Critical Optimized Conditions for Gamma-Aminobutyric Acid (GABA)-Producing Tetragenococcus Halophilus Strain KBC from a Commercial Soy Sauce Moromi in Batch Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
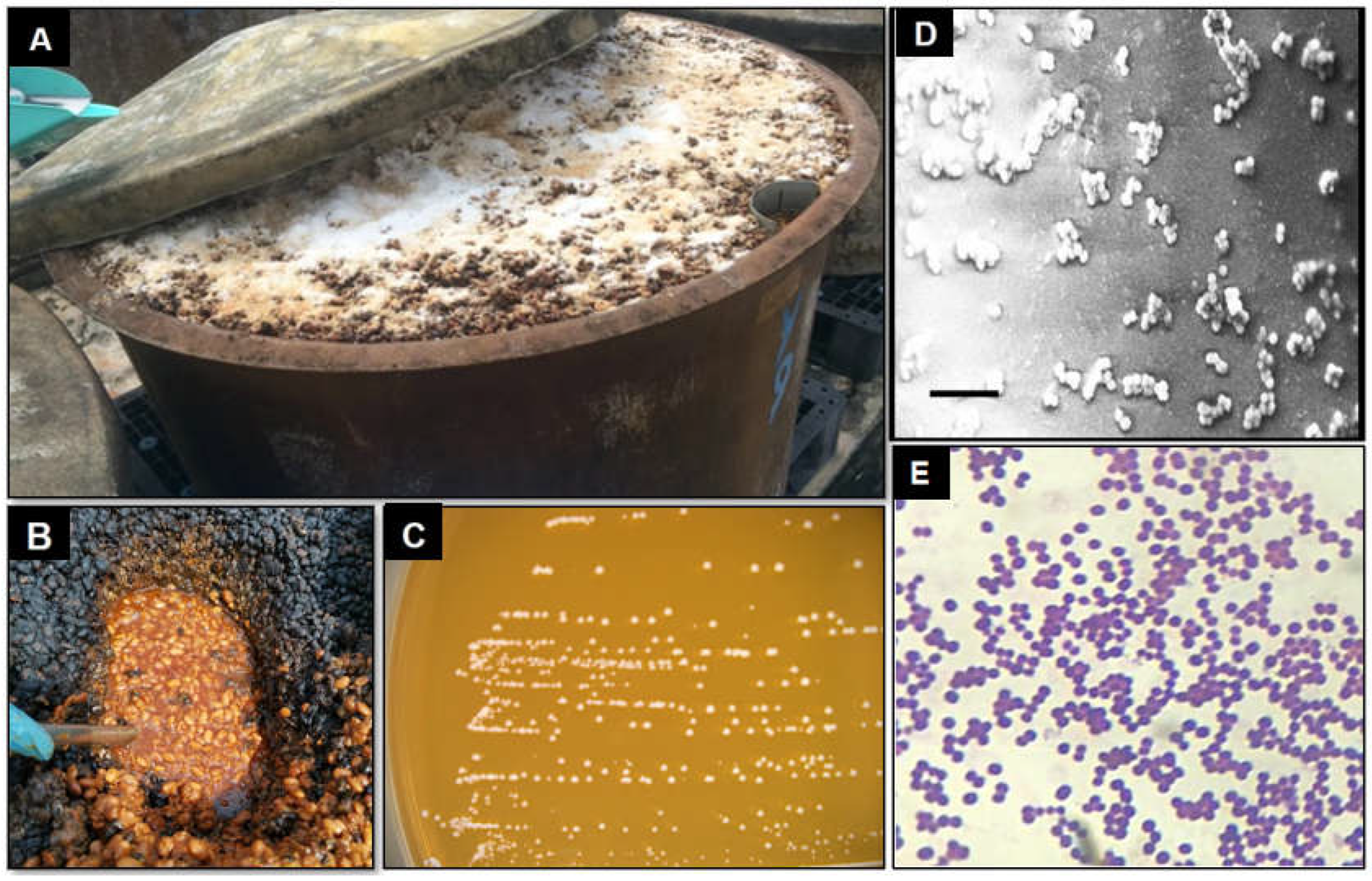
2.2. Strains
2.3. Inoculum Preparation
2.4. Growth Curve
2.5. GABA Capabilities of the Selected Strain
2.6. GABA Optimization
2.7. Analytical Methods
- GABA content
2.8. Statistical Analysis
3. Results and Discussion
3.1. Growth of Tetragenococcus Halophilus Strain KBC
3.2. GABA Capabilities of THSK
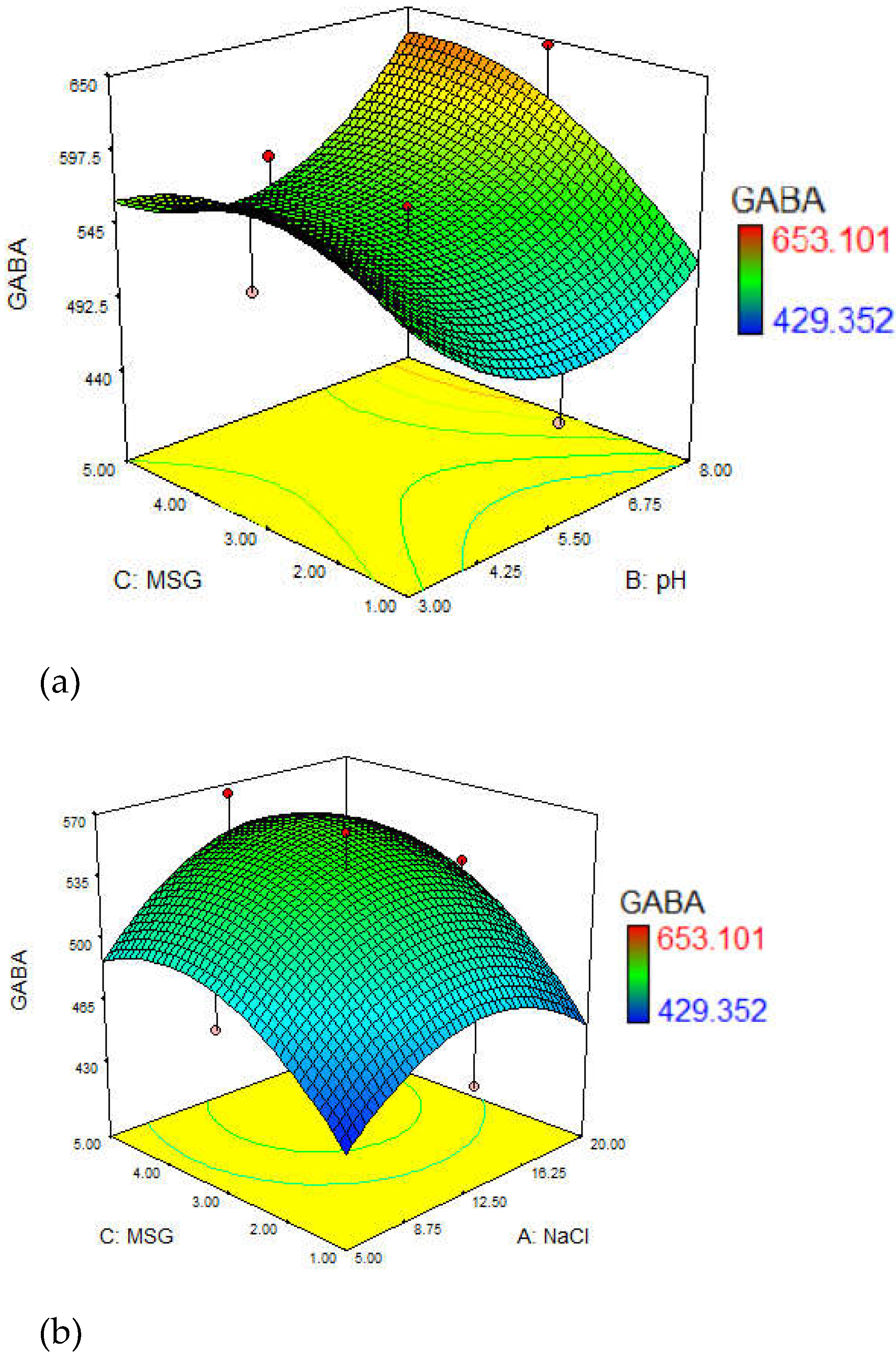
3.3. Optimization of GABA Yield
3.4. Effect of Culture Media
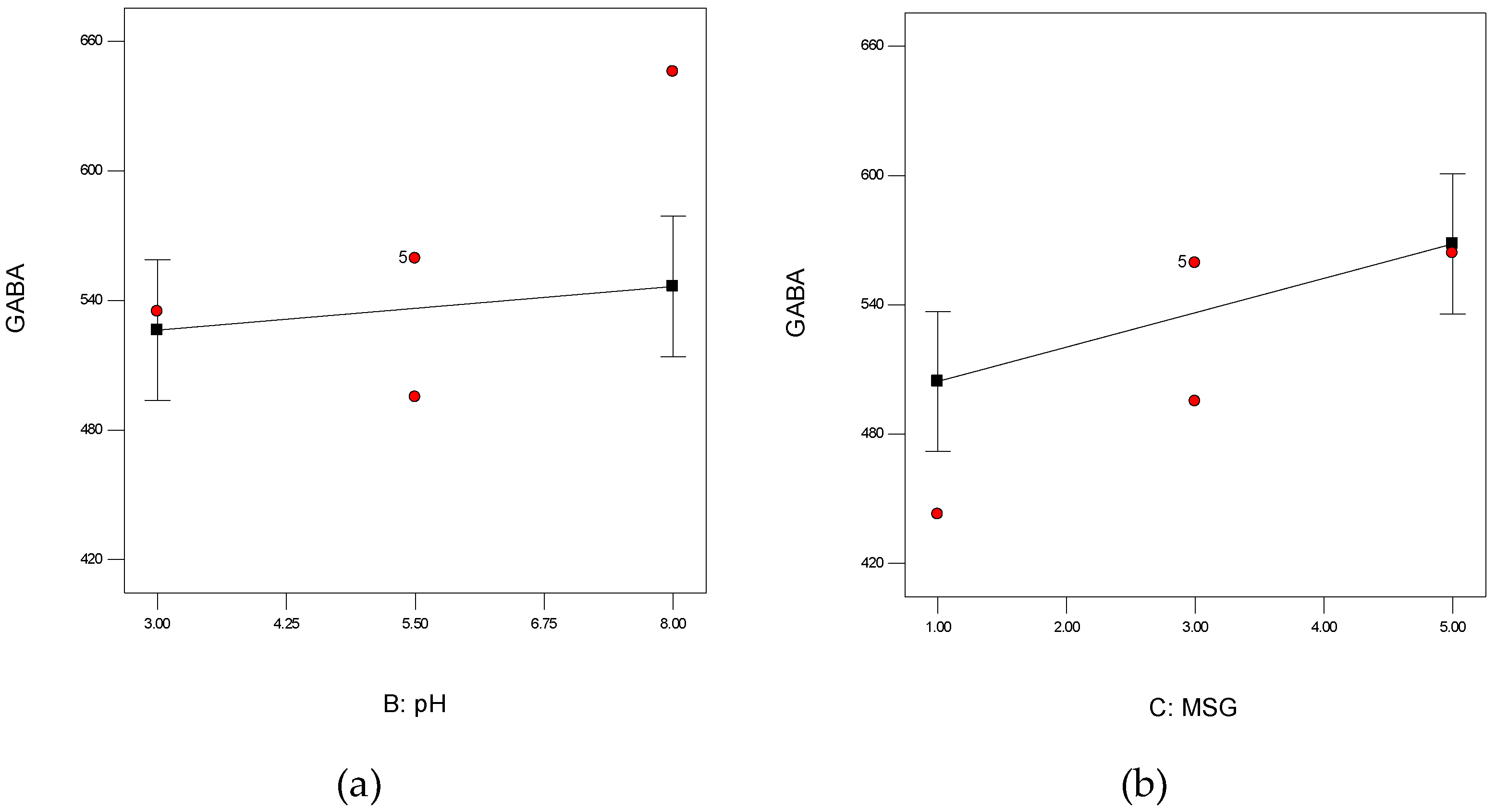
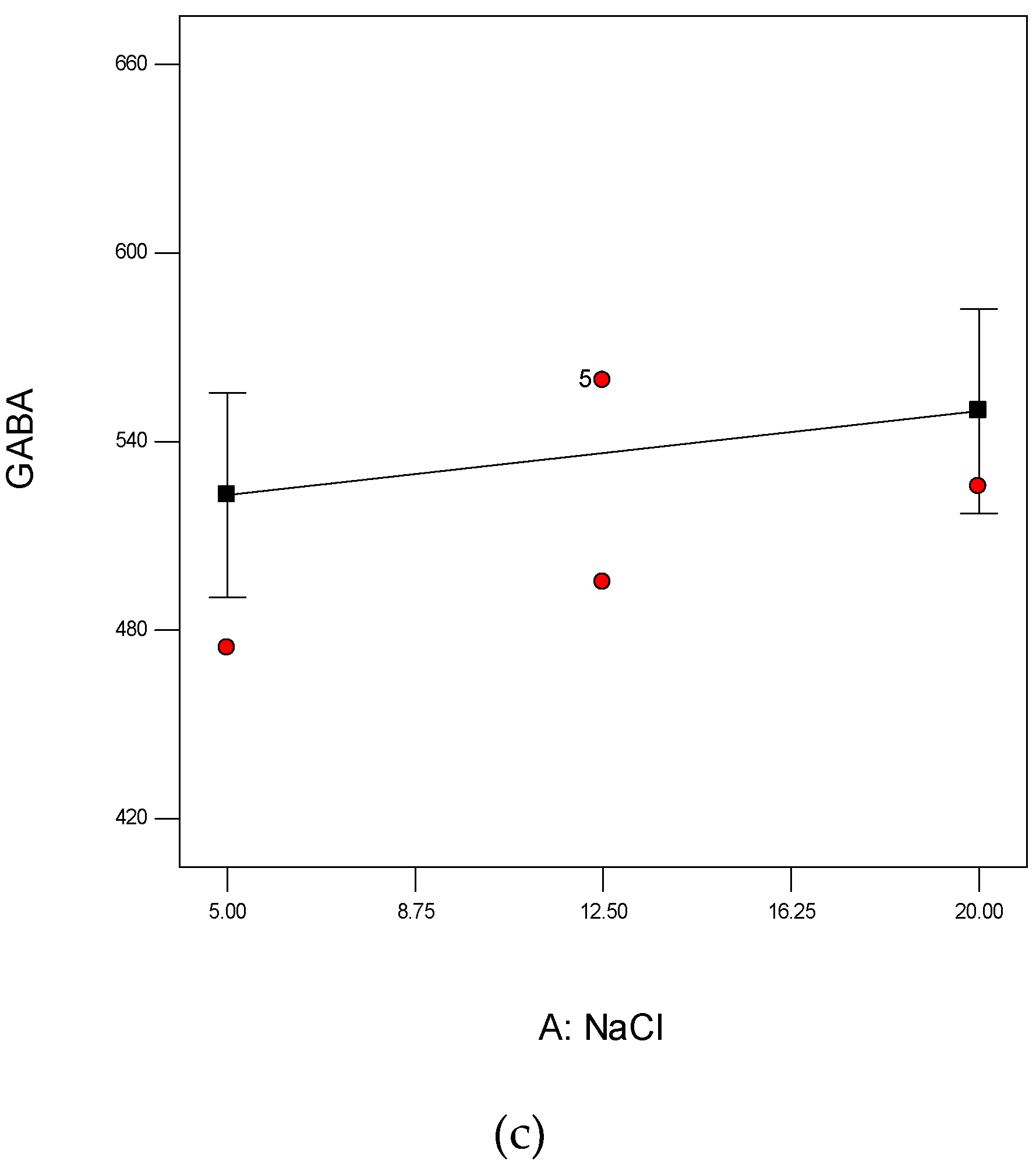
3.4.1. Effect of NaCl Concentrations
3.4.2. Effect of Initial pH
3.4.3. Effect of MSG Concentration
3.5. Highlights of Relevant Literature against the Current Work
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shimada, M.; Hasegawa, T.; Nishimura, C.; Kan, H.; Kanno, T.; Nakamura, T.; Matsubayashi, T. Anti-hypertensive effect of gamma-aminobutyric acid (GABA)-rich Chlorella on high-normal blood pressure and borderline hypertension in placebo-controlled double blind study. Clin. Exp. Hypertens. 2009, 31, 342–354. [Google Scholar] [CrossRef] [PubMed]
- Al-Kuraishy, H.M.; Hussian, N.R.; Al-Naimi, M.S.; Al-Gareeb, A.I.; Al-Mamorri, F.; Al-Buhadily, A.K. The Potential Role of Pancreatic γ-Aminobutyric Acid (GABA) in Diabetes Mellitus: A Critical Reappraisal. Int. J. Prev. Med. 2021, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S. An Updated Review on Pharmaceutical Properties of Gamma-Aminobutyric Acid. Molecules 2019, 24, 2678. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.I.; Ab Kadir, S.; Halim-Lim, S.A.; Ilham, Z.; Hajar-Azhari, S.; Saari, N. Vital parameters for high gamma-aminobutyric acid (GABA) production by an industrial soy sauce koji Aspergillus oryzae NSK in submerged-liquid fermentation. Food Sci. Biotechnol. 2019, 28, 1747–1757. [Google Scholar] [CrossRef]
- Sassi, S.; Wan-Mohtar, W.A.A.Q.I.; Jamaludin, N.S.; Ilham, Z. Recent progress and advances in soy sauce production technologies: A review. J. Food Process. Pres. 2021, 45, e15799. [Google Scholar] [CrossRef]
- Ding, W.; Cui, J.; Zhao, Y.; Han, B.; Li, T.; Zhao, P.; Xu, J.-W.; Yu, X. Enhancing Haematococcus pluvialis biomass and γ-aminobutyric acid accumulation by two-step cultivation and salt supplementation. Bioresour. Technol. 2019, 285, 121334. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.-J.; Kim, M.-J.; Kim, K.-S. Utilization of Barley or Wheat Bran to Bioconvert Glutamate to γ-Aminobutyric Acid (GABA). J. Food Sci. 2013, 78, C1376–C1382. [Google Scholar] [CrossRef]
