Abstract
The objective of this study was to see how dietary supplementation with essential oils (EOs) affected rumen fermentation, blood metabolites, growth performance and meat quality of beef cattle through a meta-analysis. In addition, a simulation analysis was conducted to evaluate the effects of EOs on the economic and environmental impact of beef production. Data were extracted from 34 peer-reviewed studies and analyzed using random-effects statistical models to assess the weighted mean difference (WMD) between control and EOs treatments. Dietary supplementation of EOs increased (p < 0.01) dry matter intake (WMD = 0.209 kg/d), final body weight (WMD = 12.843 kg), daily weight gain (WMD = 0.087 kg/d), feed efficiency (WMD = 0.004 kg/kg), hot carcass weight (WMD = 5.45 kg), and Longissimus dorsi muscle area (WMD = 3.48 cm2). Lower (p < 0.05) ruminal concentration of ammonia nitrogen (WMD = −1.18 mg/dL), acetate (WMD = −4.37 mol/100 mol) and total protozoa (WMD = −2.17 × 105/mL), and higher concentration of propionate (WMD = 0.878 mol/100 mol, p < 0.001) were observed in response to EOs supplementation. Serum urea concentration (WMD = −1.35 mg/dL, p = 0.026) and haptoglobin (WMD = −39.67 μg/mL, p = 0.031) were lower in cattle supplemented with EOs. In meat, EOs supplementation reduced (p < 0.001) cooking loss (WMD = −61.765 g/kg), shear force (WMD = −0.211 kgf/cm2), and malondialdehyde content (WMD = −0.040 mg/kg), but did not affect pH, color (L* a* and b*), or chemical composition (p > 0.05). Simulation analysis showed that EOs increased economic income by 1.44% and reduced the environmental footprint by 0.83%. In conclusion, dietary supplementation of EOs improves productive performance and rumen fermentation, while increasing the economic profitability and reducing the environmental impact of beef cattle. In addition, supplementation with EOs improves beef tenderness and oxidative stability.
1. Introduction
Antibiotics are generally used for the treatment and prevention of diseases in livestock, but in some cases, they are also used as growth promoters for ruminants and non-ruminants [1]. However, according to Argudín et al. [2], the inappropriate use of antibiotics in food animals has contributed to the emergence of bacteria that are resistant to their effects. Consequently, the use of antibiotics as growth promoters has been banned in several countries, leading to research on natural products that may have similar effects to antibiotics [3]. Important alternatives to antibiotics reported in livestock are enzymes, organic acids, probiotics, prebiotics, yeasts, and phytogenics (i.e., tannins, and saponins, among others) [3,4]. Essential oils (EOs) are among the most studied and economically relevant plant-derived products [4]. EOs are mixtures of volatile compounds extracted from plants by distillation [5] and chemically they comprise a wide diversity of low molecular weight molecules including terpenes, alcohols, aldehydes, and ketones [4].
The effects of purified EOs and plants with high EOs content have been investigated mainly in pigs and poultry [6,7,8], but information on the effects of EOs in ruminant diets is still limited. In ruminants, the effects of some bioactive metabolites of EOs (eugenol, cinnamaldehyde, and anethole) on ruminal fermentation change depending on rumen pH [9], which is influenced by the level of concentrate in the diet. Similarly, some studies [10,11] have reported better weight gain, feed efficiency, and digestibility in ruminants supplemented with EOs. In other studies, dietary supplementation of EOs had negative [12] or neutral [13] effects on nutrient digestibility and productive performance, respectively. In addition, some studies reported better color, higher tenderness, and lower lipid peroxidation in ruminant meat supplemented with EOs [14,15]. In contrast, in other studies, the dietary inclusion of EOs had no effect on the color, tenderness, and lipid peroxidation of sheep and goat meat [16,17].
Particularly in beef cattle, some studies have evaluated the effects of dietary inclusion of EOs on productive performance [18,19], ruminal fermentation [20,21], blood metabolites [22,23], and meat quality [24,25]. However, the results obtained to date are controversial and inconclusive. Therefore, to develop products containing EOs that can improve the productivity and health of beef cattle, it is necessary to identify the factors that contribute to this variability. Variations in the primary bioactive metabolite of EOs, the duration of the experimental period, the level of concentrate in the diet, and the dose of EOs appear to be factors contributing to the variability of the effects observed in beef cattle supplemented with EOs [4,26].
Several review articles [4,26,27,28] have mentioned that EOs can improve health, rumen fermentation, animal performance, and the characteristics of cattle-derived products. Furthermore, in a previously published meta-analytical study, Khiaosa-ard and Zebeli [29] reported that in ruminants (including beef cattle) the use of EOs improved some ruminal parameters. However, Khiaosa-ard and Zebeli [29] did not evaluate the variables related to productive performance, health, or meat quality of beef cattle. Meta-analysis (MA) is a statistical approach that allows combining and quantitatively synthesizing data previously published in different studies [30]. In addition, it is possible to use MA to identify sources of heterogeneity among the various studies [31]. The hypothesis of this study is that dietary supplementation of EOs will benefit productive performance and rumen parameters of beef cattle without affecting health and meat quality. The objective of this study was to perform a meta-analysis of the effects of dietary inclusion of essential oils on productive performance, ruminal parameters, blood metabolites, and meat quality of beef cattle. A second objective was to examine heterogeneity and publication bias among the studies. In addition, the results of the meta-analysis were used to evaluate the potential effect of EOs on the economic and environmental impact of beef production by simulation analysis.
2. Materials and Methods
2.1. Literature Search and Study Selection
To obtain a robust meta-analysis, PRISMA guidelines [32] were used during the processes of identification, selection, choice, and inclusion of references. The information search strategy used is shown in Figure A1. Studies that evaluated the effects of dietary supplementation of EOs on nutrient intake and digestibility, productive performance, carcass characteristics, ruminal parameters, blood metabolites, and meat quality of beef cattle were identified by systematically searching for information in the following databases: ScienceDirect, PubMed, Scopus, and Web of Science. During the search process, the following keywords were used in all databases: essential oils, beef cattle, finishing steer, finishing bull, growth performance, carcass traits, ruminal fermentation, blood metabolites, and meat quality. The results obtained during the search and selection processes were restricted to studies published between January 2010 and January 2022, and a total of 702 scientific publications were identified (Figure A1). Only articles published from 2010 to date were included because Khiaosa-ard and Zebeli [29] previously published a meta-analysis on ruminal parameters of beef cattle, and in that study, they included studies published before 2010.
All identified publications were subjected to a two-step screening process, as previously reported by Orzuna-Orzuna et al. [33,34]. First, titles and abstracts were reviewed, thereby excluding review articles, simulation articles, studies that did not use beef cattle, in vitro experiments, and studies that did not measure the variables of interest. Second, the articles analyzed had to meet some inclusion criteria, similar to those previously described by other authors [33,34,35]: (1) studies that used beef cattle housed in confinement, non-lactating, and non-pregnant conditions; (2) studies containing data on nutrient intake and digestibility, productive performance, carcass characteristics, rumen parameters, blood metabolites, and/or meat quality; (3) similarity between control and experimental treatments except for the presence of EOs; (4) present quantification or possible determination of the dose of EOs included in the diets; (5) articles written in the English language and published in peer-reviewed journals; and (6) reporting of least squares means of control and experimental treatments with at least one measure of variability (standard error or standard deviation).
2.2. Data Extraction
After going through the screening process, 54 full-text articles were evaluated, of which only 34 articles met all the inclusion criteria used (Table A1). The quantitative data used for the meta-analysis were extracted from these articles. Moreover, variables had to be reported in at least three different studies to be included [33,34,36]. Therefore, the response variables included in the present meta-analysis were: dry matter and nutrient intake and digestibility (crude protein, neutral detergent fiber, among others), daily weight gain, feed efficiency, hot and cold carcass weight and yield, backfat thickness, Longissimus dorsi muscle area, ruminal parameters (pH, ammonia nitrogen, volatile fatty acids, protozoa, among others), blood metabolites (urea, glucose, triglycerides, hemoglobin, white blood cells, and acute-phase proteins), and characteristics related to meat quality (pH, shear force, malondialdehyde content, color, and chemical composition). In addition to the response variables, the following information was extracted from the 34 selected articles: publication reference, the nutritional composition of the diet, amount of concentrate in the diet (g/kg DM), country, the dose of EOs in the diet (g/kg DM), number of replicates, duration of supplementation with EOs (days), and primary bioactive metabolite of the EOs.
Table A1 shows the complete list of articles that were included in the final database. For each of these articles, the number of replicates, treatment means, and standard deviations (SDs) were obtained. When articles did not report SD, it was calculated using the equation [37]: SD = SEM × √n, where SEM = standard error of treatment means, and n = number of replicates.
2.3. Calculations and Statistical Analysis
All meta-analysis, meta-regression, heterogeneity analysis, publication bias, and subgroup analysis data were analyzed with the “metafor” package [38] of the R statistical software (v. 4.1.2, R Core Team, Vienna, Austria). The effects of dietary inclusion of EOs in beef cattle were evaluated through weighted mean differences (WMDs) between diets without EOs (control treatments) and diets with the inclusion of EOs (experimental treatments). To calculate WMDs, treatment means were weighted by the inverse of the variance, using the methods for random effects models proposed by DerSimonian and Laird [39]. The WMD allows the interpretation of the results in the original units of measurement [40].
Descriptive statistical values of the nutritional composition of the diets were obtained using the MEANS procedure of SAS statistical software [41]. Similarly, the MIXED procedure of SAS was used to evaluate the differences in the chemical composition of the diets of the control and EO-supplemented treatments. For this, the studies were used as a random effect and Tukey’s test was applied to detect differences between treatments [33,34,42].
2.4. Heterogeneity
To evaluate variations among WMD at the trial level, the chi-square (Q) test for heterogeneity and the I2 statistic, which measures the percentage of variation due to heterogeneity, were used [31]. For the Q test, an α level of 0.10 was used because of its relatively low power [43]. On the other hand, when I2 values were negative, they were assigned a value of zero; therefore, I2 values are between 0 and 100%. I2 values below 25% indicate low heterogeneity, values between 25 and 50% indicate moderate heterogeneity, while values above 50% indicate high and significant heterogeneity [30,31,44].
2.5. Publication Bias
The evaluation of publication bias was performed with Egger’s regression asymmetry test [45]. When p ≤ 0.05 in Egger’s test was obtained, publication bias was considered to be present. The “trim-and-fill” method of Duval and Tweedie [46] was applied to variables that had statistical evidence of publication bias, to estimate the number of possible missing observations.
