Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Method
2.2.1. Fermentation of Rice Bran and Optimization of Fermentation Technology
2.2.2. DPPH Free Radical Scavenging Capacity
2.2.3. Determination of Bioactive Constituents of Water-Soluble Extracts
2.2.4. Phenolic Acid Composition
2.2.5. Antioxidant Activity in Zebrafish
Collection of Zebrafish Embryos
Exposure of Embryos to AAPH
Oxidative-Stress-Induced Intracellular ROS Production, Cell Death, and Lipid Peroxidation Measurements and Image Analysis
Antioxidant Enzyme Activity Measurements
2.3. Statistical Analysis
3. Results and Discussion
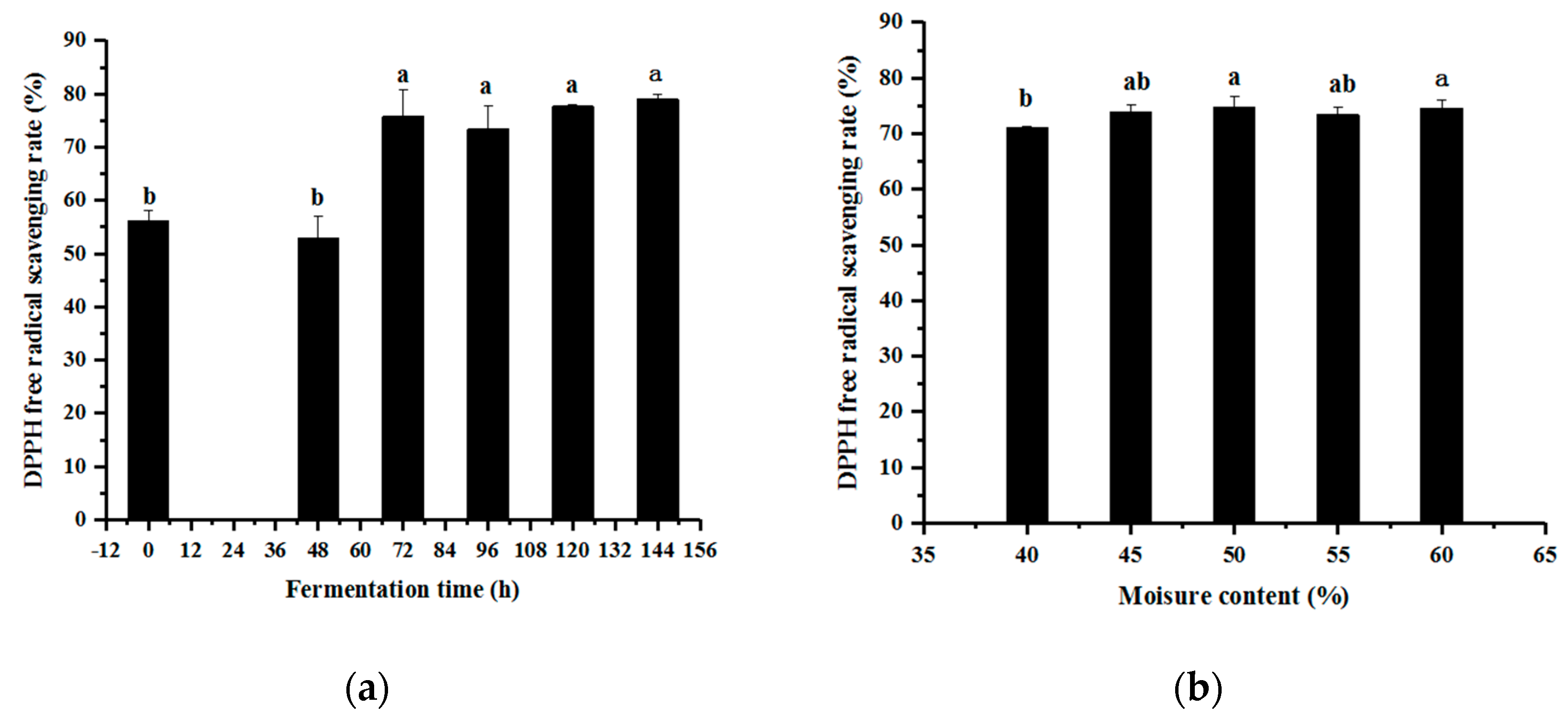
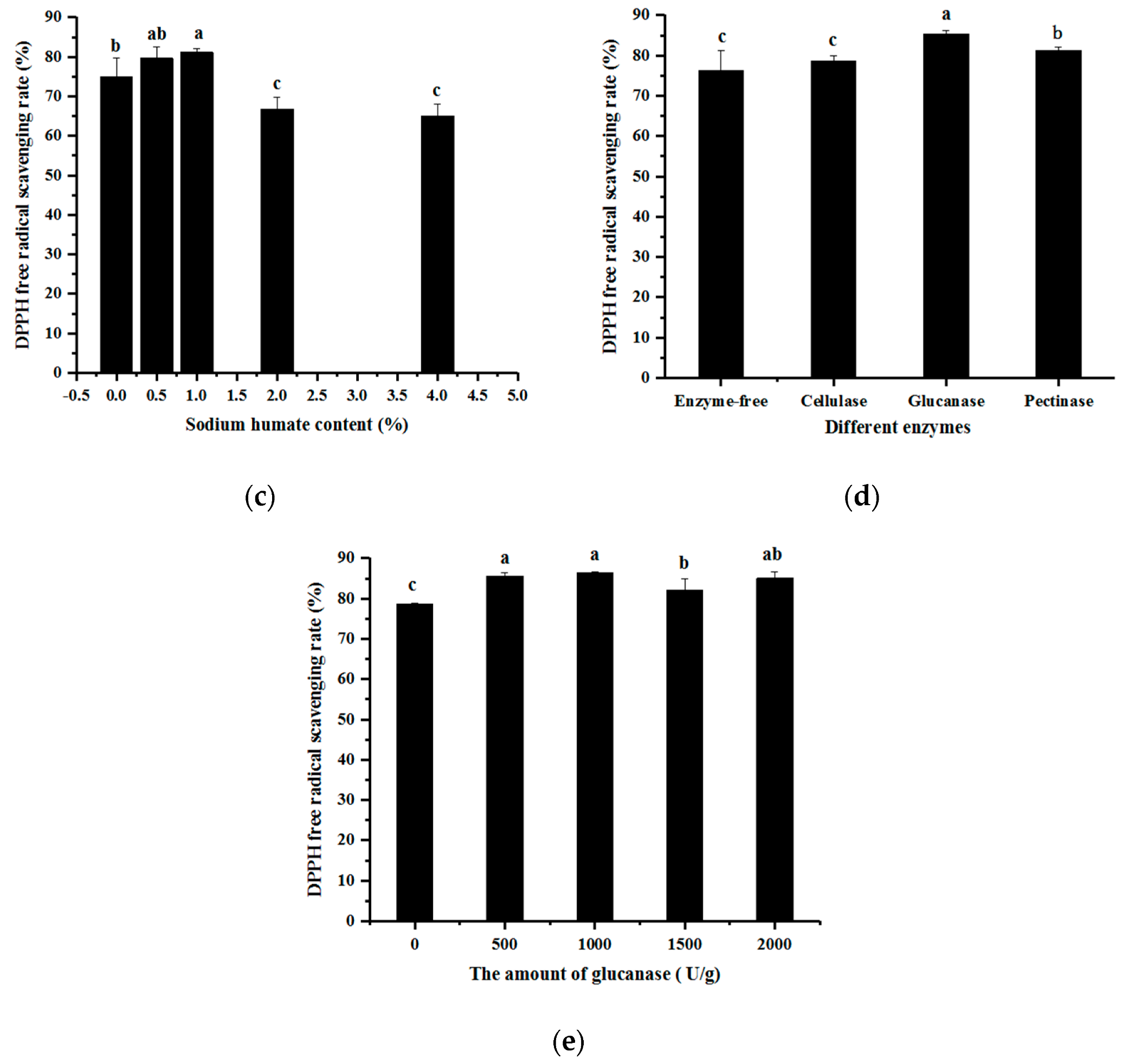
3.1. Single-Factor Experimental Results of Fermentation Technology
3.2. Analysis of Bioactive Constituents of Water-Soluble Extracts
3.3. Analysis of Water-Soluble Phenolic Acid Composition
3.4. Antioxidant Activity in Zebrafish
3.4.1. Reactive Oxygen Species Production
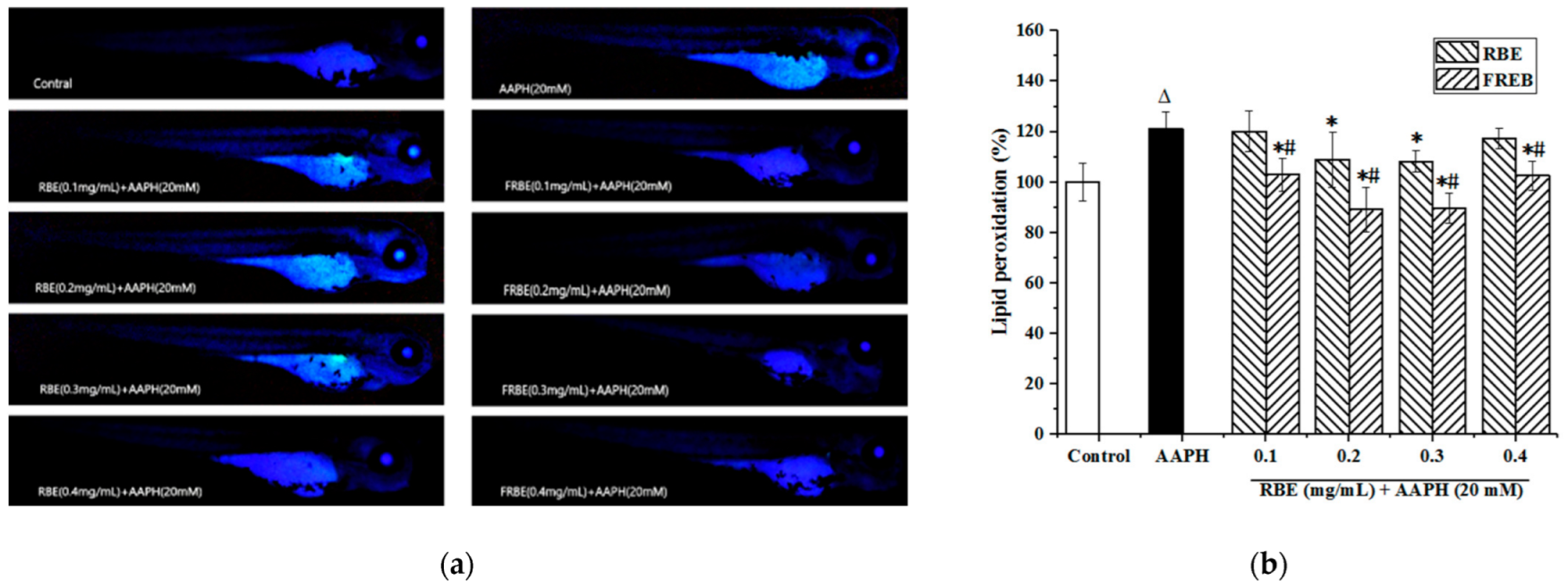
3.4.2. Lipid Peroxidation of Zebrafish Embryos
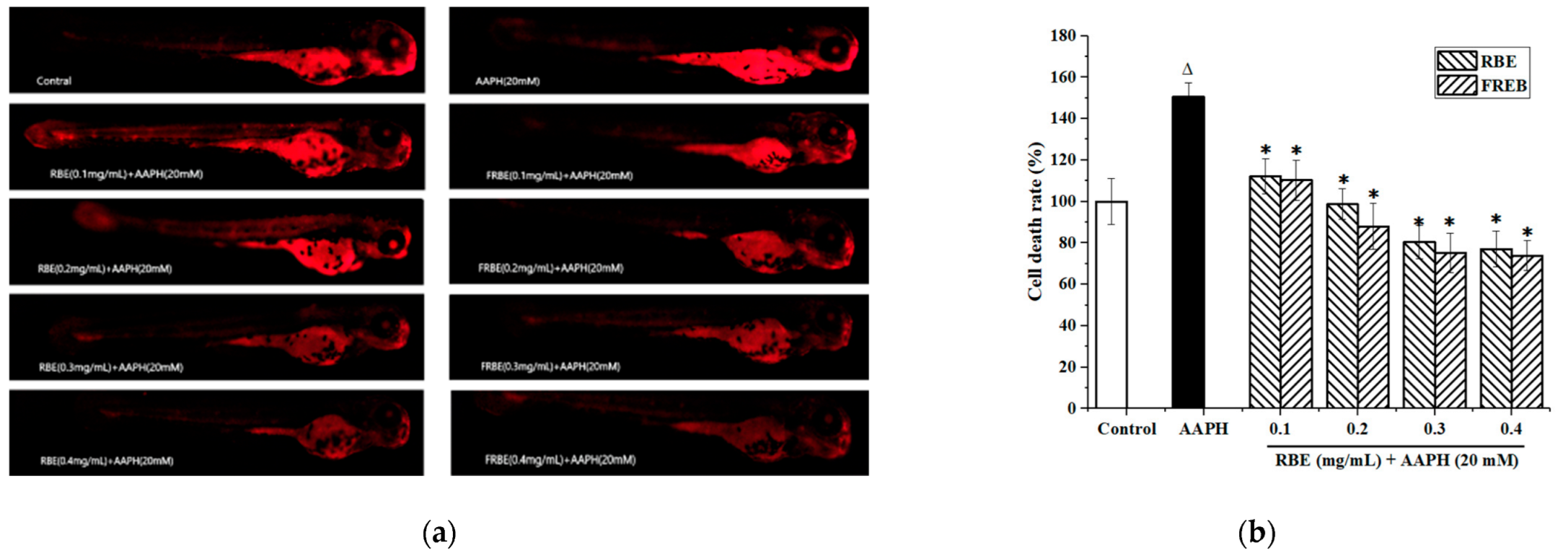
3.4.3. Cell death in Zebrafish Embryos
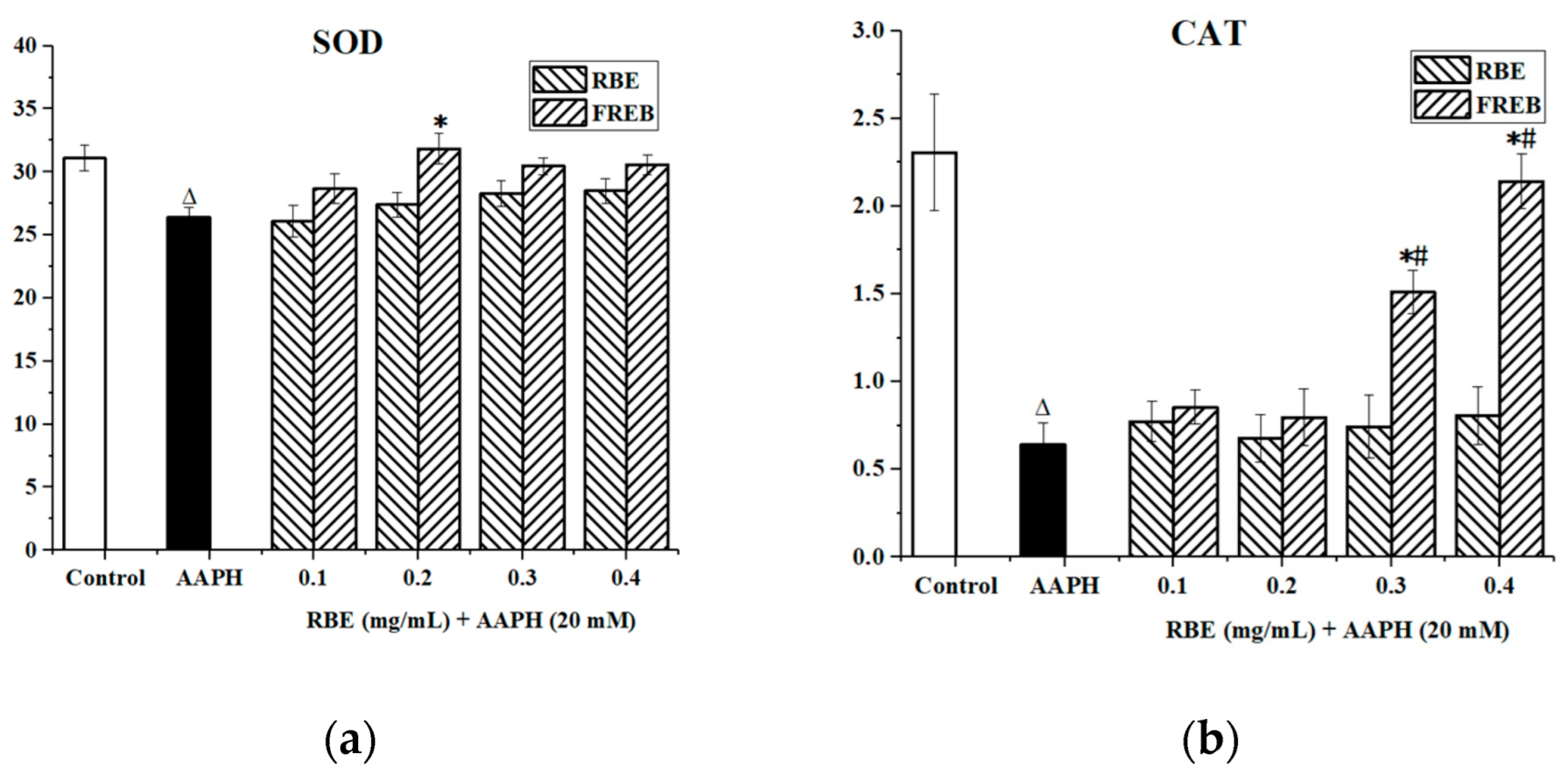
3.4.4. Enzyme Activity Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, M.; Lei, M.; Samina, N.; Chen, L.; Liu, C.; Yin, T.; Yan, X.; Wu, C.; He, H.; Yi, C. Impact of Lactobacillus plantarum 423 fermentation on the antioxidant activity and flavor properties of rice bran and wheat bran. Food Chem. 2020, 330, 127156. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ma, Y.; Dong, L.; Jia, X.; Liu, L.; Huang, F.; Chi, J.; Xiao, J.; Zhang, M.; Zhang, R. Extrusion and fungal fermentation change the profile and antioxidant activity of free and bound phenolics in rice bran together with the phenolic bioaccessibility. LWT—Food Sci. Technol. 2019, 115, 108461. [Google Scholar] [CrossRef]
- Chaiyasut, C.; Pengkumsri, N.; Sirilun, S.; Peerajan, S.; Khongtan, S.; Sivamaruthi, B.S. Assessment of changes in the content of anthocyanins, phenolic acids, and antioxidant property of Saccharomyces cerevisiae mediated fermented black rice bran. AMB Express 2017, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Wu, W. Effects of rice bran rancidity on the oxidation and structural characteristics of rice bran protein. LWT—Food Sci. Technol. 2019, 120, 108943. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Curiel, J.A.; Poutanen, K.; Katina, K. Effect of bioprocessing and particle size on the nutritional properties of wheat bran fractions. Innov. Food Sci. Emerg. Technol. 2014, 25, 19–27. [Google Scholar] [CrossRef]
- Filannino, P.; Di Cagno, R.; Gobbetti, M. Metabolic and functional paths of lactic acid bacteria in plant foods: Get out of the labyrinth. Curr. Opin. Biotechnol. 2018, 49, 64–72. [Google Scholar] [CrossRef]
- Shirakawa, H. Volatile Compounds, Sensory Profile and Phenolic Compounds in Fermented Rice Bran. Plants 2021, 10, 1073. [Google Scholar] [CrossRef]
