Abstract
One of the relevant areas in microbiology and biotechnology is the study of microorganisms that induce the destruction of different materials, buildings, and machines and lead to negative effects. At the same time, the positive ecological effects of degradation can be explained by the detoxication of industrial and agricultural wastes, chemical substances, petroleum products, xenobiotics, pesticides, and other chemical pollutants. Many of these industrial wastes include hard-to-degrade components, such as lignocellulose or plastics. The biosynthesis of natural products based on the transformation of lignocellulosic wastes is of particular interest. One of the world’s unique ecosystems is presented by Lake Baikal. This ecosystem is characterized by the highest level of biodiversity, low temperatures, and a high purity of the water. Here, we studied the ability of several psychrophilic representatives of Baikal Actinobacteria to grow on sawdust wastes and transform them into bioactive natural products. Different strains of both widely spread genus of Actinobacteria and rare genera of Actinobacteria were tested. We used the LC-MS methods to show that Actinobacteria living in sawmill wastes can produce both known and novel natural products with antibiotic activity. We demonstrated that the type of sawmill wastes and their concentration influence the Actinobacteria biosynthetic potential. We have shown for the first time that the use of Baikal psychrophilic microorganisms as a factory for biodegradation is applicable for the transformation of lignocellulosic wastes. Thus, the development of techniques for screening novel natural products leads to an elaboration on the active ingredients for novel drugs.
1. Introduction
One of the main challenges of the 21st century is the destruction of technogenic waste accumulated by humans due to the unsustainable exploitation of the ecosystem [1]. Sawdust and lignocellulose are types of technological and agricultural wastes. Technologies using sawdust as briquettes for biofuel and bioethanol are well-known, as well as the production of alcohols and gases from sawdust [2]. However, due to various economic reasons, many logging industry companies do not utilize sawdust and plant-originated wastes [3]. According to the agricultural experience in New Zealand, sawdust and lignocellulose wastes are used to treat nitrogen and fecal bacteria in winter stand-off pads on dairy farms [4]. In the study by Zorpas A.A. and Loizidou, it was demonstrated that sawdust and natural zeolite can be effective bulking agents to improve the quality of a composting product from anaerobically stabilized sewage sludge [5]. Moreover, several recent reviews related to biomass conversion into high-value chemicals were published [6,7].
Despite their useful properties and applications, sawdust and lignocellulose wastes can be hazardous and have a negative impact on ecosystems where logging takes place [8]. These particular risks and negative impacts can be detrimental for ancient ecosystems with high rates of endemics and sustainable communities of living organisms. For example, this is true for Lake Baikal in the Irkutsk Region, Russia. The region is in the center of taiga forests and the leader for wood exports. Moreover, from 1966 to 2013, the activity of the Baikalsk pulp and paper mill led to an accumulation of approx. eight mln. cubic meters of sludge lignin and wood-derived wastes. These wastes are stored close to the lake’s shoreline and its inhabitants [9]. Strong anthropogenic impacts, water level regulation, and high seismic activity in the region cause the need for taking measures aimed at ecological clearance, detoxication, and waste processing.
Microorganisms inhabit all environmental niches and participate in biodegradation and destruction processes. Actinobacteria and microscopic fungi transform hard degradable wastes [10]. Fungi can implement the degradation process quickly and efficiently [11]. However, the advantages of Actinobacteria include its high-level conversion of substrates and its great biotechnological and biosynthetic potential due to well-studied and well-applied methods of genetic engineering [12]. The ability of microorganisms to grow on hard degradable polymers, such as plastic or plant materials, can be explained by their ability to degrade these substrates and consume organic matter obtained in the processes of oxidation or hydrolysis [13,14]. It has been shown that Actinobacteria immobilized on sawdust can be used as an effective biocatalyst for the transformation and degradation of hydrocarbons [15,16].
The growth and development of microorganisms on the above substrates can lead to the synthesis of natural products with biological (in particular, antibiotic) activity. The increasing interest in developing new pharmaceuticals, biologicals, and drugs produced by microorganisms leads to the regular screening of new natural products [17]. The synergetic effects of using agricultural and technological wastes in biotechnological industry can solve at least three of humankind’s problems: the discovery of new molecules with biological activity (1), the destruction and detoxication of industrial and technological wastes (2), and the optimization of microbial cultivation parameters with a cheap and economically available source of carbohydrates (3).
Here, we tested the possibility of developing a technology of recycling sawdust/sawmill waste to produce new active ingredients of pharmaceuticals on the basis of microbial-induced degradation and biotransformation. We studied Actinobacteria as microorganisms that are suitable and convenient “microscopic cellular factories” for the production of bioactive biotechnological products.
The biosynthetic potential of Actinobacteria, which evolved in cold-water, ancient, and clean ecosystems, is of interest, both for fundamental science and for industry to develop technologies for the sustainable management of natural resources.
Actinobacterial natural products are important for the treatment of multiple infectious diseases [18]. For example, about 23,000 antibiotics have been found in various bacteria. Actinobacteria synthesize about 10,000 of them [19]. The genus Streptomyces is a classic and widespread source of natural products with biological activity. It is one of numerous genera in the soil microbial world. This genus is presented by more than 670 species and 1080 strains [20]. Other, rarer Actinobacteria genera are presented by Rhodococcus sp., Micrococcus sp., Actinomadura sp., Frankia sp., etc. Often, these rare genera are well-adapted to extreme environmental conditions [21]. As a rule, these microorganisms need a specific source of carbohydrates. Unusual mechanisms of survival, various biosynthetic mechanisms encoding the important metabolic pathways, and the synthesis of new natural products explain the uniqueness of rare genera of Actinobacteria and their little-studied biosynthetic potential [22]. The biosynthetic potential of Actinobacteria is well-described and presented by bioactive metabolites, such as antibiotics; different volatiles; siderophores; antioxidants; antitumor, antimalaria, and anti-inflammatory agents; and industrial enzymes [23]. Thus, Actinobacteria are an important source of different molecules with biological activity. However, their use for the biosynthesis of natural products from different industrial wastes is poorly understood.
2. Materials and Methods
2.1. Sampling and Isolation of Actinobacteria
Strains of Actinobacteria were isolated on solid MS media (soy flour—20 g/L, D-mannitol—20 g/L, agar—20 g/L), Czapek-Dox media (sucrose—30 g/L, KH2PO4—1 g/L, MgSO4 × 7H2O—0.5 g/L, KCl—0.5 g/L, FeSO4—0.01 g/L, NaNO3—2 g/L, agar—20 g/L), and Hutchinson nutrient media (cellulose—10 g/L, KH2PO4—1 g/L, MgSO4 × 7H2O—0.3 g/L, NaCl—0.07 g/L, CaCl2—0.007 g/L, FeCl3 × 6H2O—0.007 g/L, NaNO3—2.5 g/L, agar—20 g/L). Actinobacteria were isolated from the gastrointestinal tract of Baikal phytophagous amphipods that belong to species Acanthogammarus lappaceus longispinus [24].
Pure strains of Actinobacteria were deposited in the collection of microorganisms of Irkutsk State University (Irkutsk), and in the Russian Collection of Agricultural Microorganisms (RCAM, WDCM 966) of the Federal State All-Russian Research Institute of Agricultural Microbiology, St. Petersburg, Russia. The strains were deposited in the National Institute of Industrial Property (Act 001167 2021100641 at 13 January 2021) to obtain the national patent “Method for obtaining biologically active natural compounds”. Strains of three different genera were used to study the biotechnological potential of Actinobacteria to produce natural products when cultivated on sawdust wastes. These are the species related to genera Streptomyces sp., Rhodococcus sp., and Microbacterium sp.