2.6. Meta-Regression and Subgroup Analysis
Meta-regression analysis was used to evaluate sources of heterogeneity for variables that had Q with α ≤ 0.10 [43] or I2 greater than 50% [30]. It is not appropriate to use meta-regression when there are fewer than 10 studies reporting the variable of interest [47], so it was only applied to parameters that met this criterion. The method of moments of DerSimonian and Laird [39] was used to estimate the meta-regression because it is well established for estimating between-study variance. Categorical and continuous covariates were used in the meta-regression. The primary bioactive compound (blend, cinnamaldehyde, eugenol, thymol, capsaicin, and anethole) was used as a categorical covariate. The continuous covariates were the doses of EOs (mg/kg DM), the duration of the experimental phase (days), and the level of concentrate in the diet (g/kg DM). When the categorical covariate (primary bioactive metabolite) was significant at a level α ≤ 0.05, subgroup analysis was used to assess the WMD [33,42]. Similarly, when the meta-regression was significant (p ≤ 0.05) for EOs dose, experimental period, and concentrate level, these covariates were evaluated using the following subgroups: EOs dose (≤400 and >400 mg/kg DM), experimental period (≤90 and >90 days), and concentrate level (≤700 and >700 g/kg DM).
2.7. Simulation Analysis
Based on the results of dietary supplementation of EOs on the productive performance of beef cattle, a simulation analysis was conducted to evaluate how dietary supplementation of EOs influences the economic and environmental impact of beef production. The simulation was based on the impact of supplementing EOs in the diet of 1000 cattle to gain 200 kg of body weight each. Table A2 shows the data used for the simulation inputs. The variables included in the simulation input were previously used by Salami et al. [48]: the number of animals, daily weight gain (DWG), dry matter intake (DMI), feed efficiency (FE), target body weight, protein production, and lean meat yield. Regrading indicators of the economic impact of EOs supplementation on beef production, total feed cost (USD/1000 animals) and feeding days to slaughter were calculated. Moreover, as indicators of the environmental impact of beef production, the emission intensity attributed to the use of feed (EIAFU) was calculated, in addition to the intensity of total emissions. For this, the equations reported by Salami et al. [48] were applied:
where: FR: feed required; BW: body weight; TLWG: target live weight gain; FE: feed efficiency; DFS: days on feed to the slaughter; DWG: daily live weight gain; TFU: total feed use; DM: dry matter; FC: feed cost; EI: emission intensity; EIAFU: the EI attributed to feeding use; BPO: beef protein output; AEIAFU: the global average EI attributed to feeding use; AEINAFU: the global average EI not attributed to feeding use.
FR to gain 200 kg BW = TLWG/FE
DFS = TLWG /DWG
TFU = FR to gain 200 kg BW × (1/DM content of diet)
FC (USD) = TFU × ration cost (US$)
Total FC (USD/1000 cattle) = FC × 1000
EIAFU = BPO × AEIAFU
EI = EIAFU + (AEINAFU × BPO)
Total EI = EI × 1000
To the previously described equations, we applied the assumptions used by Salami et al. [48]:
- (1)
- Cost of the basal diet = 0.25 USD/kg DM. A cost of 0.0021 USD/kg DM was added to the cost of the diet supplemented with EOs, considering the average dose of EOs used in the present study (Table 1) and the average cost of 7.45 USD/kg reported in the literature for some of the most commonly used EOs [49,50,51].
 Table 1. Descriptive statistics of the complete dataset for the effect of EO supplementation on beef cattle diets.
Table 1. Descriptive statistics of the complete dataset for the effect of EO supplementation on beef cattle diets. - (2)
- AEIAFU = 108 kg CO2-eq/kg of protein, based on data previously reported by Gerber et al. [52], which indicated that the global average emission intensity (GAEI) of beef is 300 kg CO2-eq/ kg of protein and that on average 36% of beef emissions (BEs) were attributed to animal feed use. In addition, data on beef GAEI were used because worldwide there is wide variation in the environmental footprint of beef production systems [48].
- (3)
- Moreover, considering the above assumption (2), the remaining component of BEs was allocated to non-food use [48]: AEINAFU = 192 kg CO2-eq/kg of protein.
3. Results
3.1. Study Attributes and Excluded Studies
Table 1 shows the descriptive statistics and comparison of means for the chemical composition of the diets. No significant differences were observed between the control treatment and the EOs treatment for any of the diet components (p > 0.05). Therefore, it is possible to exclude the effects of diet components on the response of beef cattle to dietary supplementation of EOs in our dataset.
In the present meta-analysis, the included studies were conducted in 10 countries, mainly Brazil (44.2%), Canada (17.6%), and the United States of America (14.7%). The experimental doses of EOs ranged from 5 to 1200 mg/kg DM, and the duration of the experimental periods ranged from 21 to 390 days (Table 1). Based on the primary bioactive metabolite, the EOs used were grouped as follows: mixture, eugenol, thymol, cinnamaldehyde, capsaicin, and anethole. In most of the treatments (66.4%), EOs with mixtures of bioactive metabolites were used in a similar proportion, 12.3% of the treatments used EOs with cinnamaldehyde as the primary bioactive metabolite and, in 7.8% of the treatments, the primary bioactive metabolite was capsaicin. In the remaining treatments (13.5%), EOs with thymol, eugenol and anethole were used as primary bioactive metabolites. In addition, 61.7% of the treatments used diets high in concentrate (>700 g/kg DM), and 38.3% used diets with 0–700 g/kg DM of concentrate. Moreover, in most of the treatments (57.3%) the experimental phase lasted up to 90 days, and 42.7% of the treatments used experimental periods longer than 90 days.
3.2. Nutrient Intake and Digestibility
Dietary inclusion of EOs increased (p < 0.01) dry matter intake (DMI), crude protein intake (CPI), neutral detergent fiber intake (NDFI), and acid detergent fiber intake (ADFI) (Table 2). However, dietary supplementation of EOs did not affect (p > 0.05) organic matter intake (OMI).

Table 2.
Nutrient intake and digestibility of beef cattle supplemented with essential oils.
By comparison, there was no significant impact (p > 0.05) of dietary inclusion of EOs on dry matter digestibility (DMD), organic matter digestibility (OMD), crude protein digestibility (CPD), and neutral detergent fiber digestibility (NDFD). However, acid detergent fiber digestibility (ADFD) decreased in response to dietary supplementation with EOs (p < 0.05).
3.3. Growth Performance and Carcass Characteristics
Dietary inclusion of EOs increased (p < 0.05) final body weight (FBW), daily weight gain (DWG), feed efficiency (FE), hot carcass weight (HCW), cold carcass weight (CCW), and Longissimus dorsi muscle area (LMA) (Table 3). However, dietary supplementation of EOs did not affect (p > 0.05) hot carcass yield (HCY), cold carcass yield (CCY), and backfat thickness (BFT).

Table 3.
Growth performance and carcass characteristics of beef cattle supplemented with essential oils.
3.4. Ruminal Parameters and Nitrogen Balance
No significant effects (p > 0.05) of dietary inclusion of EOs on pH and ruminal butyrate concentration were observed (Table 4). Supplementation of EOs in the diet decreased (p < 0.05) ruminal concentration of ammonia nitrogen (NH3-N), total volatile fatty acids (TVFA), and acetate. However, higher (p < 0.001) ruminal propionate concentration was observed in response to dietary EOs supplementation (Table 4).

Table 4.
Ruminal parameters and nitrogen metabolism of beef cattle supplemented with essential oils.
Table 4 shows that dietary inclusion of EOs decreased total (p = 0.041), Isotricha (p = 0.004), and Dasytricha (p < 0.001) protozoan populations but did not affect Entodinium populations (p > 0.05). In addition, dietary supplementation of EOs reduced urinary nitrogen (N) excretion (p = 0.005) and increased N retention (p = 0.011). However, no significant impact (p > 0.05) of dietary inclusion of EOs on N intake, fecal N excretion, microbial ruminal nitrogen (RNM), or microbial protein supply efficiency (MPSE) was observed.
3.5. Blood Metabolites
Dietary supplementation with EOs did not affect the serum concentration of triglycerides and glucose (p > 0.05) but decreased the serum concentration of urea (p = 0.026) and non-esterified fatty acids (NEFA; p = 0.004) (Table 5).

Table 5.
Blood metabolites of beef cattle supplemented with essential oils.
By comparison, there was no significant impact (p > 0.05) of EOs dietary inclusion on the blood concentration of hemoglobin, white blood cells (WBC), lymphocytes, monocytes, and eosinophils. However, neutrophils and basophils decreased (p < 0.05) in response to dietary EOs supplementation (Table 5). In addition, plasma haptoglobin concentration decreased (p = 0.031) with dietary EOs supplementation, but no significant changes (p > 0.05) were observed in serum amyloid A (SAA) and lipopolysaccharide-binding protein (LBP) concentration.
3.6. Meat Quality
Dietary supplementation of EOs did not affect meat pH (p > 0.05), but decreased cooking loss (p = 0.009), shear force (ShF; p = 0.029), and malondialdehyde content (MDA; p = 0.008) (Table 6). In addition, there was no significant impact (p > 0.05) of EOs dietary inclusion on the lightness (L*), redness (a), yellowness (b*), and moisture, protein, fat, ash, and collagen content of meat.

Table 6.
Meat quality of beef cattle supplemented with essential oils.
3.7. Publication Bias and Meta-Regression
Table 2, Table 3, Table 4, Table 5 and Table 6 show that the presence of publication bias from Egger’s regression asymmetry test was not significant for any variable (p > 0.05).
By comparison, significant heterogeneity (Q; p ≤ 0.10) was observed for DMI, CPI, NDFI, DMD, OMD, CPD, NDFD (Table 2), ADFD, FBW, DWG, HCW, HCY, CCW, CCY, BFT, LMA (Table 3), TVFA, acetate, butyrate, total protozoa, Entodinium, CH4, urinary N, RNM (Table 4), Hb, basophils (Table 5), meat pH, cooking loss, MDA, lightness (L*), and meat moisture (Table 6). However, it is not recommended to use meta-regression when the response variable of interest is reported in less than 10 studies [47]. Therefore, this analysis was only performed for the variables DMI, NDFD, FBW, DWG, BFT, LMA, TVFA, acetate, and butyrate.
Table 7 shows that the dose of EOs explained (p < 0.05) 33.59, 20.17, 55.43 and 12.50% of the heterogeneity observed for DMI, BFT, TVFA, and acetate, respectively. In addition, the period of EOs supplementation in the diet had a significant relationship (p < 0.05) with DMI, FBW, DWG, and BFT, which explained between 3.08 and 65.50% of the observed heterogeneity. The primary bioactive metabolite did not significantly (p > 0.05) influence the variability of the evaluated response parameters (DMI, NDFD, FBW, DWG, BFT, LMA, TVFA, acetate, and butyrate). The level of concentrate in the diet explained (p < 0.05) 40.07, 33.61 and 27.56% of the observed heterogeneity for DMI, FBW, and DWG, respectively. There was no significant relationship (p > 0.05) between the covariates used (EOs dose, supplementation period, primary bioactive metabolite, and concentrate level) and the response variables NDFD, LMA, and butyrate (Table 7).

Table 7.
Meta-regression comparing the associations between covariates and measured outcomes.
3.8. Subgroup Analysis
DMI increased regardless of the dose of EOs used (p < 0.05); however, the effect was greater when doses greater than 400 mg/kg DM (WMD = 0.324 kg) were used in comparison to doses of ≤400 mg/kg DM (WMD = 0.110 kg/d) (Figure 1a). BFT increased when doses of EOs greater than 400 mg/kg DM were used (WMD = 0.368 mm; p = 0.033), but doses of ≤400 mg/kg DM did not affect BFT (Figure 1b; p = 0.089). Figure 1c shows that the ruminal concentration of TVFA was not affected by the dose of EOs in the diet (p > 0.05). Ruminal acetate concentration decreased (WMD = −3.504 mol/100 mol; p = 0.017) when low doses of EOs (≤400 mg/kg DM) were used, but doses of EOs higher than 400 mg/kg DM did not affect acetate concentration (Figure 1d; p > 0.05).

