From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fermentation Media and Kefir Grains
2.2. Experimental Plan
2.3. Sampling and Processing of Samples
2.4. Microbiological Profiling Analysis
2.5. Sensory Evaluation
2.6. Analytical Techniques
2.7. Statistical Analysis
3. Results
3.1. Effect of Temperature on the Fermentation of Milk
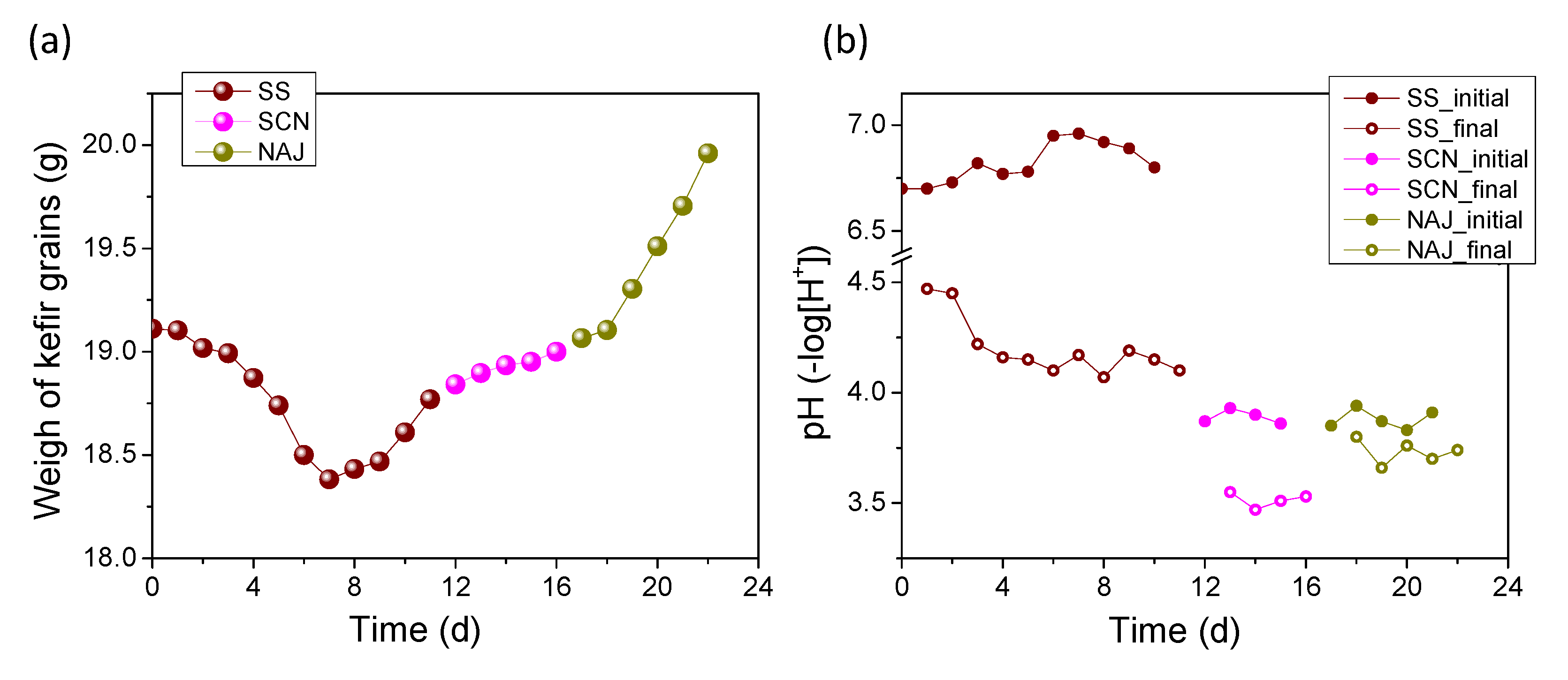
3.2. Transition from Milk to Water Kefir—Gradul Substitution of Milk from Sucrose in the Fermentation Medium
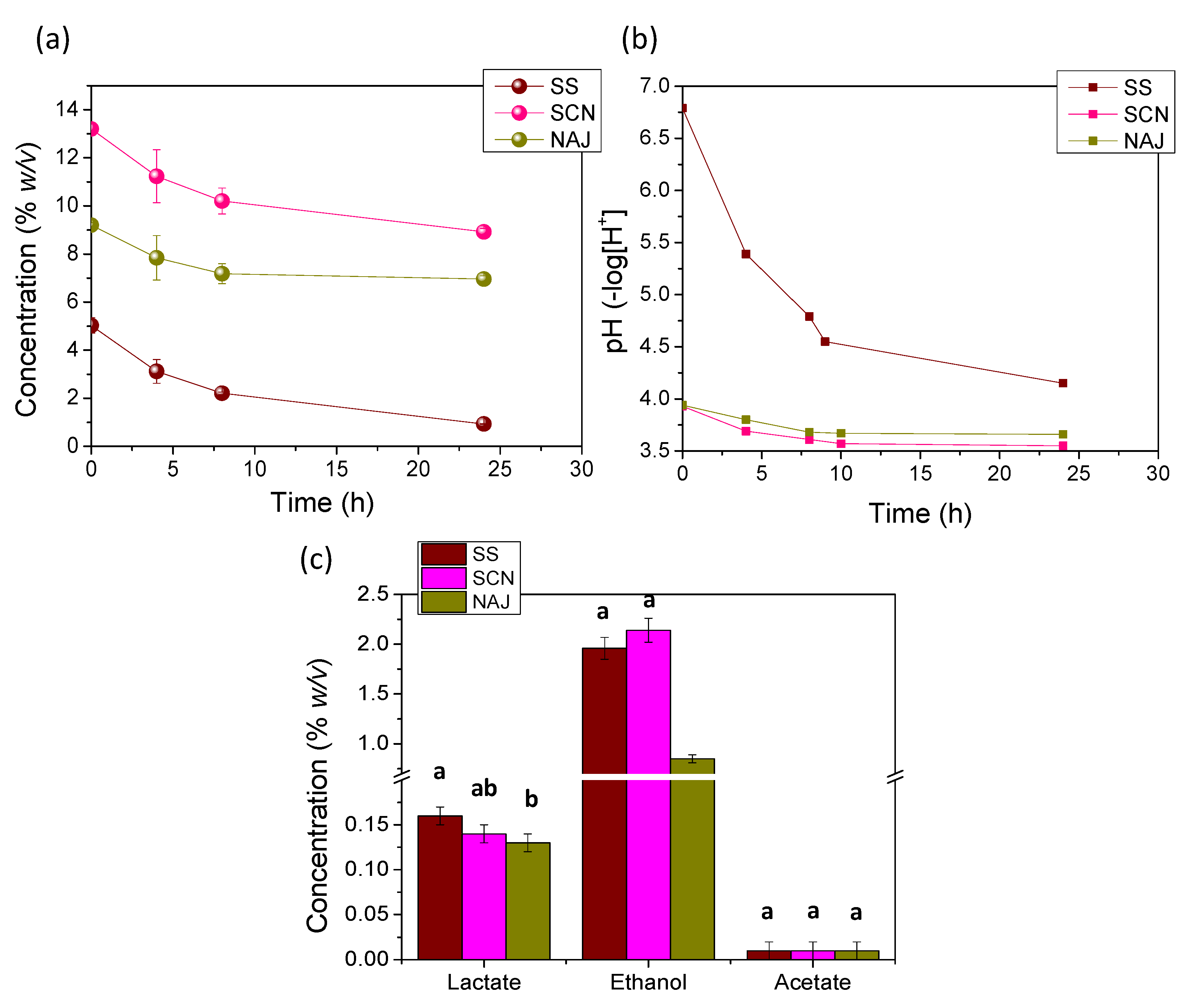
3.3. Production of Water Kefir from Sugary Substrates by the Acclimated Kefir Grains
3.4. Sensory Evaluation of the Water Kefir Beverages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Şanlier, N.; Gökcen, B.B.; Sezgin, A.C. Health benefits of fermented foods. Crit. Rev. Food Sci. Nutr. 2019, 59, 506–527. [Google Scholar] [CrossRef] [PubMed]
- Plessas, S.; Nouska, C.; Mantzourani, I.; Kourkoutas, Y.; Alexopoulos, A.; Bezirtzoglou, E. Microbiological Exploration of Different Types of Kefir Grains. Fermentation 2016, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Hertzler, S.R.; Clancy, S.M. Kefir improves lactose digestion and tolerance in adults with lactose maldigestion. J. Am. Diet. Assoc. 2003, 103, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Maria do Carmo, G.P. Milk kefir: Nutritional, microbiological and health benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Lopitz-Otsoa, F.; Rementeria, A.; Elguezabal, N.; Garaizar, J. Kefir: A symbiotic yeasts-bacteria community with alleged healthy capabilities. Rev. Iberoam. Micol. 2006, 23, 67–74. [Google Scholar] [CrossRef]
- Assadi, M.; Pourahmad, R.; Moazami, N. Use of isolated kefir starter cultures in kefir production. World J. Microbiol. Biotechnol. 2000, 16, 541–543. [Google Scholar] [CrossRef]
- Açik, M.; Çakiroğlu, F.P.; Altan, M.; BAYBO, T. Alternative source of probiotics for lactose intolerance and vegan individuals: Sugary kefir. Food Sci. Technol. 2020, 40, 523–531. [Google Scholar] [CrossRef]
