Stress Resistance and Adhesive Properties of Commercial Flor and Wine Strains, and Environmental Isolates of Saccharomyces cerevisiae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Cultivation Methods
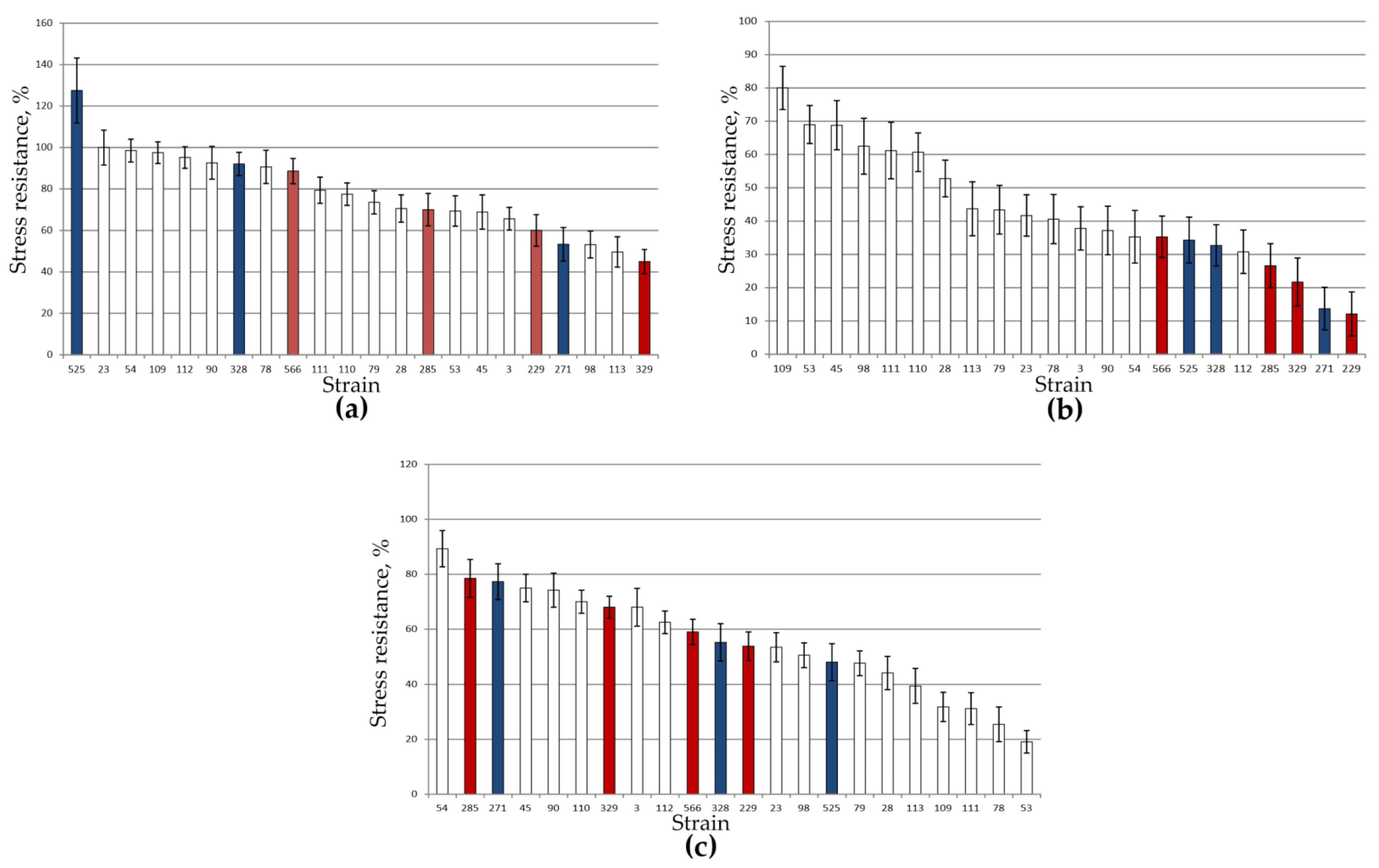
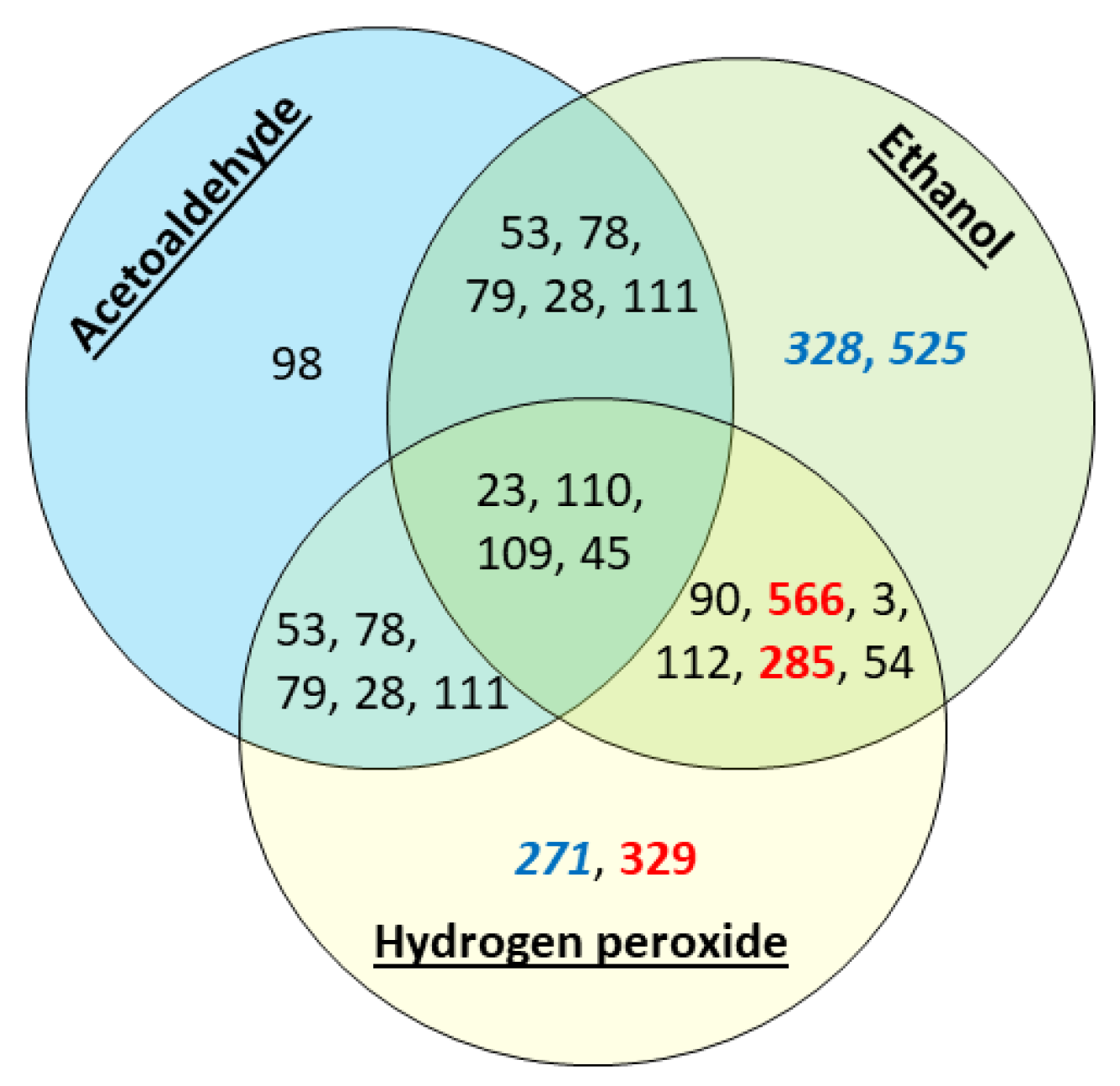
2.3. Analysis of Sensitivity for Strains to Abiotic Stresses and Toxic Compounds
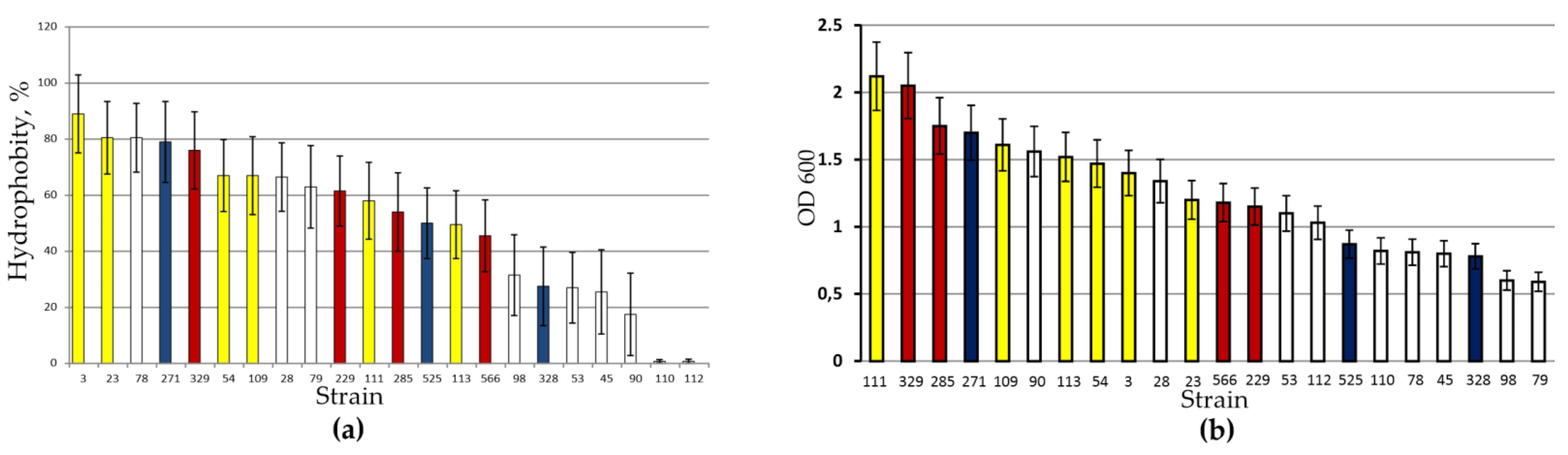
2.4. Analysis of Hydrophobicity for Yeast Cells (Isooctane Test)
2.5. Determination of Ability to Adhere to Polystyrene for Yeast Cells
2.6. Determination of the Growth Rate of Yeast Strains
2.7. Statistical Analysis
3. Results
3.1. Growth Characteristics of Strains
3.2. Stress Tolerance
3.3. Adhesive Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexandre, H. Flor yeasts of Saccharomyces cerevisiae-their ecology, genetics and metabolism. Int. J. Food Microbiol. 2013, 167, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Legras, J.L.; Moreno-Garcia, J.; Zara, S.; Zara, G.; Garcia-Martinez, T.; Mauricio, J.C.; Mannazzu, I.; Coi, A.L.; Zeidan, M.B.; Dequin, S.; et al. Flor yeast: New perspectives beyond wine aging. Front. Microbiol. 2016, 7, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Ángeles Pozo-Bayón, M.; Victoria Moreno-Arribas, M. Sherry wines. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2011; Volume 63, pp. 17–40. [Google Scholar]
- Sayenko, N.F. Sherry Wine and Technology of Its Production; Kartya Mol.: Kishinev, Moldavia, 1975. [Google Scholar]
- Eldarov, M.A.; Kishkovskaia, S.A.; Tanaschuk, T.N.; Mardanov, A.V. Genomics and biochemistry of Saccharomyces cerevisiae wine yeast strains. Biochemistry 2016, 81, 1650–1668. [Google Scholar] [CrossRef]
- Kishkovskaia, S.A.; Eldarov, M.A.; Dumina, M.V.; Tanashchuk, T.N.; Ravin, N.V.; Mardanov, A.V. Flor yeast strains from culture collection: Genetic diversity and physiological and biochemical properties. Appl. Biochem. Microbiol. 2017, 53, 359–367. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Medina, M. Aroma series as fingerprints for biological aging in fino sherry-type wines. J. Sci. Food Agric. 2007, 87, 2319–2326. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Analytical study of aromatic series in sherry wines subjected to biological aging. J. Agric. Food Chem. 2002, 50, 7356–7361. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Villafuerte, L.; Medina, M. Comparison of odour active compounds in sherry wines processed from ecologically and onventionally Brown Pedro Ximenez grapes. J. Agric. Food Chem. 2009, 57, 968–973. [Google Scholar] [CrossRef]