2.2. Identification
First, the isolated strains were identified using the mass spectrometry system for microorganism identification MALDI BIOTYPER (Bruker Daltonik GmbH, Bremen, Germany) in the Irkutsk Antiplague Research Institute of Siberia and Far East of the Federal Service for Surveillance in the Sphere of Consumers’ Rights Protection and Human Welfare. For identification, we used the method of direct application according to the Bruker recommendation [25,26]. The identification was considered successful when the identified strain was assigned to the color-scale green area.
Moreover, to verify the identification of microorganisms, we used 16S rRNA gene sequencing with basic phylogenetic analysis. To isolate total DNA, strains were grown in 10 mL of TSB (Merck, Darmstadt, Germany) nutrient medium at 8 °C for 30 days at 180 rpm. The total DNA was isolated using the QIAamp DNA Kit (Qiagen, Hilden, Germany). To identify the isolates, gene 16S rRNA was amplified by PCR with Actinobacteria-specific and universal primers. The Actinobacteria-specific primers were F-Act-235 (CGC GGC CTA TCA GCT TGT TG) and R-Act-878 (CCG TAC TCC CCA GGC GGG G) [27]. Universal eubacterial primers included 8F (AGA GTT TGA TCC TGG CTC AG) and 1492R (TAC GGY TAC CTT GTT ACG ACT T) [28]. The PCR reaction was performed using the ScreenMix 5X PCR kit (Kat.PK041L, Evrogen, Moscow, Russia). PCR was performed in the TGradient Thermocycler (Biometra, Göttingen, Germany) at a volume of 25 uL. The PCR parameters were as follows: initial denaturation at 95 °C for 5 min, followed by 25 cycles of 95 °C for 40 s, 49–52 °C for 25 s, and 72 °C for 110 s, and final elongation at 72 °C for 5 min. The PCR products were purified using the QIAquick Gel Extraction Kit (Qiagen, Hilden, Germany) and sequenced using Actinobacteria-specific or universal primers in Evrogen LLC (Moscow, Russia). Forward and reverse sequences were assembled using the Bioedit software (version 7.2.5). The obtained sequences were deposited to the GenBank with numbers OL739263-OL739265 and aligned with the bacterial 16S rRNA gene sequences using BLAST (NCBI, Bethesda, MD, USA).
Evolutionary history was inferred using the Neighbor-Joining method [29]. The nucleotide sequences obtained in the experiment were aligned with the sequences from the NCBI database that showed the greatest similarity. The percentages of replicate trees, in which the associated taxa clustered together in the bootstrap test (1000 replicates), are shown next to the branches [30]. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Evolutionary distances were computed using the Tamura–Nei method [31] and given in the units of the number of base substitutions per site. This analysis involved 33 nucleotide sequences of Streptomyces sp., 37 nucleotide sequences of Rhodococcus sp., and 49 nucleotide sequences of Microbacterium sp. All ambiguous positions were removed for each sequence pair (pairwise deletion option). In total, the final dataset includes 608 positions for Streptomyces sp., 599 positions for Rhodococcus sp., and 553 positions for Microbacterium sp. The evolutionary analysis was conducted using MEGA X [32].
2.3. Cultivation
The isolated strains were cultivated on solid minimal media (MM) (media composition: L-asparagine—0.5 g/L, K2HPO4—0.5 g/L, MgSO4 × 7H2O—0.2 g/L, FeSO4 × 7H2O—0.01 g/L, glucose—10 g/L, agar—20 g/L). Sawdust presented by pine and birch were added to MM in concentrations of 30 g/L (relatively high content) and 10 g/L (relatively low content). The size of the sawdust ranged 0.7–1.2 mm. Sawdust was added in a gradient manner (from 0 g/L to 30 g/L of sawdust). Cultivation was carried out in 20 × 20 cm square Petri dishes. We prepared three types of MM: original MM, MM with pine sawdust, and MM with birch sawdust. To prepare the gradient nutrient media, we used two sterile removable plexiglass partitions to divide the Petri dishes into three equal zones. The first zone was for MM nutrient medium with sawdust in a concentration of 30 g/L. The second zone was for MM nutrient medium with sawdust in a concentration of 10 g/L. The third zone was for original MM nutrient medium without sawdust. The first and third zones were simultaneously filled and polymerized, while the second remained empty. Then, the plexiglass partitions were removed, and the second zone was formed.
The Petri dish was conventionally divided into three equal sectors (along the gradient). The strains were inoculated in a row on the first and second sectors of the Petri dish. Only one strain was inoculated on each Petry dish. The third sector was empty and served as a control. The Petri dishes were incubated at 8 °C for 30 days. Then, the Petri dishes were divided into nine equal parts (3 × 3) using the neck of a 50 mL Falcon tube. The upper disk of each column meant that the MM media did not contain sawdust, the medium disk had a low content of sawdust in MM media (10 g/L), and the lower disk had a high level of sawdust in MM media (30 g/L).
2.4. Extraction of Natural Products
Agar disks were homogenized in 15 mL of isobutyl alcohol and sonicated for 30 min. The sample was centrifuged at 3000 rpm, transferred to new tubes, and evaporated in the needle nitrogen evaporator at 45 °C. The extracts in concentration of 0.1 mg/mL were dissolved in 1:1 mixture of methanol and DMSO (Merck, Germany) [33,34]. The dissolved samples were stored for 5–7 days at −20 °C for sedimentation and aggregation of soluble polymeric molecules. Then, the samples were centrifuged for 1 min at 16,000 rpm at +6 °C before the analysis described in Section 2.5 and Section 2.6. [34]
2.5. Assay of Antibiotic Activity of Extracts from Isolated Strains
Two bacterial cultures were chosen to test the extracts’ antibiotic activity. Test cultures were presented by model strains of Bacillus subtilis ATCC 6633 and Pseudomonas putida KT 2440.
The qualitative test for antimicrobial activity was performed using the disk diffusion method [35]. In short, 12-h bacterial cultures were inoculated on agarized LB media in a volume of 100 µL. Then, Petri dishes with test cultures were dried for 30 min. In parallel, an extract in a volume of 30 µL was used for antibiotics activity assay. Extracts were loaded on 5 mm diameter paper disks. Then, the paper disks were dried in natural conditions and placed on prepared LB media with the inoculated test culture. Petri dishes were incubated for 24 h at 37 °C until the appearance of growth inhibition zones [36]. Paper disks loaded with the methanol and DMSO mixture were used as negative control.
2.6. Estimation of Biotechnological Potential Using the LC-MS Approach
Approaches of ultra-performance liquid chromatography were used to estimate biotechnological potential. We used the Thermo Fisher Scientific Ultimate 3000 (Dionex, Waltham, MA, USA) chromatography system with ultra-high-resolution mass spectrometric detector Q-TOF maXis Impact II (Bruker Daltonik GmbH, Bremen, Germany). Crude extracts were separated in a linear gradient of acetonitrile from 5 to 95% against a 0.1% ammonium formate solution in water at 0.5 mL/min flow rate for 20 min using the Acquity UPLC BEH C18 UHPLC column (Waters, Eschborn, Germany; with the column parameters: 130 Å, 1.7 µm, 2.1 mm × 100 mm) [37,38]. Masses of natural products were detected in positive mode, with the detection range of 160–2500 m/z. Data were collected and analyzed using the Bruker Compass Data Analysis software, version 4.1 (Bruker Daltonik GmbH, Bremen, Germany). Natural products were dereplicated using the Dictionary of Natural Products (DNP) database (CRC Press, Boca Raton, FL, USA) with the following search parameters: accurate molecular mass, absorption spectra, and biological source of molecules isolation. Natural products were considered to be identified when the difference between accurate mass was approximately m/z 0.001, 10 ppm, and the biological source matched the materials deposited in the DNP database. The masses of molecules were calculated using the standard adduct protocols ([M + H], [M + Na], [M + NH4], etc.) [39]. Analysis of each sample was carried out three times.