Figure 1.
Subgroup analysis (subgroup = essential oils dose (mg/kg DM)) of the effect of essential oils in diet of the beef cattle, WMDs = weighted mean differences between essential oil treatments and control.
DMI increased when dietary supplementation with EOs lasted longer than 90 days (WMD = 0.191 kg/d; p < 0.001) but was not affected when EOs were offered for up to 90 days (WMD = 0.007 kg; p > 0.05) (Figure 2a). FBW increased regardless of the period of EO supplementation used (Figure 2b); however, the effect was greater when EOs were offered for more than 90 days (WMD = 20.16 kg; p < 0.001) compared to periods of up to 90 days (WMD = 5.38 kg; p = 0.008). Similarly, DWG increased regardless of the period of EO supplementation used (p < 0.05); however, the effect was greater when EOs were offered for more than 90 days (WMD = 0.110 kg/d) compared to periods up to 90 days (Figure 2c; WMD = 0.069 kg/d). Figure 2d shows that BFT was not affected by the period of supplementation with EOs in the diet (p > 0.05).
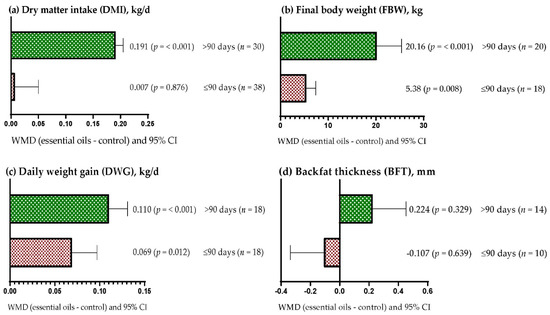
Figure 2.
Subgroup analysis (subgroup = supplementation period (days)) of the effect of essential oils in diet of the beef cattle, WMD = weighted mean differences between essential oil treatments and control.
DMI increased when EOs were supplemented in diets with more than 700 g/kg DM of concentrate (WMD = 0.194 kg/d; p < 0.001), but DMI was not affected when EOs were offered in diets with up to 700 g/kg DM of concentrate (WMD = −0.033 kg/d; p > 0.05) (Figure 3a). FBW increased (WMD = 21.02 kg; p < 0.001) when EOs were included in diets high in concentrate (>700 g/kg DM); however, it was not affected when EOs were fed in diets with less than 700 g/kg DM of concentrate (WMD = 2.48 kg; p = 0.115) (Figure 3b). DWG increased regardless of the level of concentrate used in the diets (p < 0.01; Figure 3c); however, the effect was greater when EOs were included in diets with more than 700 g/kg DM of concentrate (WMD = 0.115 kg/d) compared to diets having up to 700 g/kg DM of concentrate (WMD = 0.041 kg/d).
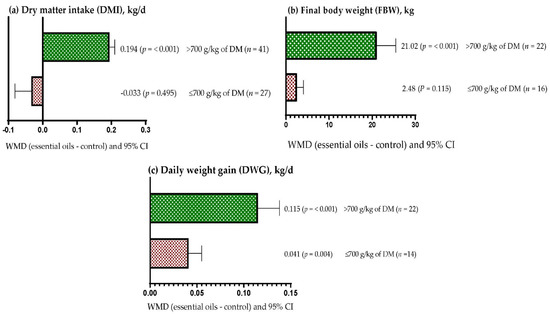
Figure 3.
Subgroup analysis (subgroup = concentrate in diet (g/kg of DM)) of the effect of essential oils in the diet of the beef cattle, WMD = weighted mean differences between essential oil treatments and control.
3.9. Environmental and Economic Impacts of Dietary Supplementation with Essential Oils in Beef Cattle Production
Simulation analysis indicated that the positive effects of dietary supplementation of EOs on DWG and FE of beef cattle improved the economic and environmental parameters of beef production. Table 8 shows that supplementation of EOs to 1000 cattle required to gain 200 kg BW, decreased 11 days of feeding days until slaughter. Moreover, dietary supplementation of EOs increased economic income (USD 4170/1000 animals) through a reduction in feed cost. In addition, dietary supplementation of EOs reduced emissions from feed used for beef production by 43 tons of CO2-eq, which contributed to a 0.83% reduction in the carbon footprint of beef production.

Table 8.
Economic and environmental impacts of essential oils (EOs) supplementation in beef cattle.
4. Discussion
4.1. Nutrient Intake and Digestibility
Some EOs have positive effects on rumen fungal and fibrolytic bacteria populations [53], which can result in increased DMD and NDFD. This can increase the rate of particle passage in the rumen and consequently increase DMI. It has been reported that EOs can improve the taste and palatability of livestock feeds [54]. In addition, dietary inclusion of EOs can increase DMI by improving feed quality, because EOs contain bioactive compounds (e.g., terpenes and terpenoids) with antioxidant and antimicrobial properties [55]. Similar effects of EOs consumption in the present study would partially explain the higher DMI observed in response to dietary supplementation of EOs. By comparison, Mucha and Witkowska [54] mentioned that EOs doses included in the diet of animals should be carefully established because the intense aroma of EOs may negatively affect the DMI of livestock. However, in the present study, a subgroup analysis revealed that DMI increased regardless of the dose of EOs used. Moreover, another subgroup analysis revealed that DMI increased significantly only when EOs were administered for more than 90 days. This suggests that beef cattle can adapt to consuming EOs, but this adaptation may require the use of EOs for prolonged periods of time.
With respect to digestibility, Zhou et al. [53] observed that dietary supplementation of EOs enhanced fungal growth in sheep rumen. In addition, in vitro [56] and in vivo [57] studies reported that EOs increase the relative abundance of fiber-degrading bacteria (Fibrobacter succinogenes, Ruminococcus albus, and R. flavefaciens) in sheep rumen fluid. Simlarly, in beef cattle, EOs have been reported to increase the relative abundance of ruminal microorganisms (Bacteroidetes, Fibrobacteres, and Firmicutes) that are positively correlated with NDFD [58], and with the concentration of β-glucosidase and cellulase in the rumen [59]. This can result in higher DMD and NDFD; however, in the present meta-analysis dietary supplementation of EOs did not affect DMD, CPD, OMD, or NDFD, but reduced ADFD. It has been mentioned that rumen protozoa play an important role in ruminal fiber degradation [60]. Therefore, the lower ADFD could be related to the reduction (−17.4%) observed in the population of rumen protozoa in cattle supplemented with EOs.
4.2. Growth Performance and Carcass Characteristics
Dietary supplementation of EOs increased DM and nutrient intake, which partially explains the higher DWG observed in the present study. Similarly, in beef cattle, Zhang et al. [59] reported that supplementation with EOs increased the relative abundance of Bacteroidetes and Firmicutes in the rumen microbiome, which have been reported to be positively correlated with higher DWG in goats [61]. Similar effects of EO consumption observed in our meta-analysis partially explain the observed increase in DWG for beef cattle supplemented with EOs. By comparison, it has been reported that low doses (260 mg/day) of EOs increase up to 30% of the length of ruminal papillae in beef cattle [59]. This can increase the area of volatile fatty acid absorption through the ruminal wall and lead to higher DWG and FE. Furthermore, in the present meta-analysis protozoan populations decreased in response to EOs supplementation. Removal of protozoa from the rumen increases the duodenal flux of microbial protein by up to 30% [62]. This can increase the metabolic availability of amino acids, which, in our case partially explains the higher DWG and FE observed in cattle supplemented with EOs.
In ruminants, the definition of the optimal supplementation period that improves animal performance is one of the main limitations of the use of EOs and other phytochemicals [28]. For example, in sheep, it has been reported that the metabolic benefits of dietary inclusion of EOs decrease over the supplementation period [42]. However, in the present meta-analysis, it was observed that FBW and DWG increased regardless of the supplementation period used, but the increase was greater when long supplementation periods (>90 days) were used. This is congruent with the higher DMI observed in the present study with supplementation of EOs for more than 90 days.
Dietary supplementation of EOs increased HCW and CCW. This may be related to the observed increase in FBW and LMA because HCW and CCW are positively correlated with FBW and LMA [63]. In addition, the increase observed for CCW may be beneficial because there is a positive correlation (r between 0.64 and 0.78) between CCW and the yield of primal cuts (ribs, sirloin, among others) in beef cattle [64].
Information on the mechanisms of action of EOs on muscle development and lipogenesis in beef cattle is still limited. However, in rats, it has been reported that some monoterpene-rich EOs can reduce skeletal muscle atrophy [65], which is characterized by reduced cross-sectional area and protein content of muscle fibers [66]. Similar effects of EO consumption observed in our meta-analysis partially explain the observed increase in LMA for cattle supplemented with EOs.
It has been mentioned that the size and number of adipocytes are related to the fat deposition process in beef cattle [67]. Ngamdokmai et al. [68] evaluated the effects of various EOs (Zingiber officinale, Piper nigrum, among others) and their primary bioactive metabolites (camphor, citral, limonene, and β-pinene, among others) on adipogenesis. In their study, they observed that all EOs and bioactive metabolites inhibited preadipocyte differentiation, and decreased lipid accumulation in maturing preadipocytes. However, in the present meta-analysis dietary supplementation of EOs did not affect BFT.
The main precursors of lipogenesis in ruminants are acetate and butyrate [69]. In the present study, subgroup analyses showed that high doses of EOs (>400 mg/kg DM) increased BFT without altering ruminal acetate concentration. This suggests that EOs can stimulate lipogenesis in ruminants through mechanisms unrelated to rumen acetate and butyrate concentration.
4.3. Ruminal Parameters and Nitrogen Balance
Rumen pH is an important parameter that can be used as an indicator of the internal homeostasis of the rumen environment [70]. In the present study, dietary inclusion of EOs did not affect rumen pH, suggesting that ruminal functions were performed under stable conditions of the rumen environment. By comparison, the lower rumen NH3-N concentration in cattle supplemented with EOs can be associated with the reduction (−17.4%) of total rumen protozoa, because Newbold et al. [62] reported that complete elimination of rumen protozoa decreases the rumen NH3-N concentration up to 26%.
In ruminants, it has been mentioned that increased rumen concentration of TVFA can be associated with increased metabolic availability of energy because rumen concentration of TVFA serves as the main source of energy for ruminants [71,72]. In the present meta-analysis, dietary supplementation of EOs reduced rumen TVFA concentration. This suggests that EOs reduce energy availability in beef cattle; however, DWG, FBW, and FE were higher in cattle supplemented with EOs. Moreover, the lower rumen TVFA concentration appears to be associated only with the observed reduction in acetate concentration, because EOs increased ruminal propionate concentration and did not affect butyrate concentration.