- Poojary, M.M.; Dellarosa, N.; Roohinejad, S.; Koubaa, M.; Tylewicz, U.; Gómez-Galindo, F.; Saraiva, J.A.; Rosa, M.D.; Barba, F.J. Influence of Innovative Processing on γ-Aminobutyric Acid (GABA) Contents in Plant Food Materials. Compr. Rev. Food Sci. F 2017, 16, 895–905. [Google Scholar] [CrossRef]
- Barla, F.; Koyanagi, T.; Tokuda, N.; Matsui, H.; Katayama, T.; Kumagai, H.; Michihata, T.; Sasaki, T.; Tsuji, A.; Enomoto, T. The γ-aminobutyric acid-producing ability under low pH conditions of lactic acid bacteria isolated from traditional fermented foods of Ishikawa Prefecture, Japan, with a strong ability to produce ACE-inhibitory peptides. Biotechnol. Rep. 2016, 10, 105–110. [Google Scholar] [CrossRef]
- Carafa, I.; Stocco, G.; Nardin, T.; Larcher, R.; Bittante, G.; Tuohy, K.; Franciosi, E. Production of Naturally γ-Aminobutyric Acid-Enriched Cheese Using the Dairy Strains Streptococcus thermophilus 84C and Lactobacillus brevis DSM 32386. Front. Microbiol. 2019, 10, 93. [Google Scholar] [CrossRef]
- Okada, T.; Sugishita, T.; Murakami, T.; Murai, H.; Saikusa, T.; Horino, T.; Onoda, A.; Kajimoto, O.; Takahashi, R.; Takahashi, T. Effect of the Defatted Rice Germ Enriched with GABA for Sleeplessness, Depression, Autonomic Disorder by Oral Administration. Nippon Shokuhin Kagaku Kogaku Kaishi 2000, 47, 596–603. [Google Scholar] [CrossRef]
- Koubaa, M.; Delbecq, F.; Roohinejad, S.; Mallikarjunan, K. Gamma-Aminobutyric Acid. In Encyclopedia of Food Chemistry; Melton, L., Shahidi, F., Varelis, P., Eds.; Academic Press: Oxford, UK, 2019; pp. 528–534. [Google Scholar]
- Gharehyakheh, S.; Rad, A.H.E.; Nateghi, L.; Varmira, K. Production of GABA-enriched honey syrup using Lactobacillus bacteria isolated from honey bee stomach. J. Food Process. Pres. 2019, 43, e14054. [Google Scholar] [CrossRef]
- Venturi, M.; Galli, V.; Pini, N.; Guerrini, S.; Granchi, L. Use of Selected Lactobacilli to Increase gamma-Aminobutyric Acid (GABA) Content in Sourdough Bread Enriched with Amaranth Flour. Foods 2019, 8, 218. [Google Scholar] [CrossRef]
- Karimian, E.; Moayedi, A.; Khomeiri, M.; Aalami, M.; Mahoonak, A.S. Application of high-GABA producingLactobacillusplantarumisolated from traditional cabbage pickle in the production of functional fermented whey-based formulate. J. Food Meas. Charact. 2020, 14, 3408–3416. [Google Scholar] [CrossRef]
