New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical and Standards
2.2. Strain and Culture Conditions
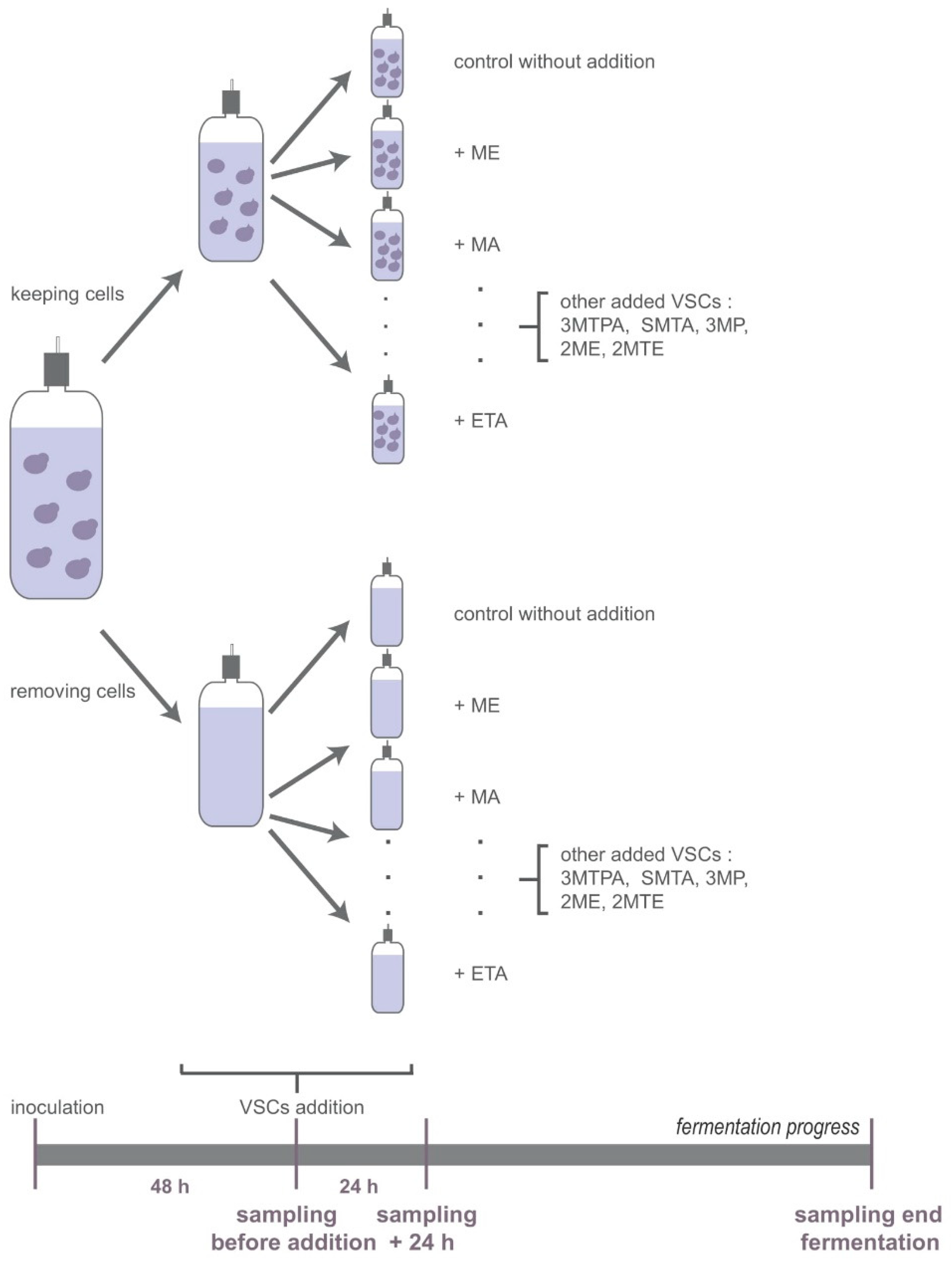
2.3. Fermentations and Sampling Procedure
2.4. VSCs Detemination
3. Results and Discussion
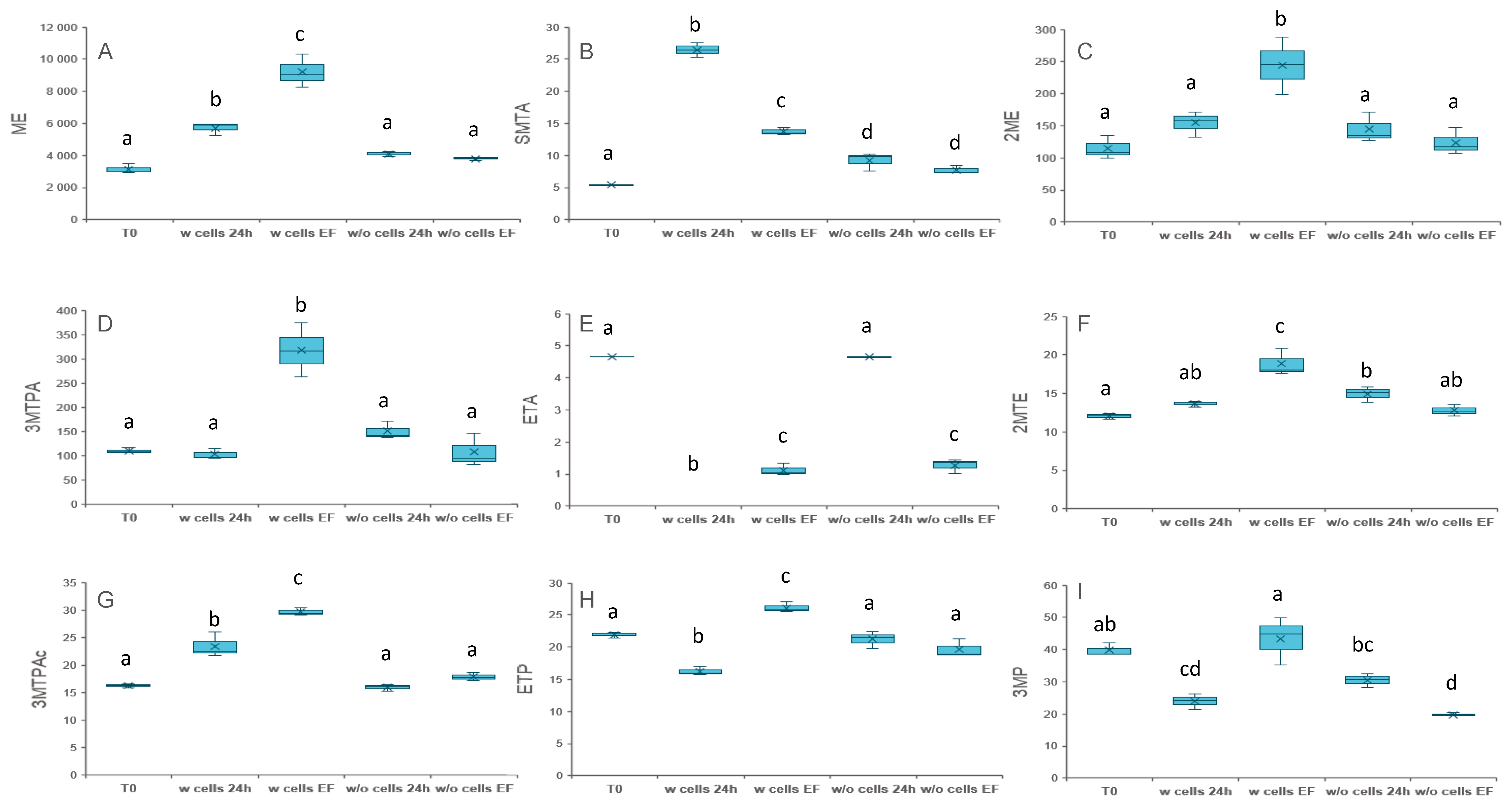
3.1. Elucidating the Origin of VSCs in Absence of Supplementation
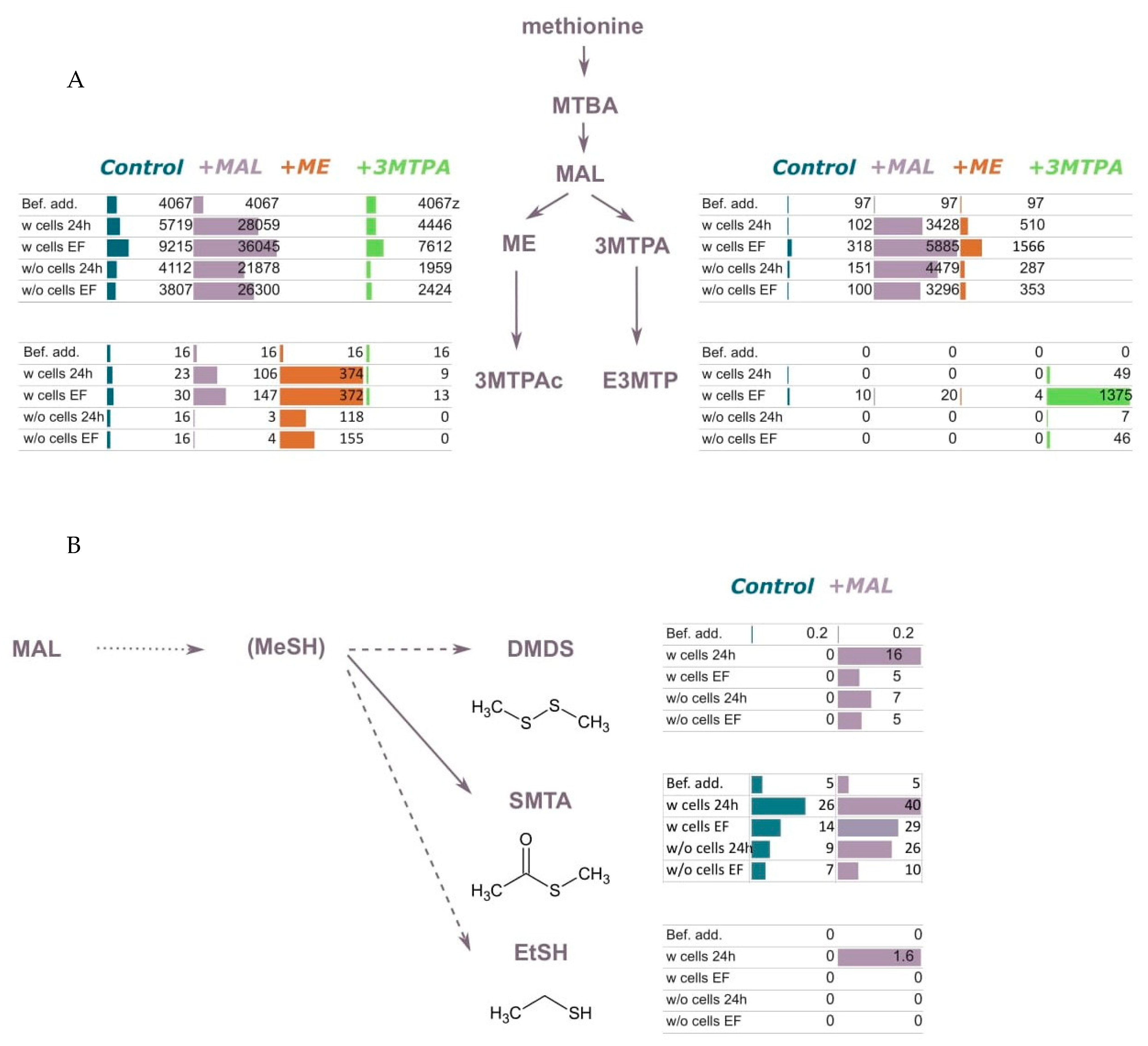
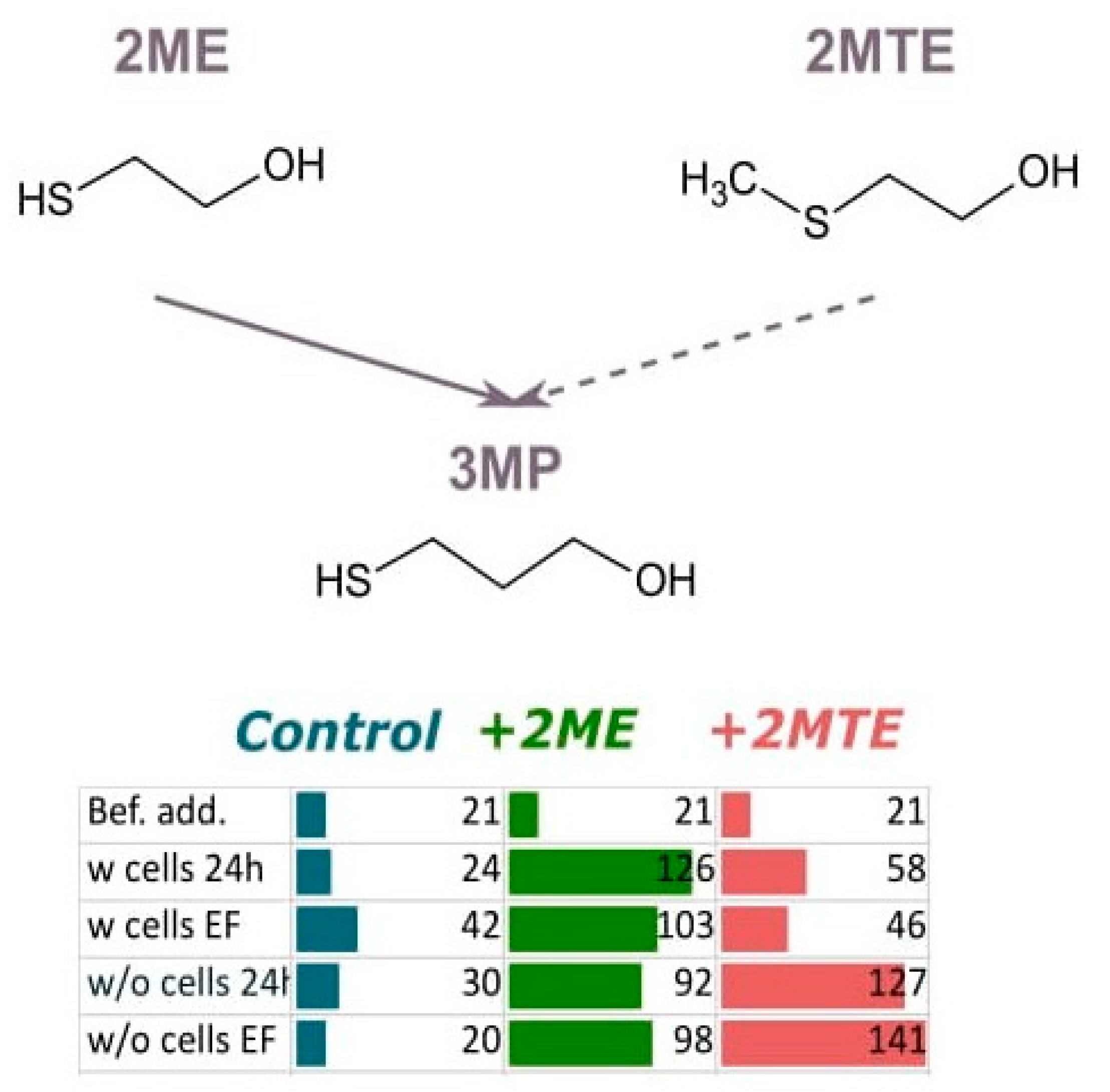
3.2. Unravelling the Chemical and Biological Connection between VSCs
3.2.1. Addition of Compounds Deriving from Methionine Catabolism
3.2.2. Addition of Thioesters
3.2.3. Addition of Compounds Deriving from Homocysteine and Cysteine Catabolism
3.3. Changes in VSCs Profile during Aging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maga, J.A.; Katz, I. The role of sulfur compounds in food flavor part III: Thiols. Crit. Rev. Food Sci. Nutr. 1976, 7, 147–192. [Google Scholar] [CrossRef]
- Smith, M.E.; Bekker, M.Z.; Smith, P.A.; Wilkes, E.N. Sources of volatile sulfur compounds in wine. Aust. J. Grape Wine Res. 2015, 21 (Suppl. S1), 705–712. [Google Scholar] [CrossRef]
- Spiropoulos, A.; Tanaka, J.; Flerianos, I.; Bisson, L.F. Characterization of hydrogen sulfide formation in commercial and natural wine isolates of saccharomyces. Am. J. Enol. Vitic. 2000, 51, 233–248. [Google Scholar]
- Roland, A.; Delpech, S.; Dagan, L.; Ducasse, M.; Cavelier, F.; Schneider, R. Innovative analysis of 3-mercaptohexan-1-ol, 3-mercaptohexylacetate and their corresponding disulfides in wine by stable isotope dilution assay and nano-liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1468, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Franco-Luesma, E.; Ferreira, V. Quantitative analysis of free and bonded forms of volatile sulfur compouds in wine. basic methodologies and evidences showing the existence of reversible cation-complexed forms. J. Chromatogr. A 2014, 1359, 8–15. [Google Scholar] [CrossRef]
