3.1. Ultrasound-Assisted Extraction Process
Following the methodology described in
Section 2.2, the ultrasound extraction was performed on the previously fermented samples. The results, according to the treatment for each of the variables studied, are presented in
Table 2.
As seen in
Table 2, the TPC was higher in samples that had ethanol-water solutions compared to those extracted with water or pure ethanol. This behavior can be attributed to the fact that alcohol-water mixtures allow a major extraction of bioactive compounds compared to the solvents individually [
35]. According to the results, the highest value of TPC was obtained under the conditions (T: 62 °C, 30 min, 50% ethanol and a solvent to sample ratio of 10:1 mL ethanol/g solid sample), obtaining 779.92 mg GAE/g d.m, which reflects better results compared to conventional methodologies such as Soxhlet extraction and maceration [
36]. It is also possible to observe that, as the temperature increased, the TPC and antioxidant activity also increased.
Ethanol was chosen as the extraction solvent because of its reported low toxicity and high ability to dissolve phenolic compounds [
37]. Both solvents contributed to the extraction process, water acting as a swelling agent and ethanol, acting on the cell wall of the substrate [
38]. The highest value obtained in the treatments performed (779.92 mg GAE/g d.m) was higher than that reported by [
39] who performed ultrasound assisted extraction on the Merr variety of pineapple peels, obtaining under the optimal conditions (5 min; 50% ethanol: water; 65% amplitude; 35:1 mL/g liquid-solid) a value of 708.10 mg GAE/g d.m.
The increase in temperature had a positive influence on the antioxidant activity. This behavior is directly related to better extraction yields of the phenolic compounds since a rupture in the matrix bonds is generated, the solvent diffusion rate is increased as well as the solubility of the compounds and, additionally, there is a greater mass transfer, which reduces the viscosity and surface tension of the solvent [
40]. Celli et al. [
41] also concluded that higher antioxidant activities are obtained at medium-high temperatures.
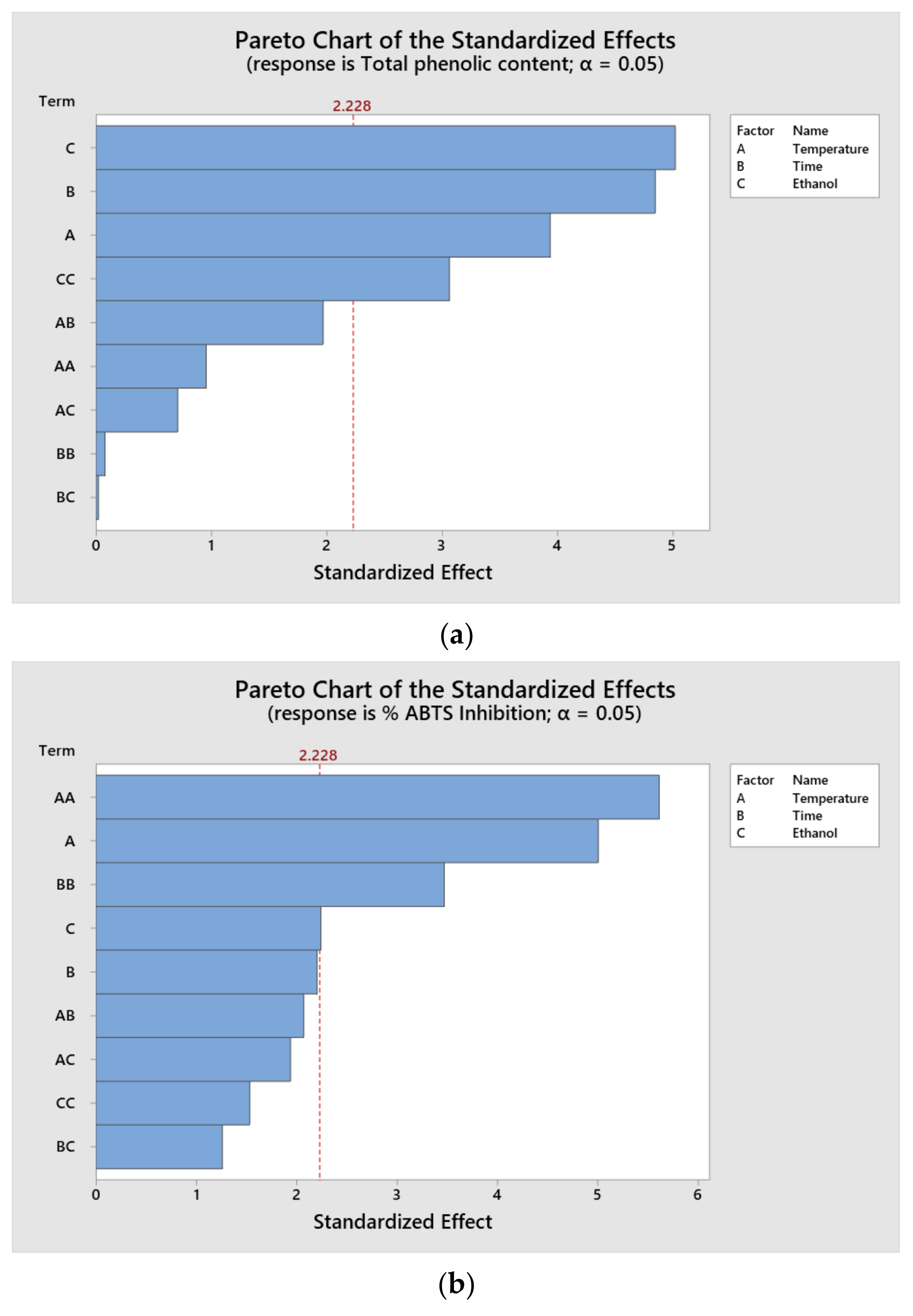
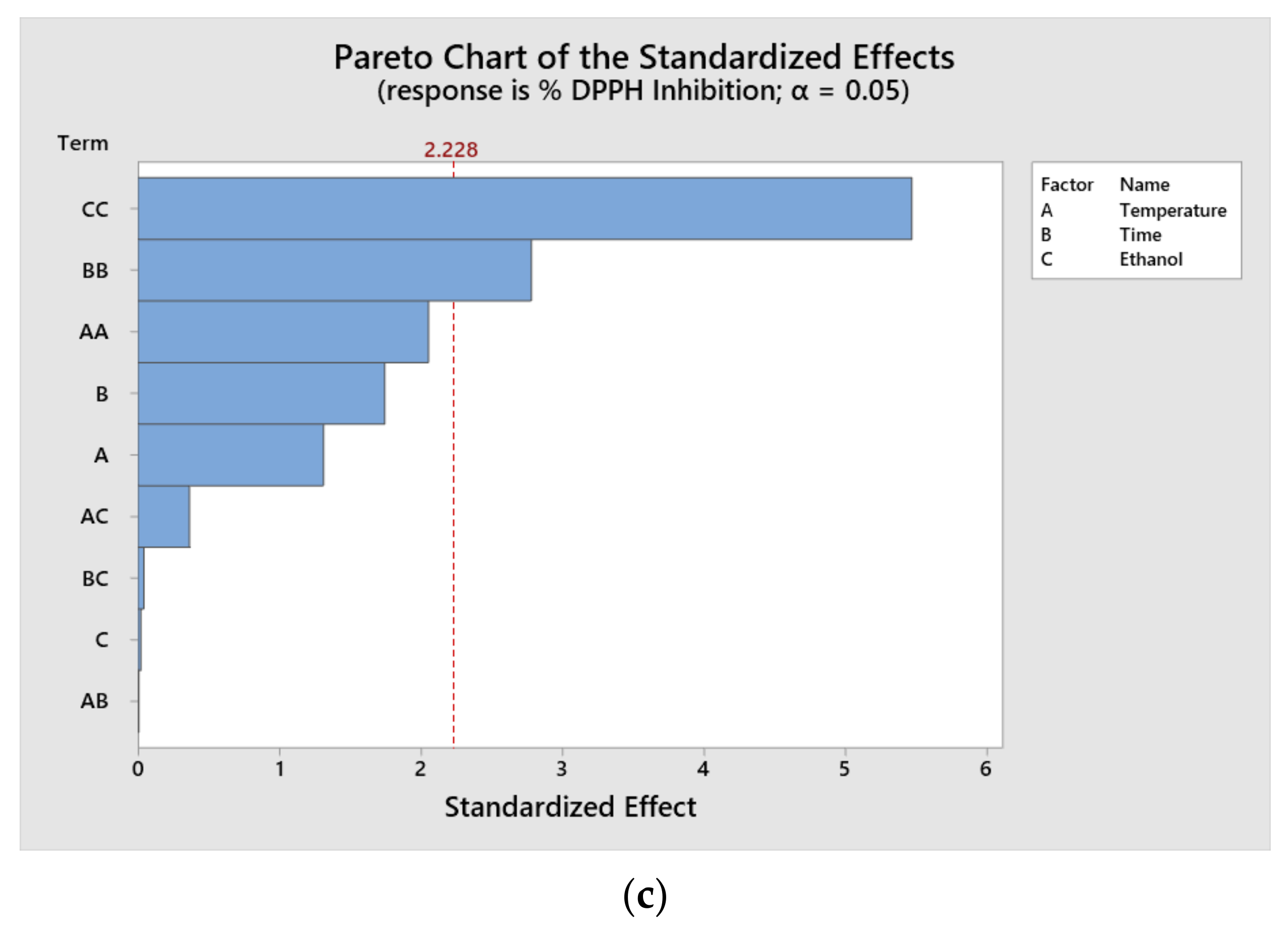
Pareto diagrams were plotted to determine which parameters had the greatest influence on the response variables of the treatments (