- Zea, L.; Moyano, L.; Moreno, J.; Cortes, B.; Medina, M. Discrimination of the aroma fraction of sherry wines obtained by oxidative and biological aging. Food Chem. 2001, 75, 79–84. [Google Scholar] [CrossRef]
- Moyano, L.; Zea, L.; Moreno, J.; Medina, M. Evaluation of the active odorants in Amontillado Sherry wines during the aging process. J. Agric. Food Chem. 2010, 58, 6900–6904. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Muñoz, M.; Cordero-Bueso, G.; Benítez-Trujillo, F.; Martínez, S.; Pérez, F.; Cantoral, J.M. Rethinking about flor yeast diversity and its dynamic in the “criaderas and soleras” biological aging system. Food Microbiol. 2020, 92, 103553. [Google Scholar] [CrossRef] [PubMed]
- Matallana, E.; Aranda, A. Biotechnological impact of stress response on wine yeast. Lett. Appl. Microbiol. 2017, 64, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Attfield, P.V. Stress tolerance: The key to effective strains of industrial baker’s yeast. Nat. Biotechnol. 1997, 15, 1351–1357. [Google Scholar] [CrossRef]
- Carrasco, P.; Querol, A.; del Olmo, M. Analysis of the stress resistance of commercial wine yeast strains. Arch. Microbiol. 2001, 175, 450–457. [Google Scholar] [CrossRef]
- Ingram, L.O.; Buttke, T.M. Effects of alcohols on micro-organisms. Adv. Microb. Physiol. 1984, 25, 253–300. [Google Scholar] [CrossRef] [PubMed]
- Leão, C.; Van Uden, N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta 1984, 774, 43–48. [Google Scholar] [CrossRef]
- Aranda, A.; Querol, A.; del Olmo, M. Correlation between acetaldehyde and ethanol resistance and expression of HSP genes in yeast strains isolated during the biological aging of sherry wines. Arch. Microbiol. 2002, 177, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Coi, A.L.; Bigey, F.; Mallet, S.; Marsit, S.; Zara, G.; Gladieux, P.; Galeote, V.; Budroni, M.; Dequin, S.; Legras, J.L. Genomic signatures of adaptation to wine biological ageing conditions in biofilm-forming flor yeasts. Mol. Ecol. 2017, 26, 2150–2166. [Google Scholar] [CrossRef]