- Fiorda, F.A.; Pereira, G.; Soccol, V.T.; Rakshit, S.K.; Pagnoncelli, M.G.B.; Vandenberghe, L.; Soccol, C.R. Microbiological, biochemical, and functional aspects of sugary kefir fermentation—A review. Food Microbiol. 2017, 66, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Guzel-Seydim, Z.B.; Gökırmaklı, Ç.; Greene, A.K. A comparison of milk kefir and water kefir: Physical, chemical, microbiological and functional properties. Trends Food Sci. Technol. 2021, 113, 42–53. [Google Scholar] [CrossRef]
- Lynch, K.M.; Wilkinson, S.; Daenen, L.; Arendt, E.K. An update on water kefir: Microbiology, composition and production. Int. J. Food Microbiol. 2021, 345, 109128. [Google Scholar] [CrossRef]
- Luning, P.A.; Vries, R.V.D.V.D.; Yuksel, D.; Ebbenhorst-Seller, T.; Wichers, H.J.; Roozen, J.P. Combined Instrumental and Sensory Evaluation of Flavor of Fresh Bell Peppers (Capsicum annuum) Harvested at Three Maturation Stages. J. Agric. Food Chem. 1994, 42, 2855–2861. [Google Scholar] [CrossRef]
- Ntaikou, I.; Siankevich, S.; Lyberatos, G. Effect of thermo-chemical pretreatment on the saccharification and enzymatic digestibility of olive mill stones and their bioconversion towards alcohols. Environ. Sci. Pollut. Res. 2020, 28, 24570–24579. [Google Scholar] [CrossRef]
- Dounavis, A.S.; Ntaikou, I.; Kamilari, M.; Lyberatos, G. Production of Bio-Based Hydrogen Enriched Methane from Waste Glycerol in a Two Stage Continuous System. Waste Biomass Valorization 2016, 7, 677–689. [Google Scholar] [CrossRef]
- Apar, D.K.; Demirhan, E.; Özel, B.; Özbek, B. Kefir Grain Biomass Production: Influence of Different Culturing Conditions and Examination of Growth Kinetic Models. J. Food Process Eng. 2016, 40, e12332. [Google Scholar] [CrossRef]
- Ribeiro, A.C.; Lemos, T.; Lopes, R.P.; Mota, M.J.; Inácio, R.S.; Gomes, A.M.P.; Sousa, S.; Delgadillo, I.; Saraiva, J.A. The Combined Effect of Pressure and Temperature on Kefir Production—A Case Study of Food Fermentation in Unconventional Conditions. Foods 2020, 9, 1133. [Google Scholar] [CrossRef] [PubMed]