Figure 1). The results indicate that the individual significant factors at a 95% confidence level were time, ethanol concentration and temperature for TPC; the quadratic terms of temperature and time for ABTS and the quadratic terms of time and ethanol for DPPH. It is important to consider that the relative influence of each parameter varies according to the nature of the extracted compound.
According to the results of the Pareto diagram, it is possible to appreciate that the values of TPC and ABTS were influenced by the time and temperature variables. It has been reported that, with an increase in the extraction time, it is possible to increase the extraction capacity for all of the components. In the case of the antioxidant capacity by DPPH, one of the most influential variables was the interaction with ethanol. This has a positive effect on extraction yields.
Time was one of the variables that positively influenced the increase in the TPC and ABTS, which can be explained as mass transfer is a time dependent process [
41]. Regarding temperature, authors such as Gullón et al. [
42] have evidenced that high temperatures favor the extraction efficiency of bioactive compounds due to the increase in acoustic cavitation, surface contact area and the decrease in viscosity and density of the solvent. Likewise, Palma et al. [
41], who performed ultrasound extraction on Eucalyptus globulus leaves concluded that high temperatures were adequate to obtain higher values in TPC. Bhuyan et al. [
43] found that temperature, followed by time and power, presented a significant influence on the extraction efficiency of phenolic compounds.
Some authors have shown that a higher ethanol concentration results in better extraction yields [
37,
44] and that ethanol-water mixtures favor the release of polyphenols [
45]. For this reason, this was one of the parameters that most affected the antioxidant activity by DPPH.
To obtain the models to find the best relationship between ethanol concentration, time and temperature that predict the behavior of the TPC, DPPH radical scavenging activity and ABTS as response variables, a surface response design was applied, using the data in
Table 2. A central composite design (
Table 1) was applied with twenty treatments, six central points and six axial points allowing the incorporation of quadratic terms in the model. To assess the quality of the model, the correlation coefficient R
2 was selected, and the root mean square error (RMSE) was estimated. The results of the ANOVA analysis of variance for each of the response variables are shown in
Table 3.