- Marín-Menguiano, M.; Romero-Sanchez, S.; Barrales, R.R.; Ibeas, J.I. Population analysis of biofilm yeasts during fino sherry wine aging in the Montilla-Moriles, D.O. region. Int. J. Food Microbiol. 2017, 244, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Charpentier, C.; Colin, A.; Alais, A.; Legras, J.L. French Jura flor yeasts: Genotype and technological diversity. Antonie Van Leeuwenhoek 2009, 95, 263–273. [Google Scholar] [CrossRef]
- Zara, G.; Zara, S.; Pinna, C.; Marceddu, S.; Budroni, M. FLO11 gene length and transcriptional level affect biofilm-forming ability of wild flor strains of Saccharomyces cerevisiae. Microbiology 2009, 155, 3838–3846. [Google Scholar] [CrossRef] [Green Version]
- Moreno-García, J.; Coi, A.L.; Zara, G.; García-Martínez, T.; Mauricio, J.C.; Budroni, M. Study of the role of the covalently linked cell wall protein (Ccw14p) and yeast glycoprotein (Ygp1p) within biofilm formation in a flor yeast strain. FEMS Yeast Res. 2018, 18. [Google Scholar] [CrossRef]
- Legras, J.L.; Galeote, V.; Bigey, F.; Camarasa, C.; Marsit, S.; Nidelet, T.; Sanchez, I.; Couloux, A.; Guy, J.; Franco-Duarte, R.; et al. Adaptation of S. cerevisiae to fermented food environments reveals remarkable genome plasticity and the footprints of domestication. Mol. Biol. Evol. 2018, 35, 1712–1727. [Google Scholar] [CrossRef]
- Eldarov, M.A.; Beletsky, A.V.; Tanashchuk, T.N.; Kishkovskaya, S.A.; Ravin, N.V.; Mardanov, A.V. Whole-genome analysis of three yeast strains used for production of sherry-like wines revealed genetic traits specific to flor yeasts. Front. Microbiol. 2018, 9, 965. [Google Scholar] [CrossRef] [Green Version]
- Moreno-García, J.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Stress responsive proteins of a flor yeast strain during the early stages of biofilm formation. Process. Biochem. 2016, 51, 578–588. [Google Scholar] [CrossRef]
- Moreno-García, J.; García-Martínez, T.; Millán, M.C.; Mauricio, J.C.; Moreno, J. Proteins involved in wine aroma compounds metabolism by a Saccharomyces cerevisiae flor-velum yeast strain grown in two conditions. Food Microbiol. 2015, 51, 1–9. [Google Scholar] [CrossRef]
- David-Vaizant, V.; Alexandre, H. Flor yeast diversity and dynamics in biologically aged wines. Front. Microbiol. 2018, 9, 2235. [Google Scholar] [CrossRef]