- Kwon, S.Y.; Garcia, C.V.; Song, Y.C.; Lee, S.P. GABA-enriched water dropwort produced by co-fermentation with Leuconostoc mesenteroides SM and Lactobacillus plantarum K154. Lwt-Food Sci. Technol. 2016, 73, 233–238. [Google Scholar] [CrossRef]
- Choi, W.C.; Reid, S.N.S.; Ryu, J.K.; Kim, Y.; Jo, Y.H.; Jeon, B.H. Effects of gamma-aminobutyric acid-enriched fermented sea tangle (Laminaria japonica) on brain derived neurotrophic factor-related muscle growth and lipolysis in middle aged women. Algae-Seoul 2016, 31, 175–187. [Google Scholar] [CrossRef]
- Harnentis, H.; Nurmiati, N.; Marlida, Y.; Adzitey, F.; Huda, N. gamma-Aminobutyric acid production by selected lactic acid bacteria isolate of an Indonesian indigenous fermented buffalo milk (dadih) origin. Vet. World 2019, 12, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Zareian, M.; Oskoueian, E.; Majdinasab, M.; Forghani, B. Production of GABA-enriched idli with ACE inhibitory and antioxidant properties using Aspergillus oryzae: The antihypertensive effects in spontaneously hypertensive rats. Food Funct. 2020, 11, 4304–4313. [Google Scholar] [CrossRef]
- Hajar-Azhari, S.; Wan-Mohtar, W.A.I.; Ab Kadir, S.; Abd Rahim, M.H.; Saari, N. Evaluation of a Malaysian soy sauce koji strain Aspergillus oryzae NSK for gamma-aminobutyric acid (GABA) production using different native sugars. Food Sci. Biotechnol. 2018, 27, 479–488. [Google Scholar] [CrossRef]
- Sahab, N.R.M.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric acid found in fermented foods and beverages: Current trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef]
- Kobayashi, W.; Kobayashi, T.; Takahashi, A.; Kumakura, K.; Matsuoka, H. Metabolism of glutamic acid to alanine, proline, and γ-aminobutyric acid during takuan-zuke processing of radish root. J. Food Sci. 2021, 86, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Shim, J.M.; Yao, Z.; Kim, J.A.; Kim, J.H. Properties of Kimchi Fermented with GABA-Producing Lactic Acid Bacteria as a Starter. J. Microbiol. Biotechnol. 2018, 28, 534–541. [Google Scholar] [CrossRef]
- Lv, X.; Liu, G.; Fan, X.; Qiao, Y.; Zhang, A.; Zhao, X.; Lin, Y.; Feng, Z. Effects of NaCl and ethanol stresses on γ-aminobutyric acid synthesis in Kocuria kristinae. Food Biosci. 2020, 37, 100702. [Google Scholar] [CrossRef]
