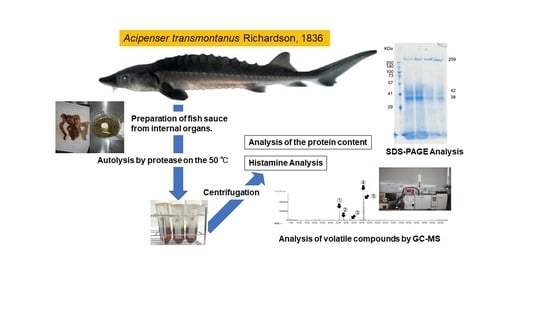
Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836
Abstract
1. Introduction
2. Materials and Methods
2.1. Proximate Analysis
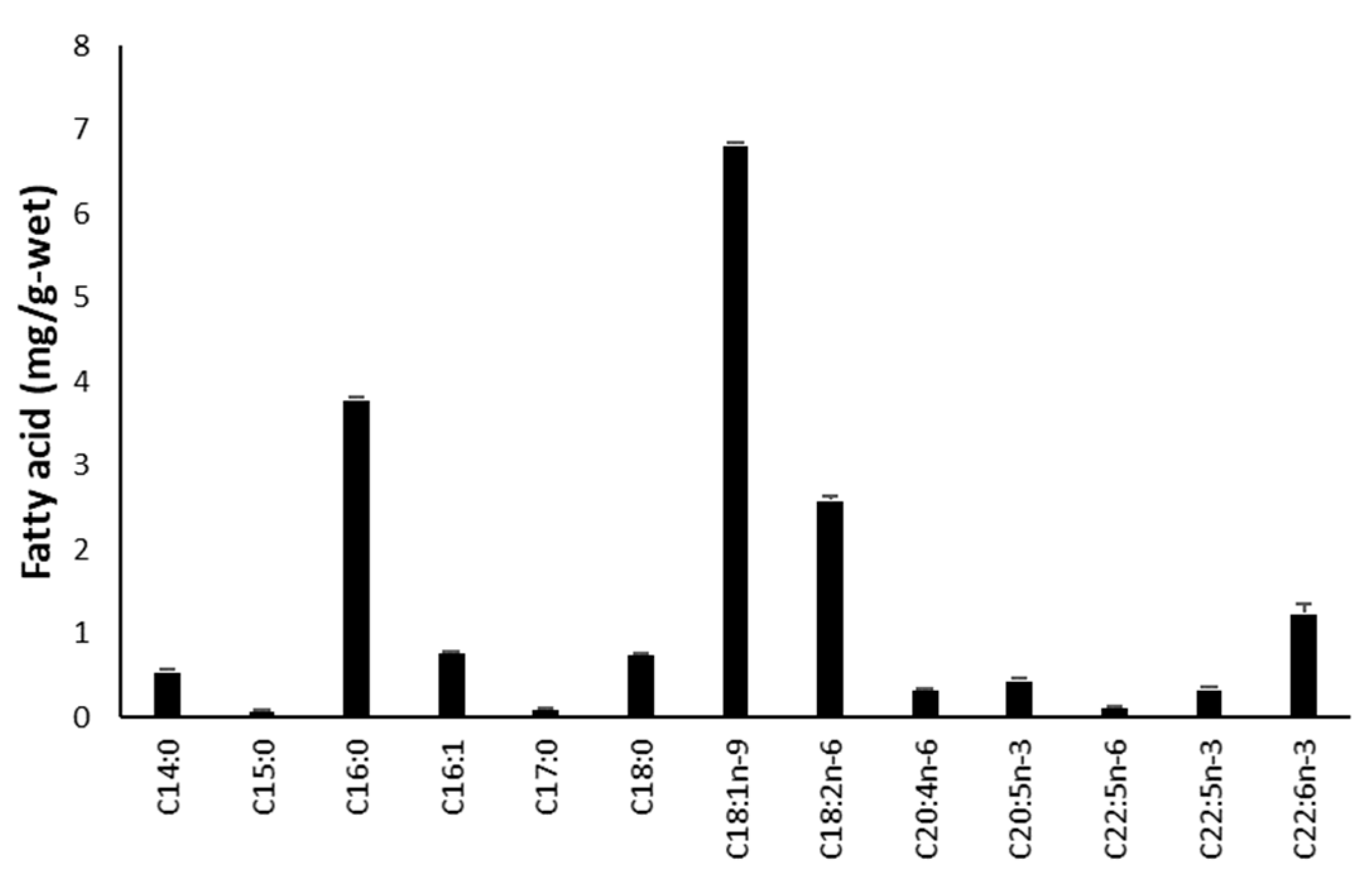
2.2. Fatty Acid Analysis
2.3. Preparation of Crude Enzyme Solution from White Sturgeon
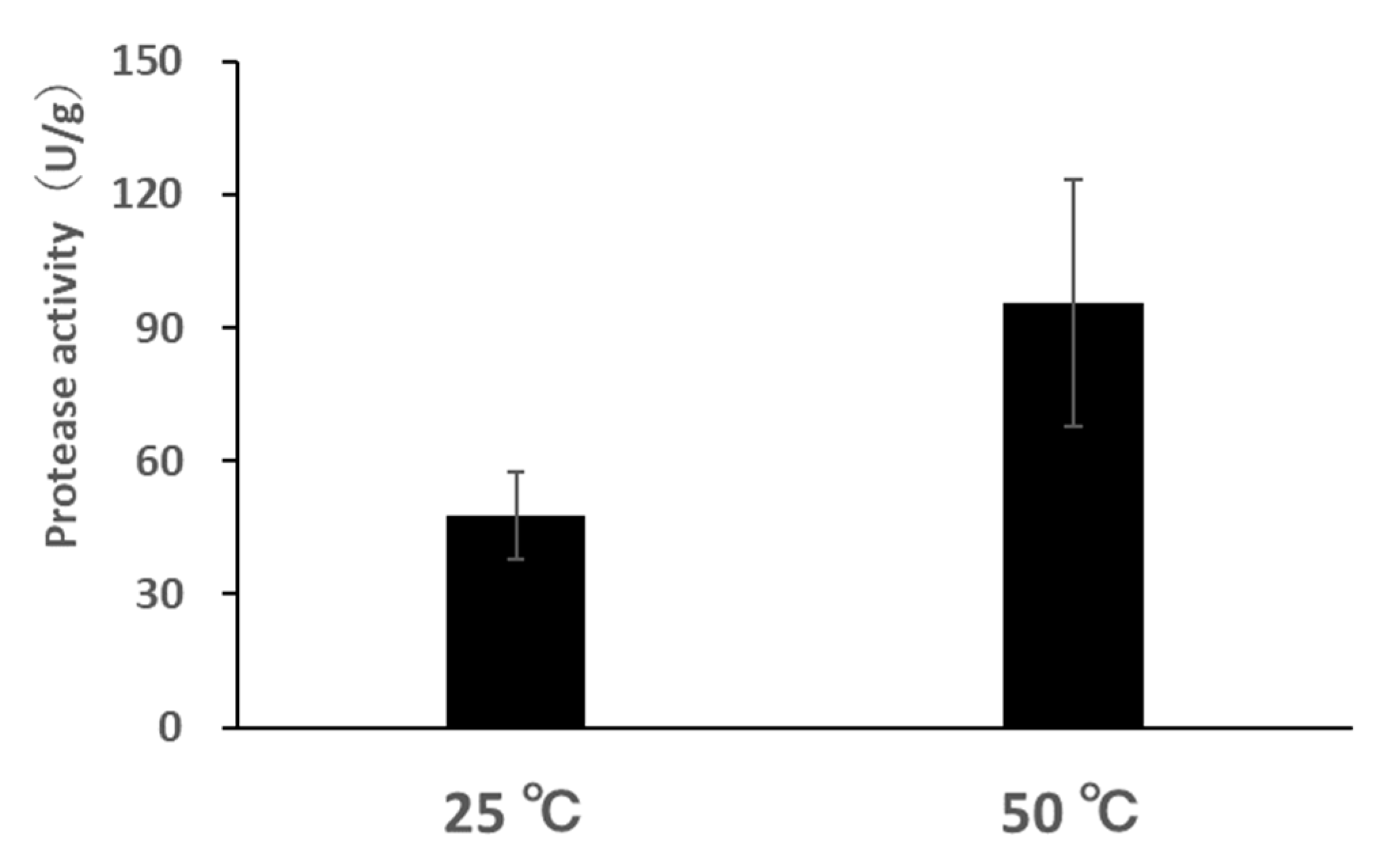
2.4. Protease Assay
2.5. Preparation of Fish Sauce from Internal Organs of White Sturgeon
2.6. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.7. Bacteria Plate Count and Histamine Analysis
2.8. Volatile Compound Analysis
2.9. Statistical Analysis
3. Results
3.1. Proximate Analysis of Minced Internal Organs
3.2. Protease Activity
3.3. Time Course of Autolysis
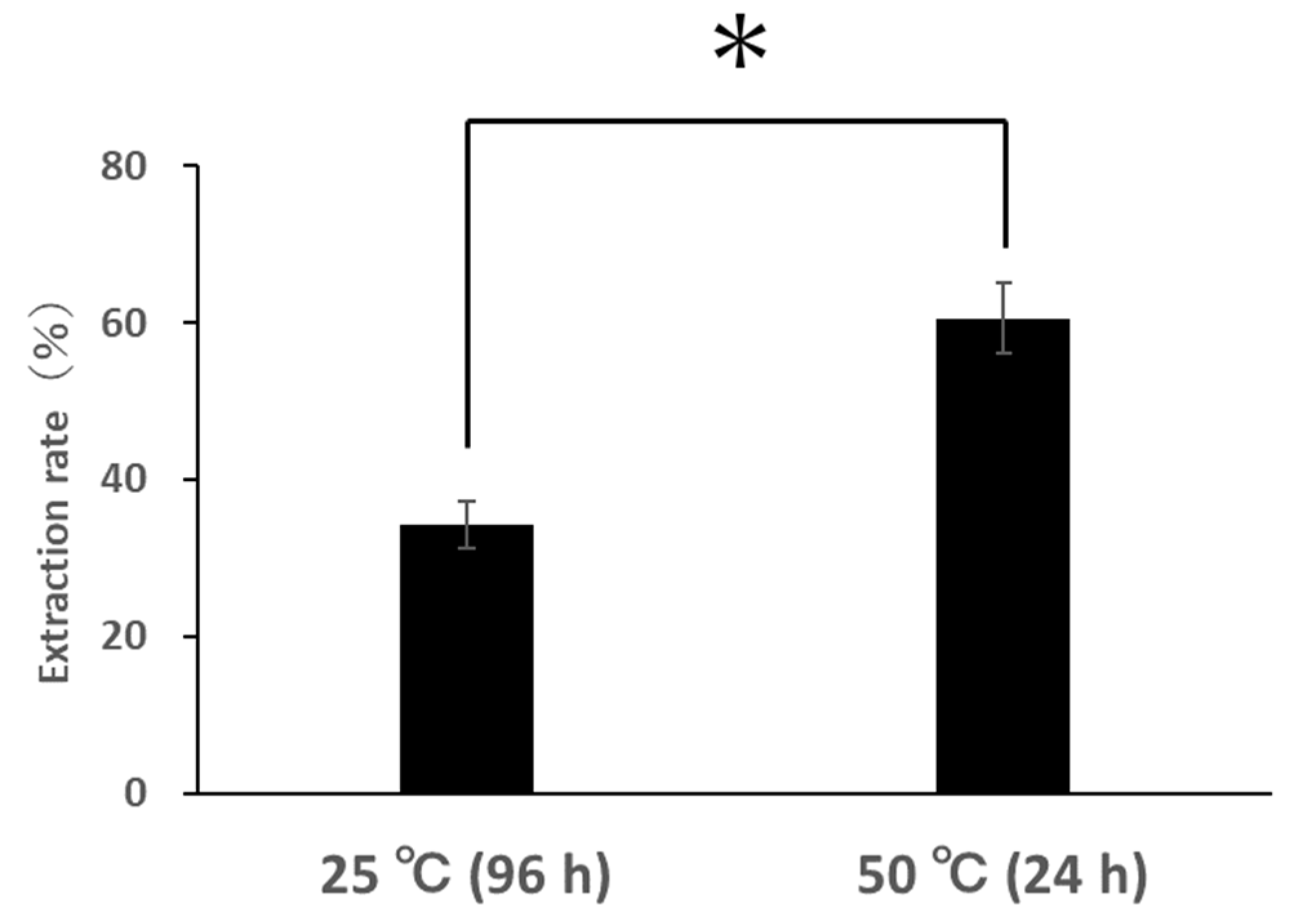
3.3.1. Changes in Extraction Rate
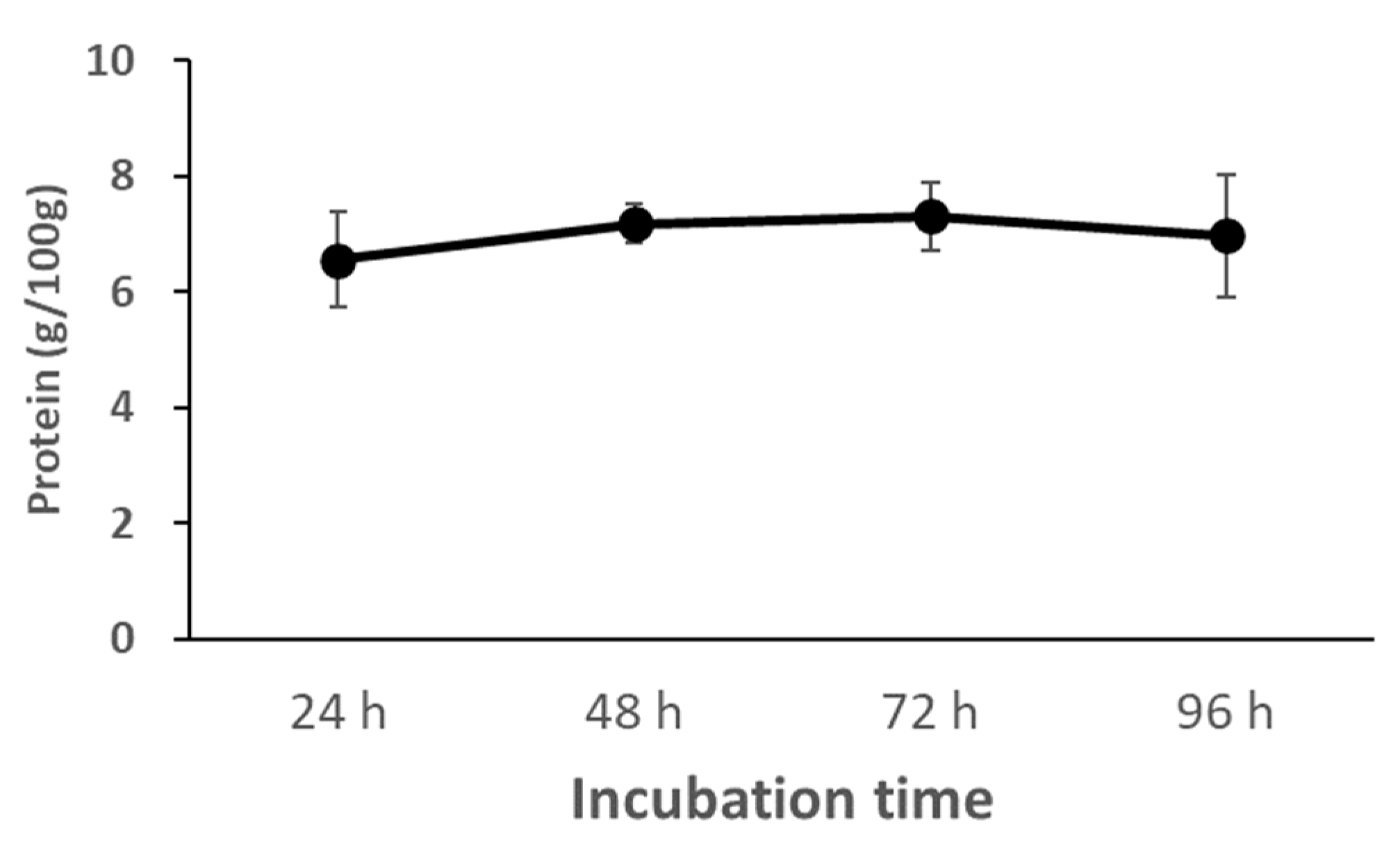
3.3.2. Changes in Protein Concentration
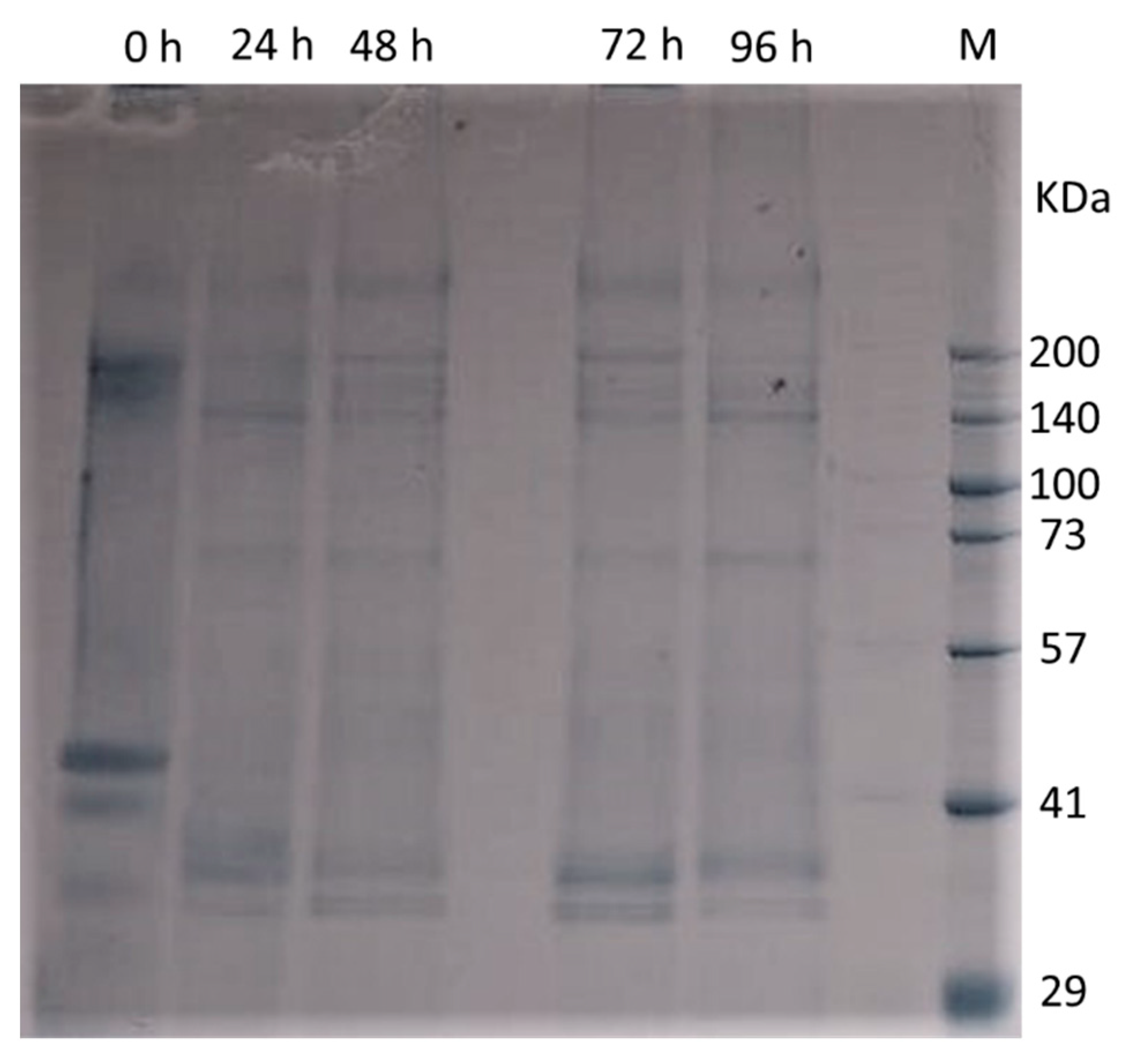
3.3.3. Distribution of Protein Molecular Size