- Kishkovskaya, S.A.; Tanashchuk, T.N.; Shalamitskiy, M.Y.; Zagoryiko, V.I.; Shiryaev, M.I.; Avdanina, D.A.; Eldarov, M.A.; Ravin, N.V.; Mardanov, A.V. Natural yeast strains of Saccharomyces cerevisiae that are promising for sherry production. Appl. Biochem. Microbiol. 2020, 56, 329–335. [Google Scholar] [CrossRef]
- Rosenberg, M.; Gutnick, D.; Rosenberg, E. Adherence of bacteria to hydrocarbons: A simple method for measuring cell-surface hydrophobicity. FEMS Microbiol. Lett. 1980, 9, 29–33. [Google Scholar] [CrossRef]
- Ferreira, D.; Galeote, V.; Sanchez, I.; Legras, J.L.; Ortiz-Julien, A.; Dequin, S. Yeast multistress resistance and lag-phase characterisation during wine fermentation. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fierro-Risco, J.; Rincón, A.M.; Benítez, T.; Codón, A.C. Overexpression of stress-related genes enhances cell viability and velum formation in Sherry wine yeasts. Appl. Microbiol. Biotechnol. 2013, 97, 6867–6881. [Google Scholar] [CrossRef] [PubMed]
- Hsu, P.-H.; Chiang, P.-C.; Liu, C.-H.; Chang, Y.-W. Characterization of cell wall proteins in Saccharomyces cerevisiae Clinical isolates elucidates Hsp150p in virulence. PLoS ONE 2015, 10, e0135174. [Google Scholar] [CrossRef] [Green Version]
- Aranda, A.; Del Olmo, M.L. Exposure of Saccharomyces cerevisiae to acetaldehyde induces sulfur amino acid metabolism and polyamine transporter genes, which depend on Met4p and Haa1p transcription factors, respectively. Appl. Environ. Microbiol. 2004, 70, 1913–1922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, V.; Radecka, D.; Aerts, G.; Verstrepen, K.J.; Lievens, B.; Thevelein, J.M. Phenotypic landscape of non-conventional yeast species for different stress tolerance traits desirable in bioethanol fermentation. Biotechnol. Biofuels 2017, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z. Spot assay for yeast. Bio-Protocol 2012, 2, e16. [Google Scholar] [CrossRef]
- Hung, C.W.; Martínez-Márquez, J.Y.; Javed, F.T.; Duncan, M.C. A simple and inexpensive quantitative technique for determining chemical sensitivity in Saccharomyces cerevisiae. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Degre, R. Selection and commercial cultivation of wine yeast and bacteria. In Wine Microbiology and Biotechnology; Fleet, G.H., Ed.; Harwood: Chur, Switzerland, 1993. [Google Scholar]
