Production of Gamma-Aminobutyric Acid by Levilactobacillus brevis CD0817 by Coupling Fermentation with Self-Buffered Whole-Cell Catalysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strain and Media
2.3. GABA Fermentation Trial
2.3.1. Preparation of Inoculum
2.3.2. Fermentation
2.4. Optimization of Whole-Cell Catalysis System
2.5. Recovery of GABA from Whole-Cell Conversion Solution
2.6. Analytic Methods
2.7. Calculations and Statistical Analyses
3. Results and Discussion
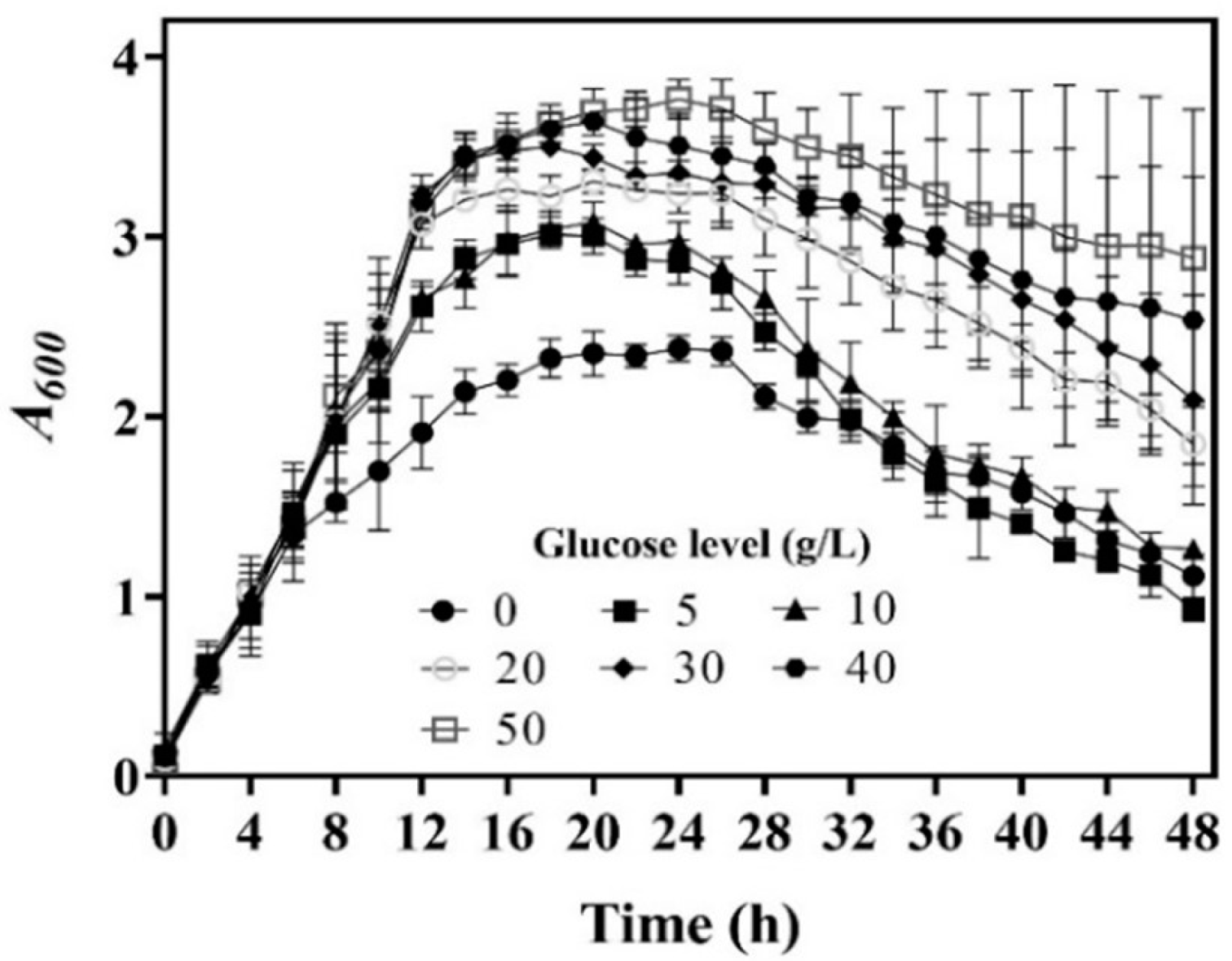
3.1. Effects of Cell Culture Method on Whole-Cell Catalysis
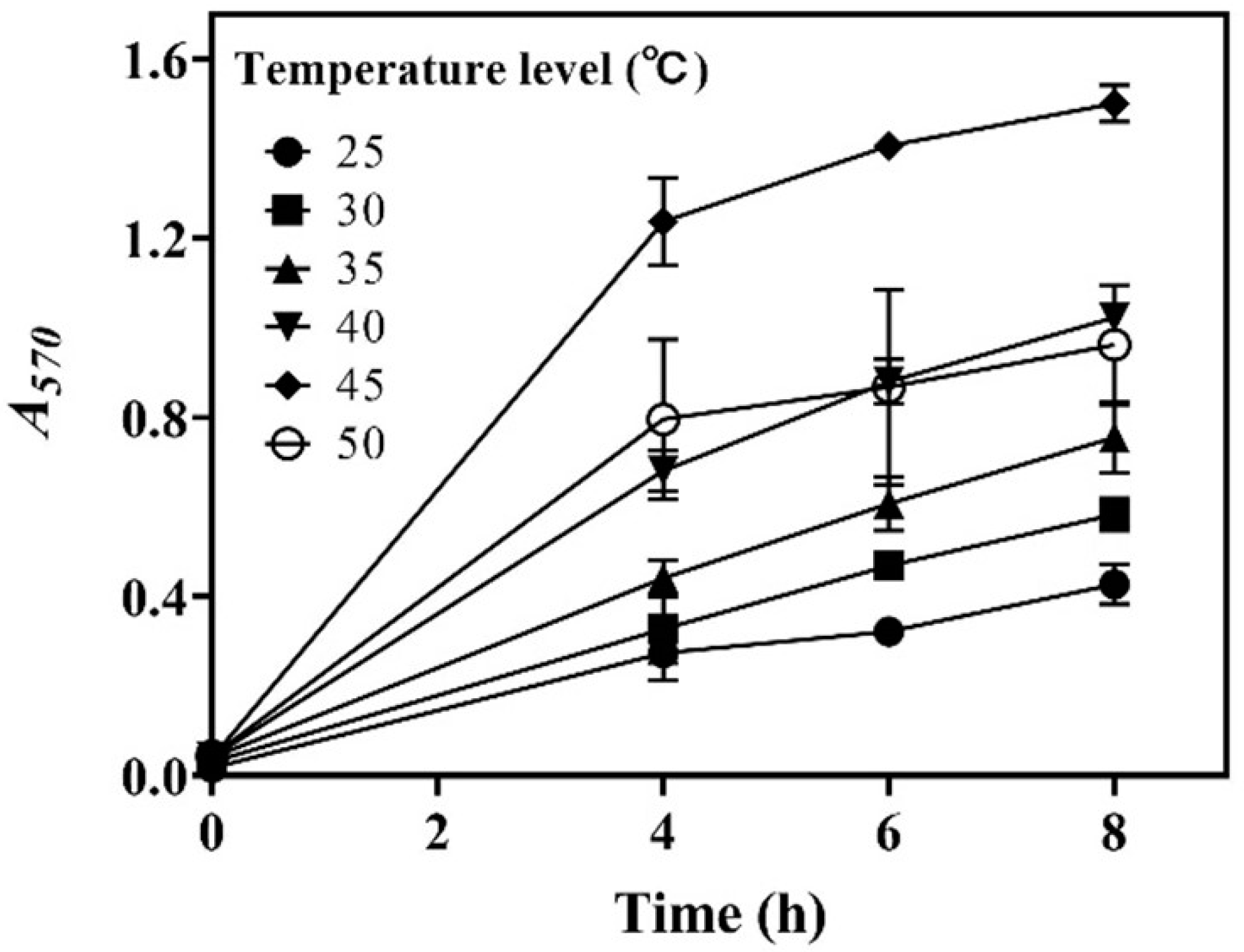
3.2. Effects of Temperature on Whole-Cell Catalysis
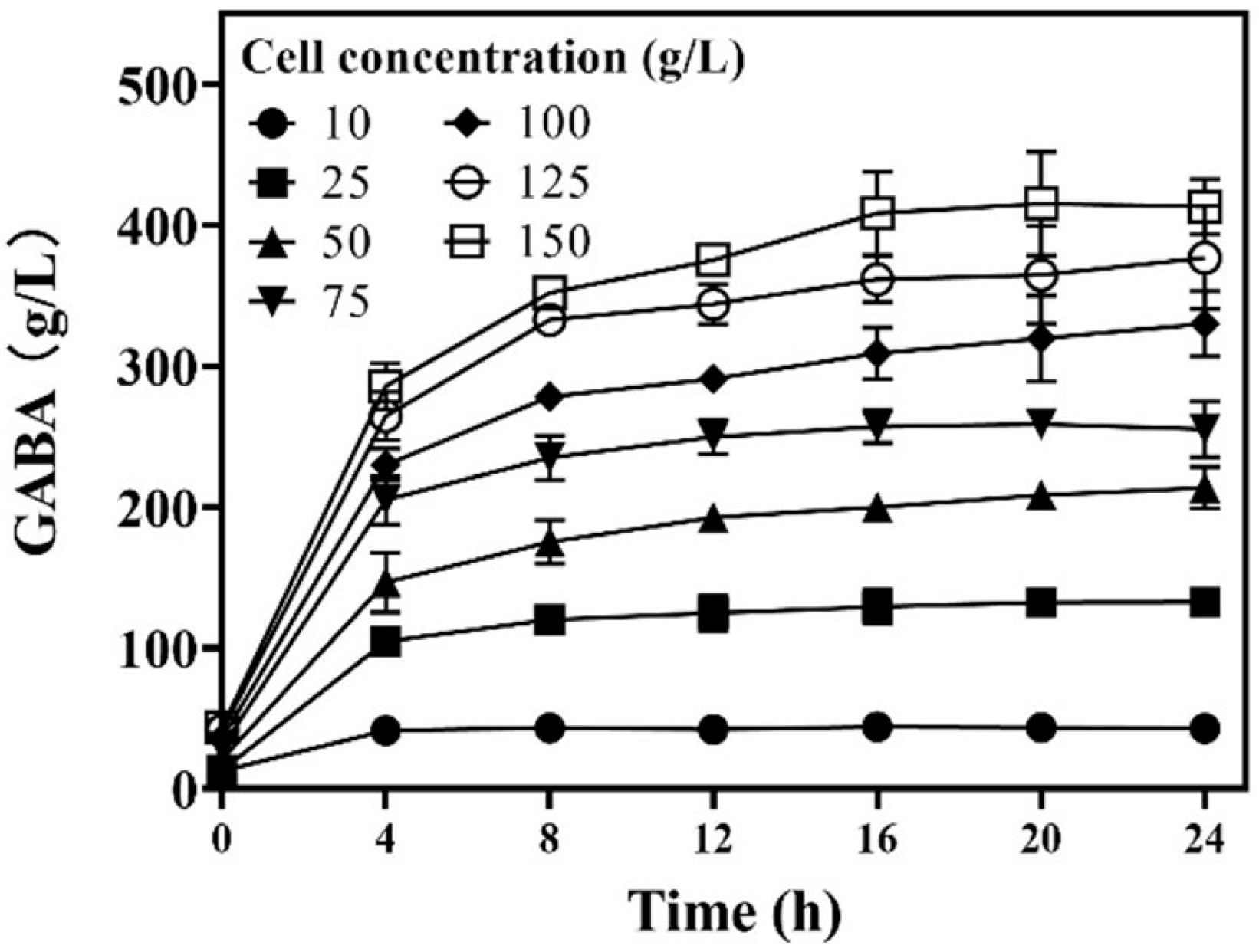
3.3. Effects of Wet Cell Level on Whole-Cell Catalysis
3.4. Recyclability of Whole-Cell System
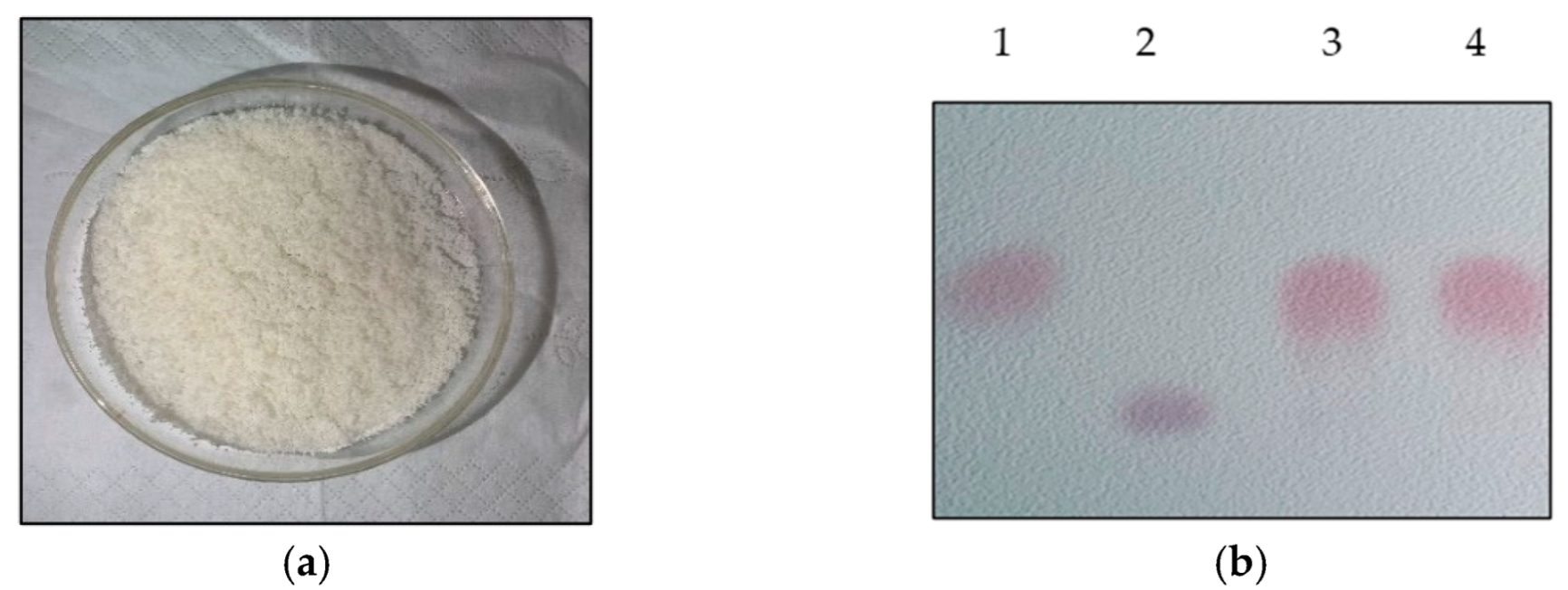
3.5. Recovery of GABA from Whole-Cell System
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, C.G.T.; Bottiglieri, T.; Snead, O.C. GABA, gamma-hydroxybutyric acid, and neurological disease. Ann. Neurol. 2003, 54, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Rui, Q.Y.; Han, S.T.; Wu, X.J.; Wang, X.Y.; Wu, P.; Shen, Y.P.; Dai, H.; Xue, Q.; Li, Y.G. Reduced GABA levels in the medial prefrontal cortex are associated with cognitive impairment in patients with NMOSD. Mult. Scler. Relat. Disord. 2022, 58, 103496. [Google Scholar] [CrossRef] [PubMed]
