Abstract
Consumers’ increasing interest in sparkling wine has enhanced the global market’s demand. The pro-technological yeasts strains selected for the formulation of microbial starter cultures are a fundamental parameter for exalting the quality and safety of the final product. Nowadays, the management of the employed microbial resource is highly requested by stakeholders, because of the increasing economic importance of this oenological sector. Here, we report an overview of the production processes of sparkling wine and the main characterisation criteria to select Saccharomyces and non-Saccharomyces strains appropriate for the preparation of commercial starter cultures dedicated to the primary and, in particular, the secondary fermentation of sparkling wines. We also focused on the possible uses of selected indigenous strains to improve the unique traits of sparkling wines from particular productive areas. In summary, the sparkling wine industry will get an important advantage from the management of autochthonous microbial resources associated with vineyard/wine microbial diversity.
1. Introduction
During the alcoholic fermentation of grape must, yeasts mainly synthesise ethanol and carbon dioxide (CO2), the latter being directly discharged into the atmosphere when the process is carried out in an open container, thus producing wines usually defined as “still”. Inversely, wines containing a significant amount of carbon dioxide are defined as sparkling wines. These beverages’ intense interest and vast consumption have led research to exploit all the resources and phases to make qualitatively better sparkling wines and improve product segmentation. In particular, researchers aim to concretise both process and product innovations, reduce the time and costs of the production process, evaluate new grape varieties, test emerging technologies, and exploit selected microbial resources [1].
According to the International Code of Oenological Practices of the OIV, sparkling wines belong to the category of special wines; they are made from grapes, musts, or wines and produce a more or less persistent foam upon uncorking. The foam results from the release of carbon dioxide (CO2) contained in the bottle at a pressure of 3.5 bar at 20 °C [2]. The production undergoes two fermentation phases: (i) in the first, the must is converted into base wine by a typical alcoholic fermentation process; (ii) in the second, which is carried out in the bottle or in the autoclave, sucrose, selected yeasts, and other ingredients are added. The latter is followed by a long period of maturation in the cellar, during which yeast autolysis takes place in situ with the release of various metabolites that enrich the product’s aromatic profile [3]. This composite role contributes to underlining why starter cultures are crucial in improving the quality and safety characteristics of these fermented alcoholic beverages [4].
In detail, in 2018, sparkling wine production reached 20 thousand hectolitres for the first time, with an overall worldwide growth of 57% compared to 2002. Almost half of the total volume produced in 2018 came from Italy (27%) and France (22%) [2]. In particular, the top five producing countries represent 80% of the worldwide production of sparkling wines. France is the leader in sparkling wine exports, followed by Italy and Spain. This significant increase has been driven by the growing demand for sparkling wines produced using the Charmat-Martinotti method (in which the product is bottled at the end of the refermentation carried out in large containers) and for sparkling wines produced using the traditional Champenoise method (where refermentation is initiated in each individual bottle) [2].
However, modern wine market trends are directing sparkling wine production towards regional Italian areas and countries that are not traditional producers. This has stemmed from recent advances in agricultural research that have allowed the production of grapes for sparkling wines even in areas (such as Southern Italy) that are not used to developing these types of effervescent wines [5]. In Apulia (Southern Italy), for example, different grape varieties are employed for sparkling wine production, being international cultivars such as Pinot Noir, Chardonnay, and Pinot Meunier [6,7] or autochthonous varieties such as Maresco, Negroamaro, and Bombino Nero [8,9].
As reported in Annex VII—Part II (categories of grapevine products) of EU Regulation 1308/2013 of the European Parliament and Council [10], sparkling wines are classified into different categories (Table 1).

Table 1.
Legislative classification of sparkling wines according to EU Reg. 1308/2013, (Regulation (EU) No 1308/2013 of the European Parliament and Council).
Therefore, sparkling wine is considered an effervescent wine, because, as opposed to still wine, it has a high content of CO2. Generally, the term ‘effervescent wines’ refers to all sparkling and carbonated wines from grapes, must, or wine [2].
The category of effervescent wines also includes sparkling wines, which are halfway between a still wine, and a sparkling wine. Semi-sparkling wines and sparkling wines represent two sides of the same coin, and they are different from both a legislative point of view and their organoleptic-sensorial characteristics.
Semi-sparkling wines have modest contents of CO2 (1–2.5 bar) dissolved in the solution, and upon opening, they present bubbles but not a persistent foam [11]. A lower alcohol content also characterises them because the grapes are usually harvested at a lower degree of ripeness.
2. Sparkling Wine Production Process
After the meticulous harvesting of the grapes with the desired specific maturing stage and their pressing, the process proceeds with the sulphitation of the must (to avoid the triggering of spontaneous fermentations), clarification with pectolytic enzymes, and inoculation of S. cerevisiae starter cultures; the latter reduces the sugars present into ethanol and CO2. The grape must ferments to produce a base wine with reduced alcohol content. Sparkling wines can be produced after a refermentation (secondary fermentation) in bottles (traditional method or Champenoise method) or in an autoclave (Charmat method). For the traditional, classic, or Champenoise method in the secondary fermentation, the liqueur de tirage is added to the base wine; it is a mixture of sucrose, adjuvants, and yeasts, such as Saccharomyces cerevisiae or Saccharomyces bayanus, a hybrid between S. uvarum and S. cerevisiae [1,12] (Figure 1).
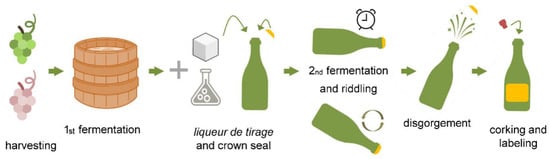
Figure 1.
An illustrative description of Champenoise sparkling wine production. Image reproduced from [13].