- Nisa, K.; Rosyida, V.T.; Nurhayati, S.W.; Indrianingsih, A.W.; Darsih, C.; Apriyana, W. Total Phenolic Contents and Antioxidant Activity of Rice Bran Fermented with Lactic Acid Bacteria. In Proceedings of the IOP Conference Series Earth and Environmental Science, Moscow, Russia, 6 June 2019; p. 251. [Google Scholar]
- Verni, M.; Rizzello, C.G.; Coda, R. Fermentation Biotechnology Applied to Cereal Industry By-Products: Nutritional and Functional Insights. Front. Nutr. 2019, 6, 42. [Google Scholar] [CrossRef] [Green Version]
- Massarolo, K.C.; de Souza, T.D.; Collazzo, C.C.; Furlong, E.B.; Soares, L.A.D.S. The impact of Rhizopus oryzae cultivation on rice bran: Gamma-oryzanol recovery and its antioxidant properties. Food Chem. 2017, 228, 43–49. [Google Scholar] [CrossRef]
- Ritthibut, N.; Oh, S.-J.; Lim, S.-T. Enhancement of bioactivity of rice bran by solid-state fermentation with Aspergillus strains. LWT—Food Sci. Technol. 2021, 135, 110273. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, R.; Deng, Y.; Zhang, Y.; Xiao, J.; Huang, F.; Wen, W.; Zhang, M. Fermentation and complex enzyme hydrolysis enhance total phenolics and antioxidant activity of aqueous solution from rice bran pretreated by steaming with α-amylase. Food Chem. 2017, 221, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Webber, D.M.; Hettiarachchy, N.S.; Li, R.; Horax, R.; Theivendran, S. Phenolic Profile and Antioxidant Activity of Extracts Prepared from Fermented Heat-Stabilized Defatted Rice Bran. J. Food Sci. 2014, 79, 2383–2391. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Rashid, N.; Jamaluddin, A.; Sharifudin, S.A.; Kahar, A.A.; Long, K. Cosmeceutical potentials and bioactive compounds of rice bran fermented with single and mix culture of Aspergillus oryzae and Rhizopus oryzae. J. Saudi Soc. Agric. Sci. 2017, 16, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.G.; Gonçalves, L.M.; Prietto, L.; Hackbart, H.S.; Furlong, E.B. Antioxidant activity and enzyme inhibition of phenolic acids from fermented rice bran with fungus Rizhopus oryzae. Food Chem. 2014, 146, 371–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, C.L.; Liu, N.; Wang, Y.; Song, M.; Wang, R.F.; Yang, Y.P.; An, X.P.; Qi, J.W. Study on Antioxidant Activity of Fermented Rice Bran Water Extract. Feed. Ind. 2021, 42, 8–13. [Google Scholar]
- Chen, Q.; Wang, R.; Wang, Y.; An, X.; Qi, J. Characterization and antioxidant activity of wheat bran polysaccharides modified by Saccharomyces cerevisiae and Bacillus subtilis fermentation. J. Cereal Sci. 2020, 97, 103157. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Qi, J. The effects of solid-state fermentation on the content, composition and in vitro antioxidant activity of flavonoids from dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Coda, R.; Kärki, I.; Nordlund, E.; Heiniö, R.-L.; Poutanen, K.; Katina, K. Influence of particle size on bioprocess induced changes on technological functionality of wheat bran. Food Microbiol. 2014, 37, 69–77. [Google Scholar] [CrossRef]