- Han, S.M.; Lee, J.S. Production and its anti-hyperglycemic effects of gamma-aminobutyric acid from the wild yeast strain Pichia silvicola UL6-1 and Sporobolomyces carnicolor 402-JB-1. Mycobiology 2017, 45, 199–203. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Cao, Y.S. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 2010, 39, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Francois, A.; Low, S.A.; Sypek, E.I.; Christensen, A.J.; Sotoudeh, C.; Beier, K.T.; Ramakrishnan, C.; Ritola, K.D.; Sharif-Naeini, R.; Deisseroth, K.; et al. A brainstem-spinal cord inhibitory circuit for mechanical pain modulation by GABA and enkephalins. Neuron 2017, 93, 822–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.L.; Shah, N.P. High gamma-aminobutyric acid production from lactic acid bacteria: Emphasis on Lactobacillus brevis as a functional dairy starter. Crit. Rev. Food Sci. Nutr. 2016, 57, 3661–3672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhakal, R.; Bajpai, V.K.; Baek, K.-H. Production of gaba (γ-aminobutyric acid) by microorganisms: A review. Braz. J. Microbiol. 2012, 43, 1230–1241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotter, P.D.; Hill, C. Surviving the acid test: responses of gram-positive bacteria to low pH. Microbiol. Mol. Biol. Rev. 2003, 67, 429–453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, P.L.C.; Waterman, S.R. Acid stress, anaerobiosis and gadCB: lessons from Lactococcus lactis and Escherichia coli. Trends Microbiol. 1998, 6, 214–216. [Google Scholar] [CrossRef]
- Gong, L.; Ren, C.; Xu, Y. GlnR negatively regulates glutamate-dependent acid resistance in Lactobacillus brevis. Appl. Environ. Microbiol. 2020, 86, e02615–e02619. [Google Scholar] [CrossRef]