- Franco-Luesma, E.; Ferreira, V. Formation and release of H2S, methanethiol, and dimethylsulfide during the anoxic storage of wines at room temperature. J. Agric. Food Chem. 2016, 64, 6317–6326. [Google Scholar] [CrossRef] [PubMed]
- Kinzurik, M.I.; Deed, R.C.; Herbst-Johnstone, M.; Slaghenaufi, D.; Guzzon, R.; Gardner, R.C.; Fedrizzi, B. Addition of volatile sulfur compounds to yeast at the early stages of fermentation reveals distinct biological and chemical pathways for aroma formation. Food Microbiol. 2020, 89, 103435. [Google Scholar] [CrossRef]
- Bely, M.; Sablayrolles, J.; Barre, P. Automatic detection of assimilable nitrogen deficiencies during alcoholic fermentation in oenological conditions. J. Ferment. Bioeng. 1990, 70, 246–252. [Google Scholar] [CrossRef]
- Denat, M.; Pérez, D.; Heras, J.M.; Querol, A.; Ferreira, V. The effects of saccharomyces cerevisiae strains carrying alcoholic fermentation on the fermentative and varietal aroma profiles of young and aged tempranillo wines. Food Chem. X 2021, 9, 100116. [Google Scholar] [CrossRef]
- Jimenez-Lorenzo, R.; Bloem, A.; Farines, V.; Sablayrolles, J.; Camarasa, C. How to modulate the formation of negative volatile sulfur compounds during wine fermentation? FEMS Yeast Res. 2021, 21, foab038. [Google Scholar] [CrossRef]
- Deed, R.C.; Hou, R.; Kinzurik, M.I.; Gardner, R.C.; Fedrizzi, B. The role of yeast ARO8, ARO9 and ARO10 genes in the biosynthesis of 3-(methylthio)-1-propanol from L-methionine during fermentation in synthetic grape medium. FEMS Yeast Res. 2019, 19, foy109. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, C.; Lejeune, I.; Tran, T.; Collin, S. Occurrence of polyfunctional thiols in fresh lager beers. J. Agric. Food Chem. 2006, 54, 5061–5068. [Google Scholar] [CrossRef] [PubMed]