- Ko, S.-C.; Cha, S.-H.; Heo, S.-J.; Lee, S.-H.; Kang, S.-M.; Jeon, Y.-J. Protective effect of Ecklonia cava on UVB-induced oxidative stress: In vitro and in vivo zebrafish model. J. Appl. Phycol. 2011, 23, 697–708. [Google Scholar] [CrossRef]
- Ji, E.C.; Kim, S.; Jin, H.A.; Youn, P.; Ryu, D.Y. Induction of oxidative stress and apoptosis by siver nanoparticles in the liver of adult zebrafish. Aquat. Toxicol. 2010, 100, 151–159. [Google Scholar]
- Walker, S.L.; Junko, A.; Mathias, J.R.; Veena, C.; Xie, X.; Martin, D.; Köster, R.W.; Parsons, M.J.; Bhalla, K.N.; Saxena, M.T.; et al. Automated Reporter Quantification In Vivo: High-Throughput Screening Method for Reporter-Based Assays in Zebrafish. PLoS ONE 2012, 7, e29916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.-Y.; Kim, E.-A.; Kim, Y.-S.; Yu, S.-K.; Choi, C.; Lee, J.-S.; Kim, Y.-T.; Nah, J.-W.; Jeon, Y.-J. Protective effects of polysaccharides from Psidium guajava leaves against oxidative stresses. Int. J. Biol. Macromol. 2016, 91, 804–811. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Lipid Peroxidation and Its Inhibition: Overview and Perspectives. J. Oleo Sci. 2001, 50, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Sangiuliano, B.; Pérez, N.M.; Moreira, D.F.; Belizário, J.E. Cell death-associated molecular-pattern molecules: Inflammatory signaling and control. Mediat. Inflamm. 