Abstract
Shameta is a traditional, Ethiopian, cereal-based fermented porridge exclusively prepared for lactating mothers. The aim of this study was to determine the microbial quality of Shameta samples collected from households of lactating mothers and to determine microbial dynamics and physicochemical changes during laboratory fermentation of Shameta. Isolation and characterization of the dominant microbes and analysis of the physicochemical properties of samples were done following standard microbiological methods and analytical techniques. Results of this study showed that the highest mean count of lactic acid bacteria (8.33 log cfu/g) was recorded in a sample from laboratory-fermented barley-based Shameta, and the lowest (5.88 log cfu/g) in Shameta made from a mixture of barley and maize (BMS). In both barley-based and maize-based laboratory-prepared Shameta, the microflora were dominated by LAB, followed by yeasts. The dominant LAB were the genus Lactobacillus (74.85%), followed by Enterococcus (15.79%). It could be concluded that Shameta collected from households of lactating mothers are fairly safe for consumption, as the stringent physicochemical conditions of the final product could inhibit the growth of pathogens. However, as Shameta is a traditional fermented porridge fed to lactating mothers, we call for a further improvement to the fermentation process by using defined starter cultures.
1. Introduction
Fermented foods are among the staple foods of humans and have been produced and consumed since the development of human civilizations [1]. In Africa, cereal-based fermented foods are staples and complementary and weaning foods for infants and young children [2]. Traditional fermented foods are prepared using locally available equipment, raw materials and microorganisms known of by old traditions, although the products and materials used vary from place to place [3,4]. Due to their preparation processes that involve uncontrolled conditions, traditional food fermentation processes involve a wide range of microorganisms and their enzymes to achieve the desirable characteristics [5]. However, lactic acid fermentation has attracted attention, as it reduces contamination by pathogenic microbes because of their well-established ability to produce lactic acid, besides other antimicrobial metabolites, that result in lowering of the pH of the fermented food products [6].
The major microbes in cereal-based fermented foods are lactic acid bacteria (LABs), yeasts and molds [7,8]. Along with other microorganisms, such as endospore forming bacteria, they play significant roles at different stages of fermentation, such as enhancing organoleptic and preservative properties while improving nutritional quality [9,10]. The growth dynamics, survival and biochemical activities of microorganisms in food are the results of stress reactions in response to the physicochemical changes in the food’s microenvironment [11]. Many studies support that LAB grow better than other microorganisms in fermented food products due to the increase in acidity; the anaerobic conditions created in the course of fermentation; and their ability to produce antimicrobial products, such as hydrogen peroxide, diacetyl, carbon dioxide, organic acids and bacteriocins [12,13].
Shameta is an indigenous traditional fermented porridge produced and consumed by lactating women in order to help them recover and gain strength after delivering babies. It is produced from a mixture of different ingredients, such as cereal grains (maize, barley, sorghum or wheat); vegetable oils, mainly rapeseed; and spices and herbs as flavor enhancers and preservatives. Unlike other fermented products in Ethiopia, Shameta is made with a double fermentation process, especially when maize is used as a major ingredient. In the first stage of fermentation, the ingredient mixture (dough) is allowed to ferment for a week and then cooked to make porridge. Cooked porridge, once cooled at room temperature, is subjected to the second fermentation process (that lasts for 14–30 days) after the addition of some other ingredients. During these two fermentations, it is expected that different changes take place in terms of microbial dynamics and physicochemical parameters.
So far, several studies have been done on describing the process, microbial dynamics and safety of various fermented foods of Ethiopia, such as Borde [14], Azo [15], Tella [16], Bubugn [17] and Wakalim [18]. However, there is a gap in the assessment of the microbial quality and growth dynamics of traditionally prepared and used Shameta in the western part of the country. Therefore, the aim of this study was to determine the microbial quality of Shameta samples collected from households of lactating mothers and to investigate the growth dynamics and identify the dominant microorganisms in Shameta fermentation under laboratory conditions. The information generated in this study could help to determine the appropriate fermentation time, proper substrate combinations and safety level of traditionally prepared Shameta, besides presenting baseline data for large scale production of the product using a defined starter culture.
2. Materials and Methods
2.1. Description of the Study Area
The study was carried out in East Wollega Zone of Oromia Regional State, located in the western part of Ethiopia at latitude of 80°31′52″ south and longitude of 360°07′51″ east. It has annual rainfall of 1150–2070 mm/year on average and a temperature range of 18.5 to 27.5 °C (Figure 1). Based on the census conducted by the Central Statistical Authority of Ethiopia [19], this zone has 17 districts and a total population of 1,213,503, of whom 606,379 are men and 607,124 are women. It has an area of 12,579.77 Km2. Some of the major crops grown in the zone are Teff (Eragrostis tef), barley (Hordeum vulgare), wheat (Triticum aestivum), faba bean (Vicia faba L), sesame, groundnut, field pea, maize, sorghum, finger millet, potato, tomato and hot pepper [20]. The zone was selected based on the popularity of the use of fermented porridge (Shameta) in the diets of lactating mothers.