In beef cattle, Zhang et al. [59] observed that EOs supplementation increases the presence of ruminal microorganisms (Proteiniphilum acetatigenes and Parabacteroides distasonis) that are positively and negatively correlated with ruminal propionate and acetate concentration, respectively. Consequently, similar effects of EOs consumption in the present study would partially explain the higher rumen propionate concentration and lower acetate concentration observed.
Guyader et al. [73] reported that rumen protozoa are associated with CH4 emissions through the equation: CH4 (dry matter intake in g/kg) = −30.7 + 8.14 × protozoa ( cells/mL). Although in the present study dietary supplementation of EOs reduced rumen protozoan populations, no significant changes in CH4 emissions were observed. However, these results should be interpreted carefully due to the low number of studies reporting this response variable.
It has been mentioned that enteric CH4 emissions represent around 43% of greenhouse effect gases emitted in beef production worldwide [52]; therefore, considerable efforts have been made to reduce CH4 emissions. In this regard, in recent years there has been increased interest in evaluating the potential of some natural additives that could be used to reduce methanogenesis [18,29]. In the present meta-analysis, dietary inclusion of EOs did not decrease CH4 emissions. However, a meta-analysis by Belanche et al. [36] reported that the use of a commercial blend of EOs (Agolin) significantly decreased CH4 emissions in dairy cows. Therefore, EOs can be considered as a possible CH4 mitigation strategy, but more in vivo research is needed on the effects of EOs on enteric methane emissions and rumen methanogenic microorganisms.
It has been reported that N plays an important role in the growth and productivity of ruminants [74]. N balance can be used to evaluate the efficiency of utilization of ingested protein in ruminants [72]. In the present study, retained N was higher in cattle supplemented with EOs. This suggests that beef cattle can better utilize ingested protein in the presence of EOs in the diet. Moreover, the higher level of retained N may be related to lower urinary N excretion observed in response to EOs supplementation. By comparison, it has been mentioned that N excreted by ruminants can pollute the environment through ammonia volatilization emissions and nitrous oxide emissions [75]. In the present study, the reduction observed for urinary N excretion suggests that EOs can be useful in reducing environmental N pollution. This is because, compared to fecal N excretion, urinary N causes more nitrous oxide emissions [76]. However, these results should be interpreted with caution because of the low number of studies that reported these response variables.
4.4. Blood Metabolites
When there is an excess of NH3-N in the rumen, it is absorbed through the rumen wall, then passes to the liver where it is converted to urea and, finally, the urea is transferred to the bloodstream [77]. Similarly, Paengkoum et al. [78] reported a positive correlation (r = 0.55) between serum urea concentration and ruminal NH3-N concentration. In the present meta-analysis, the lower serum urea concentration was observed in response to dietary supplementation of EOs, which may be associated with lower rumen NH3-N concentration observed in animals supplemented with EOs. In addition, serum glucose and NEFA concentration are considered important indicators of energy status in cattle [79]. In the present study, the serum glucose concentration was similar between treatments; however, the inclusion of EOs in the diet decreased the serum NEFA concentration. This indicates better energy balance in beef cattle supplemented with EOs compared to control treatment cattle. In addition, the dietary inclusion of EOs increased DMI; this provides more energy to the animal and partially explains the lower serum NEFA concentration observed.
Dietary supplementation of EOs reduced the percentage of neutrophils and basophils; however, these results should be interpreted with caution due to the low number of studies reporting these response variables. Blood neutrophil depletion usually occurs in the presence of bacterial disease [80]. However, it has been mentioned that neutrophils are not always related to the presence of bacterial infections because the number of neutrophils also decreases in response to other factors [81]. It has also been reported that basophils increase in response to bacterial, viral, and parasitic infections [82]. Therefore, the lower percentage of basophils in beef cattle supplemented with EOs suggests that these animals had a better health status.
According to Ceciliani et al. [83], activation of the innate immune system under conditions of inflammation, infection, and tissue injury can result in the release of acute-phase proteins (e.g., haptoglobin, SAA, and LBP) into the bloodstream. For example, plasma haptoglobin concentration increases in cattle with subacute ruminal acidosis [84]. In the present meta-analysis, dietary inclusion of EOs reduced haptoglobin concentration, suggesting that EOs can reduce the incidence of SARA. On the other hand, SAA and LBP are involved in endotoxin clearance during an acute phase response [85], and their blood concentration increases when there is endotoxin transfer to the bloodstream [83]. Therefore, in the present study, the absence of changes in the blood concentration of SAA and LBP suggests that EO supplementation does not significantly affect the transfer of endotoxins into the blood.
4.5. Meat Quality
It has been mentioned that meat pH is related to water holding capacity (WHC) and tenderness [86]. Similarly, in beef, Węglarz [87] reported negative correlations (r between −0.24 and −0.29) between pH and color parameters (L* a* and b*). In the present study, EOs supplementation did not affect meat pH, suggesting that EOs can be used in beef diets without deteriorating meat color, tenderness, and WHC. The cooking loss can also be used as an indicator of WHC in meat because there is a negative correlation (r = −0.89) between these parameters [88]. Therefore, the values observed for cooking loss in the present meta-analysis suggest that the dietary inclusion of EOs improves WHC.
Dietary supplementation of EOs reduced ShF, suggesting that EOs increase beef tenderness. Collagen content is related to ShF of beef [89]. However, in the present study, collagen content was similar between treatments. By comparison, Rowe et al. [90] observed that the presence of post-mortem oxidative conditions inactivates calpain activity and decreases myofibrillar proteolysis in beef, which decreases meat tenderness. In the present meta-analysis, lipid oxidation was lower in beef supplemented with EOs, which partially explains the lower ShF observed. In addition, tenderness has been identified as one of the main attributes that determine meat quality [91], and some consumers are willing to pay more for meat if it is guaranteed to have higher tenderness [92]. Therefore, the dietary inclusion of EOs can be used as a nutritional strategy to improve the quality, acceptability, and price of beef.
According to Falowo et al. [93], the main non-microbial cause of quality loss in meat and meat products is lipid oxidation. MDA content is used to determine the presence of lipid peroxidation in meat [94]. In the present meta-analysis, lower MDA content was observed in meat from beef cattle supplemented with EOs, indicating that dietary inclusion of EOs reduces lipid peroxidation and improves beef quality. There is limited information on the mechanisms of action of EOs on the activity of antioxidant enzymes in ruminants. However, in pigs and poultry, dietary supplementation of EOs has been reported to increase mRNA abundance and activity of superoxide dismutase, catalase, and glutathione peroxidase in smooth and skeletal muscle [95,96]. Therefore, similar effects of EOs consumption in the present study would partially explain the lower MDA content observed in the meat.
The L* in beef makes this product more attractive to consumers because the brightness of red meat is associated with fresh products [97]. In the present study, dietary supplementation of EOs did not affect the L* of meat, suggesting that dietary inclusion of EOs does not affect the appearance of beef. By comparison, a* values in meat decrease because of metmyoglobin formation [98]. Therefore, the absence of observed changes in a* in meat suggests that EOs do not affect metmyoglobin formation. Furthermore, it has been reported that in meat b* values are related to its fat content [99] and pH [100]. In the present meta-analysis, EOs supplementation did not affect the fat content and pH of meat, which would partially explain the lack of changes observed in b*.
Dietary supplementation of EOs did not affect the protein, fat, ash, and moisture content of meat. It has been mentioned that the nutritional value of meat is related to its protein, fat, vitamin, and mineral content [101]. This suggests that the dietary inclusion of EOs does not affect the nutritional value of beef. In the present meta-analysis, the lack of observed differences in the chemical composition of the meat was expected, because the nutritional composition of the diets was similar between the control and EOs-supplemented treatments.
4.6. Simulation Analysis
The environmental impact of beef cattle production systems has become a global concern [102] because beef production contributes about 41% of greenhouse gas emissions from the global livestock sector [52]. According to Capper and Hayes [103], in beef cattle, the improvement of productivity is positively related to economic and environmental sustainability. In the present study, the simulation analysis used showed that the positive effects of EOs on DWG and FE increased economic income through a 1.44% reduction in feed cost. Moreover, the benefits of dietary inclusion of EOs in DWG and FE decreased the environmental footprint by 0.83%, as a consequence of the reduction in emissions associated with bovine feed use. This suggests that dietary inclusion of EOs can improve the economic and environmental sustainability of beef production.
5. Conclusions
The results of the present meta-analysis indicate that the inclusion of EOs in beef cattle diets does not affect digestibility but improves dry matter intake, final body weight and daily weight gain, feed efficiency, carcass weight, and Longissimus dorsi muscle area. The best results for dry matter intake are obtained with EOs doses higher than 400 mg/kg DM, with supplementation periods longer than 90 days, and using diets high in concentrate (>700 g/kg DM). Similarly, the best results for final body weight and daily weight gain are achieved with EOs supplementation periods longer than 90 days and using high concentrate diets (>700 g/kg DM). Therefore, EOs can be used as natural growth promoters in beef cattle; however, more in vivo research is needed to confirm the effects of EOs on the performance and feed efficiency of beef cattle.
Additionally, EOs improve fermentation by increasing ruminal propionate concentration and reducing the concentration of ammonia nitrogen, acetate, and rumen protozoa. Moreover, the results of blood metabolites indicate that EOs do not negatively affect the health of beef cattle. Similarly, EOs do not affect the color and chemical composition of meat but reduce lipid oxidation and improve water holding capacity and tenderness. Finally, the results of the simulation analysis indicate that the addition of EOs in beef cattle diets can be used as a natural alternative to reduce the environmental impact and increase the economic profitability of beef production.
Author Contributions
Conceptualization, J.F.O.-O., G.D.-I. and A.L.-B.; methodology, J.F.O.-O. and G.D.-I.; software, L.A.M.-R. and G.D.M.-M.; validation, A.L.-B., L.A.M.-R. and G.D.M.-M.; formal analysis, J.F.O.-O. and G.D.-I.; investigation, J.F.O.-O. and G.D.-I.; resources, A.L.-B.; data curation, J.F.O.-O., I.S.-F. and G.D.-I.; writing—original draft preparation, J.F.O.-O. and G.D.-I.; writing—review and editing, A.L.-B., L.A.M.-R., G.D.M.-M. and I.S.-F.; visualization, J.F.O.-O.; supervision, A.L.-B.; project administration, A.L.-B.; funding acquisition, A.L.-B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
The first author, José Felipe Orzuna Orzuna is a Ph.D. student in the Program of Animal Production of the Universidad Autónoma Chapingo, and thanks to the CONACyT Program for the scholarship.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Figure A1.
A PRISMA flow diagram detailing the literature search strategy and study selection for the meta-analysis.

Table A1.
Summary of the studies included in the meta-analysis.
Table A1.