After further chemical-physical treatments of clarification, decanting, and filtration of the base wine, it is ready to be bottled. Afterwards, the liqueur de tirage solution is added, and it is made of 20–25 g/L of sucrose, selected strains of S. cerevisiae, grape must or wine, and bentonite. The base wine used in bottle refermentation must have specific characteristics, such as light colour, fruity aroma, low residual sugars, moderate alcohol content (10–11% v/v), low volatile acidity (acetic acid), and total acidity of 12–18 g/L tartaric acid. The wine is then bottled, and the bottles are closed with a crown cork with a plastic cylinder (bidule) underneath, which will collect the lees. Bottles are kept in special rooms equipped for the decanting of sparkling wine, in which there are minimum temperature values and scarce lighting. The refermentation in bottles is done at 12–15 °C (54–50 °F) for about 15–45 days; the fermentation process is monitored by analysing the reduction of sugar and the increase of internal pressure using an aphrometer. According to the type of sparkling wine and to the country of origin’s laws, the ageing or maturation of the product has a variable duration from 9 to 12 months. During the ageing period, sparkling wine acquires specific organoleptic-sensorial characteristics conferred by the autolytic process of yeasts, mediated by hydrolytic enzymes; the latter favour the release of polysaccharides, peptides, fatty acids, proteins, and mannoproteins into the sparkling wine. The following remuage phase (shaking of bottles) removes lees by conveying them into the bidule and, therefore, avoids the harmful effects of oxygen and biological degradation. During the remuage, the bottles are rotated daily for about 15 days until they are perpendicular to the floor. Then follows the dégorgement phase, which consists of freezing the bottle’s neck in a solution of calcium chloride and glycol; the internal pressure increases and eliminates the lees collected in the bidule. During this process, part of the liquid is lost, and for this reason, it will be added a dosing solution (liqueur d’expedition) of variable composition, giving every sparkling wine its typical organoleptic-sensorial characteristics [4,14].
The Charmat method, on the other hand, is much simpler and faster than the traditional method. This approach is made inside stainless steel and hermetically sealed autoclaves equipped with agitation mechanisms in order to mix yeasts into the base wine during froth taking uniformly. At the end of the prise de mousse phase, there is an ageing of about 20 days with the lees. Then, these are removed by filtration, and the sparkling wine is bottled in isobaric and cold conditions to avoid CO2 dispersion [4].
3. Yeast Strain for Sparkling Wine Production
Starter cultures accelerate the fermentation process, avoid stuck fermentation or the beginning of anomalous fermentations, and offer a final product with standard and constant characteristics. As for other fermented foods and beverages, it is possible to isolate and select enological yeasts by identifying strains with the best attitudes and technological characteristics to ferment effectively grape must and produce quality wines, especially enriched from the point of view of the flavour. Yeast strains with excellent technological inclinations are highly requested for the Champenoise and the Charmat methods to exalt the aromatic bouquet of the final products. In particular, for the production of traditional sparkling wines, yeasts with flocculant and autolytic capacities are highly required for the pro-technological interest and for the positive impact on the quality of sparkling wines [12].
3.1. Selection Criteria for Microbial Resources in Sparkling/Sparkling Wine
The selection of starter cultures is based on several oenological traits, which also reflect the adaptation to the unique characteristics of the grape must and sparkling wine base [4]. Alexandre and Guilloux-Benatier [15] reported that desired yeast properties for first and second fermentation are different. In any case, it is critical to identify starter cultures that can tolerate the peculiar characteristics of grape must and the harsh conditions of base wine. The fermenting grape must contain a high concentration of sugars (about 200 g/L) and sulphites, increasing the content of ethanol, glycerol, and CO2, and is characterised by a low pH (3–3.5) and a gradual depletion of nutrients. The genus Saccharomyces belongs to the phylum Ascomycota and includes seven species, including Saccharomyces cerevisiae. This species (Figure 2) is considered one of the main players during the alcoholic fermentation of musts.

Figure 2.
S. cerevisiae cells visualised using scanning electron microscopic (images on the left, reproduced from [16]). Sparkling wine bottles during the prise de mousse (image on the right, reproduced from [17]).
It adapts well to stressful must/wine environmental conditions such as low pH, high sugar concentration (osmotic stress), and progressive increase of ethanol and corresponding nutrient depletion. S. cerevisiae is a facultative anaerobic microorganism with an optimal growth temperature between 25 and 30 °C. It has a high fermentative vigour that allows it to reduce must sugars to ethanol and CO2. It is considered the most important yeast species in the winemaking/sparkling process and is used industrially as a starter culture to achieve fermentative processes of high quality. The choice of yeast to be used in the primary fermentation of grape must, which is intended to become a base wine, and also influences the sensory profile of sparkling wine [18]. S. cerevisiae strains to be used in the secondary fermentation of base wine present additional physiological and technological characteristics compared to those suggested for ‘primary’ starter cultures. The objective is to totally transform the sugars added to the base wine, by means of the liqueur de tirage, into ethanol and CO2. These starter cultures must be able to grow in a medium containing at least 10–12% v/v ethanol, a low pH (2.9–3.2), tolerate low temperatures (10–15 °C), total SO2 concentration of 50–80 mg/L, high total acidity (12–18 g/L tartaric acid), low volatile acidity (0.2–0.4 g/L), high CO2/high pressure (5–6 bars), and glycerol content of 5–20 g/L (Table 2) [19].

Table 2.
Summary of the main technological and qualitative properties of yeast strains for the production of sparkling wines.
During the selection procedure of the S. cerevisiae strain, it is also important to consider its resistance to high concentrations of acetic acid. Although the latter has no effect on glucose transport, it can acidify the cytoplasm, slow down the fermentation rate, and the yeast enolase activity. In fact, volatile acidity (expressed as g/L of acetic acid) usually varies between 0.6–0.9 g/L of acetic acid. Concentrations above 1.2–1.3 g/L can be unpleasant, and the legal limit of volatile acidity according to the European Economic Community (EEC) is approximately 1.5 g/L [27]. In addition, many researchers have emphasised in several papers the importance of flocculating [12,26] and autolytic capacities of yeasts in secondary fermentation. In general, flocculation is a peculiar characteristic of yeasts and allows for clarification of fermenting musts. This physiological character can be described as a natural aptitude of yeast strains to form compact microbial biomass that descends to the bottom of the fermentation medium [20,28] and, following froth taking, facilitates disgorgement of the deposit that accumulates in the bidule [14]. In addition, it appears that yeast flocculation is associated with improved ester production [29]. Flocculation is regulated by the expression of some genes belonging to the FLO family. The FLO5 gene has recently shown the best aptitudes to control the flocculation phenotype in a S. cerevisiae strain used in the sparkling wine production process [1]. Interspecific hybrid strains are also well suited for sparkling wine production, refermented in the bottle; these are obtained following an appropriate selection of flocculating strains of S. cerevisiae and non-flocculating strains of S. bayanus var. uvarum. Interspecific hybrids have the ability to ferment in an extensive temperature range, between 6–36 °C [21]. The autolytic capacity of the yeast is inherent to another yeast selection character: the killer phenotype; this is the release, by the killer strain, of toxic proteins [4]. Under oenological conditions, attempts are made to accelerate the autolytic process through (i) the appropriate combined selection of killer and killer-sensitive yeast strains [30] and (ii) the use of mutant yeast strains with autolytic characteristics. Mutagenesis of S. cerevisiae strains induces the accelerated release of proteins, amino acids, and polysaccharides. Sparkling wines made from selected mutant strains exhibited better foaming properties than those inoculated with non-mutant strains [31]. The autolytic capacity of yeasts is closely related to the release of volatile compounds in sparkling wine and the impact these have on the sensory profile [4,19].