For the TPC, the results show that the linear model presented a significant effect
p < 0.05 on the response variable, however, it can be observed that, although the quadratic model and the interactions did not present a significant effect (0.051 and 0.284), the quadratic factor of time did present a major influence on this variable (
p = 0.012). For the adequate prediction of this response variable, all the linear and quadratic terms and the interactions were considered, obtaining an R
2 value of 0.8883, indicating that the selected terms explain 88.83% of the variability of the response and presenting a strong correlation (0.7 < R
2 < 1) [
46]. The root mean square error was 44.36 which indicates that there is no significant difference between the values obtained and those predicted by the model. For DPPH radical inhibition, the quadratic model presented a significant effect
p < 0.05, except for the temperature (0.068). Similarly, all the terms of the variables studied were considered to guarantee the quality of the model. The R
2 value obtained was 0.8074, indicating that the selected terms explain 80.74% of the variability of the response. Likewise, the RMSE value was 2.01, indicating a good fit of the model to the experimental data.
Finally, for the inhibition percentage of ABTS radicals, both the linear and quadratic models showed a significant effect (p < 0.05). The only terms that did not present a significant effect were time in the linear model (0.060) and the quadratic factor of ethanol (0.172), however, it is not possible to exclude them because they affect the precision of the model. The interactions did not show a significant influence. The R2 value was 0.9029, which is considered a good fit and therefore can effectively represent the studied variable.
According to the results of the ANOVA table, models were proposed for each of the response variables studied, considering the coefficients of the temperature (T), time (t) and ethanol (E) components. The equations obtained are shown in
Table 4.
To observe the fit of the models to the experimental data,
Table 5 is presented.
According to the data reported in
Table 5, it is possible to observe that there is a good fit of the models with the experimental data obtained. The percentage inhibition of ABTS, the one that presented the best fit, the values of R
2 and RMSE were 90.29% and 1.43, respectively. For the TPC and percentage inhibition by DPPH the values for R
2 were 88.83% and 80.74%, indicating a strong correlation; for the RMSE 44.36 and 2.01, evidencing a good performance for the models.
3.3. Total Phenolic Content and Antioxidant Activity by DPPH and ABTS
Contour plots were made to analyze the behavior of each of the variables studied, (
Figure 3,
Figure 4 and
Figure 5). These graphs show the lowest or highest values of each variable through shade variations, allowing the identification of the region of interest and predicting at what levels the factors (temperature, time and ethanol concentration) must be to find the desired response values.
Figure 3 shows the contour plot for the behavior of the TPC under the different combinations of the analyzed factors. The blue color represents the zone where the lowest TPC values are obtained after the combined process of SSF and ultrasound, and the green color represents the zone where the highest values are obtained. It can be seen that the areas where the highest values are obtained are those where the factors of temperature, time and ethanol concentration are greater than the conditions (T: 45 °C, 30 min and 50% ethanol).
In
Figure 3 it is possible to observe that, as the time increases, there is an increase in the TPC, with the highest value (779.92 mg GAE/g d.m) at 30 min. The same behavior was observed by Bouafia et al. [
50], who reported that, at 30 min, the highest amount of phenols were obtained (40.01 ± 1.49 mg GAE/g m.s) in
Centaurea sp. extracts, and Wang et al. [
51], who used ultrasound to extract phenolic compounds from wheat bran, found that the optimal extraction conditions were at an ethanol concentration of 60%, temperature of 50 °C and a time of 29 min.
It was observed that, at temperatures above 45 °C, higher TPC was obtained. This factor has a great influence on the results, since at higher temperatures, the bonds in the substrate start to break, leading to greater extraction yields, as well as an increase in the solubility of the compounds [
52]. Bouafia et al. [
50] showed that the extraction yield of phenolic compounds from
Centaurea sp. leaves increased remarkably at a temperature range of 35–50 °C, in which the optimal value were at the highest temperatures evaluated.
As shown in
Figure 4 and
Figure 5, there was an increase in the inhibition percentage by DPPH and ABTS when the extraction time and temperature were increased. The effect of the temperature observed can be attributed to the fact that, at higher temperatures, the extraction of phenolic compounds is greater, due to solvent molecules moving faster and easier, facilitating the extraction process [
53].
According to the results in
Figure 4 and
Figure 5, it is shown that heating at moderate temperatures (50 °C to 70 °C) is adequate to soften the plant tissues and thus, release large amounts of bioactive compounds [
54]. Gónzalez-Centeno et al. [
55] observed this behavior when performing ultrasound extraction on grape pomace and these findings match with the ones shown in this research, since an increase in the inhibition percentage of DPPH and ABTS radicals at temperatures higher than 45 °C was observed.
Comparing the results obtained by the combination of these two technologies,
Table 6 shows the TPC values and antioxidant activity of the control sample (untreated peel), fermented peel, sonicated peel and fermented with sonicated peel.
According to the analysis of variance (one way ANOVA), there was a statistically significant difference (
p < 0.05) between the treatments, and the POST-ANOVA (Tukey) analysis showed that the highest value was obtained with the combination of treatments. The TPC of the control sample (30 ± 0.93 mg GAE/g d.m) was similar to that reported by Lourenço et al. [
56] who encapsulated Golden pineapple peel extracts using 80% ethanol as solvent. However, it was higher than the value reported by Azizan et al. [
57] who also worked with the MD2 variety and obtained 10.73 mg GAE/g d.m, while the percentage inhibition by DDPH (72.67%) was higher than that determined in this research (37.02%). Regarding the antioxidant activity by ABTS, the control sample presented (5.64 µmol/g d.m) a similar value to that reported by Kuskoski et al. [
58], who evaluated this property in freeze-dried pineapple products, obtaining a value of 3.4 µmol/g d.m. Martínez et al. [
59] reported a lower value (1.7 ± 0.2 µmol/g d.m) in dehydrated pineapple by-products (peel and core). Regarding the antioxidant activity of DPPH and ABTS, there was a statistically significant difference between the treatments (
p < 0.05), but there were no representative differences between the fermented and the ultrasound-fermented samples, indicating that the combination of treatments did not significantly increase the antioxidant capacity with respect to the fermented sample. It is important to highlight that all treatments presented higher values of TPC and antioxidant activity with respect to the control.