- Ivorra, C.; Pérez-Ortín, J.E.; del Olmo, M. An inverse correlation between stress resistance and stuck fermentations in wine yeasts: A molecular study. Biotechnol. Bioeng. 1999, 64, 698–708. [Google Scholar] [CrossRef]
- Zuzuarregui, A.; del Olmo, M. Analyses of stress resistance under laboratory conditions constitute a suitable criterion for wine yeast selection. Antonie Van Leeuwenhoek 2004, 85, 271–280. [Google Scholar] [CrossRef]
- Bonciani, T.; De Vero, L.; Mezzetti, F.; Fay, J.C.; Giudici, P. A multi-phase approach to select new wine yeast strains with enhanced fermentative fitness and glutathione production. Appl. Microbiol. Biotechnol. 2018, 102, 2269–2278. [Google Scholar] [CrossRef]
- Garofalo, C.; Berbegal, C.; Grieco, F.; Tufariello, M.; Spano, G.; Capozzi, V. Selection of indigenous yeast strains for the production of sparkling wines from native Apulian grape varieties. Int. J. Food Microbiol. 2018, 285, 7–17. [Google Scholar] [CrossRef]
- Taymaz-Nikerel, H.; Cankorur-Cetinkaya, A.; Kirdar, B. Genome-wide transcriptional response of Saccharomyces cerevisiae to stress-induced perturbations. Front. Bioeng. Biotechnol. 2016, 4, 17. [Google Scholar] [CrossRef] [Green Version]
- Święciło, A. Cross-stress resistance in Saccharomyces cerevisiae yeast—New insight into an old phenomenon. Cell Stress Chaperones 2016, 21, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Mardanov, A.V.; Eldarov, M.A.; Beletsky, A.V.; Tanashchuk, T.N.; Kishkovskaya, S.A.; Ravin, N.V. Transcriptome profile of yeast strain used for biological wine aging revealed dynamic changes of gene expression in course of flor development. Front. Microbiol. 2020, 11, 538. [Google Scholar] [CrossRef]
- Hope, E.A.; Dunham, M.J. Ploidy-regulated variation in biofilm-related phenotypes in natural isolates of Saccharomyces cerevisiae. G3 (Bethesda) 2014, 24, 1773–1786. [Google Scholar] [CrossRef] [Green Version]
- Tek, E.L.; Sundstrom, J.F.; Gardner, J.M.; Oliver, S.G.; Jiranek, V. Evaluation of the ability of commercial wine yeasts to form biofilms (mats) and adhere to plastic: Implications for the microbiota of the winery environment. FEMS Microbiol Ecol. 2018, 1, 94. [Google Scholar] [CrossRef] [Green Version]