Interesting research focuses on the knowledge of a specific mannoprotein synthesised by some strains of S. cerevisiae. This mannoprotein, encoded by the seripauperin PAU5 gene, directly reduces the undesirable phenomenon of gushing or churning in sparkling wines, and, therefore, possesses foam-stabilising properties. Gushing means an excessive spontaneous foam of carbonated beverages after the release of pressure when the bottle is opened, which causes severe economic losses. Arguably, this could be caused either by a contaminated raw material (e.g., due to the presence of Botrytis cinerea) or by irregularities in the production process. In particular, Frisch and co-authors [32] studied the potential gushing induced by proteins synthesised from Penicillium expansum and Pichia pastoris. However, a heat treatment (about 85 °C) could prevent protein degradation induced by fungal contamination, as fungal enzymes are not stable at high temperatures. Vogt et al. [24] demonstrated significant results about sparkling wine disgorgement following contamination of grapes by Penicillium oxalicum. PAU5 production is strain-specific and was identified analytically by testing oenological yeasts. Only a few were identified as potential high PAU5-producing strains; culturing S. cerevisiae strains under conditions different from the standard ones (higher temperatures and daylight) showed higher protein content. However, cultivating the strains under shaking conditions or in co-culture with other non-Saccharomyces species of wine interest (i.e., Metschnikowia pulcherrima or Torulaspora delbrueckii) was observed a reduction in PAU5 production compared to standard conditions [22,23,24].
Regarding the negative sensory influence of indole on sparkling wine, Dorignac and Gosselin [25] stated that it is also necessary to include this aspect among the yeast selection criteria. Following the monitoring of secondary fermentation of sparkling wine, it has been shown that (i) the ability of S. cerevisiae to produce indole is strain-specific and (ii) this trait is particularly pronounced when the viability of the culture is very low. Therefore, the authors propose selecting the strain and evaluating the indole concentration analytically. The value of CO2 released by the yeasts and dissolved in the liquid phase during the fermentation process is an additional selection parameter of fundamental importance as it enlivens the tasting of sparkling beverages or sparkling wines [33]. It is crucial to underline that, in all the trials involving secondary fermentation, the yeast must be rehydrated and acclimatised before secondary fermentation to obtain significant and different effects on the final viability of the culture.
3.2. Impact of Microbial Resources on the Sensory Quality of Sparkling Wine
The sensory properties of sparkling wine are influenced by different factors (Figure 3), such as the production method, grape variety, composition of the base wine, the yeast strain especially selected for primary and secondary fermentation, yeast autolysis after frothing, and the time of maturation of the product in contact with the lees. However, the sensory profile of sparkling wines depends more on the autolytic release of volatile compounds during the ageing phase [14]. In detail, the aroma is one of the most important indicators of sparkling wine quality belonging to the sensory aspects. The primary (pre-fermentative) aroma is derived from the grape variety, the secondary (fermentative) aroma released by yeast metabolism during the first and/or second fermentation and the tertiary (post-fermentative) aroma released by ageing during decantation [34,35].
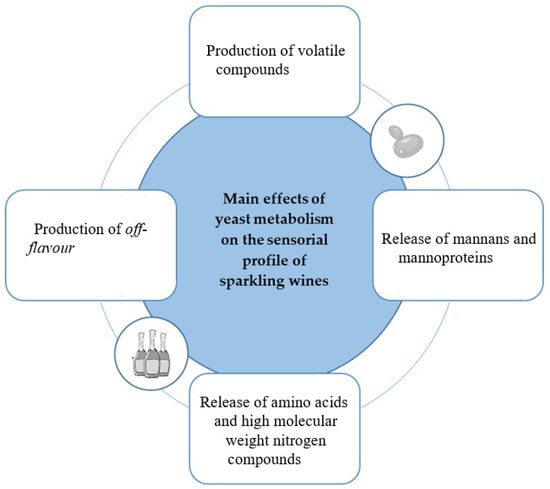
Figure 3.
Impact of yeast metabolisms on the sensory properties of sparkling wines [1,25,34,40,41].
Recently, Cotea and co-workers [36] have investigated the effects on the volatile profile of sparkling wines related to the employment of different specific commercial yeast. These authors showed a substantial influence of the strains on the development of the volatile aroma of produced wines, thus indicating that the enrichment of flavour compounds is a strain-specific character. Sensory evaluation and chemical analysis of volatile compounds by qualified experts are among the most important techniques to test the aroma profile of sparkling wines [9,37]. In effect, at the end of the second fermentation or froth taking, the long ageing in contact with yeast lees begins; a release of intracellular compounds determines an increase of free amino acids, defined as the precursors of aromatic compounds in sparkling wine. In particular, during the ageing of sparkling wine, the autolysis of S. cerevisiae takes place, and it releases a considerable number of volatile molecules: esters, higher alcohols, aldehydes, sulphur-containing compounds, carbonyl compounds, and organic acids. The release of these molecules is strain-dependent [1,14]. Autolysis is an enzymatic hydrolysis of biopolymers and is a very slow phenomenon; it is associated with yeast cell death and the release of constituents that influence the sensory properties of classic method sparkling wines. Many studies in the literature have focused on analysing the different compounds released during the autolytic process [14] and exploring additional methods to induce autolysis, facilitating the development of aged character in sparkling wine [38]. For example, enzyme preparations have been used since the 1970s/1980s to promote hydrolysis of must pectic substances, colour extraction, and flavour release. Combining killer and sensitive S. cerevisiae yeasts can also accelerate autolysis; these co-cultures of killer and sensitive yeasts positively affect the aromatic quality of sparkling wine [39]. Different scientific studies have investigated the various compositional changes that occur during the production of sparkling wines and the factors that most influence the varied sensations on the palate. Polysaccharidesderived from grapes or from the type of yeast can influence viscosity, foaming properties, and sensorial quality of sparkling wines. In reference to the microbial component, polysaccharides such as mannans and mannoproteins are released into the sparkling wine during yeast autolysis and perform functions to prevent protein haze or crystallisation of potassium bitartrate, all of which result in exceptional quality improvement of these fermented products [34].