3. Results
3.1. Identification of Isolated Strains and Their Ability to Grow on Sawdust
During the study, representatives of three genera of Actinobacteria were investigated: Streptomyces sp. (Streptomyces sp. LPB2019K190-4), Microbacterium sp. (Microbacterium sp. LPB2019K198-2), and Rhodococcus sp. (Rhodococcus sp. LPB2019K201-3). The isolated strains revealed similarity with other known species of microorganisms. According to data from the MALDI mass spectrometric identification, the Microbacterium sp. LPB2019K198-2 showed similarity with species M. liquefaciens. At the same time, the isolated Rhodococcus sp. LPB2019K201-3 was similar to species R. fascians. However, according to phylogenetic data, the isolated strain of Microbacterium sp. was closely similar to M. hydrothermale, M. proteolyticum, M. testaceum, M. zeae, and M. hominis. The Baikal strain forms another clade of the phylogenetic tree (Figure 1).
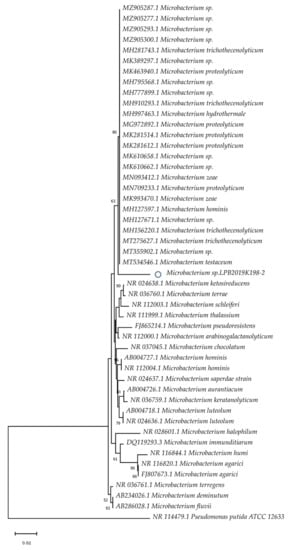
Figure 1.
Evolutionary relationships of the Baikal strain of Microbacterium sp. The phylogenetic tree was constructed using the Neighbor-Joining method. The Baikal strain is marked with the “o” symbol.
By contrast, the strain of Baikal Rhodococcus sp. formed a strict clade with such genera as R. erythropolis, R. zopfii, and R. coprophilus. It does not form a separate clade (Figure 2). The strain of Baikal Streptomyces sp. formed strict clades with S. griseolus, S. flavovirens, S. argenteolus, S. badius, and S. lunaelactis (Figure 3).
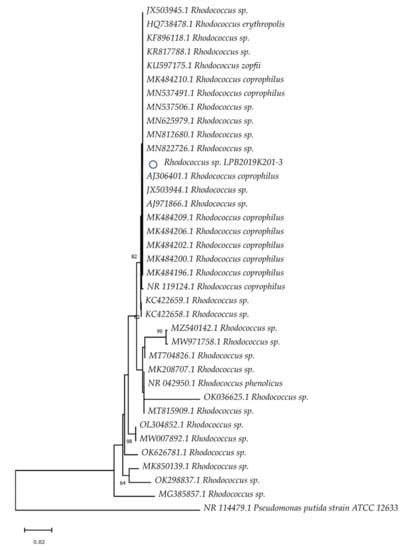
Figure 2.
Evolutionary relationships of the Baikal strain of Rhodocuccus sp. The phylogenetic tree was constructed using the Neighbor-Joining method. The Baikal strain is marked with the “o” symbol.
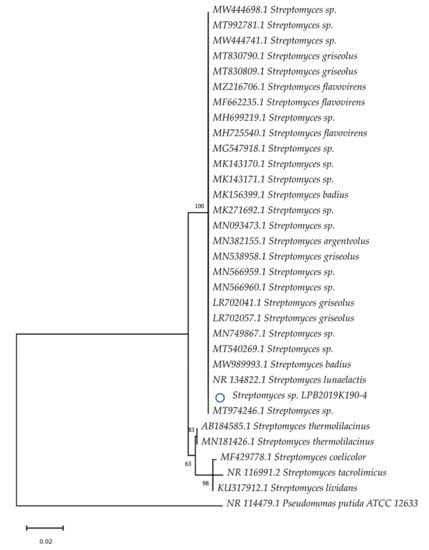
Figure 3.
Evolutionary relationships of the Baikal strain of Streptomyces sp. The phylogenetic tree was constructed using the Neighbor-Joining method. The Baikal strain is marked with the “o” symbol.
At the first stage of our study, we tested the ability of Actinobacteria to grow on nutrient media with the sawdust wastes of hardwood and softwood sawdust. The results demonstrated that Streptomyces sp. LPB2019K190-4 is capable of growing on two types of MM media. The addition of both pine and birch sawdust led to active growth of the strain.
Rare strains of Actinobacteria showed their ability to grow on MM modified by birch sawdust. The addition of pine sawdust in MM media suppressed the growth of rare genus Rhodococcus sp. and Microbacterium sp. Table 1 presents the summary characterizing the presence of strain growth.

Table 1.
Ability of isolated strains to grow on sawdust waste.
3.2. Antibiotic Activity of Isolated Strains
Table 2 presents the antibiotic activity of the strains cultivated on gradient nutrient media. The cultivation of Streptomyces sp. LPB2019K190-4 on solid nutrient media with pine sawdust induced the synthesis of natural products with antimicrobial activity. Crude extracts inhibited the growth of Gram-positive bacteria B. subtilis. We found that Streptomyces sp. LPB2019K190-4 cultivated on MM media inhibited the growth of B. subtilis, just like when cultivated on MM media with pine sawdust. The cultivation of Streptomyces sp. LPB2019K190-4 on MM media with birch sawdust did not lead to growth inhibition in tested B. subtilis and P. putida.

Table 2.
Antibacterial activity of studied strains cultivated on gradient nutrient media with sawdust waste.
Among the rare strains, we detected the antimicrobial activity of Microbacterium sp. LPB2019K198-2 cultivated on birch sawdust and did not detect any antimicrobial activity of Rhodococcus sp. LPB2019K201-3. Microbacterium sp. LPB2019K198-2 was characterized by the absence of antimicrobial activity of crude extracts when grown on MM nutrient media. However, adding birch sawdust to the nutrient media induced the occurrence of antimicrobial activity against Gram-negative bacteria P. putida. The size of the growth inhibition zones ranged from 9 mm to 12 mm (incl. 5 mm paper disk).
3.3. Mass Spectrometric Estimation of Biotechnological Potential
To estimate the biotechnological potential and determine the preliminary composition of low-molecular-weight natural products of Actinobacteria cultivated on sawdust, we analyzed the extracts using UPLC-MS. In total, we found at least 80 molecules synthesized by the studied strains under the experimental conditions. Out of the 80 natural products, 67 could not be identified using the Dictionary of Natural Products database (Figure 4). We demonstrated that adding sawdust in different amounts led to both activation and suppression of natural product synthesis. We did not find strong effects of the type of sawdust on the number of natural products synthesized by Streptomyces sp. representatives. Here, we observed that the studied strain produced from five to nine natural products under the experimental conditions. Representatives of rare strains produced from seven to fifteen natural products in the case of Rhodococcus sp., and from five to nineteen natural products in the case of Microbacterium sp. Thus, for the studied Microbacterium sp., we found that adding birch sawdust to nutrient media resulted in a fourfold increase in the number of produced molecules.