- Tanamool, V.; Hongsachart, P.; Soemphol, W. Screening and characterisation of gamma-aminobutyric acid (GABA) producing lactic acid bacteria isolated from Thai fermented fish (Plaa-som) in Nong Khai and its application in Thai fermented vegetables (Som-pak). Food Sci. Technol. 2020, 40, 483–490. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, S.; Chen, F.; He, M.; Lin, J. Isolation of high γ-aminobutyric acid-producing lactic acid bacteria and fermentation in mulberry leaf powders. Exp. Ther. Med. 2019, 18, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, H.Z.; Zhang, C.; Lu, Y.J.; Zhu, X.Y.; Lu, Z.X. Gamma-Aminobutyric acid-rich yogurt fermented by Streptococcus salivarius subsp thermophiles fmb5 apprars to have anti-diabetic effect on streptozotocin-induced diabetic mice. J. Funct. Foods 2016, 20, 267–275. [Google Scholar] [CrossRef]
- Ab Kadir, S.; Wan-Mohtar, W.A.I.; Mohammad, R.; Lim, S.A.H.; Mohammed, A.S.; Saari, N. Evaluation of commercial soy sauce koji strains of Aspergillus oryzae for gamma-aminobutyric acid (GABA) production. J. Ind. Microbiol. Biotechnol. 2016, 43, 1387–1395. [Google Scholar] [CrossRef]
- Shin Yee, C.; Sohedein, M.N.A.; Poh Suan, O.; Weng Loen, A.W.; Abd Rahim, M.H.; Soumaya, S.; Ilham, Z.; Wan-Mohtar, W.A.A.Q.I. The production of functional γ-aminobutyric acid Malaysian soy sauce koji and moromi using the trio of Aspergillus oryzae NSK, Bacillus cereus KBC, and the newly identified Tetragenococcus halophilus KBC in liquid-state fermentation. Future Foods 2021, 4, 100055. [Google Scholar] [CrossRef]
- Wan-Mohtar, W.A.I.; Sohedein, M.N.A.; Ibrahim, M.F.; Ab Kadir, S.; Suan, O.P.; Weng Loen, A.W.; Sassi, S.; Ilham, Z. Isolation, Identification, and Optimization of γ-Aminobutyric Acid (GABA)-Producing Bacillus cereus Strain KBC from a Commercial Soy Sauce moromi in Submerged-Liquid Fermentation. Processes 2020, 8, 652. [Google Scholar] [CrossRef]
- Aryuman, P.; Lertsiri, S.; Visessanguan, W.; Niamsiri, N.; Bhumiratana, A.; Assavanig, A. Glutaminase-producing Meyerozyma (Pichia) guilliermondii isolated from Thai soy sauce fermentation. Int. J. Food Microbiol. 2015, 192, 7–12. [Google Scholar] [CrossRef]
- Sharafi, S.; Nateghi, L. Optimization of gamma-aminobutyric acid production by probiotic bacteria through response surface methodology. Iran. J. Microbiol. 2020, 12, 584–591. [Google Scholar] [CrossRef] [PubMed]