Recently, in a research paper by Di Gianvito et al. [1], the potential aroma profiles of sparkling wines obtained with S. cerevisiae strains having a different degree of flocculation were tested; these were compared with S. cerevisiae commercial strains not flocculating but pulverulent (i.e., a yeast strain that has lost its flocculation ability). Yeast flocculation may be measured based on four criteria: bond strength, morphology, the extent of sedimentation, and rate of sedimentation [12]. In this study, the content of free amino acids (AAN), the release of high molecular weight nitrogen (HMWN), and the chemical-physical composition of sparkling wines were evaluated [1]. From the results obtained, it is possible to extrapolate some similarities in oenological performance between flocculating and pulverulent yeasts in terms of AAN and HMWN. Although flocculation capacity is one of the criteria for selecting starter cultures, the commercial pulverulent strain released high amounts of AAN and HMWN; this behaviour was later found in some flocculent strains. Proteins and AANs positively influenced the foam properties and characteristics in terms of volume and stability. High ester contents have been highlighted, which can be attributed to the strong autolytic capacity of some flocculant strains. Generally, ethyl esters contribute (with more than 70% to the sum of the aromatic series) to the sensory profile of sparkling wines with herbaceous, sweet, and rose scents; floral, balsamic, spicy, creamy, caramel, and toasted scents can also be included among these descriptors [40]. These aromatic descriptors were found in Spanish sparkling wines obtained by employing immobilisation systems (alginate spheres or biocapsules) of different S. cerevisiae strains. In fact, using immobilised yeasts, the aromatic quality could be improved compared to those sparkling wines made with free cells of S. cerevisiae. Lòpez de Lerma et al. [40] highlighted, in aged sparkling wines made with immobilised yeast, in addition to ethyl esters, other macro-categories of volatile compounds, such as acetates, alcohols, lactones, carbonyl compounds, and C-13 norisoprenoids. A different aromatic descriptor was identified for each of these. For example, alcohols, carbonyl compounds and organic acids released rather negative hints of burnt, plastic, or rancid; instead, octanal, nonanal, decanal, and some terpenes enriched sparkling wines with a citrus aroma. High chemical, toasted, floral, herbaceous, and fatty notes were shown in sparkling wine produced with S. cerevisiae strain bioimmobilised with filamentous fungi (Penicillium chrysogenum). However, most of the immobilised S. cerevisiae strains, with either calcium alginate systems or bioimmobilised, showed similar characteristics. Relevant were the values of the fruity series in all sparkling wines, while the floral resulted in a lower impact. Therefore, both yeast strain and immobilisation mode can impact on the sensorial quality of sparkling wines. The only shortcoming of immobilisation in alginate is that this system releases a higher concentration of calcium ions that could produce insoluble tartaric salts, thus reducing foam stability and altering organoleptic quality [40]. Conversely, recent research confirmed that biocapsules in sparkling winemaking do not negatively affect aromatic quality [37].
The presence of indole and other volatile compounds negatively affects the sensory profile of these products by releasing the off-flavour of ‘plastic’ [40]. This is a by-product of tryptophan digestion, it being an essential amino acid whose synthesis is strain-specific. Both Saccharomyces and non-Saccharomyces can produce high indole concentrations, mainly during primary fermentation. Although there are few studies in the literature about indole accumulation during secondary fermentation, this off-flavour is nevertheless common in sparkling wine [25,41].
3.3. Autochthonous Saccharomyces Starter Strains
The extensive use of starter cultures in the wine industry has been an important innovation in the last century. It is possible to reduce fermentation arrests and accelerate/guide the fermentation process [42,43,44]. This routine practice can lead to more standardised products with balanced flavours and improve the safety of the production process; however, it could cause an excessive uniformity of the characteristic flavour profile and taste determinants [45,46]. In order to improve the beneficial contribution of yeast, diversify the product, and satisfy the consumer’s different needs, researchers and winemakers have aroused strong interest in the selection and characterisation of indigenous yeasts to be used as starters in the fermentation processes of winemaking or sparkling wine. Therefore, since the 2000s, the importance of exploiting the oenological potential of indigenous yeast species present on the grape in the must or the wine has been emphasised. In fact, indigenous yeasts are isolated from a given region’s micro-biodiversity and can contribute to the pursuit of stylistic peculiarities [47], i.e., they are considered among the emerging protagonists of regionalisation trends [48].
There are numerous studies in the literature about the selection of indigenous microflora, particularly yeasts, to be used in grape must fermentation. On the contrary, few studies have been identified that have deepened the study of autochthonous yeasts in sparkling winemaking. In this case, the selection of indigenous yeasts is much more complex because the base wine, in which the specific strain of S. cerevisiae is inoculated, is considered a hostile environment for the growth of microorganisms because of its high ethanol content and the low pH [45]. For application purposes, autochthonous strains of S. cerevisiae are selected based on several technological criteria (fermentative power and vigour, SO2 and ethanol tolerance, and flocculating capacity) and qualitative characteristics (the content of acetic acid, glycerol, and H2S production) [18,49]. An interesting property highlighted for indigenous S. cerevisiae strains used in sparkling wine production is the ability to modulate the phenolic components of the final product [45]. The selection of autochthonous S. cerevisiae starter strains for sparkling wine production has been conducted in different Italian regions (Table 3).

Table 3.
Selection of autochthonous S. cerevisiae starter strains for sparkling wine production in Italy.
In particular, in some cases, the goal was to appropriately select the S. cerevisiae strain and ensure its ability to enhance the varietal properties of the grape; this is a crucial point for the production of a quality sparkling wine with a sensory profile that reflects the typicality of the grape variety [45].