2014, 2014, 821043. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Parrish, M.; Tay, I.J.J.; Li, N.; Ackerman, S.; He, F.; Kwang, J.; Chow, V.T.; Engelward, B.P. Streptococcus pneumoniae secretes hydrogen peroxide leading to DNA damage and apoptosis in lung cells. Proc. Natl. Acad. Sci. USA 2015, 112, E3421–E3430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kreuz, S.; Fischle, W. Oxidative stress signaling to chromatin in health and disease. Epigenomics 2016, 8, 843–862. [Google Scholar] [CrossRef] [Green Version]
- Parlak, V. Evaluation of apoptosis, oxidative stress responses, AChE activity and body malformations in zebrafish (Danio rerio) embryos exposed to deltamethrin. Chemosphere 2018, 207, 397–403. [Google Scholar] [CrossRef]
- Larine, K.; Eliane, C.; Da, R.M.; Santos, O.M.D.; Almeida, S.S.L.D.; Eliana, B.F. Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar]

| Soluble Bioactive Constituents | RBE | FRBE |
|---|---|---|
| Polysaccharide content (mg g−1 dry matter) | 552 ± 3 a | 450 ± 2 b |
| Polyphenol content (mg g−1 dry matter) | 16 ± 0.4 b | 32 ± 0.1 a |
| Flavonoid content (mg g−1 dry matter) | 6 ± 0.0 b | 9 ± 0.0 a |
| Protein content (mg g−1 dry matter) | 54 ± 0.5 b | 76 ± 0.4 a |
| Uronic acid (mg kg−1 dry matter) | 1 ± 0.1 b | 16 ± 0.2 a |
| Mannose (mg kg−1 dry matter) | 7617 ± 2 b | 9321 ± 3 a |