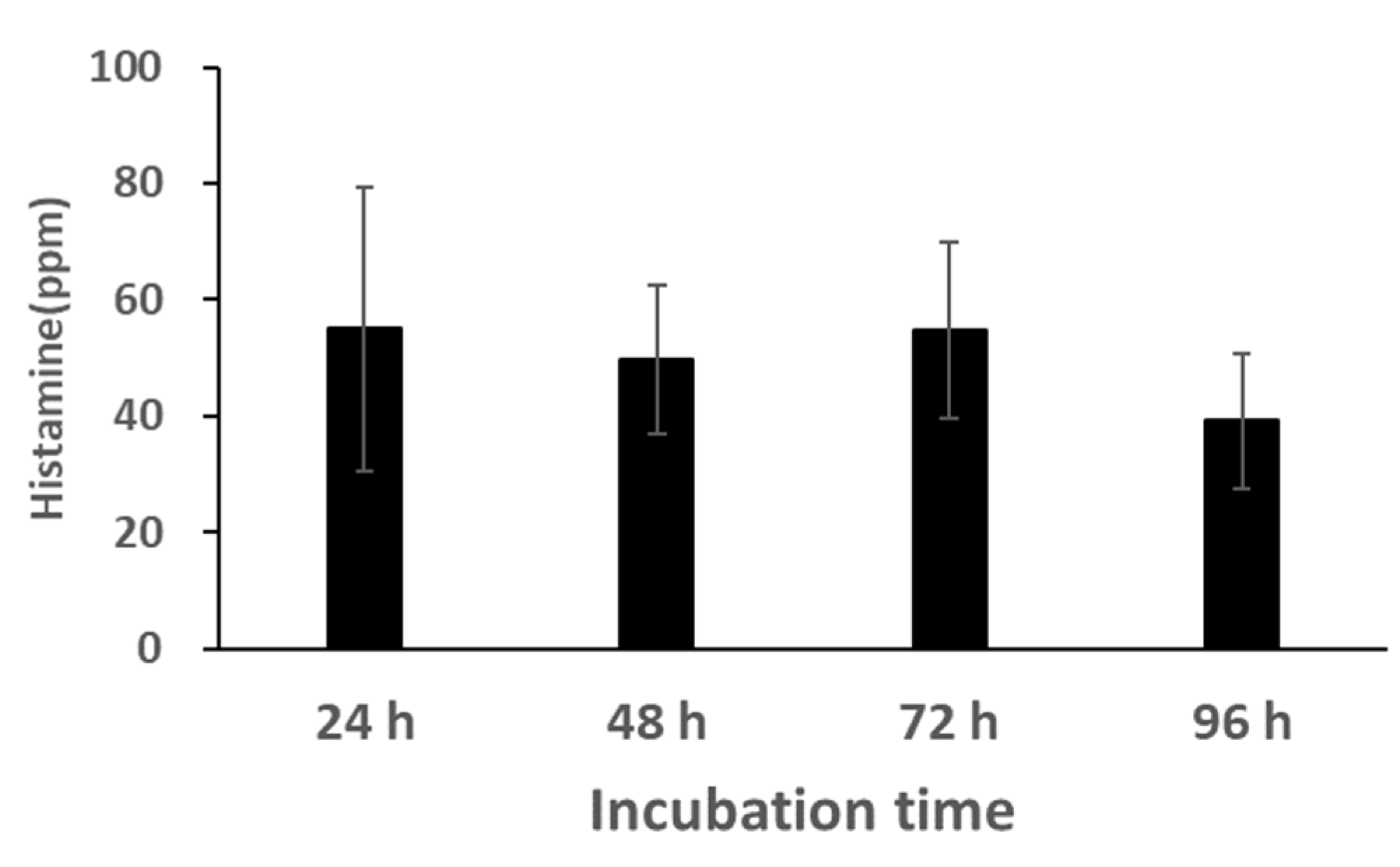
3.3.4. Bacterial Counts and Histamine Concentration
3.3.5. Volatile Compound Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Russo, G.L.; Langellotti, A.L.; Genovese, A.; Martello, A.; Sacchi, R. Volatile compounds, physicochemical and sensory characteristics of Colatura di Alici, a traditional Italian fish sauce. J. Sci. Food Agric. 2020, 9, 3755–3764. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Ishida, K.; Watanabe, T.; Endoh, K.; Watanabe, K.; Murakami, M.; Abe, H. Taste-active components in a Vietnamese fish sauce. Fish. Sci. 2002, 68, 913–920. [Google Scholar] [CrossRef]
- Uchida, M.; Ou, J.; Chen, B.W.; Yuan, C.H.; Zhang, X.H.; Cheng, S.S.; Funatsu, Y.; Kawasaki, K.I.; Satomi, M.; Fukuda, Y. Effects of soy sauce koji and lactic acid bacteria on the fermentation of fish sauce from freshwater silver carp Hypophthalmichthys molitrix. Fish. Sci. 2005, 71, 422–430. [Google Scholar] [CrossRef]