- Dzialo, M.C.; Park, R.; Steensels, J.; Lievens, B.; Verstrepen, K.J. Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol. Rev. 2017, 41 (Suppl. S1), S95–S128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etschmann, M.; Kötter, P.; Hauf, J.; Bluemke, W.; Entian, K.; Schrader, J. Production of the aroma chemicals 3-(methylthio)-1-propanol and 3-(methylthio)-propylacetate with yeasts. Appl. Microbiol. Biotechnol. 2008, 80, 579–587. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.; Van Maris Antonius, J.A.; Pronk, J.T.; Dickinson, J.R. The ehrlich pathway for fusel alcohol production: A century of research on saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Pripis-Nicolau, L.; De Revel, G.; Bertrand, A.; Maujean, A. Formation of flavor components by the reaction of amino acid and carbonyl compounds in mild conditions. J. Agric. Food Chem. 2000, 48, 3761–3766. [Google Scholar] [CrossRef]
- Landaud, S.; Helinck, S.; Bonnarme, P. Formation of volatile sulfur compounds and metabolism of methionine and other sulfur compounds in fermented food. Appl. Microbiol. Biotechnol. 2008, 77, 1191–1205. [Google Scholar] [CrossRef]
- Van Dijken, J.P.; Scheffers, W.A. Redox balances in the metabolism of sugars by yeasts. FEMS Microbiol. Rev. 1986, 1, 199–224. [Google Scholar] [CrossRef] [Green Version]
- Arfi, K.; Landaud, S.; Bonnarme, P. Evidence for distinct L-methionine catabolic pathways in the yeast geotrichum candidum and the bacterium brevibacterium linens. Appl. Environ. Microbiol. 2006, 72, 2155–2162. [Google Scholar] [CrossRef] [Green Version]
- Kinzurik, M.I.; Herbst-Johnstone, M.; Gardner, R.C.; Fedrizzi, B. Hydrogen sulfide production during yeast fermentation causes the accumulation of ethanethiol, S-ethyl thioacetate and diethyl disulfide. Food Chem. 2016, 209, 341–347. [Google Scholar] [CrossRef]