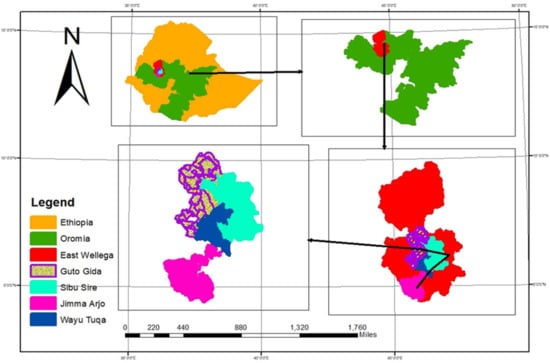
Figure 1.
Map of the study site.
2.2. Shameta Sample Collection and Laboratory-Scale Fermentation of Shameta
2.2.1. Study Population and Household Shameta Sampling
Shameta is a fermented porridge solely produced and consumed by lactating mothers of the Oromo community in the western part of Ethiopia. East Wollega Zone was purposively selected for the study based on the community’s rich experience and culture of producing and consuming Shameta. From the zone, four districts (Sibu Sire, Guto Gida, Jimma Arjo and Wayu Tuka) (Figure 1) were purposively selected for the study due to the popularity of the product among lactating mothers in those districts. Lactating mothers who gave birth in health centers during 2–16 January 2021 were considered as the study population. This was intentionally done to collect samples having more or less similar fermentation ages. Mothers who gave birth before and after the specified dates of data collection, and those who gave birth outside of health centers, were excluded from the study. A significant deviation in processing method or ingredients compared to the traditional practice were also considered exclusion criteria. A list of the mothers who gave birth in the specified period was acquired from the district heath offices. Accordingly, a study population consisting of 103 lactating mothers (35 from Sirbu Sire, 34 from Guto Gida, 23 from Jimma Arjo and 11 from Wayu Tuka) was used for the study. Based upon the proportion sampling method, a total of 27 samples were randomly collected from four districts (Sibu Sire (9), Guto Gida (9) Jimma Arjo (6) and Wayu Tuka (3)). Collection of additional data related to ingredients used, methods employed and other relevant information continued to the end of April 2021. The ethical clearance permit was secured from the Research and Ethical Clearance Board of Jimma University, College of Agriculture and Veterinary Medicine, before resuming data and Shameta sample collection. Both data and sample collections were performed in accordance with the permit secured from the board.
2.2.2. Collection of Shameta Samples
During sample collection, efforts were made to gather fresh samples based upon the delivery date of mothers (two-week interval). As indicated above, 9 samples each from Sibu Sire and Guto Gida, 6 from Jimma Arjo and 3 from Wayu Tuka districts were collected in triplicate. Based upon ingredients and fermentation time, three similar samples were combined and thoroughly mixed to group the samples into nine categories (Table 1). The samples were collected using sterilized glass bottles with a holding capacity of 500 mL each and transported using an icebox.

Table 1.
Sample collection sites and the ingredients used for the preparation of Shameta samples, East Wollega Zone, 2020.
2.2.3. Laboratory Sample Preparation
Preparation of maize: Maize grains were sorted, cleaned and thoroughly washed by immersion in cold tap water. It was stirred by hand and strained out of the water to remove impurities. The kernels were then sun dried and milled using hammer milling machine into flour to a sieve size of 0.5 mm. Finally, the maize flour was packaged and stored at room temperature in glass bottles (container) until it was used for formulation and fermentation.
Preparation of barley: Barley was cleaned by the sorting technique followed by de-hulling by mortar and pestle (made of wood) with the addition of little water to soften the bran and ease its removal. The bran was removed from the dried barely and milled using a hammer milling machine into flour to a sieve size of 0.5 mm. Finally, the maize flour was packaged and stored at room temperature in glass bottles (container) until it was used for formulation and fermentation.
Preparation of faba beans: Faba beans were sorted and cleaned, and their skin was removed via hummer mill. Then, after the skin was removed, it was milled using a hammer milling machine into flour to a sieve size of 0.5 mm. Finally, the faba bean flour was packaged and stored at room temperature in glass bottles (container) until it was used for formulation and fermentation.
Preparation of rapeseed: During preparation of rapeseed, a grinding stone was used for size reduction. The traditional sorter was used to remove unwanted materials after it was dried in the sun light. Then, the rapeseed was milled repeatedly using a mortar and pestle, until crude oil was visible in the mortar. The ground rapeseed was transferred to a clay pot (3 L holding capacity) and sealed for three days. After three days, 1 L hot water was added to crude rapeseed oil, and it was separated from the cake by pressing the mix through a cloth and sieve.
Preparation of spices: Spices such as fenugreek (Trigonella foenum-graecum), black cumin (Nigella sativa), black cardamom and white cumin (Cuminum cyminum) were roasted on a griddle made of a thick iron sheet after grinding using a spice grinding machine. However, garlic bulbs and basil leaves were only cleaned and washed with water before being added to the mixture of all ingredients. The mix was transferred to a fermentation vessel to kick of the fermentation processes.