- Perpetuini, G.; Tittarelli, F.; Schirone, M.; Di Gianvito, P.; Corsetti, A.; Arfelli, G.; Suzzi, G.; Tofalo, R. Adhesion Properties and Surface Hydrophobicity of Pichia manshurica Strains Isolated from Organic Wines. LWT 2018, 87, 385–392. [Google Scholar] [CrossRef]
- Reynolds, T.B.; Fink, G.R. Bakers’ yeast, a model for fungal biofilm formation. Science 2001, 291, 878–881. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M. Adhesion to hydrocarbons and microbial hydrophobicity-do the MATH. FEMS Microbiol. Lett. 2017, 364, 1–3. [Google Scholar] [CrossRef]
- Dimopoulou, M.; Renault, M.; Dols-Lafargue, M.; Albertin, W.; Herry, J.-M.; Bellon-Fontaine, M.-N.; Masneuf-Pomarede, I. Microbiological, Biochemical, Physicochemical Surface Properties and Biofilm Forming Ability of Brettanomyces bruxellensis. Ann. Microbiol. 2019, 69, 1217–1225. [Google Scholar] [CrossRef]
- Pinto, L.; Baruzzi, F.; Cocolin, L.; Malfeito-Ferreira, M. Emerging Technologies to Control Brettanomyces spp. in Wine: Recent Advances and Future Trends. Trends Food Sci. Technol. 2020, 99, 88–100. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y.; Yang, H. Efficacy of Low Concentration Neutralised Electrolysed Water and Ultrasound Combina-tion for Inactivating Escherichia coli ATCC 25922, Pichia pastoris GS115 and Aureobasidium pullulans 2012 on Stainless Steel Coupons. Food Control. 2017, 73, 889–899. [Google Scholar] [CrossRef]
- Perpetuini, G.; Rossetti, A.P.; Battistelli, N.; Arfelli, G.; Tofalo, R. Adhesion Properties, Biofilm Forming Potential, and Susceptibility to Disinfectants of Contaminant Wine Yeasts. Microorganisms 2021, 22, 654. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Bakalinsky, A.T.; Zara, G.; Pirino, G.; Demontis, M.A.; Budroni, M. FLO11-based model for air-liquid interfacial biofilm formation by Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2005, 71, 2934–2939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Strain ID | Genotype * | Oenological Parameters | |||||||
|---|---|---|---|---|---|---|---|---|---|
| FLO11 | ITS1-4 | YDR 379 C-A | Concentration | Flor Growth *** | Presence of “Sherry” Tones in Aroma and Taste ** | ||||
| Sugars, g/L | Volatile Acids, g/L | Aldehydes, mg/L | Alcohol, % vol. | ||||||
| Flor Strains | |||||||||
| 229 | F | F | F | 2.5 ± 0.06 | 0.66 ± 0.01 | 286.0 ± 5.9 | 11.3 ± 0.1 | +++ | + |