- Yoshinaka, R.; Sato, M.; Tsuchiya, N.; Ikeda, S. Production of fish sauce from sardine by utilization of its visceral enzymes. Bull. Jpn. Soc. Sci. Fish. 1983, 49, 463–469. [Google Scholar] [CrossRef]
- Fukami, K.; Ishiyama, S.; Yaguramaki, H.; Masuzawa, T.; Nabeta, Y.; Endo, K.; Shimoda, M. Identification of distinctive volatile compounds in fish sauce. J. Agric. Food Chem. 2002, 50, 5412–5416. [Google Scholar] [CrossRef]
- Lapsongphon, N.; Yongsawatdigul, J.; Cadwallader, K.R. Identification and characterization of the aroma-impact components of Thai fish sauce. J. Agric. Food Chem. 2015, 63, 2628–2638. [Google Scholar] [CrossRef]
- Maehashi, K.; Yamamoto, Y. Development of low-salt fish sauce using high levels of yeast. Food Preserv. Sci. 2007, 33, 315–321. [Google Scholar] [CrossRef]
- Wakinaka, T.; Iwata, S.; Takeishi, Y.; Watanabe, J.; Mogi, Y.; Tsukioka, Y.; Shibata, Y. Isolation of halophilic lactic acid bacteria possessing aspartate decarboxylase and application to fish sauce fermentation starter. Int. J. Food Microbiol. 2019, 292, 137–143. [Google Scholar] [CrossRef]
- Doyle, E.L.; Glassk, A. Sodium reduction and its effect on food safety, food quality, and human health. Compr. Rev. Food Sci. Food Saf. 2010, 9, 44–56. [Google Scholar] [CrossRef]
- Taoka, Y.; Nakamura, M.; Nagai, S.; Nagasaka, N.; Tanaka, R.; Uchida, K. Production of anserine-rich fish sauce from giant masu salmon, Oncorhynchus masou masou and γ-Aminobutyric Acid (GABA)-enrichment by Lactobacillus plantarum Strain N10. Fermentation 2019, 5, 45. [Google Scholar] [CrossRef]
- Noman, A.; Xu, Y.; AL-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Giri, A.; Osako, K.; Okamoto, A.; Ohshima, T. Olfactometric characterization of aroma active compounds in fermented fish paste in comparison with fish sauce, fermented soy paste and sauce products. Food Res. Int. 2010, 43, 1027–1040. [Google Scholar] [CrossRef]