- Gul, O.; Mortas, M.; Atalar, I.; Dervisoglu, M.; Kahyaoglu, T. Manufacture and characterization of kefir made from cow and buffalo milk, using kefir grain and starter culture. J. Dairy Sci. 2015, 98, 1517–1525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londero, A.; Hamet, M.F.; De Antoni, G.L.; Garrote, G.L.; Abraham, A.G. Kefir grains as a starter for whey fermentation at different temperatures: Chemical and microbiological characterisation. J. Dairy Res. 2012, 79, 262–271. [Google Scholar] [CrossRef]
- Syrokou, M.K.; Papadelli, M.; Ntaikou, I.; Paramithiotis, S.; Drosinos, E.H. Sugary Kefir: Microbial Identification and Biotechnological Properties. Beverages 2019, 5, 61. [Google Scholar] [CrossRef] [Green Version]
- Bourrie, B.C.T.; Willing, B.P.; Cotter, P.D. The Microbiota and Health Promoting Characteristics of the Fermented Beverage Kefir. Front. Microbiol. 2016, 7, 647. [Google Scholar] [CrossRef] [Green Version]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented Foods as a Dietary Source of Live Organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Zajšek, K.; Kolar, M.; Goršek, A. Characterisation of the exopolysaccharide kefiran produced by lactic acid bacteria entrapped within natural kefir grains. Int. J. Dairy Technol. 2011, 64, 544–548. [Google Scholar] [CrossRef]
- Gulitz, A.; Stadie, J.; Wenning, M.; Ehrmann, M.A.; Vogel, R.F. The microbial diversity of water kefir. Int. J. Food Microbiol. 2011, 151, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Laureys, D.; De Vuyst, L. Microbial Species Diversity, Community Dynamics, and Metabolite Kinetics of Water Kefir Fermentation. Appl. Environ. Microbiol. 2014, 80, 2564–2572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agarbati, A.; Ciani, M.; Canonico, L.; Galli, E.; Comitini, F. Exploitation of Yeasts with Probiotic Traits for Kefir Production: Effectiveness of the Microbial Consortium. Fermentation 2021, 8, 9. [Google Scholar] [CrossRef]
- How to Make Water Kefir with Milk Kefir Grains. Available online: https://northernhomestead.com/how-to-make-water-kefir-with-milk-kefir-grains (accessed on 10 December 2021).