- Chun, B.H.; Han, D.M.; Kim, K.H.; Jeong, S.E.; Park, D.; Jeon, C.O. Genomic and metabolic features of Tetragenococcus halophilus as revealed by pan-genome and transcriptome analyses. Food Microbiol. 2019, 83, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Link, T.; Vogel, R.F.; Ehrmann, M.A. The diversity among the species Tetragenococcus halophilus including new isolates from a lupine seed fermentation. Bmc Microbiol. 2021, 21, 320. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Liu, Z.; Xie, F.; Bilal, M.; Liu, L.; Yang, R.; Wang, Z. Microbial production of gamma-aminobutyric acid: Applications, state-of-the-art achievements, and future perspectives. Crit. Rev. Biotechnol. 2021, 41, 491–512. [Google Scholar] [CrossRef]
- Wu, Q.; Shah, N.P. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 2018, 69, 151–158. [Google Scholar] [CrossRef]
- Di Cagno, R.; Mazzacane, F.; Rizzello, C.G.; De Angelis, M.; Giuliani, G.; Meloni, M.; De Servi, B.; Gobbetti, M. Synthesis of γ-aminobutyric acid (GABA) by Lactobacillus plantarum DSM19463: Functional grape must beverage and dermatological applications. Appl. Microbiol. Biot. 2010, 86, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Rayavarapu, B.; Tallapragada, P.; Usha, M.S. Statistical optimization of γ-aminobutyric acid production by response surface methodology and artificial neural network models using Lactobacillus fermentum isolated from palm wine. Biocatal. Agric. Biotechnol. 2019, 22, 101362. [Google Scholar] [CrossRef]
- Cui, Y.; Miao, K.; Niyaphorn, S.; Qu, X. Production of Gamma-Aminobutyric Acid from Lactic Acid Bacteria: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 995. [Google Scholar] [CrossRef]
- Kanwal, S.; Rastogi, R.P.; Incharoensakdi, A. Glutamate decarboxylase activity and gamma-aminobutyric acid content in Synechocystis sp. PCC 6803 under osmotic stress and different carbon sources. J. Appl. Phycol. 2014, 26, 2327–2333. [Google Scholar] [CrossRef]
- Hussin, F.S.; Chay, S.Y.; Hussin, A.S.M.; Wan Ibadullah, W.Z.; Muhialdin, B.J.; Abd Ghani, M.S.; Saari, N. GABA enhancement by simple carbohydrates in yoghurt fermented using novel, self-cloned Lactobacillus plantarum Taj-Apis362 and metabolomics profiling. Sci. Rep. 2021, 11, 9417. [Google Scholar] [CrossRef]
- Lyu, C.; Zhao, W.; Peng, C.; Hu, S.; Fang, H.; Hua, Y.; Yao, S.; Huang, J.; Mei, L. Exploring the contributions of two glutamate decarboxylase isozymes in Lactobacillus brevis to acid resistance and γ-aminobutyric acid production. Microb. Cell Factories 2018, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, M.Y.; Ji, G.E.; Lee, Y.S.; Hwang, K.T. Production of γ-aminobutyric acid in black raspberry juice during fermentation by Lactobacillus brevis GABA100. Int. J. Food Microbiol. 2009, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Alcazar, J.; Yang, T.; Lu, Z.; Lu, Y. Optimized cultural conditions of functional yogurt for γ-aminobutyric acid augmentation using response surface methodology. J. Dairy Sci. 2018, 101, 10685–10693. [Google Scholar] [CrossRef] [PubMed]