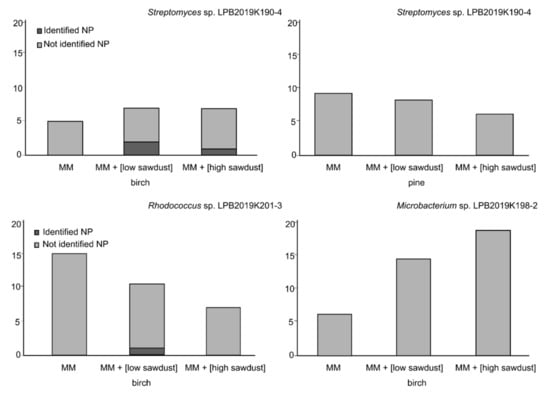
Figure 4.
Number of natural products produced by the studied Actinobacteria when cultivated on nutrient media with sawdust.
The analysis of the synthetic potential of Streptomyces sp. LPB2019K190-4 during cultivation on MM with pine sawdust revealed the induced synthesis of at least 12 natural products. Figure 5 shows the comparative mass chromatograms of molecules extracted from MM with pine sawdust added (left column, control parameters), and from MM where we cultivated Streptomyces sp. LPB2019K190-4 (right column, experimental parameters). Three pre-identified metabolites were found in the crude extracts. These natural products were Glaciapyrrol B ([M + H] 317.1989 Da, retention time (RT) 5.2 min, Δ0.0001 Da, 0.3 ppm) and N2-[5-(4-Aminophenyl)-2,4-pentadienoyl] glutamine ([M + H] 317.1408, RT 2.6 min, Δ0.003 Da, 10.2 ppm), produced by Streptomyces sp. LPB2019K190-4 at a relatively low content of pine sawdust on MM (Figure 6, Table 3). Moreover, the cultivation of Streptomyces sp. LPB2019K190-4 on nutrient media with a relatively high content of pine sawdust caused the synthesis of molecule NFAT 133 ([M + H] 276.1717 Da, RT 6.5 min, Δ0.0008 Da, 3.1 ppm) (Figure 7, Table 3).
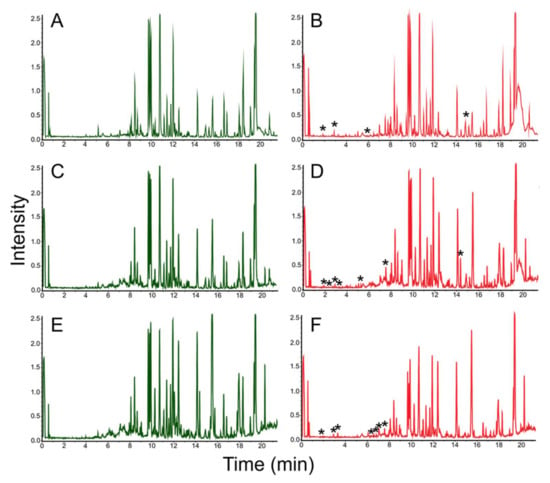
Figure 5.
Chromatograms of natural products extracted from MM with pine sawdust added (left column, control parameters) and MM (left column, experimental parameters) where Streptomyces sp. LPB2019K190-4 was cultivated: (A) MM media without sawdust (control); (B) Streptomyces sp. LPB2019K190-4 cultivated on MM media without sawdust (control); (C) MM media with a relatively low content of pine sawdust (control); (D) Streptomyces sp. LPB2019K190-4 cultivated on MM media with a relatively low content of pine sawdust; (E) MM media with a relatively high content of pine sawdust (control); (F) Streptomyces sp. LPB2019K190-4 cultivated on MM media with a relatively high content of pine sawdust. *-asterisks indicate the differences (new peaks) between MS-spectrums of control and experimental samples.
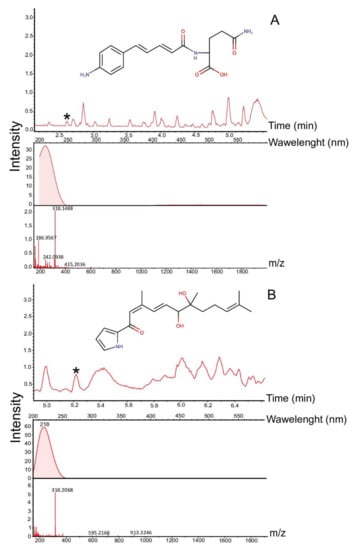
Figure 6.
Mass chromatograms of natural products subjected to bacterial destruction: (A) MS-chromatogram and MS-spectrum of N2-[5-(4-Aminophenyl)-2,4-pentadienoyl] glutamine; (B) MS-chromatogram and MS-spectrum of Glaciapyrrol B. *-asterisks indicate peaks of natural products.

Table 3.
Pre-identified natural products of Streptomyces sp. LPB2019K190-4 and Rhodococcus sp. LPB2019K201-3 cultivated on nutrient medium with sawdust added.
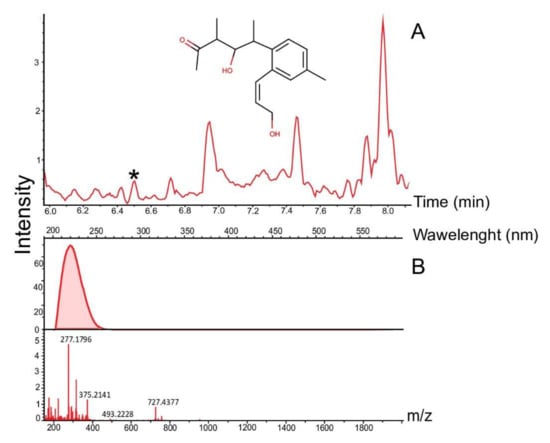
Figure 7.
Mass chromatograms of NFAT 133: (A) Mass chromatogram of NFAT 133; (B) UV and mass profile of the peak identified as NFAT 133. *-asterisk indicates peak of natural product.
The analysis of the biosynthetic potential of Streptomyces sp. LPB2019K190-4 during cultivation on MM with birch sawdust revealed the induced synthesis of at least 22 natural products (Figure 8).
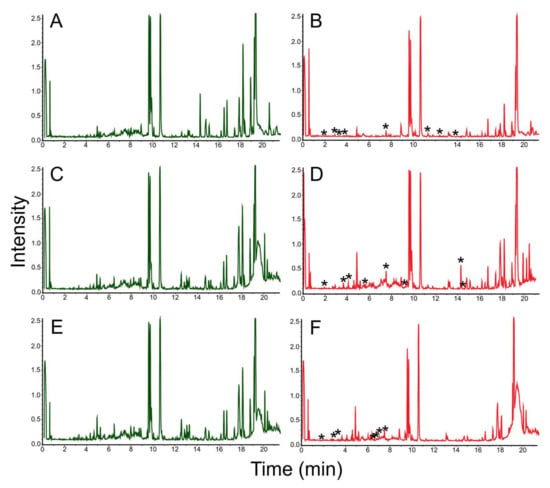
Figure 8.
Chromatograms of natural products extracted from MM media with birch sawdust added (left column, control parameters) and MM (left column, experimental parameters) where Streptomyces sp. LPB2019K190-4 was cultivated: (A) MM media without sawdust (control); (B) Streptomyces sp. LPB2019K190-4 cultivated on MM media without sawdust (control); (C) MM media with a relatively low content of birch sawdust (control); (D) Streptomyces sp. LPB2019K190-4 cultivated on MM media with a relatively low content of birch sawdust (E) MM media with a relatively high content of birch sawdust (control); (F) Streptomyces sp. LPB2019K190-4 cultivated on MM media with a relatively high content of birch sawdust. *-asterisks indicate the differences (new peaks) between MS-spectrums of control and experimental samples.