Summary of the studies included in the meta-analysis.
| Author | Country | Primary bioactive compound | Dose, mg/kg DM | C in Diet, g/kg DM | Duration, d |
|---|---|---|---|---|---|
| Afzalani et al. [104] | Indonesia | Blend (n = 4) | 200, 400, 800, 1200 | 400 (n = 4) | 90 (n = 4) |
| Alemu et al. [9] | Canada | Blend (n = 2) | 150 (n = 2) | 200 (n = 2) | 84 (n = 2) |
| Almeida et al. [105] | Brazil | Blend (n = 1) | 500 (n = 1) | 700 (n = 1) | 105 (n = 1) |
| Brand et al. [106] | Canada | Blend (n = 2) | 50, 100 | 900 (n = 4) | 112 (n = 4) |
| Carvalho et al. [107] | Brazil | Blend (n = 3) | 191, 398, 601 | 700 (n = 3) | 74 (n = 3) |
| Chapman et al. [108] | United States | Cinnamaldehyde (n = 2) | 37, 76 | 470 (n = 2) | 70 (n = 2) |
| Dorleku et al. [16] | Canada | Blend (n = 2) | 85, 337 | 850 (n = 2) | 100 (n = 2) |
| Fandiño et al. [11] | Spain | Capsaicin, blend, anethole (n = 3) | 12, 31, 62 (n = 3) | 900 (n = 9) | 96 (n = 9) |
| Filho et al. [109] | Brazil | Thymol (n = 3) | 232, 469, 965 | 500 (n = 3) | 84 (n = 3) |
| Gouvêa et al. [10] | United States | Blend (n = 1) | 120 (n = 1) | 930 (n = 1) | 154 (n = 1) |
| Guerrero et al. [110] | Brazil | Blend (n = 2) | 500, 1000 | 900 (n = 2) | 120 (n = 2) |
| Khorrami et al. [111] | Iran | Thymol, cinnamaldehyde | 500, 500 | 700 (n = 2) | 84 (n = 2) |
| Kim et al. [112] | Korea | Blend (n = 3) | 39, 79, 113 | 900 (n = 3) | 390 (n = 3) |
| Latack et al. [113] | United States | Blend (n = 2) | 110 (n = 2) | 880 (n = 2) | 216, 84 |
| Monteschio et al. [15] | Brazil | Blend (n = 4) | 500 (n = 4) | Not reported | 73 (n = 4) |
| Monteschio et al. [114] | Brazil | Blend (n = 4) | 500 (n = 4) | Not reported | 73 (n = 4) |
| Ornaghi et al. [115] | Brazil | Blend (n = 4) | 444, 865, 450, 890 | 900 (n = 4) | 187 (n = 4) |
| Ornaghi et al. [97] | Brazil | Blend (n = 4) | 153, 305, 444, 594 | 700 (n = 4) | 62 (n = 4) |
| Prado et al. [116] | Brazil | Blend (n = 1) | 442 (n = 1) | 500 (n = 1) | 115 (n = 1) |
| Pukrop et al. [117] | United States | Blend (n = 1) | 104 (n = 1) | 860 (n = 1) | 167 (n = 1) |
| Rivaroli et al. [118] | Brazil | Blend (n = 2) | 500, 1000 | 900 (n = 2) | 120 (n = 2) |
| Souza et al. [119] | Brazil | Blend (n = 4) | 789, 640, 678, 644 | 750 (n = 2) | 73 (n = 2) |
| Teobaldo et al. [12] | Brazil | Blend (n = 2) | 150, 300 | 280 (n = 2) | 76 (n = 2) |
| Tomkins et al. [120] | Australia | Blend (n = 2) | 185, 370 | 0 (n = 2) | 200 (n = 2) |
| Torrecilhas et al. [121] | Brazil | Eugenol (n = 2), cinnamaldehyde (n = 2) | 450, 880, 450, 880 | 900 (n = 4) | 187 (n = 4) |
| Vakili et al. [122] | Iran | Thymol, cinnamaldehyde | 617, 641 | 850 (n = 2) | 45 (n = 2) |
| Valero et al. [123] | Brazil | Blend (n = 1) | 550 (n = 1) | 550 (n = 1) | 55 (n = 1) |
| Wanapat et al. [124] | Thailand | Blend (n = 3) | 17, 25, 40 | 220 (n = 3) | 84 (n = 3) |
| Westphalen et al. [14] | United States | Capsaicin (n = 4) | 15, 5, 10, 15 | 900 (n = 4) | 84 (n = 3), 80 |
| Wu et al. [125] | China | Blend (n = 1) | 26 (n = 1) | 400 (n = 1) | 240 (n = 1) |
| Yang et al. [126] | Canada | Cinnamaldehyde (n = 3) | 37, 79, 184 | 850 (n = 3) | 84 (n = 3) |
| Yang et al. [85] | Canada | Eugenol (n = 3) | 42, 81, 166 | 850 (n = 3) | 84 (n = 3) |
| Yang et al. [13] | Canada | Cinnamaldehyde (n = 3) | 47, 98, 208 | 900 (n = 3) | 112 (n = 3) |
| Zotti et al. [12] | Brazil | Blend (n = 2) | 400 (n = 2) | 923 (n = 2) | 21 (n = 2) |
DM: dry matter; C: concentrate; d: days; n: number of treatments.

Table A2.
Simulation inputs were used for the economic and environmental impacts of essential oils (EOs) supplementation in beef cattle.
Table A2.
Simulation inputs were used for the economic and environmental impacts of essential oils (EOs) supplementation in beef cattle.
| Item | Control | EOs | Difference | % Change |
|---|---|---|---|---|
| Number of animals | 1000 | 1000 | ||
| DMI (kg DM/d/animal) | 8.40 | 8.40 | ||
| DWG (kg/d/animal) 1 | 1.213 | 1.300 | +0.087 | +7.2 |
| FE (kg DWG/kg DMI/animal) | 0.173 | 0.177 | +0.004 | +2.3 |
| TLWG (kg/animal) | 200 | 200 | ||
| LMY (kg/animal) 2 | 82 | 82 | ||
| BPO (kg/animal) 3 | 17.2 | 17.2 |
DMI: dry matter intake; DWG: daily weight gain; FE: feed efficiency; TLWG; target live weight gain; LMY: lean meat yield; BPO: beef protein output; 1: DWG of beef cattle fed EOs diets was corrected based on the current meta-analysis results which showed an average increase of +87 g/d/head in the DWG of beef cattle fed EOs diets. 2: meat yield was calculated as a proportion of the TLWG (200 kg). According to Holland et al. [127], the average LMY of beef cattle was assumed to be 41% of body weight. 3: BPO was calculated as a proportion of the LMY. Previous studies [128,129] have reported that beef contains an average of 21% protein.
References
- Iskandar, K.; Molinier, L.; Hallit, S.; Sartelli, M.; Catena, F.; Coccolini, F.; Hardcastle, T.C.; Roques, C.; Salameh, P. Drivers of Antibiotic Resistance Transmission in Low- and Middle-Income Countries from a “One Health” Perspective—A Review. Antibiotics 2020, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Deplano, A.; Meghraoui, A.; Dodémont, M.; Heinrichs, A.; Denis, O.; Nonhoff, C.; Roisin, S. Bacteria from Animals as a Pool of Antimicrobial Resistance Genes. Antibiotics 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Khan, A.; Naz, S.; Ullah, Q.; Laudadio, V.; Tufarelli, V.; Ragni, M. Potential Applications of Moringa oleifera in Poultry Health and Production as Alternative to Antibiotics: A Review. Antibiotics 2021, 10, 1540. [Google Scholar] [CrossRef] [PubMed]
- Nehme, R.; Andrés, S.; Pereira, R.B.; Ben Jemaa, M.; Bouhallab, S.; Ceciliani, F.; López, S.; Rahali, F.Z.; Ksouri, R.; Pereira, D.M.; et al. Essential Oils in Livestock: From Health to Food Quality. Antioxidants 2021, 10, 330. [Google Scholar] [CrossRef]
- Irawan, A.; Hidayat, C.; Jayanegara, A.; Ratriyanto, A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: A meta-analysis. Anim. Biosci. 2021, 34, 1499–1513. [Google Scholar] [CrossRef]
- Zeng, Z.; Zhang, S.; Wang, H.; Piao, X. Essential oil and aromatic plants as feed additives in non-ruminant nutrition: A review. J. Anim. Sci. Biotechnol. 2015, 6, 7. [Google Scholar] [CrossRef]
- Khan, R.U.; Fatima, A.; Naz, S.; Ragni, M.; Tarricone, S.; Tufarelli, V. Perspective, Opportunities and Challenges in Using Fennel (Foeniculum vulgare) in Poultry Health and Production as an Eco-Friendly Alternative to Antibiotics: A Review. Antibiotics 2022, 11, 278. [Google Scholar] [CrossRef]
- Ma, J.; Long, S.; Wang, J.; Gao, J.; Piao, X. Microencapsulated essential oils combined with organic acids improves immune antioxidant capacity and intestinal barrier function as well as modulates the hindgut microbial community in piglets. J. Anim. Sci. Biotechnol. 2022, 13, 16. [Google Scholar] [CrossRef]
- Cardozo, P.W.; Calsamiglia, S.; Ferret, A.; Kamel, C. Screening for the effects of natural plant extracts at different pH on in vitro rumen microbial fermentation of a high-concentrate diet for beef cattle1. J. Anim. Sci. 2005, 83, 2572–2579. [Google Scholar] [CrossRef]
- Estrada-Angulo, A.; Arteaga-Wences, Y.J.; Castro-Pérez, B.I.; Urías-Estrada, J.D.; Gaxiola-Camacho, S.; Angulo-Montoya, C.; Ponce-Barraza, E.; Barreras, A.; Corona, L.; Zinn, R.A.; et al. Blend of Essential Oils Supplemented Alone or Combined with Exogenous Amylase Compared with Virginiamycin Supplementation on Finishing Lambs: Performance, Dietary Energetics, Carcass Traits, and Nutrient Digestion. Animals 2021, 11, 2390. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, K.; Li, C.; Wu, J.; Davis, D.; Casper, D.; Jiang, H.; Jiao, T.; Wang, X.; Wang, J. Dietary supplementation with Essential-oils-cobalt for improving growth performance, meat quality and skin cell capacity of goats. Sci. Rep. 2018, 8, 11634. [Google Scholar] [CrossRef]
- Teobaldo, R.W.; De Paula, N.F.; Zervoudakis, J.T.; Fonseca, M.A.; Cabral, L.S.; Martello, H.F.; Rocha, J.K.L.; Ribeiro, I.J.; Mundim, A.T. Inclusion of a blend of copaiba, cashew nut shell and castor oil in the protein-energy supplement for grazing beef cattle improves rumen fermentation, nutrient intake and fibre digestibility. Anim. Prod. Sci. 2020, 60, 1039–1050. [Google Scholar] [CrossRef]
- Arteaga-Wences, Y.J.; Estrada-Angulo, A.; Ríos-Rincón, F.G.G.; Castro-Pérez, B.; Mendoza-Cortéz, D.A.; Manriquez-Núñez, O.M.; Barreras, A.; Corona-Gochi, L.; Zinn, R.A.; Perea-Domínguez, X.P.; et al. The effects of feeding a standardized mixture of essential oils vs monensin on growth performance, dietary energy and carcass characteristics of lambs fed a high-energy finishing diet. Small Rumin. Res. 2021, 205, 106557. [Google Scholar] [CrossRef]
- Parvar, R.; Ghoorchi, T.; Kashfi, H.; Parvar, K. Effect of Ferulago angulata (Chavil) essential oil supplementation on lamb growth performance and meat quality characteristics. Small Rumin. Res. 2018, 167, 48–54. [Google Scholar] [CrossRef]