- Phuengjayaem, S.; Kuncharoen, N.; Booncharoen, A.; Ongpipattanakul, B.; Tanasupawat, S. Genome analysis and optimization of γ-aminobutyric acid (GABA) production by lactic acid bacteria from plant materials. J. Gen. Appl. Microbiol. 2021, 67, 150–161. [Google Scholar] [CrossRef] [PubMed]
- Quillin, S.J.; Tran, P.; Prindle, A. Potential Roles for Gamma-Aminobutyric Acid Signaling in Bacterial Communities. Bioelectricity 2021, 3, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, K.; Jiang, Y.; Feng, X.; Li, L.; Dang, F.; Zhang, W.; Man, C. Transcriptomic response to GABA-producing Lactobacillus plantarum CGMCC 1.2437T induced by L-MSG. PLoS ONE 2018, 13, e0199021. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Lü, F.X.; Lu, Z.X.; Bie, X.M.; Jiao, Y.; Sun, L.J.; Yu, B. Production of gamma-aminobutyric acid by Streptococcus salivarius subsp. thermophilus Y2 under submerged fermentation. Amino Acids 2008, 34, 473–478. [Google Scholar] [CrossRef]
- Heider, S.A.E.; Wendisch, V.F. Engineering microbial cell factories: Metabolic engineering of Corynebacterium glutamicum with a focus on non-natural products. Biotechnol. J. 2015, 10, 1170–1184. [Google Scholar] [CrossRef]
- Shi, F.; Jiang, J.; Li, Y.; Li, Y.; Xie, Y. Enhancement of γ-aminobutyric acid production in recombinant Corynebacterium glutamicum by co-expressing two glutamate decarboxylase genes from Lactobacillus brevis. J. Ind. Microbiol. Biotechnol. 2013, 40, 1285–1296. [Google Scholar] [CrossRef]


| Variables | Range and Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| pH | 3.0 | 5.0 | 8.0 |
| MSG (g/L) | 1.0 | 3.0 | 5.0 |
| NaCl (%) | 5.0 | 12.5 | 20.0 |
| Run | Variables | Actual * | Predicted | ||
|---|---|---|---|---|---|
| pH | MSG (g/L) | NaCl (%) | GABA (mg/L) | GABA (mg/L) | |
| 1 | 8.00 | 5.00 | 20.00 | 653.10 ± 50.05 | 591.70 |
| 2 | 8.00 | 3.00 | 12.50 | 645.81 ± 32.38 | 546.44 |
| 3 | 3.00 | 3.00 | 12.50 | 534.77 ± 4.66 | 526.22 |
| 4 | 3.00 | 1.00 | 5.00 | 573.77 ± 6.94 | 480.97 |
| 5 | 5.50 | 3.00 | 20.00 | 525.58 ± 1.93 | 549.70 |
| 6 | 8.00 | 1.00 | 20.00 | 532.02 ± 6.34 | 527.91 |
| 7 | 5.50 | 3.00 | 5.00 | 474.23 ± 18.08 | 522.97 |