- Isogai, A.; Kanda, R.; Hiraga, Y.; Nishimura, T.; Iwata, H.; Goto-Yamamoto, N. Screening and identification of precursor compounds of dimethyl trisulfide (DMTS) in japanese sake. J. Agric. Food Chem. 2009, 57, 189–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pripis-Nicolau, L.; de Revel, G.; Bertrand, A.; Lonvaud-Funel, A. Methionine catabolism and production of volatile sulfur compounds by oenococcus oeni. J. Appl. Microbiol. 2004, 96, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Jia, K.; Xia, S.; Xu, Y.; Liu, R.; Li, H.; Tang, Y. Regulating ehrlich and demethiolation pathways for alcohols production by the expression of ubiquitin-protein ligase gene HUWE1. Sci. Rep. 2016, 6, 20828. [Google Scholar] [CrossRef] [PubMed]
- Hébert, A.; Beckerich, J.M.; Landaud, S.; Bonnarme, P. Sulfur metabolism of the cheese-ripening yeast yarrowia lipolytica. In Yarrowia Lipolytica; Springer: Berlin/Heidelberg, Germany, 2013; pp. 165–184. [Google Scholar]
- Xu, Y.; Jia, K.; Tang, Y. Regulatory networks governing methionine catabolism into volatile organic sulfur-containing compounds in clonostachys rosea. Appl. Environ. Microbiol. 2018, 84, 1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamagata, S.; D’andrea, R.J.; Fujisaki, S.; Isaji, M.; Nakamura, K. Cloning and bacterial expression of the CYS3 gene encoding cystathionine gamma-lyase of saccharomyces cerevisiae and the physicochemical and enzymatic properties of the protein. J. Bacteriol. 1993, 175, 4800–4808. [Google Scholar] [CrossRef] [Green Version]
- Ouellette, R.J.; Rawn, J.D. Principles of Organic Chemistry; Academic Press: Waltham, MA, USA, 2015. [Google Scholar]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Copper (II)-mediated hydrogen sulfide and thiol oxidation to disulfides and organic polysulfanes and their reductive cleavage in wine: Mechanistic elucidation and potential applications. J. Agric. Food Chem. 2017, 65, 2564–2571. [Google Scholar] [CrossRef]
- Moreira, N.; De Pinho, P.G.; Santos, C.; Vasconcelos, I. Volatile sulfur compounds composition of monovarietal white wines. Food Chem. 2010, 123, 1198–1203. [Google Scholar] [CrossRef]