- Ortuño, J.; Serrano, R.; Bañón, S. Incorporating rosemary diterpenes in lamb diet to improve microbial quality of meat packed in different environments. Anim. Sci. J. 2017, 88, 1436–1445. [Google Scholar] [CrossRef]
- Moura, L.V.; Oliveira, E.R.; Fernandes, A.R.M.; Gabriel, A.M.A.; Silva, L.H.X.; Takiya, C.S.; Cônsolo, N.R.B.; Rodrigues, G.C.G.; Lemos, T.; Gandra, J.R. Feed efficiency and carcass traits of feedlot lambs supplemented either monensin or increasing doses of copaiba (Copaifera spp.) essential oil. Anim. Feed Sci. Technol. 2017, 232, 110–118. [Google Scholar] [CrossRef]
- Serrano, R.; Jordán, M.J.; Bañón, S. Use of dietary rosemary extract in ewe and lamb to extend the shelf life of raw and cooked meat. Small Rumin. Res. 2014, 116, 144–152. [Google Scholar] [CrossRef]
- Alemu, A.W.; Romero-Pérez, A.; Araujo, R.C.; Beauchemin, K.A. Effect of Encapsulated Nitrate and Microencapsulated Blend of Essential Oils on Growth Performance and Methane Emissions from Beef Steers Fed Backgrounding Diets. Animals 2019, 9, 21. [Google Scholar] [CrossRef]
- Gouvêa, V.N.; Duff, G.C.; Sowers, C.A.; Barnes, M.L. Effects of supplemental phytomolecules on growth performance, carcass characteristics and liver abnormalities of finishing beef steers. J. Appl. Anim. Res. 2021, 49, 324–329. [Google Scholar] [CrossRef]
- Fandiño, I.; Ferret, A.; Calsamiglia, S. Dose and combinations of anise oil and capsicum oleoresin as rumen fermentation modifiers in vitro and in vivo with high concentrate diets fed to Holstein beef heifers. Anim. Feed Sci. Technol. 2020, 260, 114363. [Google Scholar] [CrossRef]
- Zotti, C.A.; Silva, A.P.; Carvalho, R.; Marino, C.T.; Rodrigues, P.H.M.; Silva, L.F.P.; McAllister, T.A.; Leme, P.R. Monensin and a blend of castor oil and cashew nut shell liquid used in a high-concentrate diet abruptly fed to Nellore cattle1. J. Anim. Sci. 2017, 95, 4124–4138. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ametaj, B.N.; Benchaar, C.; He, M.L.; Beauchemin, K.A. Cinnamaldehyde in feedlot cattle diets: Intake, growth performance, carcass characteristics, and blood metabolites1. J. Anim. Sci. 2010, 88, 1082–1092. [Google Scholar] [CrossRef]
- Westphalen, M.F.; Carvalho, P.H.V.; Oh, J.; Hristov, A.N.; Staniar, W.B.; Felix, T.L. Effects of feeding rumen-protected Capsicum oleoresin on growth performance, health status, and total tract digestibility of growing beef cattle. Anim. Feed Sci. Technol. 2021, 271, 114778. [Google Scholar] [CrossRef]
- de Oliveira Monteschio, J.; de Souza, K.A.; Vital, A.C.P.; Guerrero, A.; Valero, M.V.; Kempinski, E.M.B.C.; Barcelos, V.C.; Nascimento, K.F.; do Prado, I.N. Clove and rosemary essential oils and encapsuled active principles (eugenol, thymol and vanillin blend) on meat quality of feedlot-finished heifers. Meat Sci. 2017, 130, 50–57. [Google Scholar] [CrossRef]
- Dorleku, J.B.; Wang, L.M.; Zhou, Z.Y.; Mandell, I.; Bohrer, B.M. Effects of feeding two different blends of essential oils to finishing steers on growth performance, carcass characteristics, meat quality, meat composition, and shelf life. Can. J. Anim. Sci. 2021, 101, 507–526. [Google Scholar] [CrossRef]
- Benchaar, C.; Calsamiglia, S.; Chaves, A.V.; Fraser, G.R.; Colombatto, D.; McAllister, T.A.; Beauchemin, K.A. A review of plant-derived essential oils in ruminant nutrition and production. Anim. Feed Sci. Technol. 2008, 145, 209–228. [Google Scholar] [CrossRef]
- Simitzis, P.E. Enrichment of Animal Diets with Essential Oils—A Great Perspective on Improving Animal Performance and Quality Characteristics of the Derived Products. Medicines 2017, 4, 35. [Google Scholar] [CrossRef]
- Kholif, A.E.; Olafadehan, O.A. Essential oils and phytogenic feed additives in ruminant diet: Chemistry, ruminal microbiota and fermentation, feed utilization and productive performance. Phytochem. Rev. 2021, 20, 1087–1108. [Google Scholar] [CrossRef]
- Khiaosa-Ard, R.; Zebeli, Q. Meta-analysis of the effects of essential oils and their bioactive compounds on rumen fermentation characteristics and feed efficiency in ruminants1. J. Anim. Sci. 2013, 91, 1819–1830. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.T.; Rothstein, H.R. Introduction to Meta-Analysis, 1st ed.; John Wiley & Sons: Chichester, UK, 2009; p. 413. [Google Scholar]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Hernández-García, P.A. Effects of Dietary Tannins’ Supplementation on Growth Performance, Rumen Fermentation, and Enteric Methane Emissions in Beef Cattle: A Meta-Analysis. Sustainability 2021, 13, 7410. [Google Scholar] [CrossRef]
- Orzuna-Orzuna, J.F.; Dorantes-Iturbide, G.; Lara-Bueno, A.; Mendoza-Martínez, G.D.; Miranda-Romero, L.A.; Lee-Rangel, H.A. Growth Performance, Meat Quality and Antioxidant Status of Sheep Supplemented with Tannins: A Meta-Analysis. Animals 2021, 11, 3184. [Google Scholar] [CrossRef] [PubMed]
- Lean, I.J.; Rabiee, A.R.; Duffield, T.F.; Dohoo, I.R. Invited review: Use of meta-analysis in animal health and reproduction: Methods and applications. J. Dairy Sci. 2009, 92, 3545–3565. [Google Scholar] [CrossRef]
- Belanche, A.; Newbold, C.J.; Morgavi, D.P.; Bach, A.; Zweifel, B.; Yáñez-Ruiz, D.R. A Meta-analysis Describing the Effects of the Essential oils Blend Agolin Ruminant on Performance, Rumen Fermentation and Methane Emissions in Dairy Cows. Animals 2020, 10, 620. [Google Scholar] [CrossRef]
- Higgins, J.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; John Wiley and Sons, Ltd.: Chichester, UK, 2019; pp. 143–176. [Google Scholar]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Appuhamy, J.R.N.; Strathe, A.B.; Jayasundara, S.; Wagner-Riddle, C.; Dijkstra, J.; France, J.; Kebreab, E. Anti-methanogenic effects of monensin in dairy and beef cattle: A meta-analysis. J. Dairy Sci. 2013, 96, 5161–5173. [Google Scholar] [CrossRef]
- SAS (Statistical Analysis System). SAS/STAT User’s Guide (Release 6.4); SAS Institute: Cary, NC, USA, 2017. [Google Scholar]
- Torres, R.N.S.; Moura, D.C.; Ghedini, C.P.; Ezequiel, J.M.B.; Almeida, M.T.C. Meta-analysis of the effects of essential oils on ruminal fermentation and performance of sheep. Small Rumin. Res. 2020, 189, 106148. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Altman, D.G. Systematic Reviews in Health Care, 2nd ed.; MBJ Publishing Group: London, UK, 2001; pp. 109–121. [Google Scholar]
- Lean, I.J.; Thompson, J.M.; Dunshea, F.R. A Meta-Analysis of Zilpaterol and Ractopamine Effects on Feedlot Performance, Carcass Traits and Shear Strength of Meat in Cattle. PLoS ONE 2014, 9, e115904. [Google Scholar] [CrossRef]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. A Nonparametric “Trim and Fill” Method of Accounting for Publication Bias in Meta-Analysis. J. Am. Stat. Assoc. 2000, 95, 89–98. [Google Scholar] [CrossRef]
- Littell, J.H.; Corcoran, J.; Pillai, V. Systematic Reviews and Meta-Analysis, 1st ed.; Oxford University Press: Oxford, UK, 2008; pp. 111–132. [Google Scholar]
- Salami, S.A.; Moran, C.A.; Warren, H.E.; Taylor-Pickard, J. A Meta-Analysis of the Effects of Slow-Release Urea Supplementation on the Performance of Beef Cattle. Animals 2020, 10, 657. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Techno-economic and environmental assessment of essential oil extraction from Citronella (Cymbopogon winteriana) and Lemongrass (Cymbopogon citrus): A Colombian case to evaluate different extraction technologies. Ind. Crop. Prod. 2014, 54, 175–184. [Google Scholar] [CrossRef]
- Moncada, J.; Tamayo, J.A.; Cardona, C.A. Techno-economic and environmental assessment of essential oil extraction from Oregano (Origanum vulgare) and Rosemary (Rosmarinus officinalis) in Colombia. J. Clean. Prod. 2016, 112, 172–181. [Google Scholar] [CrossRef]
- Ortiz-Sanchez, M.; Solarte-Toro, J.C.; Orrego-Alzate, C.E.; Acosta-Medina, C.D.; Cardona-Alzate, C.A. Integral use of orange peel waste through the biorefinery concept: An experimental, technical, energy, and economic assessment. Biomass- Convers. Biorefinery 2021, 11, 645–659. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; FAO: Rome, Italy, 2013. [Google Scholar]
- Zhou, R.; Wu, J.; Zhang, L.; Liu, L.; Casper, D.P.; Jiao, T.; Liu, T.; Wang, J.; Lang, X.; Song, S.; et al. Effects of oregano essential oil on the ruminal pH and microbial population of sheep. PLoS ONE 2019, 14, e0217054. [Google Scholar] [CrossRef]
- Mucha, W.; Witkowska, D. The Applicability of Essential Oils in Different Stages of Production of Animal-Based Foods. Molecules 2021, 26, 3798. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Patra, A.K.; Yu, Z. Essential oils affect populations of some rumen bacteria in vitro as revealed by microarray (RumenBactArray) analysis. Front. Microbiol. 2015, 6, 297. [Google Scholar] [CrossRef]
- Naseri, V.; Kafilzadeh, F.; Jahani-Azizabadi, H. Effects of Pistacia atlantica gum essential oil on ruminal methanogen, protozoa, selected bacteria species and fermentation characteristics in sheep. Small Rumin. Res. 2022, 209, 106650. [Google Scholar] [CrossRef]