Generally, the performance of natives is compared to that of commercial strains of S. cerevisiae, such as the active dry yeast DV10. In a recent study by Tufariello et al. [51], the sensory profile of sparkling wines obtained with autochthonous strains of S. cerevisiae (isolated in Salento, Apulia, Italy) was compared with those obtained with DV10. In particular, DV10 released high concentrations of gluconic acid, which negatively influenced sparkling wine’s foaming properties, while this was not found in sparkling wines obtained from autochthonous S. cerevisiae strains. In addition, the use of selected indigenous strains also led to high contents of volatile compounds (release of rose hints and fruity notes), while low volatile acidity values, high glycerol content, and an interesting phenolic profile were also identified [45]. Other previous studies, in addition to monitoring the analytical contribution in terms of volatile compounds, selected indigenous strains of S. cerevisiae by including genotypic and technological screening [18,49]; this was always discriminated from the performance of commercial strains of S. cerevisiae. In fact, values of low fermentative vigour, low flocculating capacity, and reduced volatile acidity of indigenous strains, isolated both in Apulia and in the Lombardy area (Italy), were identified compared to commercial ones. In particular, S. cerevisiae indigenous strains isolated from Apulian grape berries were tested for tolerance to different stresses (effect of pH, different concentrations of ethanol, and total SO2). By monitoring fermentation at 6 °C and 12 °C, most of the autochthonous strains produced very low CO2 values and low autolytic and flocculant capacities, killer activity, resistance to pH 3.5, and tolerance to ethanol concentrations between 6% and 12% v/v and to different SO2 concentrations (100, 150, and 200 mg/L). Moreover, low volatile acidity and pressure values between 5–6 bar were highlighted. The largest number of native S. cerevisiae strains reported high glycerol concentrations and were identified as low H2S hydrogen sulphide producers. In contrast, in the study by Vigentini et al. [49], native strains isolated Oltrepò Pavese released low glycerol concentrations and high H2S productions. However, increased resistance to 12% v/v ethanol, reduced ability to tolerate high concentrations (300 mg/L) of SO2, and low volatile acidity were identified. Native starter cultures were also recently selected for the champenoise method, having a higher resistance to low pH values and the highest ethanol concentration [18], low acetic acid, H2S, and higher glycerol content [49]. Recently, Alfonzo and co-workers selected indigenous strains of S. cerevisiae for the secondary fermentation of Grillo base wine [50]. The four strains showed good fermentation strength, resistance to sulphur dioxide, ability to referment wine at high total acidity and very low pH, and absence of the production of undesired off-flavours. These investigations revealed a high level of genomic diversity within the S. cerevisiae species and demonstrated the possibility of recovering native strains in an environment that presents technological and qualitative characteristics adequate to the traditional method; native strains obtain a rating comparable to that of conventional starter cultures and, in some cases, even higher.
In reference to carbonated sparkling wine, which falls into the same category as sparkling wines, no studies were found regarding the criteria for selecting starter cultures to be used in the refermentation process or concerning the impact that commercial or native yeast might have on the sensory profile. As a whole, the above findings justify the hypothesis that selected native S. cerevisiae starter strains are able to differentiate regional sparkling wines and link them with their own area of production. Only Culbert et al. [34] identified significantly higher concentrations of organic acids and ethyl esters in sparkling wines compared to sparkling wines made using the traditional method.
Saccharomyces non-cerevisiae strains have also been suggested by Bozdoğan and co-workers [52,53] as another possibility for exalting the enological properties and the sensorial complexity of sparkling wines. The authors employed S. bayanus and S. oviformis Osterwalder (currently assimilated to the species S. bayanus) in free and immobilised form, the latter after immobilisation in alginate beads, to promote the secondary fermentation of Emir and Drimit base wines. Significant differences in free amino acids and amino acids content were detected as a consequence of the ageing time, and the yeast strains used [52,53].
3.4. Role of Non-Saccharomyces in Sparkling Wine Production
Although many yeast starter strains for sparkling wine production are commercially available nowadays, together with the attention to the selection of indigenous S. cerevisiae, there is a growing interest in non-Saccharomyces yeasts able to exalt the sensory properties of the final products [13]. However, the beneficial effects of non-Saccharomyces (e.g., Torulaspora delbrueckii, Pichia kluyveri, Lachancea thermotolerans, and Metschnikowia pulcherrima) on still wines have been widely discussed in the literature; there is little scientific evidence regarding their impact in the production of sparkling wines (Table 4).

Table 4.
Properties of the selection of non-Saccharomyces starter strains for the secondary fermentation of base wine.
Gonzalez-Royo and collaborators [54] have assayed two strains of T. delbrueckii and M. pulcherrima to produce Macabeo base wine in sequential inoculations with S. cerevisiae. The authors indicated that the sparkling wine obtained by using M. pulcherrima-enhanced its foam persistence and aromatic profile by increasing smoky and flowery notes. The employment of T. delbrueckii was also shown to lower volatile acidity, raise glycerol concentration, and improve the wine foaming properties, thanks to the autolysis of the non-Saccharomyces species cells in the base wine [55]. The differences highlighted from a sensorial point of view have directed researchers to investigate the use of these yeasts in the traditional method of sparkling wines. The possibility of employing Saccharomycodes ludwigii and Schizosaccharomyces pombe in the sparkling wine process was interesting and positive. In fact, they can modify the characteristics of colour, acidity, volatile compounds, and biogenic amines of the final product [56]. By performing, instead, a sequential inoculation of T. delbrueckii and S. cerevisiae, products with high protein content and improved foaming properties can be obtained. This was reported in a Spanish paper aimed at finding out whether sparkling wines obtained with a sequential inoculum could present better properties than those produced with a conventional inoculum. The results confirmed that sequential inoculation has a significant impact on the sensorial profile of sparkling wines. In particular, these microbial resources result in higher foam heights than conventional inoculum, as T. delbrueckii may have released a high protein content [55] and high amounts of ethylpropanoate, isobutyric, and butanoic acids, alcohols, and phenols [57].
It is also possible to employ a single inoculum of T. delbrueckii in order to enhance the aromatic complexity of sparkling wines, as confirmed by a 2018 paper in which the sole use of T. delbrueckii resulted in high ester production and the best score for aromatic descriptors [58]. On the contrary, Velázquez et al. [57] advised against single inoculation of T. delbrueckii under strict conditions such as those of froth taking (high pressure and high alcohol content). These yeasts do not complete the secondary fermentation of sparkling wine and produce rather sweet products with low CO2 production and, therefore, low pressure. The organoleptic quality of T. delbrueckii base wines has been judged to be unsuitable for sparkling winemaking. In addition to co-inoculation, sequential inoculation, and conventional inoculation, there was some improvement in the aromatic characteristics of sparkling wines by employing interspecific hybrids [21].
Therefore, further research should be done in order to confirm the various contradictory results reported in the literature; it is necessary to further investigate the use of non-Saccharomyces in the production process of sparkling/sparkling wines.
It is important to underline that some non-Saccharomyces can have a pronounced detrimental effect on sparkling productions. Zygosaccharomyces species, for example, are considered cellar contaminants, as they produce high quantities of acetic acid and represent a problem, especially for sweet and sparkling wines [59].
4. Lactic Acid Bacteria
If yeasts are the absolute protagonists of the fermentation processes of sparkling wines, the role of lactic acid bacteria is often neglected and little elucidated. Lactic acid bacteria promote malolactic fermentation (MLF), which usually occurs after the completion of the alcoholic fermentation. MLF has relevant effects on wine’s chemical composition and organoleptic properties [60]. One of the benefits of inducing MLF in base wines consists of potential bioprotection effects against harmful or spoilage microorganisms [42], also contributing to microbiological stabilisation by lowering the nutrients in base wine [61]. Moreover, the early induction of MLF in base wines can be particularly effective in sparkling wines produced with the classical method, since it can prevent a late and undesired MLF with consequent formation of hazing [62]. The role of lactic acid bacteria on other aspects related to sensory properties and foamability is debated and represents one of the field’s future perspectives.