| 285 | F | F | - | 1.3 ± 0.06 | 0.48 ± 0.03 | 211.0 ± 9.4 | 11.3 ± 0.1 | +++ | + |
| 329 | F | F | F | 1.2 ± 0.06 | 0.52 ± 0.02 | 179.6 ± 2.6 | 11.4 ± 0.1 | +++ | + |
| 566 | W | F | F | 0.8 ± 0.15 | 0.60 ± 0.02 | 352.0 ± 8.4 | 11.1 ± 0.1 | +++ | + |
| Wine Strains | |||||||||
| 271 | W | F | F | 3.7 ± 0.15 | 0.10 ± 0.01 | 343.2 ± 20.8 | 9.5 ± 0.1 | +++ | + |
| 328 | W | F | - | 4.2 ± 0.10 | 0.46 ± 0.01 | 132 ± 6.8 | 11.4 ± 0.1 | +++ | + |
| 525 | W | F | F | 2.7 ± 0.06 | 0.59 ± 0.05 | 149.6 ± 9.1 | 11.7 ± 0.1 | + | + |
| Environmental Strains | |||||||||
| 3 | F/W | F | F | 1.9 ± 0.12 | 0.78 ± 0.04 | 65.9 ± 4.0 | 12.3 ± 0.1 | +++ | + |
| 23 | F | F | F | 2.3 ± 0.15 | 0.46 ± 0.03 | 105.6 ± 6.4 | 11.9 ± 0.1 | +++ | + |
| 28 | W | F | W | 0.9 ± 0.06 | 0.35 ± 0.02 | 73.0 ± 4.4 | 12.8 ± 0.2 | - | - |
| 45 | W | W | F | 1.9 ± 0.06 | 0.64 ± 0.02 | 84.5 ± 5.1 | 12.2 ± 0.1 | - | - |
| 53 | F/W | W | W | 1.1 ± 0.10 | 0.21 ± 0.01 | 34.3 ± 2.1 | 12.9 ± 0.1 | + | - |
| 54 | F/W | W | F | 0.1 ± 0.06 | 0.59 ± 0.02 | 148.7 ± 9.0 | 12.7 ± 0.2 | +++ | + |
| 78 | W | W | F | 0.6 ± 0.06 | 0.75 ± 0.03 | 154.9 ± 9.4 | 12.6 ± 0.1 | + | + |
| 79 | W | W | F | 0.2 ± 0.10 | 0.66 ± 0.02 | 91.5 ± 5.5 | 12.3 ± 0.0 | - | - |
| 90 | F | W | W | 1.3 ± 0.06 | 0.30 ± 0.02 | 74.5 ± 4.5 | 12.7 ± 0.1 | - | - |
| 98 | W | W | W | 1.3 ± 0.06 | 1.10 ± 0.10 | 183.9 ± 6.6 | 13.0 ± 0.1 | - | - |
| 109 | F/W | F | F | 0.3 ± 0.06 | 0.72 ± 0.03 | 283.4 ± 17.2 | 12.7 ± 0.2 | +++ | + |
| 110 | F | W | F | 0.7 ± 0.06 | 0.90 ± 0.04 | 253.4 ± 15.4 | 12.5 ± 0.1 | + | + |
| 111 | F/W | F | F | 0.6 ± 0.12 | 0.66 ± 0.02 | 279.8 ± 17.0 | 11.9 ± 0.1 | +++ | + |
| 112 | W | W | F | 1.6 ± 0.06 | 0.54 ± 0.03 | 176.0 ± 10.7 | 12.3 ± 0.1 | - | - |
| 113 | F/W | F | F | 1.2 ± 0.10 | 0.87 ± 0.03 | 176.0 ± 10.7 | 12.6 ± 0.1 | +++ | + |
| Strain ID | Lag Phase Duration, h | Exponential Growth (from–to, h) | Exponential Growth Phase Duration, h | Specific Growth Rate, μ, h−1 | Generation Time, h:min | Generation Time, h:min |
|---|---|---|---|---|---|---|
| Flor Strains | ||||||
| 229 | 7 | 7–26 | 19 | 0.315 | 2:19 | 2:11 |
| 285 | 15 | 15–35 | 20 | 0.266 | 2:59 | 2:35 |
| 329 | 5 | 5–35 | 30 | 0.219 | 3:15 | 3:09 |
| 566 | 9 | 9–22 | 13 | 0.363 | 1:91 | 1:55 |
| Wine Strains | ||||||
| 271 | 5 | 5–29 | 24 | 0.266 | 2:59 | 2:35 |