- Converting Milk Kefir Grains into Water Kefir Grains. Available online: https://www.thecheesemaker.com/converting-milk-kefir-grains-into-water-kefir-grains (accessed on 10 December 2021).
- Salazar Alzate, B.C.; Rodríguez, M.C.; Campuzano, O.M. Identification of some kefir microorganisms and optimization of their production in sugarcane juice. Rev. Fac. Nac. Agron. Medellín 2016, 69, 7935–7943. [Google Scholar] [CrossRef]
- Zongo, O.; Cruvellier, N.; Leray, F.; Bideaux, C.; Lesage, J.; Zongo, C.; Traoré, Y.; Savadogo, A.; Guillouet, S. Physicochemical composition and fermentation kinetics of a novel Palm Sap-based Kefir Beverage from the fermentation of Borassus aethiopum Mart. fresh sap with kefir grains and ferments. Sci. Afr. 2020, 10, e00631. [Google Scholar] [CrossRef]
- Ben Atitallah, I.; Ntaikou, I.; Antonopoulou, G.; Alexandropoulou, M.; Brysch-Herzberg, M.; Nasri, M.; Lyberatos, G.; Mechichi, T. Evaluation of the non-conventional yeast strain Wickerhamomyces anomalus (Pichia anomala) X19 for enhanced bioethanol production using date palm sap as renewable feedstock. Renew. Energy 2020, 154, 71–81. [Google Scholar] [CrossRef]