5. Conclusions
The sensorial and chemical quality of sparkling wine can be improved by adopting the management of appropriate starter cultures that could also permit the enhancement of both production efficiency and product safety. Indeed, new autochthonous S. cerevisiae strains and/or non-S. cerevisiae and/or non-Saccharomyces yeast starters can play an important role as novel tools for innovating the sparkling wine productive chain. Significant progress will be made by assessing the advantage associated with the industrial exploitation of microbial biodiversity derived from the vineyard/wine environments. Moreover, the application of autochthonous yeast starters has already been demonstrated to promote process innovation with evident market opportunities for wineries [63]. However, further studies based on applying the “omics” approach are still needed to develop innovative procedures for utilising the huge prospects associated with natural microbial biodiversity. Indeed, these challenges will be met by adopting the above genetic approaches to characterise novel microbial consortia, together with the improvement of adaptive evolution strategies, such as directed evolution. The generation of innovative starter strains will supply new tools to the wine industry, and it will light the way for the production of the future sparkling wines.
Author Contributions
Conceptualisation, F.G., M.T., C.B., M.F., N.D.S., G.S., P.R., P.V., F.B. and V.C.; writing—original draft preparation, F.G. and V.C.; writing—review and editing, F.G., M.T., C.B., M.F., N.D.S., G.S., P.R., P.V., F.B. and V.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was partially supported by the Apulia Region projects: “Innovazione nella tradizione: tecnologie innovative per esaltare le qualità dei vini autoctoni spumante della murgia barese—INVISPUBA”and “Spumantizzazione e frizzantatura per il rilancio della vitivinicoltura dell’areale Centro Nord della regione Puglia—SPUMAPULIA” (P:S:R. Puglia 2014/2020-Misura 16.2). We would like to thank Domenico Genchi, Massimo Franchi, Leone D’Amico, and Vittorio Falco of the Institute of Sciences of Food Production—CNR for their skilled technical support provided during the realisation of this work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Di Gianvito, P.; Arfelli, G.; Suzzi, G.; Tofalo, R. 11—New Trends in Sparkling Wine Production: Yeast Rational Selection. In Alcoholic Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 347–386. ISBN 978-0-12-815269-0. [Google Scholar]
- OIV Focus—The Global Sparkling Wine Market. Available online: https://www.oiv.int/public/medias/7291/oiv-sparkling-focus-2020.pdf (accessed on 17 May 2022).
- Cebollero, E.; Gonzalez, R. Induction of Autophagy by Second-Fermentation Yeasts during Elaboration of Sparkling Wines. Appl. Environ. Microbiol. 2006, 72, 4121–4127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garofalo, C.; Arena, M.P.; Laddomada, B.; Cappello, M.S.; Bleve, G.; Grieco, F.; Beneduce, L.; Berbegal, C.; Spano, G.; Capozzi, V. Starter Cultures for Sparkling Wine. Fermentation 2016, 2, 21. [Google Scholar] [CrossRef]
- 3 2/2010 Enometrica. Available online: http://eum.unimc.it/it/enometrica/242-3-22010-enometrica (accessed on 3 May 2022).
- Buxaderas, S.; López-Tamames, E. Chapter 1—Sparkling Wines: Features and Trends from Tradition. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 1–45. [Google Scholar]
- Sardaro, R.; Bozzo, F.; Petrillo, F.; Fucilli, V. Measuring the Financial Sustainability of Vine Landraces for Better Conservation Programmes of Mediterranean Agro-Biodiversity. Land Use Policy 2017, 68, 160–167. [Google Scholar] [CrossRef]
- Fanelli, V.; Volpicella, M.; Giampetruzzi, A.; Saldarelli, P.; Leoni, C.; Ceci, L.R.; Di Rienzo, V.; Venerito, P.; Taranto, F.; Giannini, P.; et al. Valorization of Autochthonous Apulian Grapevine Cultivars for Spumante Production. Acta Hortic. 2019, 1248, 457–462. [Google Scholar] [CrossRef]
- Tufariello, M.; Pati, S.; D’Amico, L.; Bleve, G.; Losito, I.; Grieco, F. Quantitative Issues Related to the Headspace-SPME-GC/MS Analysis of Volatile Compounds in Wines: The Case of Maresco Sparkling Wine. LWT-Food Sci. Technol. 2019, 108, 268–276. [Google Scholar] [CrossRef]
- The Common Organisation of Agricltural Markets in the EU. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=LEGISSUM:0302_1 (accessed on 28 April 2022).