- Bekker, M.Z.; Wilkes, E.N.; Smith, P.A. Evaluation of putative precursors of key ‘reductive’compounds in wines post-bottling. Food Chem. 2018, 245, 676–686. [Google Scholar] [CrossRef]
- Helinck, S.; Spinnler, H.E.; Parayre, S.; Dame-Cahagne, M.; Bonnarme, P. Enzymatic versus spontaneous S-methylthioester synthesis in Geotricum candidum. FEMS Microbiol. Lett. 2000, 193, 237–241. [Google Scholar] [CrossRef]
- Carrión, O.; Pratscher, J.; Curson, A.R.; Williams, B.T.; Rostant, W.G.; Murrell, J.C.; Todd, J.D. Methanethiol-dependent dimethylsulfide production in soil environments. ISME J. 2017, 11, 2379–2390. [Google Scholar] [CrossRef] [Green Version]
- De Mora, S.J.; Eschenbruch, R.; Knowles, S.J.; Spedding, D.J. The formation of dimethyl sulphide during fermentation using a wine yeast. Food Microbiol. 1986, 3, 27–32. [Google Scholar] [CrossRef]
- Segurel, M.A.; Razungles, A.J.; Riou, C.; Trigueiro, M.G.; Baumes, R.L. Ability of possible DMS precursors to release DMS during wine aging and in the conditions of heat-alkaline treatment. J. Agric. Food Chem. 2005, 53, 2637–2645. [Google Scholar] [CrossRef] [PubMed]
- North, J.A.; Miller, A.R.; Wildenthal, J.A.; Young, S.J.; Tabita, F.R. Microbial pathway for anaerobic 5′-methylthioadenosine metabolism coupled to ethylene formation. Proc. Natl. Acad. Sci. USA 2017, 114, E10455–E10464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekowska, A.; Ashida, H.; Danchin, A. Revisiting the Methionine Salvage Pathway and Its Paralogues; Wiley: New York, NY, USA, 2019. [Google Scholar] [CrossRef]
- Kreitman, G.Y.; Danilewicz, J.C.; Jeffery, D.W.; Elias, R.J. Reaction mechanisms of metals with hydrogen sulfide and thiols in model wine. Part 1: Copper-catalyzed oxidation. J. Agric. Food Chem. 2016, 64, 4095–4104. [Google Scholar] [CrossRef]
- Vela, E.; Hernandez-Orte, P.; Franco-Luesma, E.; Ferreira, V. Micro-oxygenation does not eliminate hydrogen sulfide and mercaptans from wine; it simply shifts redox and complex-related equilibria to reversible oxidized species and complexed forms. Food Chem. 2018, 243, 222–230. [Google Scholar] [CrossRef]