- Laureys, D.; Leroy, F.; Hauffman, T.; Raes, M.; Aerts, M.; Vandamme, P.; De Vuyst, L. The Type and Concentration of Inoculum and Substrate as Well as the Presence of Oxygen Impact the Water Kefir Fermentation Process. Front. Microbiol. 2021, 12. [Google Scholar] [CrossRef]
- Pidoux, M. The microbial flora of sugary kefir grain (the gingerbeer plant): Biosynthesis of the grain fromLactobacillus hilgardii producing a polysaccharide gel. World J. Microbiol. Biotechnol. 1989, 5, 223–238. [Google Scholar] [CrossRef]
- Laureys, D.; De Vuyst, L. The water kefir grain inoculum determines the characteristics of the resulting water kefir fermentation process. J. Appl. Microbiol. 2017, 122, 719–732. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, T.; Ye, T.; Yang, X.; Xue, Y.; Shen, Y.; Zhang, Q.; Zheng, X. Effect of lactic acid bacteria and yeasts on the structure and fermentation properties of Tibetan kefir grains. Int. Dairy J. 2020, 114, 104943. [Google Scholar] [CrossRef]
- Georgalaki, M.; Zoumpopoulou, G.; Anastasiou, R.; Kazou, M.; Tsakalidou, E. Lactobacillus kefiranofaciens: From Isolation and Taxonomy to Probiotic Properties and Applications. Microorganisms 2021, 9, 2158. [Google Scholar] [CrossRef]