It is possible to observe that both the TPC and the antioxidant activity increased in the samples submitted to the two processes (SSF and UAE), TPC was 28 times higher compared to the control, the percentage inhibition by DPPH was 1.7 times higher and the percentage inhibition by ABTS 1.3 times. The increment in the TPC and the antioxidant activity could be attributed to the combined process of SSF and ultrasound. Fermentation allows the production of various hydrolyzing enzymes such as β-glucosidase, α-amylase, xylanase, esterases, etc., which are associated with the release of phenolic compounds soluble in water and/or organic solvents used for extraction [
35]. In addition to the enzymatic release of these compounds, other biochemical pathways could be involved, increasing the phenolic content and antioxidant activities in pineapple peels. Similarly, other water-soluble bioactive compounds such as small xylooligosaccharides, peptides, etc., produced through the fermentation process could contribute to enhance the antioxidant properties of the substrate [
60]. On the other hand, the acoustic and mechanical effects that occur during the ultrasound method generate the formation and subsequent decomposition of bubbles in the liquid extraction medium. The dynamically formed cavitation bubbles that implode in the surrounding liquid disintegrate the cell wall of the substrate, thereby releasing phenolic compounds that were not released during the fermentation process. It is relevant to highlight that these effects can only occur in liquids and solid materials containing liquids [
39]. These beneficial effects have been evidenced in a wide variety of plant substrates [
61,
62].
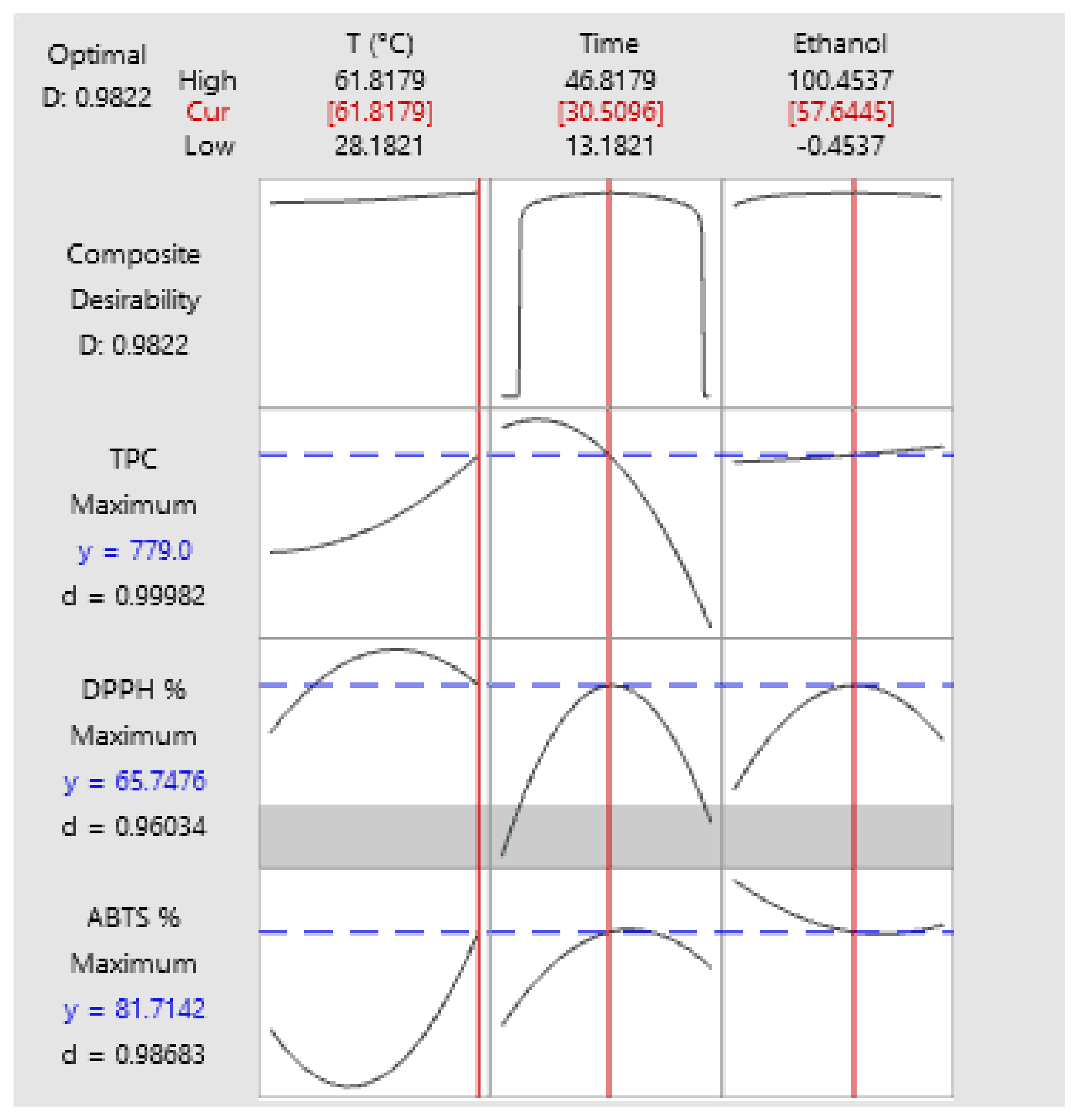
To optimize the process, a desirability function (d) was obtained, represented in the following graphs. The d value is equivalent to the degree of desirability of the response; values close to 1 indicate that the response is desirable. To apply this function, we sought to maximize the value of TPC and antioxidant activity (DPPH and ABTS).
Figure 6 shows the graph of the desirability compound. The response found presented a high desirability value (d = 0.980622), which therefore, achieves an overall optimal response. The appropriate factors for the process, as found in the function, were temperature at 62 °C, 58% ethanol and 32 min, obtaining 866.26 mg GAE/g d.m for TPC, 63.53% inhibition for DPPH and 80.06% for ABTS; values very similar to those obtained by the program.
As shown in
Figure 6, the temperature evaluated between 28 and 62 °C, presented an ascending value until it reached a maximum at 62 °C. For the ethanol concentration, on the other hand, it reached a maximum at 50% and then declined. In the case of temperature, thermal effects are associated with increased cell wall disruption, which enhances the release of certain compounds.