- Ugliano, M.; Dieval, J.; Siebert, T.E.; Kwiatkowski, M.; Aagaard, O.; Vidal, S.; Waters, E.J. Oxygen consumption and development of volatile sulfur compounds during bottle aging of two shiraz wines. Influence of pre-and postbottling controlled oxygen exposure. J. Agric. Food Chem. 2012, 60, 8561–8570. [Google Scholar] [CrossRef]
- Vela, E.; Hernández-Orte, P.; Castro, E.; Ferreira, V.; Lopez, R. Effect of bentonite fining on polyfunctional mercaptans and other volatile compounds in sauvignon blanc wines. Am. J. Enol. Vitic. 2017, 68, 30–38. [Google Scholar] [CrossRef]
- Oliveira, I.; Ferreira, V. Modulating fermentative, varietal and aging aromas of wine using non-saccharomyces yeasts in a sequential inoculation approach. Microorganisms 2019, 7, 164. [Google Scholar] [CrossRef] [Green Version]
- Fedrizzi, B.; Zapparoli, G.; Finato, F.; Tosi, E.; Turri, A.; Azzolini, M.; Versini, G. Model aging and oxidation effects on varietal, fermentative, and sulfur compounds in a dry botrytized red wine. J. Agric. Food Chem. 2011, 59, 1804–1813. [Google Scholar] [CrossRef]
- Ye, D.; Zheng, X.; Xu, X.; Wang, Y.; Duan, C.; Liu, Y. Evolutions of volatile sulfur compounds of cabernet sauvignon wines during aging in different oak barrels. Food Chem. 2016, 202, 236–246. [Google Scholar] [CrossRef]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic higher alcohols in wine: Implication on aroma and palate attributes during chardonnay aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef] [PubMed]
- Rauhut, D.; Kürbel, H.; Dittrich, H.H.; Grossman, M. Properties and differences of commercial yeast strains with respect to their formation of sulfur compounds. In The Composition of Musts: Influence on Stuck Fermentations; Lallemand: Montréal, QC, Canada, 1997; pp. 65–70. [Google Scholar]