- Adamberg, K. The effect of temperature and pH on the growth of lactic acid bacteria: A pH-auxostat study. Int. J. Food Microbiol. 2003, 85, 171–183. [Google Scholar] [CrossRef]
- Miranda-Castilleja, D.E.; Aldrete-Tapia, J.A.; Arvizu-Medrano, S.M.; Hernades-Iturriaga, M.; Soto-Muñoz, L.; Martinez-Peniche, R.A. Growth Kinetics for the Selection of Yeast Strains for Fermented Beverages. In Yeast—Industrial Applications Conversion; InTech: London, UK, 2017. [Google Scholar] [CrossRef] [Green Version]
- Mehinagic, E.; Royer, G.; Symoneaux, R.; Jourjon, A.F.; Prost, C. Characterization of Odor-Active Volatiles in Apples: Influence of Cultivars and Maturity Stage. J. Agric. Food Chem. 2006, 54, 2678–2687. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Corona, O.; Guarcello, R.; Francesca, N.; Germana, M.A.; Erten, H.; Moschetti, G.; Settanni, L. Development of new non-dairy beverages from Mediterranean fruit juices fermented with water kefir microorganisms. Food Microbiol. 2016, 54, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, P.; Wojdyło, A. Bioactive compounds and sensory attributes of sour cherry puree sweetened with natural sweeteners. Int. J. Food Sci. Technol. 2014, 50, 585–591. [Google Scholar] [CrossRef]