- Yanohara, T.; Matsuura, Y.; Masaki, H.; Ohhashi, Y.; Yamamoto, M.; Tashima, R.; Kuroda, A.; Hosoda, R. Aroma characteristics of fish sauce made from internal organs of sturgeon. Bull. Minamikyushu Univ. 2021, 51, 25–28. [Google Scholar]
- Ganeko, N.; Shoda, M.; Hirohata, I.; Bhadra, A.; Ishida, T.; Matsuda, H.; Takamura, H.; Matoba, T. Analysis of volatile flavor compounds of sardine (Sardinops melanostica) by solid phase microextraction. J. Food Sci. 2008, 73, S83–S88. [Google Scholar] [CrossRef]
- Kato, A.; Kodani, Y. Development of the fish sauce using Koji. Bull. Soc. Sea Water Sci. Jpn. 2016, 70, 303–307. [Google Scholar]
- Utagawa, T. Rapid fermentation of fish sauce and its use. J. Brew. Soc. Jpn. 2012, 107, 477–484. [Google Scholar] [CrossRef][Green Version]
- AOAC International. AOAC International Official Methods of Analysis, 18th ed.; AOAC International: Washington, DC, USA, 2005. [Google Scholar]
- Folch, J.; Ascoli, I.; Lees, M.; Meath, J.A.; Lebaron, N. Preparation of lipid extracts from brain tissue. J. Biol. Chem. 1951, 195, 833–841. [Google Scholar] [CrossRef]
- Nagano, N.; Sakaguchi, K.; Taoka, Y.; Okita, Y.; Honda, D.; Ito, M.; Hayashi, M. Detection of genes involved in fatty acid elongation and desaturation in Thraustochytrid marine eukaryotes. J. Oleo Sci. 2011, 60, 475–481. [Google Scholar] [CrossRef]
- Hirata, K.; Hirokado, M.; Uematsu, Y.; Nakajima, K.; Kazama, M. Investigation on assay for the proteolytic activity of protease and papain preparations for food manufacturing. Food Hyg. Saf. Sci. 1994, 35, 380–384. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kagawa, Y. Standard Tables of Food Composition in Japan, 8th ed.; Ministry of Education, Culture, Sports, Science and Technology: Tokyo, Japan, 2020.