| 8 | 5.50 | 5.00 | 12.50 | 564.00 ± 11.68 | 568.22 |
| 9 | 5.50 | 3.00 | 12.50 | 559.36 ± 5.69 | 536.33 |
| 10 | 5.50 | 3.00 | 12.50 | 559.36 ± 5.69 | 536.33 |
| 11 | 5.50 | 3.00 | 12.50 | 559.36 ± 5.69 | 536.33 |
| 12 | 5.50 | 3.00 | 12.50 | 559.36 ± 5.69 | 536.33 |
| 13 | 8.00 | 1.00 | 5.00 | 429.35 ± 11.23 | 501.19 |
| 14 | 3.00 | 5.00 | 20.00 | 490.00 ± 6.34 | 571.48 |
| 15 | 3.00 | 5.00 | 5.00 | 575.18 ± 4.88 | 544.75 |
| 16 | 3.00 | 1.00 | 20.00 | 489.78 ± 45.53 | 507.69 |
| 17 | 5.50 | 3.00 | 12.50 | 559.36 ± 5.69 | 536.33 |
| 18 | 5.50 | 1.00 | 12.50 | 442.75 ± 142.34 | 504.44 |
| 19 | 5.50 | 3.00 | 12.50 | 495.17 ± 151.52 | 536.33 |
| 20 | 8.00 | 5.00 | 5.00 | 504.32 ± 40.4 | 564.97 |
| Source | Sum of Squares | Df | Mean Square | F Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Model | 51340.71 | 9 | 5704.52 | 4.82 | 0.0109 | significant |
| A-NaCl | 1785.70 | 1 | 1785.70 | 1.51 | 0.2475 | |
| B-pH | 1022.27 | 1 | 1022.27 | 0.86 | 0.3746 | |
| C-MSG | 10171.51 | 1 | 10171.51 | 8.59 | 0.0150 | |
| AB | 22116.60 | 1 | 22116.60 | 18.69 | 0.0015 | |
| AC | 252.18 | 1 | 252.18 | 0.21 | 0.6543 | |
| BC | 4724.24 | 1 | 4724.24 | 3.99 | 0.0737 | |
| A2 | 3591.31 | 1 | 3591.31 | 3.03 | 0.1121 | |
| B2 | 8092.15 | 1 | 8092.15 | 6.84 | 0.0258 | |
| C2 | 2935.42 | 1 | 2935.42 | 2.48 | 0.1464 | |
| Residual | 11836.08 | 10 | 1183.61 | |||
| Lack of Fit | 8402.14 | 5 | 1680.43 | 2.45 | 0.1742 | not significant |
| Pure Error | 3433.95 | 5 | 686.79 | |||
| Cor Total | 63,176.79 | 19 | ||||
| Standard Deviation = 34.40 | Mean = 536.33 | Adequate Precision = 0.6440 | ||||
| R2 = 0.8127 | Adjusted R2 = 0.6440 | |||||
| Microorganism | Isolation Source | Culture Media | GABA Production mg/L | Optimization Method | Reference |
|---|---|---|---|---|---|
| Tetragenococcus halophilus strain KBC | Soy sauce moromi | MRS broth and 5% NaCl | 293.4 | Unoptimized | This study |
| Tetragenococcus halophilus strain KBC | Soy sauce moromi | MRS broth, MSG 5 g/L, and 20% NaCl | 653.1 | Response surface methodology | This study |
| Tetragenococcus halophilus strain KBC | Soy sauce moromi | Soybeans and 20% molasses | 159.0 | Unoptimized | [29] |
| Bacillus cereus strain KBC | Soy sauce moromi | MRS broth | 532.7 | Unoptimized | [30] |
| Bacillus cereus strain KBC | Soy sauce moromi | MRS broth and MSG (5 g/L) | 3393.0 | Response surface methodology | [30] |
| Bacillus cereus strain KBC | Soy sauce moromi | Soybeans and 5% molasses | 161.0 | Unoptimized | [29] |