- Yilmaz, L.; Yilsay, T.; Bayizit, A.A. The sensory characteristics of berry-flavoured kefir. Czech J. Food Sci. 2011, 24, 26–32. [Google Scholar] [CrossRef] [Green Version]
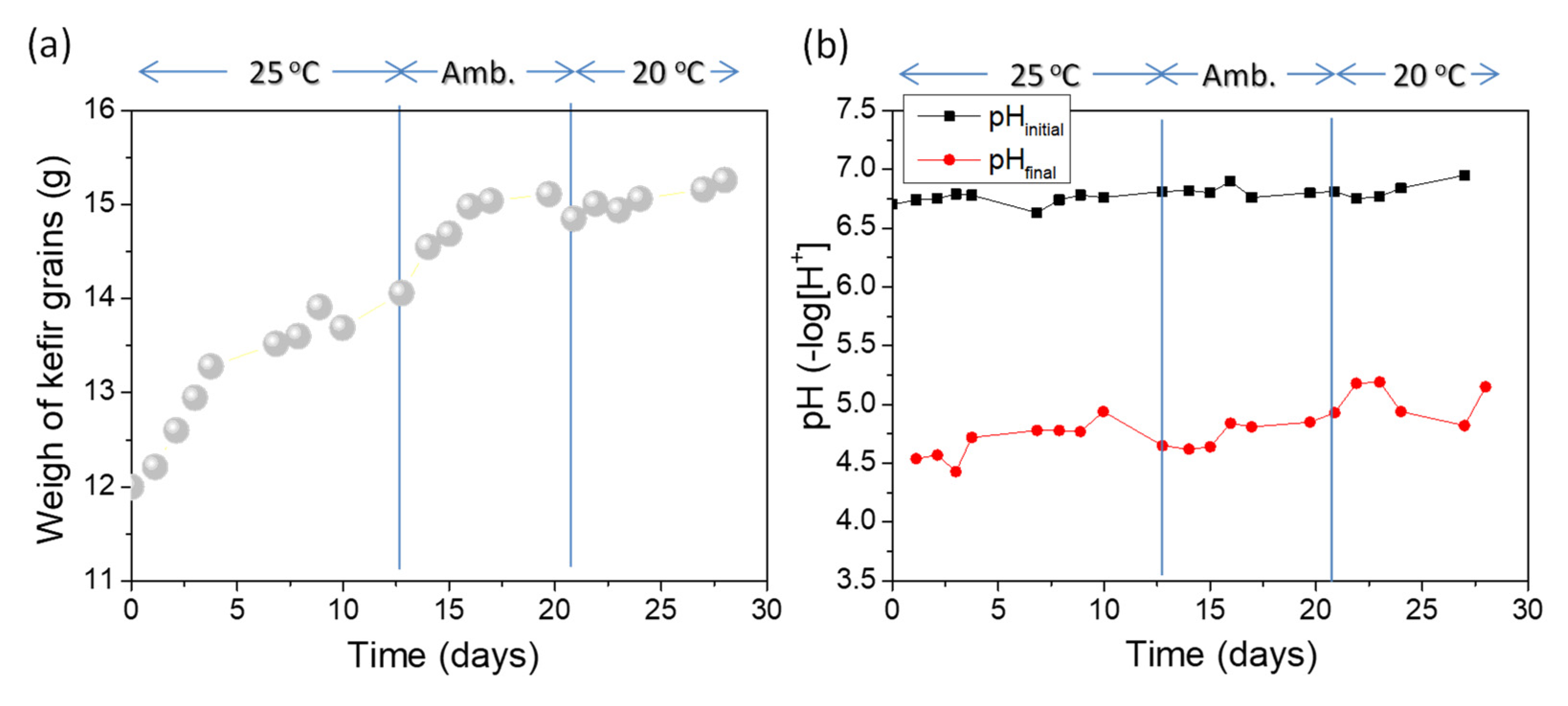
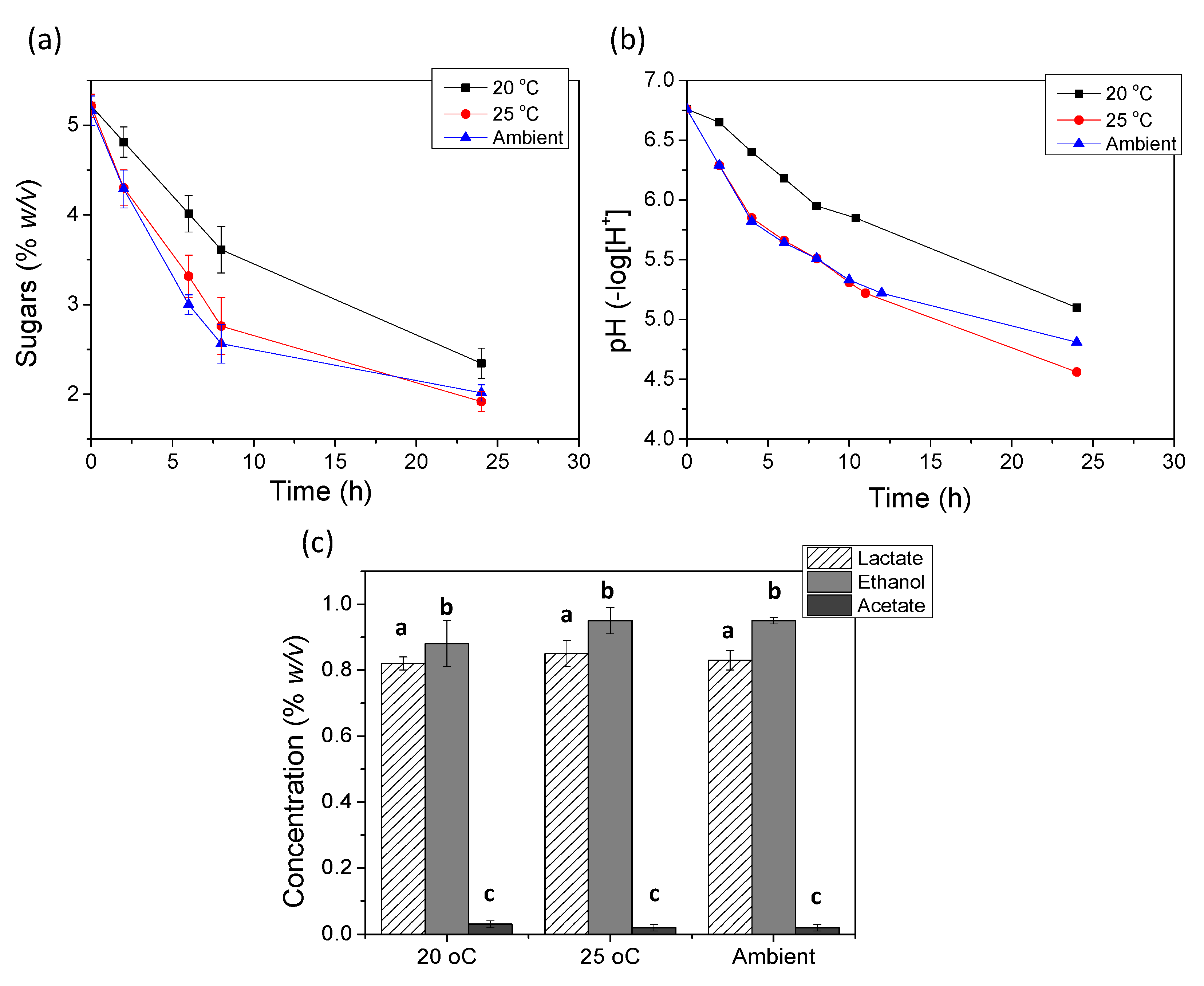

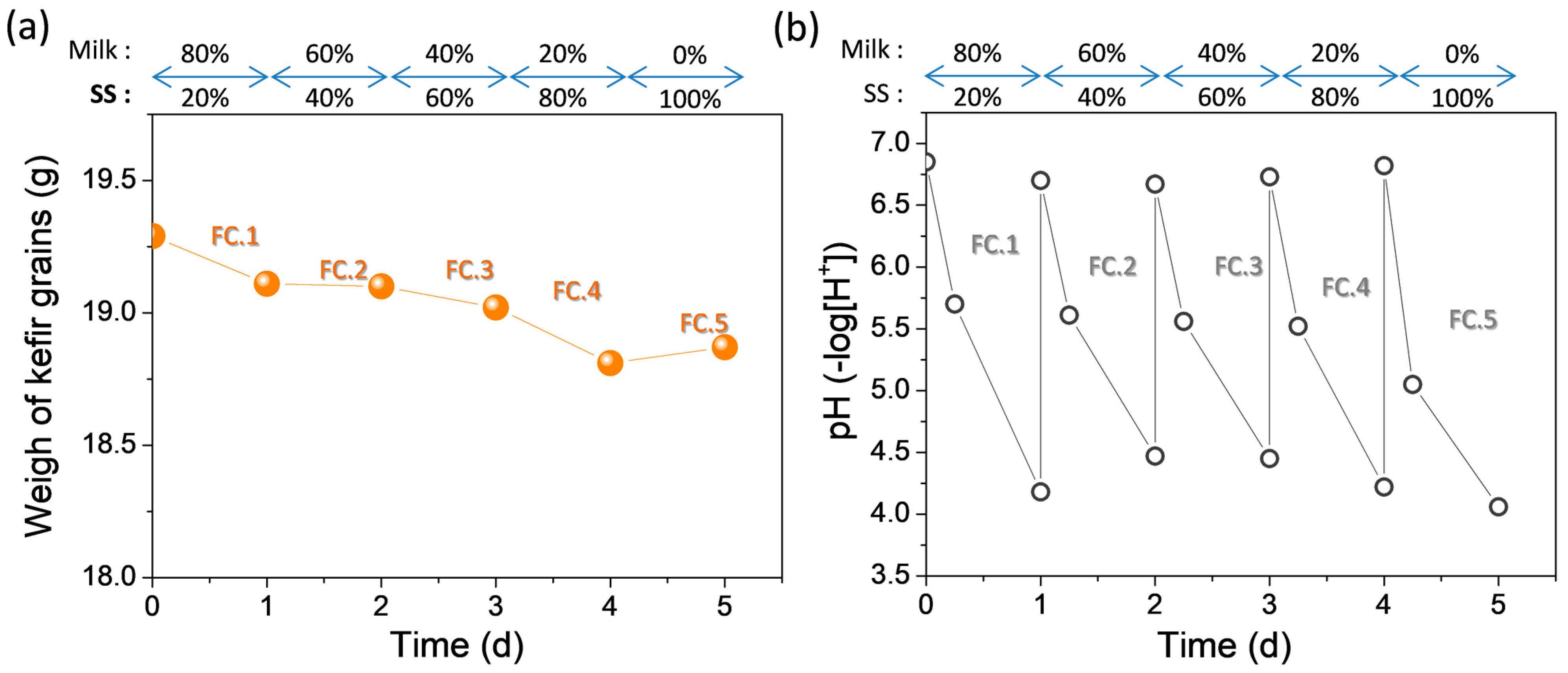
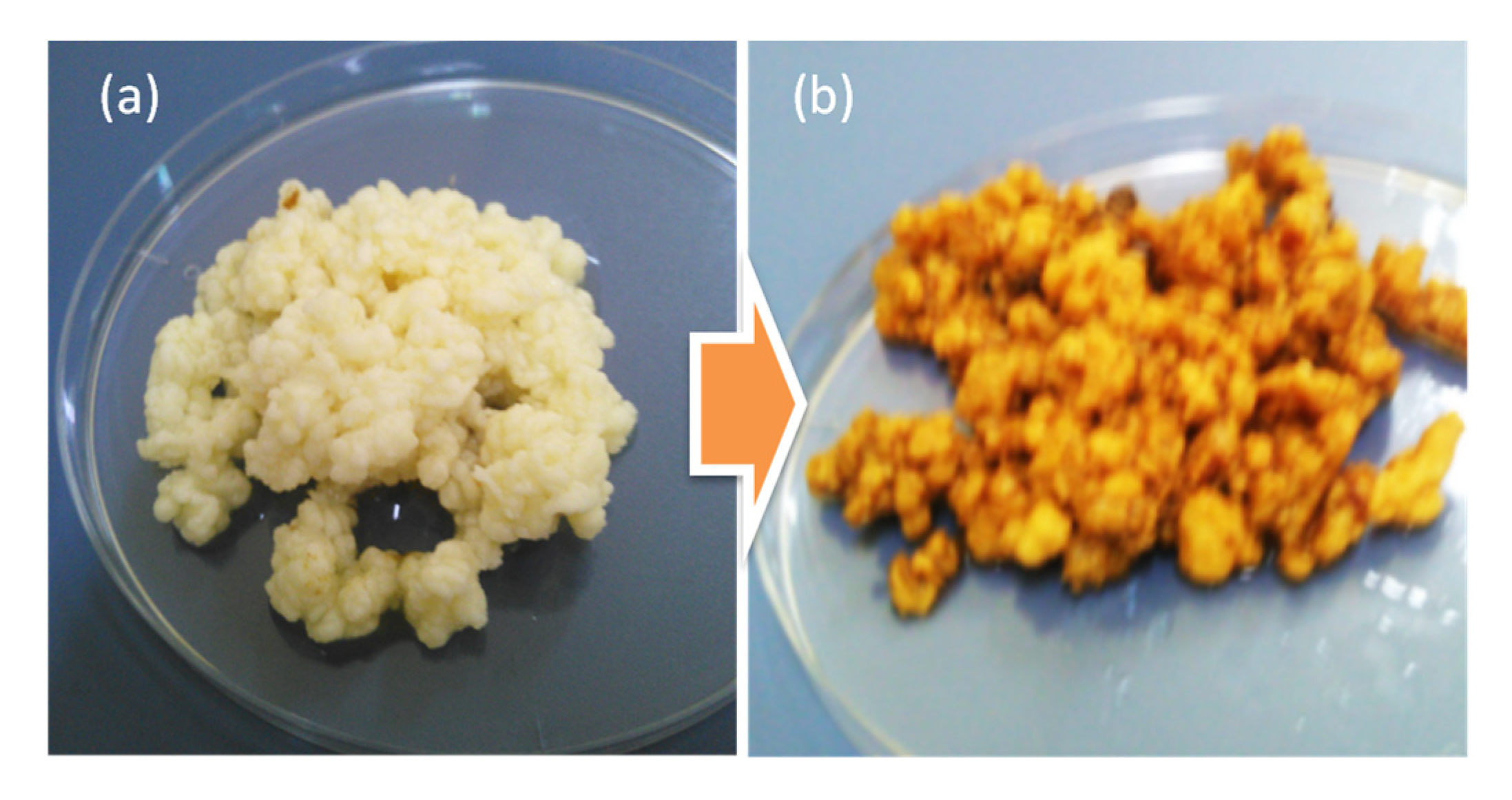


| Fermentation Temperature | Total WI (g) | Average Mean WIR (g/Day) | Mean Final pH |
|---|---|---|---|
| 25 °C | 2.06 ± 0.02 | 0.28 ± 0.16 | 4.67 ± 0.15 ab |
| Ambient | 0.95 ± 0.01 | 0.19 ± 0.12 | 4.78 ± 0.15 ac |
| 20 °C | 0.22 ± 0.02 | 0.08 ± 0.05 | 4.94 ± 0.16 bc |
| Fermentation Temperature | Final Concentration of Sugars (% w/v) | Consumption of Sugars (% of Initial) |
|---|---|---|
| 20 °C | 2.34 ± 0.16 a | 0.56 ± 0.01 a |
| 25 °C | 1.91 ± 0.11 | 0.64 ± 0.02 |
| Ambient | 2.15 ± 0.09 a | 0.59 ± 0.02 a |
| Fermentation Temperature | Total WI (g) | Average Mean WIR (g/d) | Mean Final pH |
|---|---|---|---|
| SS | 0.34 ± 0.01 * | 0.10 ± 0.08 a,* | 4.20 ± 0.13 |
| SCN | 0.16 ± 0.01 | 0.04 ± 0.02 a | 3.52 ± 0.03 |