With respect to
Figure 6, it was possible to observe that the higher the temperature, the better results obtained for TPC and antioxidant activity. Authors such as Shivamathi et al. [
63] reported a better performance when obtaining pectin with ultrasound from pineapple peels when the temperature was evaluated in a range of 50–70 °C. The research realized by Yahya et al. (2020) [
39], who used ultrasound on pineapple peels, showed an increase in the TPC when increasing the ethanol concentration up to a maximum of 50%; at higher values, it began to decrease due to the intermiscibility and similarity of the extracted components with the solvent mixture. Also, these results are similar to those reported by Živković et al. (2018) [
38] and Xu et al. (2017) [
37] who performed ultrasound extraction on pomegranate peels and
Limonium sinuatum flowers, respectively. According to other research conducted, when water was used as extraction solvent, lower extraction yields of phenolic compounds were obtained [
64], as presented in this work. As reported by Do et al. [
65], the polarity of the solvent plays a relevant role in increasing the solubility of phenolic compounds. Particularly, ethanol is one of the solvents that increases solubility in solutes that present low polarity; for this reason, using water in different proportions of ethanol leads to the formation of a medium that achieves a better extraction [
66].
3.4. Identification of Bioactive Compounds by High Performance Liquid Chromatography (HPLC)
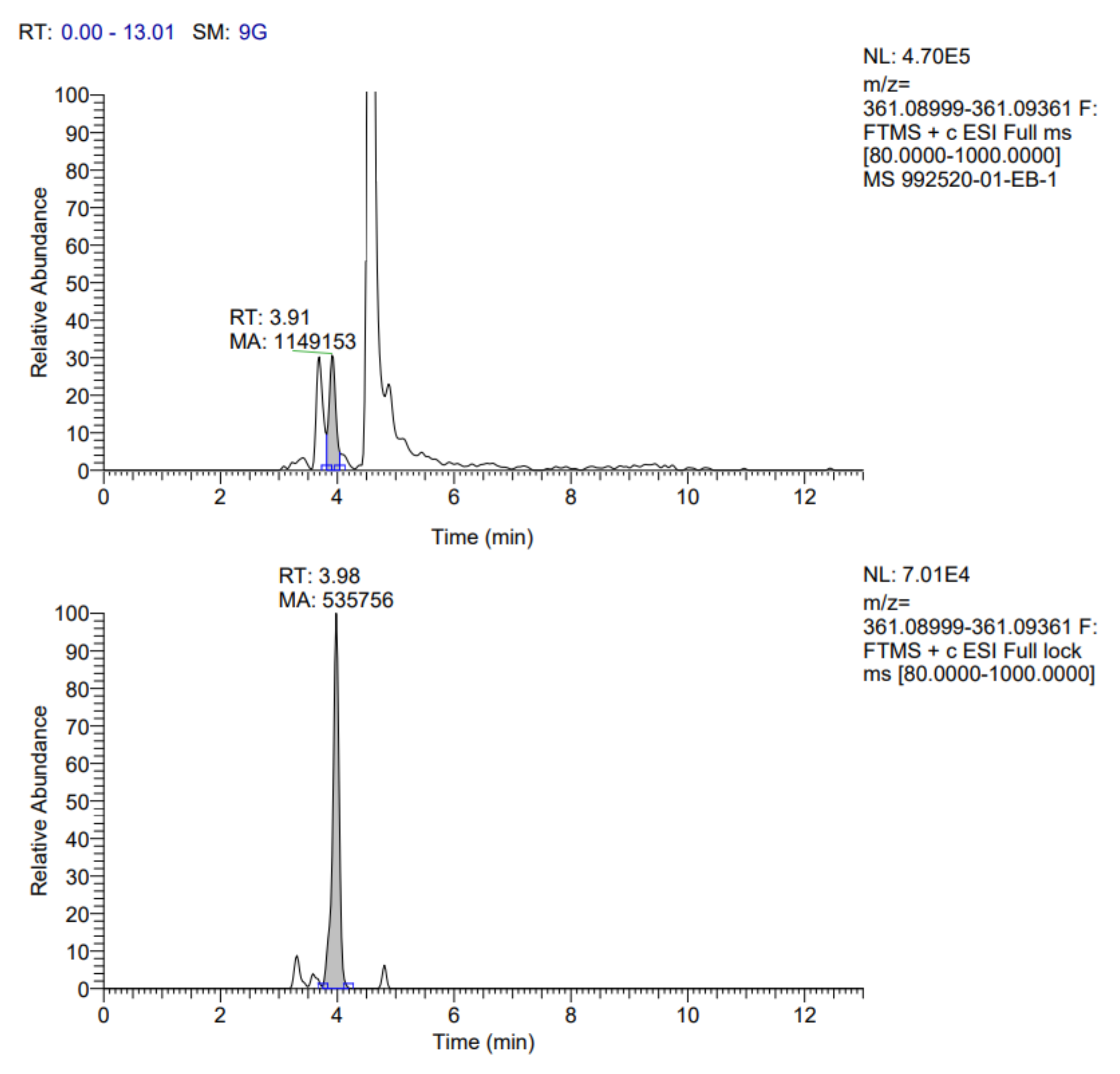
The identification profile of the polyphenolic compounds of the fermented pineapple peel subjected to the ultrasound process was obtained by UHPLC analysis, following the methodology described previously. The identified compounds in the extracts obtained by optimized conditions are presented in
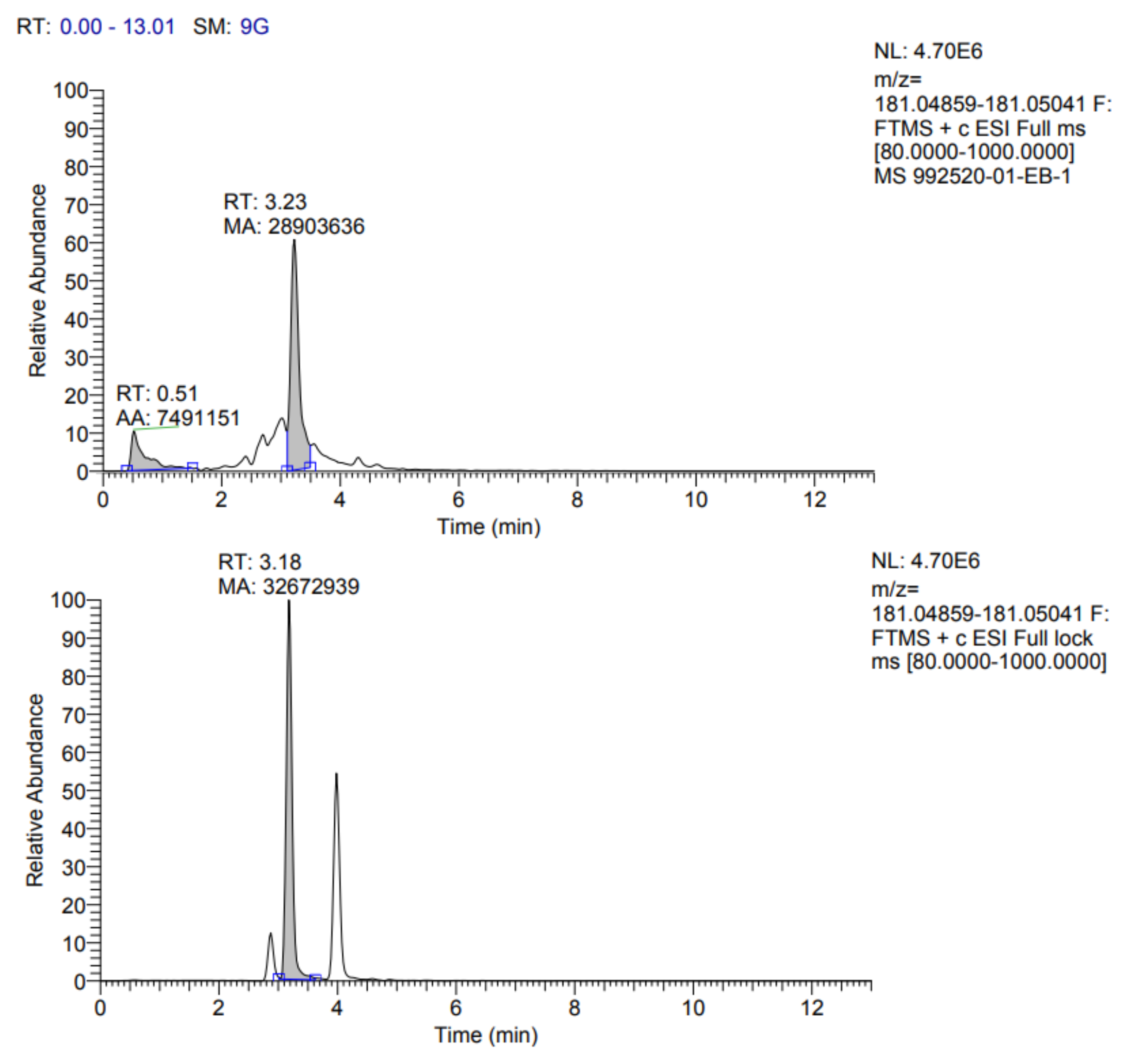
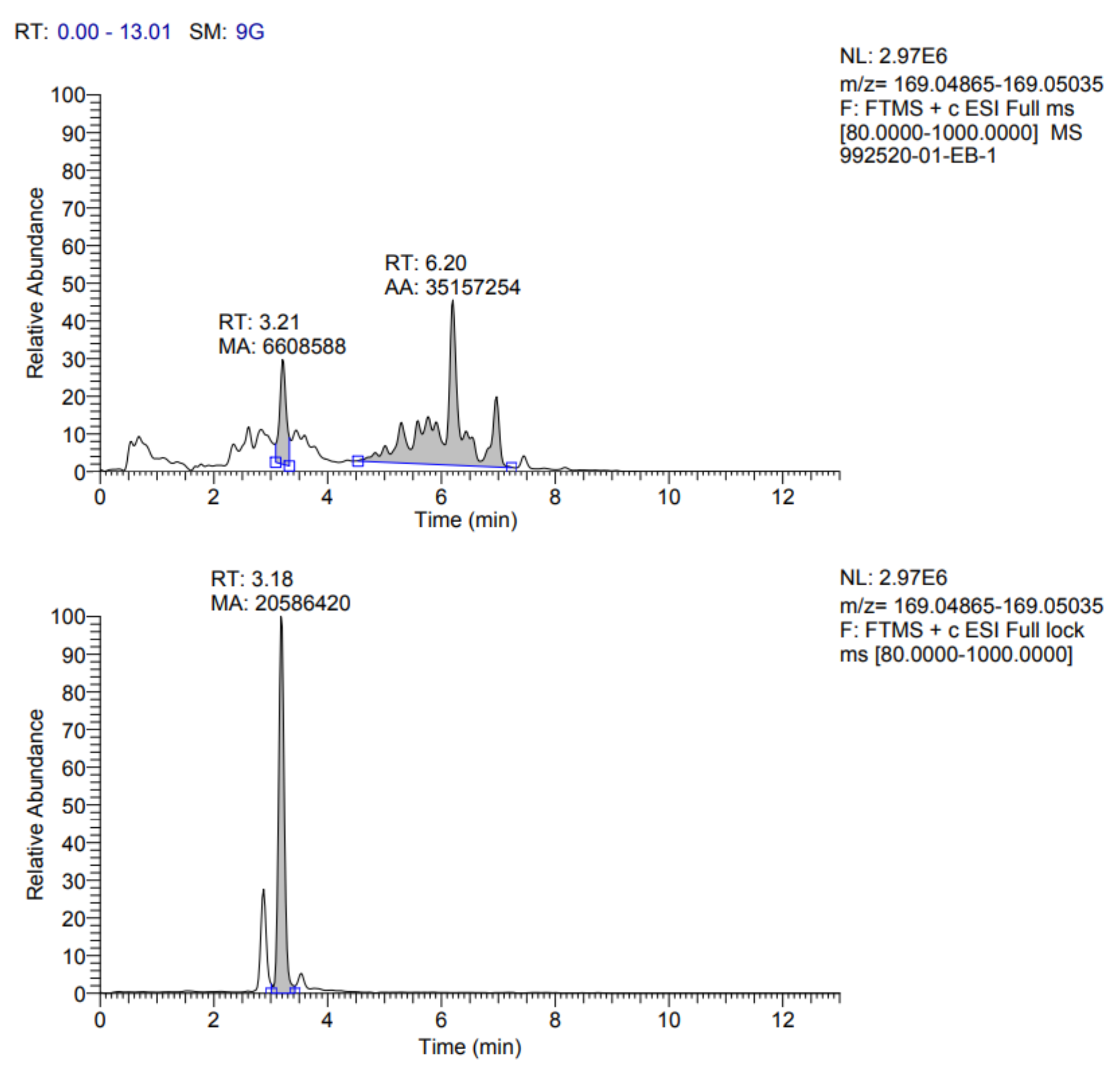
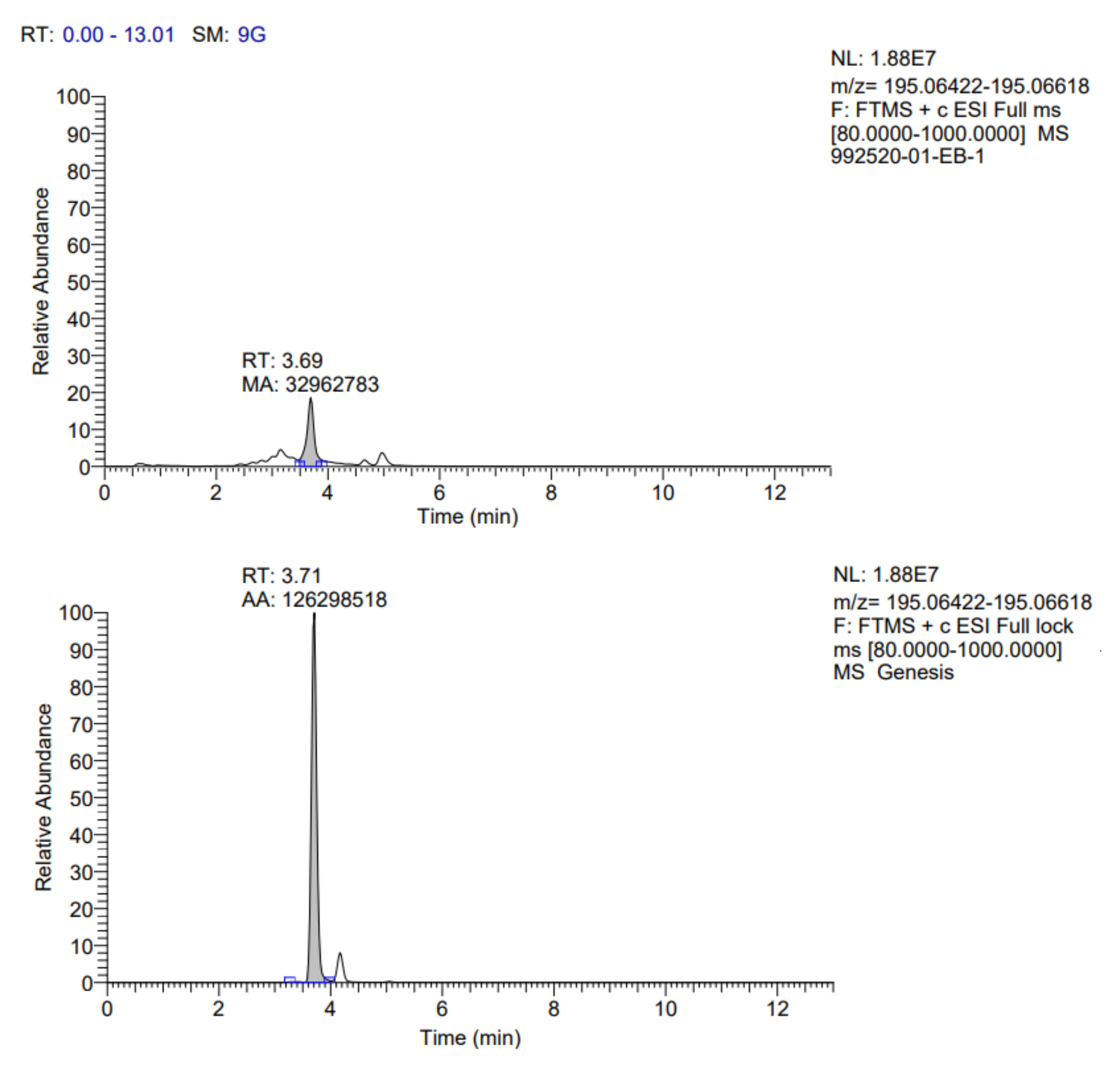
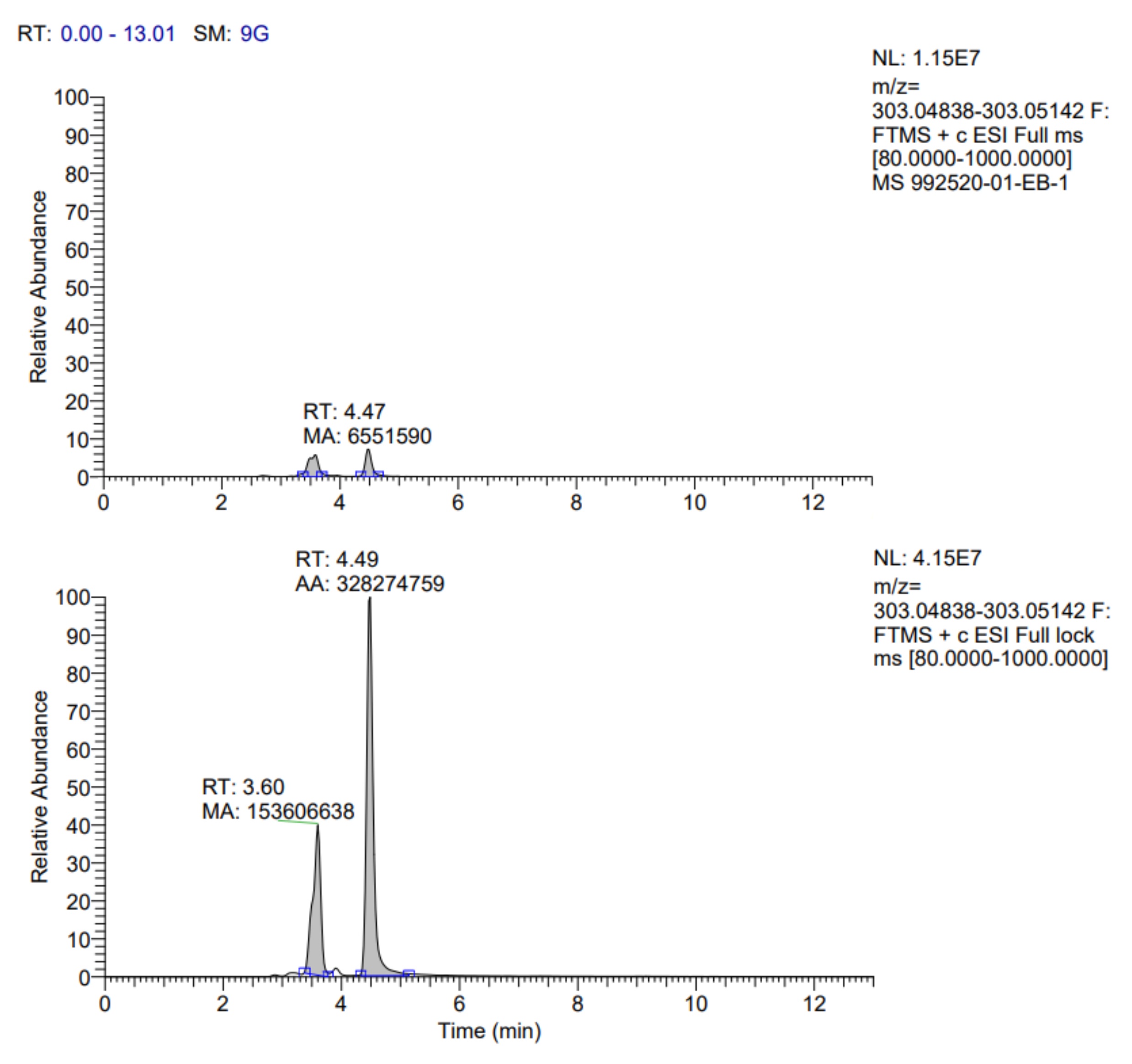
Table 7. In addition, the fragmentograms of the compounds identified and quantified in the extract can be found in
Appendix A.
Concerning the TPC, different values are often observed when applying the same processing method, mainly due to the difference in cultivars, maturity stage, sample heterogeneity, processing, as well as the extraction conditions and techniques used in each research [
67]. Phenolic compounds account for more than half of the total antioxidant capacity present in fruits, demonstrating the efficiency of these to scavenge free radicals and some reactive oxygen species [
68].
According to the results reported in
Table 7, it is possible to observe that ten compounds were quantified, including rosmarinic acid, caffeic acid, vanillic acid, coumaric acid, ferulic acid, quercetin 3-glucoside, rutin, quercetin, kaempferol 3-glucoside and gallic acid.