| Aspergillus oryzae NSK | Soy sauce koji | Glutamic acid | 194.0 | Unoptimized | [28] |
| Aspergillus oryzae NSK | Soy sauce koji | Cane molasses | 354.1 | One-factor-at-a-time optimization | [20] |
| Aspergillus oryzae NSK | Soy sauce koji | Sucrose, yeast extract, glutamic acid, and C8:N3 | 3278.3 | One-factor-at-a-time optimization | [4] |
| Aspergillus oryzae NSK | Soy sauce koji | Soybeans and organic wheat flour | 1290.0 | Unoptimized | [29] |
| Lactobacillus fermentum | Palm wine | Soymilk | 5340.0 | Response surface methodology | [38] |
| Streptococcus thermophilus 84C | Nostrano cheese | Milk | 84.0 | Unoptimized | [10] |
| Lactobacillus plantarum L10-11 | Fermented fish (Plaa-som) | MRS broth | 15740.0 | One-factor-at-a-time optimization | [25] |
| Lactobacillus delbrueckii ssp. bulgaricus | NP | MRS broth | 36.1 | Response surface methodology | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sassi, S.; Ilham, Z.; Jamaludin, N.S.; Halim-Lim, S.A.; Shin Yee, C.; Weng Loen, A.W.; Poh Suan, O.; Ibrahim, M.F.; Wan-Mohtar, W.A.A.Q.I. Critical Optimized Conditions for Gamma-Aminobutyric Acid (GABA)-Producing Tetragenococcus Halophilus Strain KBC from a Commercial Soy Sauce Moromi in Batch Fermentation. Fermentation 2022, 8, 409. https://doi.org/10.3390/fermentation8080409
Sassi S, Ilham Z, Jamaludin NS, Halim-Lim SA, Shin Yee C, Weng Loen AW, Poh Suan O, Ibrahim MF, Wan-Mohtar WAAQI. Critical Optimized Conditions for Gamma-Aminobutyric Acid (GABA)-Producing Tetragenococcus Halophilus Strain KBC from a Commercial Soy Sauce Moromi in Batch Fermentation. Fermentation. 2022; 8(8):409. https://doi.org/10.3390/fermentation8080409
Chicago/Turabian StyleSassi, Soumaya, Zul Ilham, Nazzatush Shimar Jamaludin, Sarina Abdul Halim-Lim, Chong Shin Yee, Alan Wong Weng Loen, Ooi Poh Suan, Mohamad Faizal Ibrahim, and Wan Abd Al Qadr Imad Wan-Mohtar. 2022. "Critical Optimized Conditions for Gamma-Aminobutyric Acid (GABA)-Producing Tetragenococcus Halophilus Strain KBC from a Commercial Soy Sauce Moromi in Batch Fermentation" Fermentation 8, no. 8: 409. https://doi.org/10.3390/fermentation8080409
APA StyleSassi, S., Ilham, Z., Jamaludin, N. S., Halim-Lim, S. A., Shin Yee, C., Weng Loen, A. W., Poh Suan, O., Ibrahim, M. F., & Wan-Mohtar, W. A. A. Q. I. (2022). Critical Optimized Conditions for Gamma-Aminobutyric Acid (GABA)-Producing Tetragenococcus Halophilus Strain KBC from a Commercial Soy Sauce Moromi in Batch Fermentation. Fermentation, 8(8), 409. https://doi.org/10.3390/fermentation8080409