- Carrascosa, A.V.; Martinez-Rodriguez, A.; Cebollero, E.; González, R. Chapter 2—Saccharomyces Yeasts II: Secondary Fermentation. In Molecular Wine Microbiology; Carrascosa, A.V., Muñoz, R., González, R., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 33–49. ISBN 978-0-12-375021-1. [Google Scholar]
- Perpetuini, G.; Di Gianvito, P.; Arfelli, G.; Schirone, M.; Corsetti, A.; Tofalo, R.; Suzzi, G. Biodiversity of Autolytic Ability in Flocculent Saccharomyces Cerevisiae Strains Suitable for Traditional Sparkling Wine Fermentation. Yeast 2016, 33, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Raymond Eder, M.L.; Rosa, A.L. Non-Conventional Grape Varieties and Yeast Starters for First and Second Fermentation in Sparkling Wine Production Using the Traditional Method. Fermentation 2021, 7, 321. [Google Scholar] [CrossRef]
- Torresi, S.; Frangipane, M.T.; Anelli, G. Biotechnologies in Sparkling Wine Production. Interesting Approaches for Quality Improvement: A Review. Food Chem. 2011, 129, 1232–1241. [Google Scholar] [CrossRef]
- Alexandre, H.; Guilloux-Benatier, M. Yeast Autolysis in Sparkling Wine—A Review. Aust. J. Grape Wine Res. 2006, 12, 119–127. [Google Scholar] [CrossRef]
- Alderees, F.; Mereddy, R.; Were, S.; Netzel, M.E.; Sultanbawa, Y. Anti-Yeast Synergistic Effects and Mode of Action of Australian Native Plant Essential Oils. Appl. Sci. 2021, 11, 10670. [Google Scholar] [CrossRef]
- Cilindre, C.; Henrion, C.; Coquard, L.; Poty, B.; Barbier, J.-E.; Robillard, B.; Liger-Belair, G. Does the Temperature of the Prise de Mousse Affect the Effervescence and the Foam of Sparkling Wines? Molecules 2021, 26, 4434. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of Indigenous Yeast Strains for the Production of Sparkling Wines from Native Apulian Grape Varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Borrull, A.; Poblet, M.; Rozès, N. New Insights into the Capacity of Commercial Wine Yeasts to Grow on Sparkling Wine Media. Factor Screening for Improving Wine Yeast Selection. Food Microbiol. 2015, 48, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Kemp, B.; Alexandre, H.; Robillard, B.; Marchal, R. Effect of Production Phase on Bottle-Fermented Sparkling Wine Quality. J. Agric. Food Chem. 2015, 63, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Coloretti, F.; Zambonelli, C.; Tini, V. Characterization of Flocculent Saccharomyces Interspecific Hybrids for the Production of Sparkling Wines. Food Microbiol. 2006, 23, 672–676. [Google Scholar] [CrossRef]
- Mann, M.A.; Frisch, L.M.; Vogel, R.F.; Niessen, L. Influence of Fermentation Conditions on the Secretion of Seripauperin 5 (PAU5) by Industrial Sparkling Wine Strains of Saccharomyces Cerevisiae. Food Res. Int. 2021, 139, 109912. [Google Scholar] [CrossRef]
- Kupfer, V.M.; Vogt, E.I.; Siebert, A.K.; Meyer, M.L.; Vogel, R.F.; Niessen, L. Foam-Stabilizing Properties of the Yeast Protein PAU5 and Evaluation of Factors That Can Influence Its Concentration in Must and Wine. Food Res. Int. 2017, 102, 111–118. [Google Scholar] [CrossRef]
- Vogt, E.I.; Kupfer, V.M.; Vogel, R.F.; Niessen, L. Evidence of Gushing Induction by Penicillium Oxalicum Proteins. J. Appl. Microbiol. 2017, 122, 708–718. [Google Scholar] [CrossRef]
- Dorignac, E.; Gosselin, Y. Important Considerations for Yeast Preparation and Indole Production in Sparkling Winemaking. Aust. N. Z. Grapegrow. Winemak. 2020, 674, 65–67. [Google Scholar] [CrossRef]
- Alfonzo, A.; Francesca, N.; Mercurio, V.; Prestianni, R.; Settanni, L.; Spanò, G.; Naselli, V.; Moschetti, G. Use of Grape Racemes from Grillo Cultivar to Increase the Acidity Level of Sparkling Base Wines Produced with Different Saccharomyces Cerevisiae Strains. Yeast 2020, 37, 475–486. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of Mixed Torulaspora Delbrueckii-Saccharomyces Cerevisiae Culture on High-Sugar Fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Tofalo, R.; Perpetuini, G.; Di Gianvito, P.; Schirone, M.; Corsetti, A.; Suzzi, G. Genetic Diversity of FLO1 and FLO5 Genes in Wine Flocculent Saccharomyces Cerevisiae Strains. Int. J. Food Microbiol. 2014, 191, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Soares, E.V. Flocculation in Saccharomyces Cerevisiae: A Review. J. Appl. Microbiol. 2011, 110, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todd, B.E.N.; Fleet, G.H.; Henschke, P.A. Promotion of Autolysis Through the Interaction of Killer and Sensitive Yeasts: Potential Application in Sparkling Wine Production. Am. J. Enol. Vitic. 2000, 51, 65–72. [Google Scholar]
- Nunez, Y.P.; Carrascosa, A.V.; González, R.; Polo, M.C.; Martínez-Rodríguez, A.J. Effect of Accelerated Autolysis of Yeast on the Composition and Foaming Properties of Sparkling Wines Elaborated by a Champenoise Method. J. Agric. Food Chem. 2005, 53, 7232–7237. [Google Scholar] [CrossRef]
- Frisch, L.M.; Mann, M.A.; Marek, D.N.; Baudrexl, M.; Vogel, R.F.; Niessen, L. Studies on the Gushing Potential of Penicillium Expansum. Food Res. Int. 2021, 139, 109915. [Google Scholar] [CrossRef]
- Liger-Belair, G. Effervescence in Champagne and Sparkling Wines: From Grape Harvest to Bubble Rise. Eur. Phys. J. Spec. Top. 2017, 226, 3–116. [Google Scholar] [CrossRef]
- Culbert, J.A.; McRae, J.M.; Condé, B.C.; Schmidtke, L.M.; Nicholson, E.L.; Smith, P.A.; Howell, K.S.; Boss, P.K.; Wilkinson, K.L. Influence of Production Method on the Chemical Composition, Foaming Properties, and Quality of Australian Carbonated and Sparkling White Wines. J. Agric. Food Chem. 2017, 65, 1378–1386. [Google Scholar] [CrossRef]
- Di Gianvito, P.; Tesnière, C.; Suzzi, G.; Blondin, B.; Tofalo, R. FLO5 Gene Controls Flocculation Phenotype and Adhesive Properties in a Saccharomyces Cerevisiae Sparkling Wine Strain. Sci. Rep. 2017, 7, 10786. [Google Scholar] [CrossRef]
- Cotea, V.V.; Focea, M.C.; Luchian, C.E.; Colibaba, L.C.; Scutarașu, E.C.; Marius, N.; Zamfir, C.I.; Popîrdă, A. Influence of Different Commercial Yeasts on Volatile Fraction of Sparkling Wines. Foods 2021, 10, 247. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an Electronic Nose and Volatilome Analysis to Differentiate Sparkling Wines Obtained under Different Conditions of Temperature, Ageing Time and Yeast Formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef] [PubMed]