- CODEX STAN CXS 302–2011; Codex Alimentarius Commission. Standard for Fish Sauce. CODEX: Rome, Italy, 2011.
- Siscovick, D.S.; Barringer, T.A.; Fretts, A.M.; Wu, J.H.; Lichtenstein, A.H.; Costello, R.B.; Kris-Etherton, P.M.; Jacobson, T.A.; Engler, M.B.; Alger, H.M.; et al. Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease. Circulation 2017, 135, e867–e884. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, T.; Hamaguchi, M.; Yokoyama, S. Change of volatile compounds in fresh fish meat during ice storage. J. Food Sci. 2011, 76, C1319–C1325. [Google Scholar] [CrossRef] [PubMed]
- Furutani, A.; Satomi, M. Study on proteolytic process of two fish sauce mashes prepared from deep sea smelt and waste from Kamaboko processing using soy sauce Koji mold. J. Brew. Soc. Jpn. 2013, 108, 802–812. [Google Scholar] [CrossRef]
- Zhang, L.; Xue, Y.; Xu, J.; Li, Z.; Xue, C. Effects of high-temperature treatment (≥100 ℃) on Alaska pollock (Theragra chalcogramma) surimi gels. J. Food Eng. 2013, 115, 115–120. [Google Scholar] [CrossRef]
- Tang, S.; Feng, G.; Gao, R.; Ren, J.; Zhou, X.; Wang, H.; Xu, H.; Zhao, Y.; Zeng, M. Thermal gel degradation (modori) in sturgeon (Acipenseridae) surimi gels. J. Food Sci. 2019, 84, 3601–3607. [Google Scholar] [CrossRef]
- Auerswald, L.; Morren, C.; Lopata, A.L. Histamine levels in seventeen species of fresh and processed South African seafood. Food Chem. 2006, 98, 231–239. [Google Scholar] [CrossRef]
- Satomi, M. Scombroid (Histamine) Poisoning Associated with Seafood. In Seafood Safety and Quality; Bari, M.L., Yamazaki, K., Eds.; CRC Press: Boca Raton, FL, USA, 2018; pp. 224–260. [Google Scholar]
- Fujii, T. Scombroid fish poisoning. Jpn. J. Food Microbiol. 2006, 23, 61–71. [Google Scholar] [CrossRef]
- Kita, J.; Okada, M.; Akamaru, H.; Kinoshita, M. Electronic Nose. J. Jpn. Assoc. Odor Environ. 2006, 37, 172–178. [Google Scholar] [CrossRef][Green Version]
- Shimoda, M.; Peralta, R.R.; Osajima, Y. Headspace gas analysis of fish sauce. J. Agric. Food Chem. 1996, 44, 3601–3605. [Google Scholar] [CrossRef]
- Sato, Y.; Takagi, S.; Inomata, E.; Agatsuma, Y. Odor-active volatile compounds from the gonads of the sea urchin Mesocentrotus nudus in the wild in Miyagi Prefecture, Tohoku, Japan. Food Nutr. Sci. 2019, 10, 860–875. [Google Scholar]
- Wanakhachornkrai, P.; Lertsiri, S. Comparison of determination method for volatile compounds in Thai soy sauce. Food Chem. 2003, 83, 619–629. [Google Scholar] [CrossRef]
- Wu, S.; Yang, J.; Dong, H.; Liu, Q.; Li, X.; Zeng, X.; Bai, W. Key aroma compounds of Chinese dry-cured Spanish mackerel (Scomberomorus niphonius) and their potential metabolic mechanisms. Food Chem. 2021, 342, 128381. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, K.; Kaneko, S.; Nishimura, O. Identification and characterization of volatile components causing the characteristic flavor in Miso (Japanese fermented soybean paste) and heat-processed Miso products. J. Agric. Food Chem. 2013, 61, 11968–11973. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, Z.; Liu, N.; Zhao, H.; Cui, C.; Zhao, M. Changes in fatty acid composition and lipid profile during koji fermentation and their relationships with soy sauce flavour. Food Chem. 2014, 158, 438–444. [Google Scholar] [CrossRef]
- Feng, X.; Li, X.; Zhang, C.; Kong, X.; Chen, Y.; Hua, Y. Formation mechanism of hexanal and (E)-2-hexenal during soybean [Glycine max (L.) Merr] processing based on the subcellular and molecular levels. J. Agric. Food Chem. 2022, 70, 289–300. [Google Scholar] [CrossRef]