| 328 | 4 | 4–19.5 | 16 | 0.424 | 1:63 | 1:37 |
| 525 | 5 | 4.5–25 | 21 | 0.338 | 2:04 | 2:02 |
| Environmental Strains | ||||||
| 3 | 7 | 7–20 | 13 | 0.425 | 1:63 | 1:38 |
| 23 | 6 | 6–24 | 18 | 0.348 | 1:98 | 1:59 |
| 28 | 5 | 5–20 | 15 | 0.463 | 1:49 | 1:29 |
| 45 | 5 | 5–20 | 15 | 0.462 | 1:49 | 1:29 |
| 53 | 4 | 4–18 | 14 | 0.483 | 1:43 | 1:25 |
| 54 | 9 | 9–24 | 15 | 0.401 | 1:72 | 1:43 |
| 78 | 6 | 6–24 | 18 | 0.393 | 1:76 | 1:45 |
| 79 | 5 | 5–25 | 20 | 0.362 | 1:91 | 1:54 |
| 90 | 7 | 7–20 | 13 | 0.489 | 1:41 | 1:24 |
| 98 | 2 | 2–17 | 15 | 0.506 | 1:36 | 1:21 |
| 109 | 8 | 8–24 | 16 | 0.383 | 1:80 | 1:48 |
| 110 | 3 | 3–21 | 18 | 0.464 | 1:49 | 1:29 |
| 112 | 4 | 3.5–20.5 | 17 | 0.442 | 1:56 | 1:33 |
| 113 | 4 | 4–24 | 20 | 0.389 | 1:78 | 1:46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eldarov, M.A.; Avdanina, D.A.; Ivanova, E.; Shalamitskiy, M.Y.; Tanashchuk, T.N.; Vybornaya, T.; Ravin, N.V.; Kishkovskaya, S.A.; Mardanov, A.V. Stress Resistance and Adhesive Properties of Commercial Flor and Wine Strains, and Environmental Isolates of Saccharomyces cerevisiae. Fermentation 2021, 7, 188. https://doi.org/10.3390/fermentation7030188
Eldarov MA, Avdanina DA, Ivanova E, Shalamitskiy MY, Tanashchuk TN, Vybornaya T, Ravin NV, Kishkovskaya SA, Mardanov AV. Stress Resistance and Adhesive Properties of Commercial Flor and Wine Strains, and Environmental Isolates of Saccharomyces cerevisiae. Fermentation. 2021; 7(3):188. https://doi.org/10.3390/fermentation7030188
Chicago/Turabian StyleEldarov, Michail A., Daria A. Avdanina, Elena Ivanova, Maksim Y. Shalamitskiy, Tatiana N. Tanashchuk, Tatiana Vybornaya, Nikolai V. Ravin, Svetlana A. Kishkovskaya, and Andrey V. Mardanov. 2021. "Stress Resistance and Adhesive Properties of Commercial Flor and Wine Strains, and Environmental Isolates of Saccharomyces cerevisiae" Fermentation 7, no. 3: 188. https://doi.org/10.3390/fermentation7030188
APA StyleEldarov, M. A., Avdanina, D. A., Ivanova, E., Shalamitskiy, M. Y., Tanashchuk, T. N., Vybornaya, T., Ravin, N. V., Kishkovskaya, S. A., & Mardanov, A. V. (2021). Stress Resistance and Adhesive Properties of Commercial Flor and Wine Strains, and Environmental Isolates of Saccharomyces cerevisiae. Fermentation, 7(3), 188. https://doi.org/10.3390/fermentation7030188