- Bentley, R.; Chasteen, T.G. Environmental VOSCs––Formation and degradation of dimethyl sulfide, methanethiol and related materials. Chemosphere 2004, 55, 291–317. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, B.; Magno, F.; Moser, S.; Nicolini, G.; Versini, G. Concurrent quantification of light and heavy sulfur volatiles in wine by headspace solid-phase microextraction coupled with gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. Int. J. Devoted Rapid Dissem. Up—Minute Res. Mass Spectrom. 2007, 21, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Loscos, N.; Ségurel, M.; Dagan, L.; Sommerer, N.; Marlin, T.; Baumes, R. Identification of S-methylmethionine in petit manseng grapes as dimethyl sulphide precursor in wine. Anal. Chim. Acta 2008, 621, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Fedrizzi, B.; Magno, F.; Badocco, D.; Nicolini, G.; Versini, G. Aging effects and grape variety dependence on the content of sulfur volatiles in wine. J. Agric. Food Chem. 2007, 55, 10880–10887. [Google Scholar] [CrossRef]
- Beloqui, A.A.; Bertrand, A. Study of sulfur compounds in wine: Preliminary results. Ital. J. Food Sci. 1995, 3, 279–288. [Google Scholar]
- Silva Ferreira, A.C.; Hogg, T.; Guedes de Pinho, P. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J. Agric. Food Chem. 2003, 51, 1377–1381. [Google Scholar] [CrossRef]
- Mestres, M.; Busto, O.; Guasch, J. Analysis of Organic Sulfur Compounds in Wine Aroma. J. Chromatogr. A 2000, 881, 569–581. [Google Scholar] [CrossRef]
- Lytra, G.; Tempere, S.; Marchand, S.; de Revel, G.; Barbe, J. How do esters and dimethyl sulphide concentrations affect fruity aroma perception of red wine? demonstration by dynamic sensory profile evaluation. Food Chem. 2016, 194, 196–200. [Google Scholar] [CrossRef]

 ), Viognier wine (
), Viognier wine (  ) and synthetic wine obtained with a synthetic grape enriched with methionine and cysteine (