- Zhao, A.; Hu, X.; Pan, L.; Wang, X. Isolation and characterization of a gamma-aminobutyric acid producing strain Lactobacillus buchneri WPZ001 that could efficiently utilize xylose and corncob hydrolysate. Appl. Microbiol. Biotechnol. 2015, 99, 3191–3200. [Google Scholar] [CrossRef]
- Komatsuzaki, N.; Shima, J.; Kawamoto, S.; Momose, H.; Kimura, T. Production of gamma-aminobutyric acid (GABA) by Lactobacillus paracasei isolated from traditional fermented foods. Food Microbiol. 2005, 22, 497–504. [Google Scholar] [CrossRef]
- Laroute, V.; Mazzoli, R.; Loubiere, P.; Pessione, E.; Cocaign-Bousquet, M. Environmental conditions affecting GABA production in Lactococcus lactis NCDO 2118. Microorganisms 2021, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Zhu, Y.; Wang, L.; Sun, T.; Pan, H.; Li, H. pH auto-sustain-based fermentation supports efficient gamma-aminobutyric acid production by Lactobacillus brevis CD0817. Fermentation 2022, 8, 208. [Google Scholar] [CrossRef]
- Gong, L.C.; Ren, C.; Xu, Y. Deciphering the crucial roles of transcriptional regulator GadR on gamma-aminobutyric acid production and acid resistance in Lactobacillus brevis. Microb. Cell Fact. 2019, 18, 108. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, T.; Hayashi, H.; Abe, K. Exchange of glutamate and gamma-aminobutyrate in a Lactobacillus strain. J. Bacteriol. 1997, 179, 3362–3364. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Qiu, T.; Huang, G.; Cao, Y. Production of gamma-aminobutyric acid by Lactobacillus brevis NCL912 using fed-batch fermentation. Microb. Cell Fact. 2010, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Liu, X.; Fu, J.; Wang, S.; Chen, Y.; Chang, K.; Li, H. Substrate sustained release-based high efficacy biosynthesis of GABA by Lactobacillus brevis NCL912. Microb. Cell Fact. 2018, 17, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, H.X.; Qiu, T.; Chen, Y.; Cao, Y.S. Separation of gamma-aminobutyric acid from fermented broth. J. Ind. Microbiol. Biotechnol. 2011, 38, 1955–1959. [Google Scholar] [CrossRef]
- Gao, Q.; Duan, Q.; Wang, D.P.; Zhang, Y.Z.; Zheng, C.Y. Separation and purification of gamma-aminobutyric acid from fermentation broth by flocculation and chromatographic methodologies. J. Agric. Food Chem. 2013, 61, 1914–1919. [Google Scholar] [CrossRef]
- Shi, X.; Chang, C.; Ma, S.; Cheng, Y.; Zhang, J.; Gao, Q. Efficient bioconversion of L-glutamate to gamma-aminobutyric acid by Lactobacillus brevis resting cells. J. Ind. Microbiol. Biotechnol. 2017, 44, 697–704. [Google Scholar] [CrossRef]
- Sun, L.H.; Li, S.N.; Gong, Y.Q. Synthesis of γ-aminobutyric acid by whole cells of Lactobacillus brevis DLF-19076. Food Sci. Technol. 2019, 44, 31–36. (In Chinese) [Google Scholar]
- Gao, D.D.; Chang, K.P.; Ding, G.T.; Wu, H.J.; Chen, Y.H.; Jia, M.Y.; Liu, X.H.; Wang, S.X.; Jin, Y.Y.; Pan, H.; et al. Genomic insights into a robust gamma-aminobutyric acid-producer Lactobacillus brevis CD0817. AMB Express 2019, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Li, H.X.; Cao, Y.S. Pre-staining thin layer chromatography method for amino acid detection. Afr. J. Biotechnol. 2010, 9, 8679–8681. [Google Scholar]