- Wurzenberger, M.; Grosch, W. The enzymic oxidative breakdown of linoleic acid in mushrooms (Psalliota bispora). Z. Lebensm.-Unters.-Forsch. A 1982, 175, 186–190. [Google Scholar] [CrossRef]
- Assaf, S.; Hadar, Y.; Dosoretz, C.G. 1-octen-3-ol and 13-hydroperoxylinoleate are products of distinct pathways in the oxidative breakdown of linoleic acid by Pleurotus pulmonarius. Enzym. Microb. Technol. 1997, 21, 484–490. [Google Scholar] [CrossRef]
- Tasaki, Y.; Kobayashi, D.; Sato, R.; Hayashi, S.; Joh, T. Variations in 1-octen-3- ol and lipoxygenase gene expression in the oyster mushroom Pleurotus ostreatus according to fruiting body development, tissue specificity, maturity, and postharvest storage. Mycoscience 2019, 60, 170–176. [Google Scholar] [CrossRef]
- Ding, A.; Zhu, M.; Qian, X.; Shi, L.; Huang, H.; Xiong, G.; Wang, L. Effect of fatty acids on the flavor formation of fish sauce. LWT-Food Sci. Technol. 2020, 134, 110259. [Google Scholar] [CrossRef]
- Mansur, M.A.; Bhadra, A.; Takamura, H.; Matoba, T. Volatile flavor compounds of some sea fish and prawn species. Fish. Sci. 2003, 69, 864–866. [Google Scholar] [CrossRef][Green Version]

| Retention Time (min) | 0 h | 24 h | 48 h | 72 h | 96 h | |
|---|---|---|---|---|---|---|
| 9.0 | Ethanol | 0.002 ± 0.004 | 15.001 ± 24.061 | 1.914 ± 0.557 | 3.665 ± 1.160 | 2.788 ± 1.914 |
| 12.4 | 1-Penten-3-one | 0.056 ± 0.062 | 0.352 ± 0.139 | 0.114 ± 0.009 | 0.102 ± 0.026 | 0.053 ± 0.010 |
| 15.6 | Hexanal | 2.352 ± 2.681 | 9.877 ± 11.198 | 0.667 ± 0.588 | 0.439 ± 0.002 | 0.858 ± 0.874 |
| 20.8 | 1-Penten-3-ol | 2.275 ± 0.441 | 7.135 ± 9.662 | 1.237 ± 0.259 | 1.041 ± 0.180 | 0.934 ± 0.353 |
| 26.7 | 1-Pentanol | 0.332 ± 0.162 | 6.439 ± 6.923 | 2.466 ± 0.240 | 3.557 ± 0.041 | 3.660 ± 0.232 |
| 35.5 | 3-Hexen-1-ol | 0.005 ± 0.009 | 7.308 ± 12.658 | - | - | 0.013 ± 0.011 |
| 39.5 | Methional | 0.005 ± 0.003 | 6.257 ± 10.648 | 0.641 ± 1.068 | 0.008 ± 0.0004 | 0.043 ± 0.058 |
| 39.8 | 1-Octen-3-ol | 0.716 ± 0.491 | 3.023 ± 2.305 | 0.959 ± 1.653 | 3.068 ± 0.742 | 2.471 ± 0.088 |
| 39.9 | Acetic acid | - | - | 0.083 ± 0.144 | 0.223 ± 0.315 | - |
| 40.0 | Furfural | - | 0.133 ± 0.201 | 0.033 ± 0.057 | 0.044 ± 0.027 | - |
| 50.2 | Butanoic acid | - | 4.944 ± 8.564 | 0.235 ± 0.407 | 0.683 ± 0.967 | 0.424 ± 0.734 |
| 57.0 | Pentanoic acid | 0.001 ± 0.002 | 0.887 ± 0.244 | 0.002 ± 0.003 | 0.471 ± 0.616 | 0.070 ± 0.122 |
| 64.0 | Hexanoic acid | - | 0.922 ± 1.213 | 0.002 ± 0.004 | 0.852 ± 1.205 | 0.102 ± 0.169 |
| 74.6 | Octanoic acid | - | 0.341 ± 0.202 | 0.005 ± 0.008 | 0.002 ± 0.002 | 0.011 ± 0.019 |
| 79.7 | Nonanoic acid | - | 0.472 ± 0.635 | 0.005 ± 0.004 | 0.006 ± 0.009 | 0.022 ± 0.038 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanohara, T.; Taoka, Y.; Yamamoto, M. Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836. Fermentation 2022, 8, 238. https://doi.org/10.3390/fermentation8050238
Yanohara T, Taoka Y, Yamamoto M. Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836. Fermentation. 2022; 8(5):238. https://doi.org/10.3390/fermentation8050238
Chicago/Turabian StyleYanohara, Taishi, Yousuke Taoka, and Mizuki Yamamoto. 2022. "Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836" Fermentation 8, no. 5: 238. https://doi.org/10.3390/fermentation8050238
APA StyleYanohara, T., Taoka, Y., & Yamamoto, M. (2022). Rapid Production of Fish Sauce from the Internal Organs of White Sturgeon, Acipenser transmontanus Richardson, 1836. Fermentation, 8(5), 238. https://doi.org/10.3390/fermentation8050238