) and synthetic wine obtained with a synthetic grape enriched with methionine and cysteine (  ). Samples were collected after 21, 42 and 63 days of incubation. Experiments were conducted in triplicate and mean values are reported. Raw data are available in supplementary data.
). Samples were collected after 21, 42 and 63 days of incubation. Experiments were conducted in triplicate and mean values are reported. Raw data are available in supplementary data.
 ), Viognier wine (
), Viognier wine (  ) and synthetic wine obtained with a synthetic grape enriched with methionine and cysteine (
) and synthetic wine obtained with a synthetic grape enriched with methionine and cysteine (  ). Samples were collected after 21, 42 and 63 days of incubation. Experiments were conducted in triplicate and mean values are reported. Raw data are available in supplementary data.
). Samples were collected after 21, 42 and 63 days of incubation. Experiments were conducted in triplicate and mean values are reported. Raw data are available in supplementary data.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez-Lorenzo, R.; Farines, V.; Sablayrolles, J.-M.; Camarasa, C.; Bloem, A. New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging. Fermentation 2022, 8, 139. https://doi.org/10.3390/fermentation8040139
Jiménez-Lorenzo R, Farines V, Sablayrolles J-M, Camarasa C, Bloem A. New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging. Fermentation. 2022; 8(4):139. https://doi.org/10.3390/fermentation8040139
Chicago/Turabian StyleJiménez-Lorenzo, Rafael, Vincent Farines, Jean-Marie Sablayrolles, Carole Camarasa, and Audrey Bloem. 2022. "New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging" Fermentation 8, no. 4: 139. https://doi.org/10.3390/fermentation8040139
APA StyleJiménez-Lorenzo, R., Farines, V., Sablayrolles, J.-M., Camarasa, C., & Bloem, A. (2022). New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging. Fermentation, 8(4), 139. https://doi.org/10.3390/fermentation8040139