Rosmarinic acid (RA) was the main compound identified; this one belongs to the group of hydroxycinnamic acids and was isolated for the first time from the leaves of
Rosmarinus officinalis, although it can be found in many other plant species [
69]. RA is structurally formed from an esterification reaction between caffeic and 3,4-dihydroxyphenyl acids and is currently available in powder form. This acid has been widely investigated because it has been shown to act as an anti-aging, anti-allergic, anti-diabetic, anti-microbial, anti-depressant, anti-inflammatory agent, anti-cancer agent, and its hepatoprotective, nephroprotective, cardioprotective and neuroprotective properties have been well recognized and mainly associated with its antioxidant potential [
70,
71].
These identified compounds are similar to those reported by Li et al. [
72], who found them in pineapple peel extracts using methanol as solvent (gallic acid, catechin, epicatechin and ferulic acid), however, catechin and epicatechin were found below the limit of quantification. Yahya et al. [
39] identified in ethanolic extracts of pineapple peels obtained by ultrasound assisted extraction the following compounds: gallic acid, catechin and quercetin. Yapo et al. [
73] identified eight phenolic compounds, all belonging to the family of hydroxybenzoic and hydroxycinnamic acids, which matches with the results found. In the work of Campos et al. [
74] where they identified the compounds present in the liquid residues of pineapple peels, they reported chlorogenic acid, caffeic acid, syringaldehyde and ferulic acid, of which the only one not reported is syringaldehyde, however, these differences may be attributed to the fact that the extracts were not obtained under the same conditions. Likewise, the compounds identified are consistent with four of the five compounds quantified by Lourenço et al. [
75], who identified: gallic, chlorogenic, caffeic, p-coumaric and ferulic acid in samples of pineapple peel extracts obtained by conventional extraction with 80% ethanol.
The combined process of SSF with ultrasound allowed us to increase the bioactive compounds present in pineapple peel extracts, compared to those reported by Lubaina et al. [
76], who quantified by HPLC, ethanol extracts of the Mauritius variety pineapple peels (the most popular cultivar grown in Kerala, India), reporting 0.068 ug/mg of caffeic acid, 0.533 ug/mg of p-coumaric acid and 1.828 ug/mg of ferulic acid, while in this research 7.0 ug/mg of caffeic acid, 2.60 ug/mg of p-coumaric acid and 2.10 ug/mg of ferulic acid were obtained. These compounds are of great interest because they present beneficial properties for health; for example, caffeic acid is an antioxidant which boosts immunity and controls lipid levels in blood; p-coumaric acid is recognized for its antioxidant behavior reducing the formation of carcinogenic nitrosamines in the stomach [
77] and ferulic acid is well-known for its physiological functions such as antimicrobial, anti-inflammatory and anti-cancer activities [
76].