- Xu, L.; Wen, L.; Ge, Y.; Wan, G.; Qu, M.; Xue, F. Metagenomic Insights Into the Effects of Rare-Earth Elements Supplementation on Rumen Digestibility and Meat Quality of Beef Cattle. Front. Microbiol. 2020, 11, 1933. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, J.; Lei, Y.; Bai, Y.; Jia, L.; Li, Z.; Liu, T.; Xu, Y.; Sun, J.; Wang, Y.; et al. Oregano Essential Oils Promote Rumen Digestive Ability by Modulating Epithelial Development and Microbiota Composition in Beef Cattle. Front. Nutr. 2021, 8, 871. [Google Scholar] [CrossRef]
- Williams, A.G.; Coleman, G.S. The Rumen Protozoa, 1st ed.; Springer: New York, NY, USA, 1992; pp. 1–425. [Google Scholar]
- Min, B.R.; Gurung, N.; Shange, R.; Solaiman, S.; Shange, R. Potential role of rumen microbiota in altering average daily gain and feed efficiency in meat goats fed simple and mixed pastures using bacterial tag-encoded FLX amplicon pyrosequencing1. J. Anim. Sci. 2019, 97, 3523–3534. [Google Scholar] [CrossRef]
- Newbold, C.J.; De La Fuente, G.; Belanche, A.; Ramos-Morales, E.; McEwan, N.R. The Role of Ciliate Protozoa in the Rumen. Front. Microbiol. 2015, 6, 1313. [Google Scholar] [CrossRef]
- Kwon, K.-M.; Nogoy, K.M.C.; Jeon, H.-E.; Han, S.-J.; Woo, H.-C.; Heo, S.-M.; Hong, H.K.; Lee, J.-I.; Lee, D.H.; Choi, S.H. Market weight, slaughter age, and yield grade to determine economic carcass traits and primal cuts yield of Hanwoo beef. J. Anim. Sci. Technol. 2022, 64, 143–154. [Google Scholar] [CrossRef]
- Seo, H.-W.; Van Ba, H.; Seong, P.-N.; Kim, Y.-S.; Kang, S.-M.; Seol, K.-H.; Kim, J.-H.; Moon, S.-S.; Choi, Y.-M.; Cho, S.-H. Relationship between body size traits and carcass traits with primal cuts yields in Hanwoo steers. Anim. Biosci. 2021, 34, 127–133. [Google Scholar] [CrossRef]
- Ryu, Y.; Lee, D.; Jung, S.H.; Lee, K.-J.; Jin, H.; Kim, S.J.; Lee, H.M.; Kim, B.; Won, K.-J. Sabinene Prevents Skeletal Muscle Atrophy by Inhibiting the MAPK–MuRF-1 Pathway in Rats. Int. J. Mol. Sci. 2019, 20, 4955. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, G.; Tan, X.; Zhang, X.; Liu, X.; Wei, C. Gallic acid as a key substance to inhibit proliferation and adipogenesis in bovine subcutaneous adipocyte. Anim. Biotechnol. 2020, 1–7. [Google Scholar] [CrossRef]
- Ngamdokmai, N.; Paracha, T.U.; Waranuch, N.; Chootip, K.; Wisuitiprot, W.; Suphrom, N.; Insumrong, K.; Ingkaninan, K. Effects of Essential Oils and Some Constituents from Ingredients of Anti-Cellulite Herbal Compress on 3T3-L1 Adipocytes and Rat Aortae. Pharmaceuticals 2021, 14, 253. [Google Scholar] [CrossRef] [PubMed]
- Zambell, K.L.; Fitch, M.D.; Fleming, S.E. Acetate and Butyrate Are the Major Substrates for De Novo Lipogenesis in Rat Colonic Epithelial Cells. J. Nutr. 2003, 133, 3509–3515. [Google Scholar] [CrossRef] [PubMed]
- Suriyapha, C.; Cherdthong, A.; Suntara, C.; Polyorach, S. Utilization of Yeast Waste Fermented Citric Waste as a Protein Source to Replace Soybean Meal and Various Roughage to Concentrate Ratios on In Vitro Rumen Fermentation, Gas Kinetic, and Feed Digestion. Fermentation 2021, 7, 120. [Google Scholar] [CrossRef]
- Supapong, C.; Cherdthong, A. Effect of Sulfur and Urea Fortification of Fresh Cassava Root in Fermented Total Mixed Ration on the Improvement Milk Quality of Tropical Lactating Cows. Vet. Sci. 2020, 7, 98. [Google Scholar] [CrossRef]
- Chanjula, P.; Suntara, C.; Cherdthong, A. The Effects of Oil Palm Fronds Silage Supplemented with Urea-Calcium Hydroxide on Rumen Fermentation and Nutrient Digestibility of Thai Native-Anglo Nubian Goats. Fermentation 2021, 7, 218. [Google Scholar] [CrossRef]
- Guyader, J.; Eugène, M.; Nozière, P.; Morgavi, D.P.; Doreau, M.; Martin, C. Influence of rumen protozoa on methane emission in ruminants: A meta-analysis approach. Animal 2014, 8, 1816–1825. [Google Scholar] [CrossRef]
- Hristov, A.; Bannink, A.; Crompton, L.; Huhtanen, P.; Kreuzer, M.; McGee, M.; Nozière, P.; Reynolds, C.; Bayat, A.-R.; Yáñez-Ruiz, D.; et al. Invited review: Nitrogen in ruminant nutrition: A review of measurement techniques. J. Dairy Sci. 2019, 102, 5811–5852. [Google Scholar] [CrossRef]
- Singh, B.P.; Cowie, A.L.; Chan, K.Y. Soil Health and Climate Change, 1st ed.; Springer: Berlin, Germany, 2011; p. 403. [Google Scholar]
- Wecking, A.R.; Wall, A.M.; Liáng, L.L.; Lindsey, S.B.; Luo, J.; Campbell, D.I.; Schipper, L.A. Reconciling annual nitrous oxide emissions of an intensively grazed dairy pasture determined by eddy covariance and emission factors. Agric. Ecosyst. Environ. 2020, 287, 106646. [Google Scholar] [CrossRef]
- Abdoun, K.A.; Stumpff, F.; Martens, H. Ammonia and urea transport across the rumen epithelium: A review. Anim. Health Res. Rev. 2006, 7, 43–59. [Google Scholar] [CrossRef]
- Paengkoum, P.; Chen, S.; Paengkoum, S. Effects of crude protein and undegradable intake protein on growth performance, nutrient utilization, and rumen fermentation in growing Thai-indigenous beef cattle. Trop. Anim. Health Prod. 2019, 51, 1151–1159. [Google Scholar] [CrossRef]
- Ran, T.; Shen, Y.Z.; Saleem, A.M.; AlZahal, O.; Beauchemin, K.A.; Yang, W.Z. Using ruminally protected and nonprotected active dried yeast as alternatives to antibiotics in finishing beef steers: Growth performance, carcass traits, blood metabolites, and fecal Escherichia coli. J. Anim. Sci. 2018, 96, 4385–4397. [Google Scholar] [CrossRef]
- Roland, L.; Drillich, M.; Iwersen, M. Hematology as a diagnostic tool in bovine medicine. J. Vet. Diagn. Investig. 2014, 26, 592–598. [Google Scholar] [CrossRef]
- Honda, T.; Uehara, T.; Matsumoto, G.; Arai, S.; Sugano, M. Neutrophil left shift and white blood cell count as markers of bacterial infection. Clin. Chim. Acta 2016, 457, 46–53. [Google Scholar] [CrossRef]
- Chauhan, J.; Stavraka, C.; Grandits, M.; Palhares, L.C.G.F.; Josephs, D.H.; Lacy, K.E.; Spicer, J.; Bax, H.J.; Karagiannis, S.N. Clinical and Translational Significance of Basophils in Patients with Cancer. Cells 2022, 11, 438. [Google Scholar] [CrossRef]
- Ceciliani, F.; Ceron, J.J.; Eckersall, P.D.; Sauerwein, H. Acute phase proteins in ruminants. J. Proteom. 2012, 75, 4207–4231. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef]
- Yang, W.Z.; Benchaar, C.; Ametaj, B.N.; Beauchemin, K.A. Dose response to eugenol supplementation in growing beef cattle: Ruminal fermentation and intestinal digestion. Anim. Feed Sci. Technol. 2010, 158, 57–64. [Google Scholar] [CrossRef]
- Jankowiak, H.; Cebulska, A.; Bocian, M. The relationship between acidification (pH) and meat quality traits of polish white breed pigs. Eur. Food. Res. Technol. 2021, 247, 2813–2820. [Google Scholar] [CrossRef]
- Węglarz, A. Meat quality defined based on pH and colour depending on cattle category and slaughter season. colour and pH as determinants of meat quality dependent on cattle category and slaughter season. Czech J. Anim. Sci. 2010, 55, 548–556. [Google Scholar] [CrossRef]
- Ablikim, B.; Liu, Y.; Kerim, A.; Shen, P.; Abdurerim, P.; Zhou, G.H. Effects of breed, muscle type, and frozen storage on physico-chemical characteristics of lamb meat and its relationship with tenderness. CyTA—J. Food 2016, 14, 109–116. [Google Scholar] [CrossRef]
- Li, X.; Ha, M.; Warner, R.D.; Dunshea, F.R. Meta-analysis of the relationship between collagen characteristics and meat tenderness. Meat Sci. 2022, 185, 108717. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Oxidative environments decrease tenderization of beef steaks through inactivation of μ-calpain1. J. Anim. Sci. 2004, 82, 3254–3266. [Google Scholar] [CrossRef]
- Font-I-Furnols, M.; Guerrero, L. Consumer preference, behavior and perception about meat and meat products: An overview. Meat Sci. 2014, 98, 361–371. [Google Scholar] [CrossRef]
- Realini, C.E.; i Furnols, M.F.I.; Sañudo, C.; Montossi, F.; Oliver, M.A.; Guerrero, L. Spanish, French and British consumers’ acceptability of Uruguayan beef, and consumers’ beef choice associated with country of origin, finishing diet and meat price. Meat Sci. 2013, 95, 14–21. [Google Scholar] [CrossRef]
- Falowo, A.B.; Fayemi, P.O.; Muchenje, V. Natural antioxidants against lipid–protein oxidative deterioration in meat and meat products: A review. Food Res. Int. 2014, 64, 171–181. [Google Scholar] [CrossRef]
- Amaral, A.B.; da Silva, M.V.; Lannes, S.C.D.S. Lipid oxidation in meat: Mechanisms and protective factors—A review. Food Sci. Technol. 2018, 38, 1–15. [Google Scholar] [CrossRef]
- Akbarian, A.; Michiels, J.; Golian, A.; Buyse, J.; Wang, Y.; De Smet, S. Gene expression of heat shock protein 70 and antioxidant enzymes, oxidative status, and meat oxidative stability of cyclically heat-challenged finishing broilers fedOriganum compactum andCurcuma xanthorrhiza essential oils. Poult. Sci. 2014, 93, 1930–1941. [Google Scholar] [CrossRef]
- Tian, Q.; Piao, X. Essential Oil Blend Could Decrease Diarrhea Prevalence by Improving Antioxidative Capability for Weaned Pigs. Animals 2019, 9, 847. [Google Scholar] [CrossRef]