| Ribose (mg kg−1 dry matter) | 1590 ± 2 a | 91 ± 3 b |
| Rhamnose (mg kg−1 dry matter) | 597 ± 2 a | 107 ± 3 b |
| Glucose (mg kg−1 dry matter) | 145,909 ± 13 a | 13,241 ± 7 b |
| Galactose (mg kg−1 dry matter) | 11,450 ± 5 a | 1091 ± 7 b |
| Xylose (mg kg−1 dry matter) | 23,721 ± 13 a | 1259 ± 11 b |
| Arabinose (mg kg−1 dry matter) | 24,258 ± 4 a | 1336 ± 3 b |
| Fucose (mg kg−1 dry matter) | 324 ± 1 a | 36 ± 1 |
| Compound Content | RBE | FRBE |
|---|---|---|
| Catechinic acid (mg kg−1 dry matter) | 4 ± 0.3 b | 10 ± 0.3 a |
| Caffeic acid (mg kg−1 dry matter) | 1 ± 0.1 b | 6 ± 0.3 a |
| Ferulic acid (mg kg−1 dry matter) | 641 ± 1 b | 721 ± 1 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, N.; Wang, Y.; An, X.; Qi, J. Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation. Fermentation 2022, 8, 212. https://doi.org/10.3390/fermentation8050212
Liu N, Wang Y, An X, Qi J. Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation. Fermentation. 2022; 8(5):212. https://doi.org/10.3390/fermentation8050212
Chicago/Turabian StyleLiu, Na, Yuan Wang, Xiaoping An, and Jingwei Qi. 2022. "Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation" Fermentation 8, no. 5: 212. https://doi.org/10.3390/fermentation8050212
APA StyleLiu, N., Wang, Y., An, X., & Qi, J. (2022). Study on the Enhancement of Antioxidant Properties of Rice Bran Using Mixed-Bacteria Solid-State Fermentation. Fermentation, 8(5), 212. https://doi.org/10.3390/fermentation8050212