The cultivation of Rhodococcus sp. LPB2019K201-3 on birch wastes induced the synthesis of 25 natural products (Figure 9, Table 3). The presence of a natural product known as octahydro-7α-methyl-1-(1-methyl-2-isopropyl)-5-oxo-1H-indene-4-propanoic acid ([M + H] 294.1801 Da, RT 14.4 min, Δ0.003 Da, 10.2 ppm) was preliminarily identified in crude extract (Figure 10, Table 3). Rhodococcus sp. LPB2019K201-3 produced this molecule in a relatively low amount of birch sawdust in MM media. The strain did not produce the above natural product when cultivated with a high content of birch sawdust.
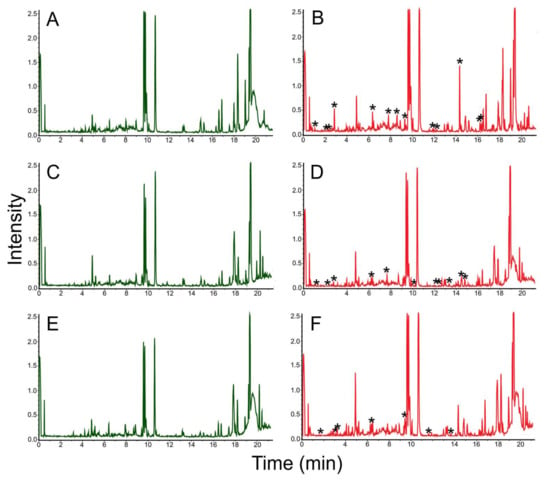
Figure 9.
Chromatograms of natural products extracted from MM with birch sawdust added (left column, control parameters) and MM (left column, experimental parameters) where Rhodococcus sp. LPB2019K201-3 was cultivated: (A) MM media without sawdust (control); (B) Rhodococcus sp. LPB2019K201-3 cultivated on MM media without sawdust (control); (C) MM media with a relatively low content of birch sawdust (control); (D) Rhodococcus sp. LPB2019K201-3 cultivated on MM media with a relatively low content of birch sawdust; (E) MM media with a relatively high content of birch sawdust (control); (F) Rhodococcus sp. LPB2019K201-3 cultivated on MM media with a relatively high content of birch sawdust. *-asterisks indicate the differences (new peaks) between MS-spectrums of control and experimental samples.
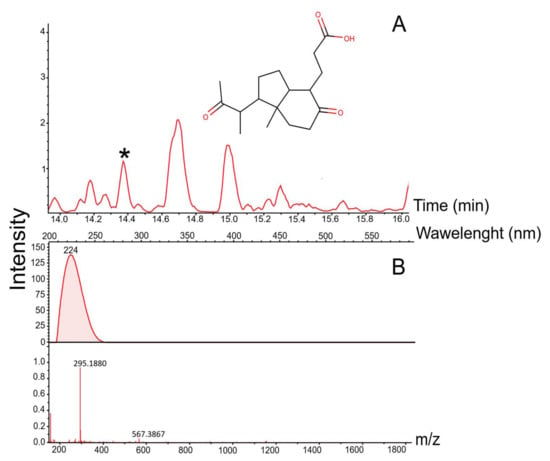
Figure 10.
Mass chromatogram of the natural product identified as Octahydro-7α-methyl-1-(1-methyl-2-oxopropyl)-5-oxo-1H-indein-4-propionic acid: (A) Chromatogram of Octahydro-7α-methyl-1-(1-methyl-2-oxopropyl)-5-oxo-1H-indein-4-propionic acid; (B) UV and mass profile of the peak identified as otahydro-7α-methyl-1-(1-methyl-2-oxopropyl)-5-oxo-1H-indein-4-propionic acid. *-asterisk indicates peak of natural product.
The presence of birch sawdust in nutrient media induced the synthesis of at least 30 natural products in Microbacterium sp. LPB2019K198-2 (Figure 11). However, all these molecules cannot be identified at this stage of our study.
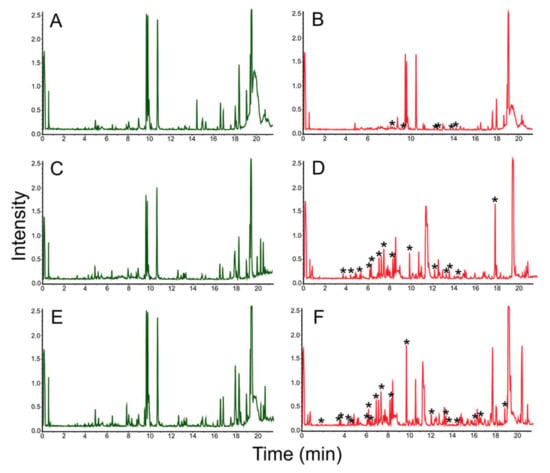
Figure 11.
Chromatograms of natural products extracted from MM with birch sawdust added (left column, control parameters) and MM (left column, experimental parameters) where Microbacterium sp. LPB2019K198-2 was cultivated: (A) MM media without sawdust (control); (B) Microbacterium sp. LPB2019K198-2 cultivated on MM media without sawdust (control); (C) MM media with a relatively low content of birch sawdust (control); (D) Microbacterium sp. LPB2019K198-2 cultivated on MM media with a relatively low content birch sawdust; (E) MM media with a relatively high content of birch sawdust (control); (F) Microbacterium sp. LPB2019K198-2 cultivated on MM media with a relatively high content of birch sawdust. *-asterisks indicate the differences (new peaks) between MS-spectrums of control and experiment.
4. Discussion
One of the current topics in biotechnology is the study of the ability of microorganisms to degrade organic matter of natural and anthropogenic origin [45,46]. The total annual scope of sawmill waste is measured in millions of cubic meters that are not used. These wastes occupy vast territories and pollute the environment. Simultaneously, sawdust and lignocellulosic wastes can be used as biosorbents [47,48,49], but for the unique and ancient ecosystem of Lake Baikal, this is not suitable. Among the number of existing technologies for the detoxication of materials and agricultural wastes, one of the crucial ecological technologies is the utilization of sawmill waste using microorganisms involved with biological destruction [50].
Lake Baikal is one of the freshwater ecosystems characterized by a great number of endemic organisms, low positive temperature, and a high level of biodiversity. Many studies describing the diversity of its microbial communities were performed in the Baikal region [51,52,53]. Our study is one of the first devoted to the synthesis of natural products by the cultivation of Baikal Actinobacteria on plant-derived polymers and sawmill waste. As destructors, Actinobacteria can simultaneously consume complex degraded plant polymers and produce valuable natural products.
In our study, we used three wild strains of Baikal psychrophilic Actinobacteria to demonstrate the possibility of using sawmill waste as a component of the nutrient medium required for the synthesis of natural products. During the experiments, we showed that Streptomyces sp. LPB2019K190-4 grew on both pine and birch sawdust, whereas rare strains presented by Rhodococcus sp. LPB2019K201-3 and Microbacterium sp. LPB2019K198-2 could only grow on nutrient media with birch sawdust. Based on this, we suppose that Streptomyces sp. LPB2019K190-4 has a specific adaptation that helps this strain (genus) to grow on nutrient medium containing pine resin. The resin contains natural products with multiple antimicrobial activities. This singularity of the Streptomyces genus is also reported in other studies [54,55,56]. For example, Actinobacteria of the Streptomyces genus were found on the surface of black pine roots [57]. Representatives of this genus of Actinobacteria are a part of the microbial community in the soils in pine forests [58].