- Gnoinski, G.B.; Schmidt, S.A.; Close, D.C.; Goemann, K.; Pinfold, T.L.; Kerslake, F.L. Novel Methods to Manipulate Autolysis in Sparkling Wine: Effects on Yeast. Molecules 2021, 26, E387. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, S.J.; De Leonardis, A.; Lustrato, G.; Testa, B.; Iorizzo, M. Yeast Autolysis in Sparkling Wine Aging: Use of Killer and Sensitive Saccharomyces Cerevisiae Strains in Co-Culture. Recent Pat. Biotechnol. 2015, 9, 223–230. [Google Scholar] [CrossRef] [PubMed]
- López de Lerma, N.; Peinado, R.A.; Puig-Pujol, A.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Influence of Two Yeast Strains in Free, Bioimmobilized or Immobilized with Alginate Forms on the Aromatic Profile of Long Aged Sparkling Wines. Food Chem. 2018, 250, 22–29. [Google Scholar] [CrossRef] [Green Version]
- Arevalo-Villena, M.; Bartowsky, E.J.; Capone, D.; Sefton, M.A. Production of Indole by Wine-Associated Microorganisms under Oenological Conditions. Food Microbiol. 2010, 27, 685–690. [Google Scholar] [CrossRef]
- Berbegal, C.; Spano, G.; Fragasso, M.; Grieco, F.; Russo, P.; Capozzi, V. Starter Cultures as Biocontrol Strategy to Prevent Brettanomyces Bruxellensis Proliferation in Wine. Appl. Microbiol. Biotechnol. 2018, 102, 569–576. [Google Scholar] [CrossRef] [Green Version]
- Berbegal, C.; Spano, G.; Tristezza, M.; Grieco, F.; Capozzi, V. Microbial Resources and Innovation in the Wine Production Sector. S. Afr. J. Enol. Vitic. 2017, 38, 156–166. [Google Scholar] [CrossRef]
- Berbegal, C.; Fragasso, M.; Russo, P.; Bimbo, F.; Grieco, F.; Spano, G.; Capozzi, V. Climate Changes and Food Quality: The Potential of Microbial Activities as Mitigating Strategies in the Wine Sector. Fermentation 2019, 5, 85. [Google Scholar] [CrossRef] [Green Version]
- Tufariello, M.; Rizzuti, A.; Palombi, L.; Ragone, R.; Capozzi, V.; Gallo, V.; Mastrorilli, P.; Grieco, F. Non-Targeted Metabolomic Approach as a Tool to Evaluate the Chemical Profile of Sparkling Wines Fermented with Autochthonous Yeast Strains. Food Control 2021, 126, 108099. [Google Scholar] [CrossRef]
- Grieco, F.; Tristezza, M.; Vetrano, C.; Bleve, G.; Panico, E.; Grieco, F.; Mita, G.; Logrieco, A. Exploitation of Autochthonous Micro-Organism Potential to Enhance the Quality of Apulian Wines. Ann. Microbiol. 2011, 61, 67–73. [Google Scholar] [CrossRef]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef] [Green Version]
- Lappa, I.K.; Kachrimanidou, V.; Pateraki, C.; Koulougliotis, D.; Eriotou, E.; Kopsahelis, N. Indigenous Yeasts: Emerging Trends and Challenges in Winemaking. Curr. Opin. Food Sci. 2020, 32, 133–143. [Google Scholar] [CrossRef]
- Vigentini, I.; Barrera Cardenas, S.; Valdetara, F.; Faccincani, M.; Panont, C.A.; Picozzi, C.; Foschino, R. Use of Native Yeast Strains for In-Bottle Fermentation to Face the Uniformity in Sparkling Wine Production. Front. Microbiol. 2017, 8, 1225. [Google Scholar] [CrossRef] [PubMed]
- Alfonzo, A.; Francesca, N.; Matraxia, M.; Craparo, V.; Naselli, V.; Mercurio, V.; Moschetti, G. Diversity of Saccharomyces Cerevisiae Strains Associated to Racemes of Grillo Grape Variety. FEMS Microbiol. Lett. 2020, 367, fnaa079. [Google Scholar] [CrossRef] [PubMed]
- Tufariello, M.; Chiriatti, M.A.; Grieco, F.; Perrotta, C.; Capone, S.; Rampino, P.; Tristezza, M.; Mita, G.; Grieco, F. Influence of Autochthonous Saccharomyces Cerevisiae Strains on Volatile Profile of Negroamaro Wines. LWT-Food Sci. Technol. 2014, 58, 35–48. [Google Scholar] [CrossRef]
- Bozdogan, A.; Canbas, A. Influence of Yeast Strain, Immobilisation and Ageing Time on the Changes of Free Amino Acids and Amino Acids in Peptides in Bottle-Fermented Sparkling Wines Obtained from Vitis Vinifera Cv. Emir. Int. J. Food Sci. Technol. 2011, 46, 1113–1121. [Google Scholar] [CrossRef]
- Bozdogan, A.; Canbas, A. The Effect of Yeast Strain, Immobilisation, and Ageing Time on the Amount of Free Amino Acids and Amino Acids in Peptides of Sparkling Wines Obtained from Cv. Dimrit Grapes. S. Afr. J. Enol. Vitic. 2012, 33, 257–263. [Google Scholar] [CrossRef]
- González-Royo, E.; Pascual, O.; Kontoudakis, N.; Esteruelas, M.; Esteve-Zarzoso, B.; Mas, A.; Canals, J.M.; Zamora, F. Oenological Consequences of Sequential Inoculation with Non-Saccharomyces Yeasts (Torulaspora Delbrueckii or Metschnikowia Pulcherrima) and Saccharomyces Cerevisiae in Base Wine for Sparkling Wine Production. Eur. Food Res. Technol. 2015, 240, 999–1012. [Google Scholar] [CrossRef]
- Medina-Trujillo, L.; González-Royo, E.; Sieczkowski, N.; Heras, J.; Canals, J.M.; Zamora, F. Effect of Sequential Inoculation (Torulaspora Delbrueckii/Saccharomyces Cerevisiae) in the First Fermentation on the Foaming Properties of Sparkling Wine. Eur. Food Res. Technol. 2017, 243, 681–688. [Google Scholar] [CrossRef]
- Ivit, N.N.; Loira, I.; Morata, A.; Benito, S.; Palomero, F.; Suárez-Lepe, J.A. Making Natural Sparkling Wines with Non-Saccharomyces Yeasts. Eur. Food Res. Technol. 2018, 244, 925–935. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Ramírez, M. Using Torulaspora Delbrueckii Killer Yeasts in the Elaboration of Base Wine and Traditional Sparkling Wine. Int. J. Food Microbiol. 2019, 289, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora Delbrueckii for Secondary Fermentation in Sparkling Wine Production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage Yeasts in the Wine Industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef]
- Liu, S.-Q. A Review: Malolactic Fermentation in Wine—Beyond Deacidification. J. Appl. Microbiol. 2002, 92, 589–601. [Google Scholar] [CrossRef]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Alexandre, H. Yeasts and Sparkling Wine Production. In Yeasts in the Production of Wine; Romano, P., Ciani, M., Fleet, G.H., Eds.; Springer: New York, NY, USA, 2019; pp. 395–432. ISBN 978-1-4939-9782-4. [Google Scholar]
- Petrontino, A.; Frem, M.; Fucilli, V.; Tricarico, G.; Bozzo, F. Health-Nutrients and Origin Awareness: Implications for Regional Wine Market-Segmentation Strategies Using a Latent Analysis. Nutrients 2022, 14, 1385. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).