | 13.69 | 7.74 | 5.44 | |
| B4 (24 h) | 6.71 | 41.19 | 89.08 | 74.2 | 14.85 | 7.16 | 5.00 | |
| B5 (48 h) | 6.57 | 22.21 | 105.84 | 73.69 | 15.04 | 6.90 | 4.90 | |
| A6 (7:3) | B1 (6 h) | 6.93 | 11.36 | 24.46 | 48.34 | 9.23 | 5.72 | 5.24 |
| B2 (9 h) | 6.9 | 13.56 | 33.71 | 72.87 | 13.87 | 8.66 | 5.26 | |
| B3 (12 h) | 6.91 | 15.78 | 48.70 | 74.37 | 13.76 | 7.88 | 5.41 | |
| B4 (24 h) | 6.82 | 18.95 | 66.67 | 72.91 | 14.79 | 7.78 | 4.93 | |
| B5 (48 h) | 6.84 | 26.06 | 100.59 | 74.13 | 14.27 | 7.00 | 5.20 | |
| A7 (8:2) | B1 (6 h) | 6.95 | 13.47 | 36.59 | 73.97 | 13.5 | 7.98 | 5.40 |
| B2 (9 h) | 6.89 | 14.62 | 44.60 | 73.01 | 14.06 | 8.52 | 5.20 | |
| B3 (12 h) | 6.83 | 13.67 | 41.78 | 72.86 | 14.51 | 8.12 | 5.02 | |
| B4 (24 h) | 6.83 | 16.93 | 59.85 | 73.24 | 15.19 | 7.32 | 4.83 | |
| B5 (48 h) | 6.76 | 19.61 | 100.32 | 62.48 | 14.50 | 7.46 | 4.29 | |
| A | A1 (2:8) | 6.70 d | 20.32 abc | 60.61 | 72.61 a | 12.81 c | 9.69 a | 5.74 |
| A2 (3:7) | 6.76 cd | 22.94 abc | 60.71 | 70.41 ab | 13.36 b | 9.99 a | 5.30 | |
| A3 (4:6) | 6.76 cd | 19.36 abc | 65.18 | 70.94 ab | 13.33 b | 9.62 a | 5.36 | |
| A4 (5:5) | 6.76 cd | 25.53 ab | 63.24 | 71.65 ab | 13.38 b | 9.29 a | 5.37 | |
| A5 (6:4) | 6.81 bc | 27.68 a | 61.15 | 73.90 a | 13.90 a | 7.87 b | 5.30 | |
| A6 (7:3) | 6.88 a | 17.14 bc | 53.98 | 73.45 a | 13.97 bc | 7.38 b | 5.23 | |
| A7 (8:2) | 6.87 ab | 15.66 c | 56.40 | 73.14 ab | 14.29 a | 7.92 b | 4.92 | |
| B | B1 (6 h) | 6.89 a | 17.83 | 35.78 b | 71.91 | 12.11 c | 9.23 a | 5.64 a |
| B2 (9 h) | 6.86 a | 19.91 | 43.14 cd | 71.63 | 12.97 c | 9.75 a | 5.54 a | |
| B3 (12 h) | 6.85 a | 21.65 | 48.27 c | 72.77 | 13.02 bc | 9.11 a | 5.61 a | |
| B4 (24 h) | 6.69 b | 22.83 | 76.31 b | 73.65 | 14.73 ab | 8.08 b | 4.98 b | |
| B5 (48 h) | 6.66 b | 23.95 | 103.93 a | 70.52 | 14.85 a | 7.69 b | 4.78 c | |
| SEM | 0.006 | 0.909 | 0.888 | 0.866 | 0.164 | 0.091 | 0.020 | |
| p-value | A | <0.001 | <0.001 | 0.057 | 0.008 | 0.033 | <0.001 | 0.510 |
| B | <0.001 | 0.297 | <0.001 | 0.420 | 0.002 | <0.001 | <0.001 | |
| A × B | 0.014 | 0.033 | 0.197 | 0.325 | 0.066 | 0.028 | 0.055 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Z.; Liu, H.; Xu, J.; Liu, T.; Wang, C.; Zheng, C. Evaluation of Gas Production, Fermentation Parameters, and Nutrient Degradability in Different Proportions of Sorghum Straw and Ammoniated Wheat Straw. Fermentation 2022, 8, 415. https://doi.org/10.3390/fermentation8080415
Wang J, Zhang Z, Liu H, Xu J, Liu T, Wang C, Zheng C. Evaluation of Gas Production, Fermentation Parameters, and Nutrient Degradability in Different Proportions of Sorghum Straw and Ammoniated Wheat Straw. Fermentation. 2022; 8(8):415. https://doi.org/10.3390/fermentation8080415
Chicago/Turabian StyleWang, Jing, Zhendong Zhang, Huihui Liu, Jianfeng Xu, Ting Liu, Cailian Wang, and Chen Zheng. 2022. "Evaluation of Gas Production, Fermentation Parameters, and Nutrient Degradability in Different Proportions of Sorghum Straw and Ammoniated Wheat Straw" Fermentation 8, no. 8: 415. https://doi.org/10.3390/fermentation8080415
APA StyleWang, J., Zhang, Z., Liu, H., Xu, J., Liu, T., Wang, C., & Zheng, C. (2022). Evaluation of Gas Production, Fermentation Parameters, and Nutrient Degradability in Different Proportions of Sorghum Straw and Ammoniated Wheat Straw. Fermentation, 8(8), 415. https://doi.org/10.3390/fermentation8080415