The analysis of published data shows that Actinobacteria of rare genera, such as Rhodococcus sp. and Microbacterium sp., can also be endophytes of pine species. This has been shown for at least four pine species growing in the forests of South Korea, where representatives of these rare genera have been found [59]. As we have shown, the Baikal rare genera of Actinobacteria are not capable of growing on nutrient media containing pine sawdust. This can be explained by the high selective sensitivity of the strains to natural products of resins and the limited adaptations that allow them to be resistant to pine resin.
Moreover, this research has found that Streptomyces sp. LPB2019K190-4 cultivated on nutrient media with sawdust is characterized by antimicrobial activity. We observed the strain’s antimicrobial activity under all tested conditions: cultivation on MM and MM modified by pine sawdust. Furthermore, the absence of antimicrobial activity in the crude extracts obtained from samples of the control nutrient medium (with sawdust, but without strain growth on the surface of the Petri Dish) and, simultaneously, the presence of antimicrobial activity in the experiment indicates the diffusion of resins and their penetration to the MM sector on experimental plates where the strain was grown. Thus, in the case of Streptomyces sp., we detected the chemical induction of the synthesis of natural products. Thus, there is a high probability that it is the molecules from pine resins that induce the synthesis of bacterial natural products.
The cultivation of Streptomyces sp. LPB2019K190-4 on MM media with pine sawdust promoted the synthesis of Glaciapyrrol B. This probably resulted in the growth inhibition of Gram-positive bacteria B. subtilis. Literature analysis showed that Glaciapyrrol B is a pyrrole sesquiterpenoid and has antibiotic activity against Micrococcus luteus and B. subtilis [41]. Moreover, in the extracts of Streptomyces sp. LPB2019K190-4 cultivated on nutrient media with sawdust added, we found a natural product that can be identified as NFAT 133. NFAT 133 is a trisubstituted aromatic molecule with antidiabetic potential due to its inhibiting NFAT-dependent transcription [43,60].
The cultivation of a rare genus of Actinobacteria on the minimal nutrient medium with sawdust caused both the activation and the inhibition of the synthesis of natural products. The strain of Microbacterium sp. revealed a strong positive effect and demonstrated the fourfold induction of the synthesis of molecules when cultivated in the presence of sawdust. Thus, the use of sawdust as a nutrient medium component led to a species-specific exhibition of biosynthetic potential.
Moreover, natural products produced by rare Actinobacteria within the experiment are not reported in the largest database titled Dictionary of Natural Products. Thus, there is a high probability of revealing new chemical molecules obtained due to the synthesis of natural products by Actinobacteria.
The singularity of rare genera can be explained by the relatively low level of knowledge about them and the new experimental design used in this research. Studies of the adaptation mechanisms and metabolic pathways of Actinobacteria cultivated on nutrient media with sawmill waste may have great biotechnological potential for both biomedical and bioremediation processes. Here, we demonstrated a preliminary estimation of the biotechnological potential of Baikal psychrophilic Actinobacteria for the production of natural products when cultivated on nutrient media containing sawmill waste. At the same time, a thorough understanding of the role of microorganisms and their impact on anthropogenic waste can be helpful for developing a technology for transforming anthropogenic waste into valuable biopharmaceutical products.
The analysis of problems of bioethanol production, as described in [61,62], leads to the necessity for testing Actinobacteria as biological agents for the detoxication of toxic compounds from bioethanol precursors. The use of Actinobacteria in intermediate processes in the technological scheme of bioethanol synthesis may lead to the development of safe and environmentally friendly technologies for the deep conversion of plant materials. Thus, the performed study and recently published reviews [6,7] can open a new era of using microorganisms in the industry and contribute to the sustainable development of the planet.
Author Contributions
E.V.P., M.E.D., M.M.M. and N.A.I. performed the experiments and analyzed the data. E.V.P. and N.A.I. isolated the strains. D.V.A.-G., E.V.P. and A.Y.B. collected samples from Lake Baikal and performed the experiments. E.V.P., A.S.O. and M.E.D. identified the strains. D.V.A.-G. and E.V.P. planned the experiments, analyzed the data, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
The study was carried out with the primary financial support of the Russian Foundation for Basic Research No 18-29-05051 and Grant of the President of the Russian Federation No MK-1245.2021.1.4. Partly, the research was funded by the Ministry of Science and Higher Education of the Russian Federation (the competition aimed at the creation of new laboratories in scientific organizations in the interests of the Regional Scientific Education Centre “Baikal”, Internal Theme 091-21-114, State registration 121111100025-5 at 11 November 2021). The study was performed in the laboratory of Pharmaceutical Biotechnology, created in ISU, and supported by GreenTechBaikal LLC and Project of RSF 20-76-00001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data presented in this research are available in the main body of the manuscript.
Acknowledgments
We thank all researchers and students at the Institute of Biology at ISU (especially Uliana Vasilyeva), Helmholtz Institute for Pharmaceutical Research Saarland (HIPS), and Saarland University (especially group of Actinobacteria Metabolic Engineering), and Nadezda Dolgikh for their technical help, and accomplishment of current research.
Conflicts of Interest
All authors declared no conflict of interest.
References
- Kundungal, H.; Gangarapu, M.; Sarangapani, S.; Patchaiyappan, A.; Devipriya, S.P. Efficient Biodegradation of Polyethylene (HDPE) Waste by the Plastic-Eating Lesser Waxworm (Achroia Grisella). Environ. Sci. Pollut. Res. 2019, 26, 18509–18519. [Google Scholar] [CrossRef] [PubMed]
- Molino, A.; Larocca, V.; Chianese, S.; Musmarra, D. Biofuels Production by Biomass Gasification: A Review. Energies 2018, 11, 811. [Google Scholar] [CrossRef]
- Mollica, G.J.G.; Balestieri, J.A.P. Is It Worth Generating Energy with Garbage? Defining a Carbon Tax to Encourage Waste-to-Energy Cycles. Appl. Therm. Eng. 2020, 173, 115195. [Google Scholar] [CrossRef]