| NAJ | 0.89 ± 0.01 | 0.18 ± 0.08 a | 3.73 ± 0.05 |
| Substrate | Final Concentration of Sugars (% w/v) | Consumed Sugars (% w/v) | Consumption of Sugars (% of Initial) |
|---|---|---|---|
| SS | 0.93 ± 0.11 | 4.10 ± 0.37 a | 0.82 ± 0.09 |
| SCN | 8.92 ± 0.10 | 4.28 ± 0.28 ab | 0.32 ± 0.02 |
| NAJ | 6.96 ± 0.07 | 2.24 ± 0.19 b | 0.24 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzavaras, D.; Papadelli, M.; Ntaikou, I. From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation 2022, 8, 135. https://doi.org/10.3390/fermentation8030135
Tzavaras D, Papadelli M, Ntaikou I. From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation. 2022; 8(3):135. https://doi.org/10.3390/fermentation8030135
Chicago/Turabian StyleTzavaras, Dimitris, Marina Papadelli, and Ioanna Ntaikou. 2022. "From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages" Fermentation 8, no. 3: 135. https://doi.org/10.3390/fermentation8030135
APA StyleTzavaras, D., Papadelli, M., & Ntaikou, I. (2022). From Milk Kefir to Water Kefir: Assessment of Fermentation Processes, Microbial Changes and Evaluation of the Produced Beverages. Fermentation, 8(3), 135. https://doi.org/10.3390/fermentation8030135