- Li, H.X.; Qiu, T.; Cao, Y.S.; Yang, J.Y.; Huang, Z.B. Pre-staining paper chromatography method for quantification of gamma-aminobutyric acid. J. Chromatogr. A 2009, 1216, 5057–5060. [Google Scholar] [CrossRef]
- Bremer, E.; Kramer, R. Responses of microorganisms to osmotic stress. Annu. Rev. Microbiol. 2019, 73, 313–334. [Google Scholar] [CrossRef]
- Choi, S.I.; Lee, J.W.; Park, S.M.; Lee, M.Y.; Ji, G.E.; Park, M.S.; Heo, T.R. Improvement of gamma-aminobutyric acid (GABA) production using cell entrapment of Lactobacillus brevis GABA 057. J. Microbiol. Biotechnol. 2006, 16, 562–568. [Google Scholar]
- Huang, J.; Mei, L.H.; Wu, H.; Lin, D.Q. Biosynthesis of gamma-aminobutyric acid (GABA) using immobilized whole cells of Lactobacillus brevis. World J. Microbiol. Biotechnol. 2007, 23, 865–871. [Google Scholar] [CrossRef]
- Wu, Q.L.; Shah, N.P. Restoration of GABA production machinery in Lactobacillus brevis by accessible carbohydrates, anaerobiosis and early acidification. Food Microbiol. 2018, 69, 151–158. [Google Scholar] [CrossRef]
- Pourbaferani, M.; Modiri, S.; Norouzy, A.; Maleki, H.; Heidari, M.; Alidoust, L.; Derakhshan, V.; Zahiri, H.S.; Noghabi, K.A. A newly characterized potentially probiotic strain, Lactobacillus brevis MK05, and the toxicity effects of its secretory proteins against MCF-7 breast cancer cells. Probiotics Antimicrob. Proteins 2021, 13, 982–992. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, N.; Werlinger, P.; Suh, D.A.; Lee, H.; Cho, J.H.; Cheng, J. Probiotic characterization of Lactobacillus brevis MJM60390 and in vivo assessment of its antihyperuricemic activity. J. Med. Food 2022, 25, 367–380. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Sun, T.; Jia, M.; Wang, L.; Wei, C.; Pei, J.; Lin, Z.; Wang, S. Production of Gamma-Aminobutyric Acid by Levilactobacillus brevis CD0817 by Coupling Fermentation with Self-Buffered Whole-Cell Catalysis. Fermentation 2022, 8, 321. https://doi.org/10.3390/fermentation8070321
Li H, Sun T, Jia M, Wang L, Wei C, Pei J, Lin Z, Wang S. Production of Gamma-Aminobutyric Acid by Levilactobacillus brevis CD0817 by Coupling Fermentation with Self-Buffered Whole-Cell Catalysis. Fermentation. 2022; 8(7):321. https://doi.org/10.3390/fermentation8070321
Chicago/Turabian StyleLi, Haixing, Tianyi Sun, Mengya Jia, Lingqin Wang, Cheng Wei, Jinfeng Pei, Zhiyu Lin, and Shuixing Wang. 2022. "Production of Gamma-Aminobutyric Acid by Levilactobacillus brevis CD0817 by Coupling Fermentation with Self-Buffered Whole-Cell Catalysis" Fermentation 8, no. 7: 321. https://doi.org/10.3390/fermentation8070321
APA StyleLi, H., Sun, T., Jia, M., Wang, L., Wei, C., Pei, J., Lin, Z., & Wang, S. (2022). Production of Gamma-Aminobutyric Acid by Levilactobacillus brevis CD0817 by Coupling Fermentation with Self-Buffered Whole-Cell Catalysis. Fermentation, 8(7), 321. https://doi.org/10.3390/fermentation8070321