- Luo, J.; Donnison, A.; Ross, C.; Bolan, N.; Ledgard, S.; Clark, D.; Qiu, W. Sawdust and Bark to Treat Nitrogen and Faecal Bacteria in Winter Stand-Off Pads on A Dairy Farm. N. Z. J. Agr. Res. 2008, 51, 331–340. [Google Scholar] [CrossRef]
- Zorpas, A.A.; Loizidou, M. Sawdust and Natural Zeolite as A Bulking Agent for Improving Quality of a Composting Product from Anaerobically Stabilized Sewage Sludge. Bioresour. Technol. 2008, 99, 7545–7552. [Google Scholar] [CrossRef]
- Cho, E.J.; Trinh, L.T.P.; Song, Y.; Lee, Y.G.; Bae, H.J. Bioconversion of Biomass Waste into High Value Chemicals. Bioresour. Technol. 2020, 298, 122386. [Google Scholar] [CrossRef]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Seelan, J.S.S. Value-Added Metabolites from Agricultural Waste and Application of Green Extraction Techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Hajam, M.E.; Plavan, G.I.; Kandri, N.I.; Dumitru, G.; Nicoara, M.N.; Zerouale, A.; Faggio, C. Evaluation of Softwood and Hardwood Sawmill Wastes Impact on the Common Carp “Cyprinus Carpio” and Its Aquatic Environment: An Oxidative Stress Study. Environ. Toxicol. Pharmacol. 2020, 75, 103327. [Google Scholar] [CrossRef]
- Samarin, E.N.; Kravchenko, N.S.; Zerkal, O.V.; Chernov, M.S.; Rodkina, I.A. Grouting of Waste of the Baikal Pulp and Paper Mill to Reduce the Technogenic Impact to the Baikal Lake Ecosystem. J. Geosci. Environ. Prot. 2020, 8, 112–118. [Google Scholar] [CrossRef]
- Amin, D.H.; Abdallah, N.A.; Abolmaaty, A.; Tolba, S.; Wellington, E.M.H. Microbiological and Molecular Insights on Rare Actinobacteria Harboring Bioactive Prospective. Bull. Natl. Res. Cent. 2020, 44, 5. [Google Scholar] [CrossRef]
- Wurzbacher, C.; Kerr, J.; Grossart, H.P. Aquatic fungi. In The Dynamical Processes of Biodiversity: Evolution and Spatial Distribution; Few Study Cases; Grillo, O., Venora, G., Eds.; InTech Open: Rijeka, Croatia, 2011; pp. 227–258. [Google Scholar]
- Wang, C.; Dong, D.; Wang, H.; Müller, K.; Qin, Y.; Wang, H.; Wu, W. Metagenomic Analysis of Microbial Consortia Enriched from Compost: New Insights into the Role of Actinobacteria in Lignocellulose Decomposition. Biotechnol. Biofuels 2016, 9, 22. [Google Scholar] [CrossRef]
- Zheng, Y.; Yanful, E.K.; Bassi, A.S. A Review of Plastic Waste Biodegradation. Crit. Rev. Biotechnol. 2005, 25, 243–250. [Google Scholar] [CrossRef]
- Hazarika, J.; Khwairakpam, M. Evaluation of Biodegradation Feasibility through Rotary Drum Composting Recalcitrant Primary Paper Mill Sludge. Waste Manag. 2018, 76, 275–283. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Kuyukina, M.S.; Krivoruchko, A.V.; Plekhov, O.A.; Naimark, O.B.; Podorozhko, E.A.; Lozinsky, V.I. Biosurfactant-Enhanced Immobilization of Hydrocarbon-Oxidizing Rhodococcus Ruber on Sawdust. Appl. Microbiol. Biotechnol. 2013, 97, 5315–5327. [Google Scholar] [CrossRef]
- Ivshina, I.B.; Vikhareva, E.V.; Richkova, M.I.; Mukhutdinova, A.N.; Karpenko, J.N. Biodegradation of Drotaverine Hydrochloride by Free and Immobilized Cells of Rhodococcus Rhodochrous IEGM 608. World J. Microbiol. Biotechnol. 2012, 28, 2997–3006. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural Product Drug Discovery in the Genomic Era: Realities, Conjectures, Misconceptions, and Opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Zhang, L.; Demain, A.L. Natural Products: Drug Discovery and Therapeutic Medicine; Humana Press: Totowa, NJ, USA, 2005. [Google Scholar]
- Salwan, R.; Sharma, V. Molecular and Biotechnological Aspects of Secondary Metabolites in Actinobacteria. Microbiol. Res. 2020, 231, 126374. [Google Scholar] [CrossRef]
- List of Prokaryotic Names with Standing in Nomenclature. Available online: https://lpsn.dsmz.de/genus/streptomyces (accessed on 11 April 2022).
- Dhakal, D.; Pokhrel, A.R.; Shrestha, B.; Sohng, J.K. Marine Rare Actinobacteria: Isolation, Characterization, and Strategies for Harnessing Bioactive Compounds. Front. Microbiol. 2017, 8, 1106. [Google Scholar] [CrossRef]
- Hui, M.L.Y.; Tan, L.T.H.; Letchumanan, V.; He, Y.W.; Fang, C.M.; Chan, K.G.; Law, J.W.F.; Lee, L.H. The Extremophilic Actinobacteria: From Microbes to Medicine. Antibiotics 2021, 10, 682. [Google Scholar] [CrossRef]
- Lee, L.H.; Goh, B.H.; Chan, K.G. Editorial: Actinobacteria: Prolific Producers of Bioactive Metabolites. Front. Microbiol. 2020, 11, 1612. [Google Scholar] [CrossRef]
- Takhteev, V.V. On the Current State of Taxonomy of the Baikal Lake Amphipods (Crustacea, Amphipoda) and the Typological Ways of Constructing Their System. Arthsel 2019, 28, 374–402. [Google Scholar] [CrossRef]
- Kostrzewa, M. Application of the MALDI Biotyper to Clinical Microbiology: Progress and Potential. Expert Rev. Proteomic 2018, 15, 193–202. [Google Scholar] [CrossRef]
- Schulthess, B.; Bloemberg, G.V.; Zbinden, A.; Mouttet, F.; Zbinden, R.; Böttger, E.C.; Hombach, M. Evaluation of the Bruker MALDI Biotyper for Identification of Fastidious Gram-Negative Rods. J. Clin. Microbiol. 2016, 54, 543–548. [Google Scholar] [CrossRef]
- Stach, J.E.M.; Maldonado, L.A.; Ward, A.C.; Goodfellow, M.; Bull, A.T. New Primers for the Class Actinobacteria: Application to Marine and Terrestrial Environments. Environ. Microbiol. 2003, 5, 828–841. [Google Scholar] [CrossRef]
- Shieh, J.C.; Martin, H.; Millar, J.B.A. Evidence for a Novel MAPKKK-Independent Pathway Controlling the Stress Activated Sty1/Spc1 MAP Kinase in FIssion Yeast. J. Cell Sci. 1998, 111, 799–807. [Google Scholar]
- The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Mol. Biol. Evol. 1987, 4, 406–425. [CrossRef]
- Felsenstein, J. Phylogenies and the Comparative Method. Am. Nat. 1985, 125, 171–185. [Google Scholar] [CrossRef]
- Tamura, K.; Nei, M. Estimation of the Number of Nucleotide Substitutions in the Control Region of Mitochondrial DNA in Humans and Chimpanzees. Mol. Biol. Evol. 1993, 10, 512–526. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Sticher, O. Natural Product Isolation. Nat. Prod. Rep. 2008, 25, 517. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L. (Eds.) Natural Products Isolation. Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 864. [Google Scholar] [CrossRef]
- Mahajan, G.B.; Balachandran, L. Sources of Antibiotics: Hot Springs. Biochem. Pharmacol. 2017, 134, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.; Bortolotto, V.; Araujo, S.; Poetini, M.; Sehn, C.; Neto, J.É.; Zeni, G.; Prigol, M. Antimicrobial Effect of 2-Phenylethynyl-Butyltellurium in Escherichia Coli and Its Association with Oxidative Stress. J. Microbiol. Biotechnol. 2018, 28, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Voitsekhovskaia, I.; Paulus, C.; Dahlem, C.; Rebets, Y.; Nadmid, S.; Zapp, J.; Axenov-Gribanov, D.; Rückert, C.; Timofeyev, M.; Kalinowski, J.; et al. New Aquayamycin-Type Angucyclines Isolated from Lake Baikal Derived Streptomyces sp. IB201691-2A. Microorganisms 2020, 8, 680. [Google Scholar] [CrossRef] [PubMed]