- Ornaghi, M.G.; Guerrero, A.; Vital, A.C.P.; de Souza, K.A.; Passetti, R.A.C.; Mottin, C.; Castilho, R.D.A.; Sañudo, C.; Prado, I.N.D. Improvements in the quality of meat from beef cattle fed natural additives. Meat Sci. 2020, 163, 108059. [Google Scholar] [CrossRef]
- Fruet, A.P.B.; Giotto, F.M.; Fonseca, M.A.; Nörnberg, J.L.; De Mello, A.S. Effects of the Incorporation of Tannin Extract from Quebracho Colorado Wood on Color Parameters, Lipid Oxidation, and Sensory Attributes of Beef Patties. Foods 2020, 9, 667. [Google Scholar] [CrossRef]
- Calnan, H.; Jacob, R.; Pethick, D.; Gardner, G. Factors affecting the colour of lamb meat from the longissimus muscle during display: The influence of muscle weight and muscle oxidative capacity. Meat Sci. 2014, 96, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Jimenez-Badillo, M.R.; Rodrigues, S.S.Q. Effect of sex and carcass weight on carcass traits and meat quality in goat kids of Cabrito Transmontano. Span. J. Agric. Res. 2011, 9, 753–760. [Google Scholar] [CrossRef]
- Toldrá, F. Lawrie’s Meat Science, 8th ed.; Woodhead Publishing Limited: Cambridge, UK, 2017; p. 713. [Google Scholar]
- Rotz, C.A.; Asem-Hiablie, S.; Place, S.; Thoma, G. Environmental footprints of beef cattle production in the United States. Agric. Syst. 2019, 169, 1–13. [Google Scholar] [CrossRef]
- Capper, J.L.; Hayes, D.J. The environmental and economic impact of removing growth-enhancing technologies from U.S. beef production. J. Anim. Sci. 2012, 90, 3527–3537. [Google Scholar] [CrossRef]
- Zein, M.; Jamarun, N.; Musnandar, E. Effect of Increasing Doses of Essential Oil Extracted from Berastagi Orange (Citrus sinensis L.) Peels on Performance, Rumen Fermentation and Blood Metabolites in Fattening Bali Cattle. Pak. J. Nutr. 2015, 14, 480–486. [Google Scholar] [CrossRef]
- Almeida, M.T.C.; Paschoaloto, J.R.; Perez, H.L.; Carvalho, V.B.; Junior, A.C.H.; Favaro, V.R.; Blair, H.T.; Ezequiel, J.M.B. Effect of adding crude glycerine to diets with feed additives on the feed intake, ruminal degradability, volatile fatty acid concentrations and in vitro gas production of feedlot Nellore cattle. J. Anim. Physiol. Anim. Nutr. 2019, 103, 988–996. [Google Scholar] [CrossRef]
- Brand, T.; Hünerberg, M.; McAllister, T.A.; He, M.; Saleem, A.M.; Shen, Y.; Miller, B.; Yang, W. Impact of a phytogenic feed additive on growth performance, feed intake, and carcass traits of finishing steers. Transl. Anim. Sci. 2019, 3, 1162–1172. [Google Scholar] [CrossRef]
- Carvalho, V.M.; Ávila, V.A.D.; Bonin, E.; Matos, A.M.; Prado, R.M.D.; Castilho, R.A.; Silva, R.R.; Filho, B.A.D.A.; Prado, I.N.D. Effect of extracts from baccharis, tamarind, cashew nut shell liquid and clove on animal performance, feed efficiency, digestibility, rumen fermentation and feeding behavior of bulls finished in feedlot. Livest. Sci. 2020, 244, 104361. [Google Scholar] [CrossRef]
- Chapman, C.E.; Chester-Jones, H.; Ziegler, D.; Clapper, J.A.; Erickson, P.S. Effects of cinnamaldehyde or monensin on performance of weaned Holstein dairy heifers. J. Dairy Sci. 2017, 100, 1712–1719. [Google Scholar] [CrossRef]
- Filho, E.S.C.; Júnior, L.C.R.; Ezequiel, J.M.B.; Salles, M.S.V.; Almeida, M.T.C.; Perez, H.L.; Suguino, E.; van Cleef, E.H.C.B. Effect of thyme essential oil supplementation on feed intake, apparent digestibility, rumen fermentation, blood parameters and in vitro methane yield of Nellore cattle. Livest. Sci. 2020, 244, 104349. [Google Scholar] [CrossRef]
- Guerrero, A.; Rivaroli, D.C.; Sañudo, C.; Campo, M.M.; Valero, M.V.; Jorge, A.M.; Prado, I.N. Consumer acceptability of beef from two sexes supplemented with essential oil mix. Anim. Prod. Sci. 2018, 58, 1700–1707. [Google Scholar] [CrossRef]
- Khorrami, B.; Vakili, A.R.; Mesgaran, M.D.; Klevenhusen, F. Thyme and cinnamon essential oils: Potential alternatives for monensin as a rumen modifier in beef production systems. Anim. Feed Sci. Technol. 2015, 200, 8–16. [Google Scholar] [CrossRef]
- Kim, S.C.; Adesogan, A.T.; Shin, J.H. Effects of dietary addition of wormwood (Artemisia montana Pampan) silage on growth performance, carcass characteristics, and muscle fatty acid profiles of beef cattle. Anim. Feed Sci. Technol. 2012, 177, 15–22. [Google Scholar] [CrossRef]
- Latack, B.C.; Montano, M.F.; Zinn, R.A.; Salinas-Chavira, J. Effects of a blend of cinnamaldehyde-eugenol and capsicum (Xtract® Ruminant 7065) and ionophore on performance of finishing Holstein steers and on characteristics of ruminal and total tract digestion. J. Appl. Anim. Res. 2021, 49, 185–193. [Google Scholar] [CrossRef]
- Monteschio, J.O.; Vargas-Junior, F.M.; Almeida, F.L.; Pinto, L.A.D.M.; Kaneko, I.N.; Almeida, A.A.; Freitas, L.W.; Alves, S.P.; Bessa, R.J.; Prado, I.N. The effect of encapsulated active principles (eugenol, thymol and vanillin) and clove and rosemary essential oils on the structure, collagen content, chemical composition and fatty acid profile of Nellore heifers muscle. Meat Sci. 2019, 155, 27–35. [Google Scholar] [CrossRef]
- Ornaghi, M.G.; Passetti, R.A.C.; Torrecilhas, J.A.; Mottin, C.; Vital, A.C.P.; Guerrero, A.; Sañudo, C.; Campo, M.d.M.; Prado, I.N. Essential oils in the diet of young bulls: Effect on animal performance, digestibility, temperament, feeding behaviour and carcass characteristics. Anim. Feed Sci. Technol. 2017, 234, 274–283. [Google Scholar] [CrossRef]
- Prado, I.N.; Cruz, O.T.B.; Valero, M.V.; Zawadzki, F.; Eiras, C.E.; Rivaroli, D.C.; Prado, R.M.; Visentainer, J.V. Effects of glycerin and essential oils (Anacardium occidentale and Ricinus communis) on the meat quality of crossbred bulls finished in a feedlot. Anim. Prod. Sci. 2016, 56, 2105–2114. [Google Scholar] [CrossRef]
- Pukrop, J.; Campbell, B.; Schoonmaker, J. Effect of essential oils on performance, liver abscesses, carcass characteristics and meat quality in feedlot steers. Anim. Feed Sci. Technol. 2019, 257, 114296. [Google Scholar] [CrossRef]
- Rivaroli, D.C.; Guerrero, A.; Valero, M.V.; Zawadzki, F.; Eiras, C.E.; del Mar Campo, M.D.M.; Sañudo, C.; Jorge, A.; do Prado, I.N. Effect of essential oils on meat and fat qualities of crossbred young bulls finished in feedlots. Meat Sci. 2016, 121, 278–284. [Google Scholar] [CrossRef]
- de Souza, K.A.; Monteschio, J.D.O.; Mottin, C.; Ramos, T.R.; Pinto, L.A.d.M.; Eiras, C.E.; Guerrero, A.; do Prado, I.N. Effects of diet supplementation with clove and rosemary essential oils and protected oils (eugenol, thymol and vanillin) on animal performance, carcass characteristics, digestibility, and ingestive behavior activities for Nellore heifers finished in feedlot. Livest. Sci. 2019, 220, 190–195. [Google Scholar] [CrossRef]
- Tomkins, N.W.; Denman, S.E.; Pilajun, R.; Wanapat, M.; McSweeney, C.S.; Elliott, R. Manipulating rumen fermentation and methanogenesis using an essential oil and monensin in beef cattle fed a tropical grass hay. Anim. Feed Sci. Technol. 2015, 200, 25–34. [Google Scholar] [CrossRef]
- Torrecilhas, J.A.; Ornaghi, M.G.; Passetti, R.A.C.; Mottin, C.; Guerrero, A.; Ramos, T.R.; Vital, A.C.P.; Sañudo, C.; Malheiros, E.B.; Prado, I.N.D. Meat quality of young bulls finished in a feedlot and supplemented with clove or cinnamon essential oils. Meat Sci. 2021, 174, 108412. [Google Scholar] [CrossRef]
- Vakili, A.R.; Khorrami, B.; Mesgaran, M.D.; Parand, E. The Effects of Thyme and Cinnamon Essential Oils on Performance, Rumen Fermentation and Blood Metabolites in Holstein Calves Consuming High Concentrate Diet. Asian-Australasian J. Anim. Sci. 2013, 26, 935–944. [Google Scholar] [CrossRef]
- Valero, M.V.; Torrecilhas, J.A.; Zawadzki, F.; Bonafé, E.G.; Madrona, G.S.; Prado, R.M.D.; Passetti, R.A.C.; Rivaroli, D.C.; Visentainer, J.V.; Prado, I.N.D. Propolis or cashew and castor oils effects on composition of Longissimus muscle of crossbred bulls finished in feedlot. Chil. J. Agric. Res. 2014, 74, 445–451. [Google Scholar] [CrossRef][Green Version]
- Wanapat, M.; Kang, S.; Khejornsart, P.; Wanapat, S. Effects of Plant Herb Combination Supplementation on Rumen Fermentation and Nutrient Digestibility in Beef Cattle. Asian-Australas. J. Anim. Sci. 2013, 26, 1127–1136. [Google Scholar] [CrossRef]
- Wu, J.; Bai, Y.; Lang, X.; Wang, C.; Shi, X.; Casper, D.P.; Zhang, L.; Liu, H.; Liu, T.; Gong, X.; et al. Dietary supplementation with oregano essential oil and monensin in combination is antagonistic to growth performance of yearling Holstein Bulls. J. Dairy Sci. 2020, 103, 8119–8129. [Google Scholar] [CrossRef]
- Yang, W.Z.; Ametaj, B.N.; Benchaar, C.; Beauchemin, K.A. Dose response to cinnamaldehyde supplementation in growing beef heifers: Ruminal and intestinal digestion1. J. Anim. Sci. 2010, 88, 680–688. [Google Scholar] [CrossRef]
- Holland, R.; Loveday, D.; Ferguson, K. How Much Meat to Expect from a Beef Carcass; University of Tennessee Institute of Agriculture: Tennessee, TN, USA, 2014. [Google Scholar]
- Pereira, P.M.d.C.C.; Vicente, A.F.d.R. B Meat nutritional composition and nutritive role in the human diet. Meat Sci. 2013, 93, 586–592. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).