- Axenov-Gribanov, D.V.; Morgunova, M.M.; Vasilieva, U.A.; Gamaiunov, S.V.; Dmitrieva, M.E.; Pereliaeva, E.V.; Belyshenko, A.Y.; Luzhetskyy, A.N. Composition of Nutrient Media and Temperature of Cultivation Imposes Effect on the Content of Secondary Metabolites of Nocardiopsis Sp. Isolated from a Siberian Cave. 3 Biotech 2021, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Błajet-Kosicka, A.; Kosicki, R.; Twarużek, M.; Grajewski, J. Determination of Moulds and Mycotoxins in Dry Dog and Cat Food Using Liquid Chromatography with Mass Spectrometry and Fluorescence Detection. Food Addit. Contam. B 2014, 7, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Buckingham, J. (Ed.) Dictionary of Natural Products, Supplement 4; CRC Press: Boca Raton, FL, USA, 1997. [Google Scholar]
- Macherla, V.R.; Liu, J.; Bellows, C.; Teisan, S.; Nicholson, B.; Lam, K.S.; Potts, B.C.M. Glaciapyrroles A, B, and C, Pyrrolosesquiterpenes from a Streptomyces sp. Isolated from an Alaskan Marine Sediment. J. Nat. Prod. 2005, 68, 780–783. [Google Scholar] [CrossRef]
- Burres, N.S.; Premachandran, U.; Hoselton, S.; Cwik, D.; Hochlowski, J.E.; Ye, Q.; Sunga, G.N.; Karwowski, J.P.; Jackson, M.; Whittern, D.N.; et al. Simple Aromatics Identified with a NFAT–lacZ Transcription Assay for the Detection of Immunosuppressants. J. Antibiot. 1995, 48, 380–386. [Google Scholar] [CrossRef][Green Version]
- Kulkarni-Almeida, A.A.; Brahma, M.K.; Padmanabhan, P.; Mishra, P.D.; Parab, R.R.; Gaikwad, N.V.; Thakkar, C.S.; Tokdar, P.; Ranadive, P.V.; Nair, A.S.; et al. Fermentation, Isolation, Structure, and Antidiabetic Activity of NFAT-133 Produced by Streptomyces strain PM0324667. AMB Express 2011, 1, 42. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, L.; Komaki, H.; Oku, N.; Igarashi, Y. Absolute Configuration of NFAT-133, an Aromatic Polyketide with Immunosuppressive and Antidiabetic Activity from Actinomycetes. J. Antibiot. 2016, 69, 69–71. [Google Scholar] [CrossRef]
- Yi, H.; Li, M.; Huo, X.; Zeng, G.; Lai, C.; Huang, D.; An, Z.; Qin, L.; Liu, X.; Li, B.; et al. Recent Development of Advanced Biotechnology for Wastewater Treatment. Crit. Rev. Biotechnol. 2020, 40, 99–118. [Google Scholar] [CrossRef]
- Tkachuk, N.; Zelena, L. The Impact of Bacteria of The Genus Bacillus Upon the Biodamage/Biodegradation of Some Metals And Extensively Used Petroleum-Based Plastics. Corros. Mater. Degrad. 2021, 2, 531–553. [Google Scholar] [CrossRef]
- Suteu, D.; Zaharia, C. Sawdust as biosorbent for removal of dyes from wastewaters. Kinetic and thermodynamic study. Chem. Bull. Politeh. 2011, 56, 85–88. [Google Scholar]
- Suteu, D.; Zaharia, C.; Badeanu, M. Agriculture wastes used as sorbents for dyes removal from aqueous environments. Seed 2010, 8, 140–145. [Google Scholar]
- Suteu, D.; Bilba, D.; Zaharia, C.; Popescu, A. Removal of dyes from textile wastewater by sorption onto ligno-cellulosic materials. Sci. Study Res. 2008, 9, 293–302. [Google Scholar]
- Yadav, B.; Pandey, A.; Kumar, L.R.; Tyagi, R.D. Bioconversion of Waste (Water)/Residues to Bioplastics- A Circular Bioeconomy Approach. Bioresour. Technol. 2020, 298, 122584. [Google Scholar] [CrossRef] [PubMed]
- Babich, O.; Shevchenko, M.; Ivanova, S.; Pavsky, V.; Zimina, M.; Noskova, S.; Anohova, V.; Chupakhin, E.; Sukhikh, S. Antimicrobial Potential of Microorganisms Isolated from the Bottom Sediments of Lake Baikal. Antibiotics 2021, 10, 927. [Google Scholar] [CrossRef] [PubMed]
- Zemskaya, T.I.; Cabello-Yeves, P.J.; Pavlova, O.N.; Rodriguez-Valera, F. Microorganisms of Lake Baikal—The Deepest and Most Ancient Lake on Earth. Appl. Microbiol. Biotechnol. 2020, 104, 6079–6090. [Google Scholar] [CrossRef]
- Suslova, M.Y.; Pestunova, O.S.; Sukhanova, E.V.; Shtykova, Y.R.; Kostornova, T.Y.; Khanaev, I.V.; Sakirko, M.V.; Parfenova, V.V. Role of Cultured Microorganisms from Biofilms Formed on Rocky Substrates in the Lake Baikal Self-Purification System. Microbiology 2018, 87, 817–824. [Google Scholar] [CrossRef]
- Chauhan, P.S. Role of Various Bacterial Enzymes in Complete Depolymerization of Lignin: A Review. Biocat. Agric. Biotechnol. 2020, 23, 101498. [Google Scholar] [CrossRef]
- Vilanova, C.; Marín, M.; Baixeras, J.; Latorre, A.; Porcar, M. Selecting Microbial Strains from Pine Tree Resin: Biotechnological Applications from a Terpene World. PLoS ONE 2014, 9, e100740. [Google Scholar] [CrossRef]
- Chang, R.; Rohindra, D.; Lata, R.; Kuboyama, K.; Ougizawa, T. Development of Poly(ε-Caprolactone)/Pine Resin Blends: Study of Thermal, Mechanical, and Antimicrobial Properties: Development of Poly(ε-Caprolactone)/Pine Resin Blends. Polym. Eng. Sci. 2019, 59, E32–E41. [Google Scholar] [CrossRef]
- Sakoda, S.; Aisu, K.; Imagami, H.; Matsuda, Y. Comparison of Actinomycete Community Composition on the Surface and Inside of Japanese Black Pine (Pinus Thunbergii) Tree Roots Colonized by the Ectomycorrhizal Fungus Cenococcum Geophilum. Microb. Ecol. 2019, 77, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Satomi, M.; Fukui, Y.; Matsunobu, S.; Morifuku, Y.; Enokida, Y. Streptomyces Abietis Sp. Nov., a Cellulolytic Bacterium Isolated from Soil of a Pine Forest. Int. J. Syst. Evol. Microbiol. 2013, 63, 4754–4759. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ponpandian, L.N.; Kim, H.; Jeon, J.; Hwang, B.S.; Lee, S.K.; Park, S.C.; Bae, H. Distribution and Diversity of Bacterial Endophytes from Four Pinus Species and Their Efficacy as Biocontrol Agents for Devastating Pine Wood Nematodes. Sci. Rep. 2019, 9, 12461. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, C.S.; Kate, A.S.; Desai, D.C.; Ghosh, A.R.; Kulkarni-Almeida, A.A. NFAT-133 Increases Glucose Uptake in L6 Myotubes by Activating AMPK Pathway. Eur. J. Pharmacol. 2015, 769, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.P.; Huang, S.H.; Ting, Y.; Hsu, H.Y.; Cheng, K.C. Evaluation of Detoxified Sugarcane Bagasse Hydrolysate by Atmospheric Cold Plasma for Bacterial Cellulose Production. Int. J. Biol. Macromol. 2022, 204, 136–143. [Google Scholar] [CrossRef]
- Lin, S.P.; Kuo, T.C.; Wang, H.T.; Ting, Y.; Hsieh, C.W.; Chen, Y.K.; Cheng, K.C. Enhanced Bioethanol Production Using Atmospheric Cold Plasma-Assisted Detoxification of Sugarcane Bagasse Hydrolysate. Bioresour. Technol. 2020, 313, 123704. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).