Barley-based Shameta fermentation: In this study, the major ingredient used for laboratory fermentation of Shameta was barley (Hordeum vulgare) (10.44 kg), as maize (Zea mays) (0.6 kg) was added only to improve the appearance (white color) of the final product. The barley was mixed with maize before milling, which was followed by sieving the flour and cooking it. The flour was cooked for about 2:30 h, and rape seed oil (0.9 kg) was added before the porridge matured. Different spices (0.06 kg) were added after the porridge was cooked very well. Then, the porridge was allowed to cool overnight at room temperature and transferred to a fermenting vessel in which garlic bulbs and basil leaves were added in a stratified manner in between each stratum of cooked and cooled porridge, with the layers being of equal volumes (Figure 2).
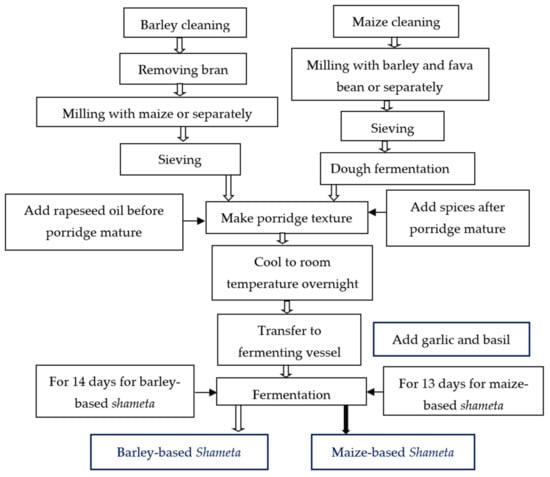
Figure 2.
Flow diagram of traditional barley and maize-based Shameta preparation.
Maize-based Shameta fermentation: As its name indicates, the major ingredient used for maize-based Shameta preparation was maize (Zea mays) (9.72 kg). Barley (Hordeum vulgare) (0.6 kg) and faba beans (Vicia faba L.) (0.6 kg) were added as minor or supplementary ingredients. Maize was mixed with barley and faba beans before milling and sieving of the flour. The flour was cooked for about 1:30 h, and rape seed oil was added before the porridge matured. The spices were added to the matured porridge. Then, the porridge was allowed to cool overnight at room temperature before transferring it to fermenting vessel, in which garlic bulbs and basil leaves were added in between strata or layers of cooked and cooled porridge intermittently, as mentioned above for barley-based Shameta fermentation (Figure 2).
2.3. Determination of pH and Titratable Acidity (TA)
The pH was measured using a digital portable pH meter (pH-013, China) after homogenizing five grams of the sample in 20 mL distilled water, followed by pipetting and measuring of 10 mL of the homogenized sample into a beaker [21]. The TA of Shameta was determined by homogenizing 2.5 g of sample in 10 mL of distilled water and filtering the mixture through Whatman No. 1 filter paper [22]. In the filtrate, 3 to 5 drops of 1% (wt/v) phenolphthalein indicator was added, and the sample was titrated with freshly prepared 0.1 mol/L NaOH solution until a faint pink color persisted for 30 s. The TA value, in terms of lactic acid as a dominant organic acid in the product, was determined using the following equation:
where, N = normality of titrant (mEq/mL), VNaOH = volume of titrant (mL), Eq. wt = equivalent weight of predominant acid (mg/mEq, which is 90.08 for lactic acid), Vs = volume of sample (mL) and 1000 = a factor for converting mg to grams.
2.4. Moisture Content Determination
A standard method of the Association of Official Analytical Chemists [23], particularly official method 925.09, was used to determine the moisture contents of Shameta samples.
2.5. Enumeration of Microbial Groups
The counting and characterization of microbial groups were carried out following standard microbiological methods [24]. For Shameta fermented under laboratory conditions but following the traditional preparation techniques, samples were drawn at 0 h and then every 24 h for 10 days, and finally on day 14 to examine the microbial dynamics of barley-based Shameta. Similarly, samples were drawn at 0 h and then every 24 h for the first 13 days to examine the microbial dynamics of maize-based Shameta. For both Shameta samples collected from households of lactating mothers and laboratory-prepared Shameta, each 10 g sample was aseptically transferred into 90 mL of sterile peptone water and homogenized in a flask at 100 rpm for 10 min on a shaker (Compact shaker, D-72379 Hechingen, Germany). After homogenization, 1 mL of each sample was aseptically transferred into 9 mL of pre-sterilized peptone water and mixed thoroughly using a vortex mixer. Then, the homogenate was serially diluted up to 10−7, and 0.1 mL volume aliquots of appropriate dilutions were spread-plated on respective pre-sterilized and surfaced dried agar media. Finally, the inoculated plates were incubated at appropriate temperatures and for various durations, after which counts (enumeration) were carried out as described below:
2.5.1. Aerobic Mesophilic Bacteria (AMB) Counts
A 0.1 mL aliquot from the appropriate dilution was spread-plated in duplicate on plate count agar (PCA) and incubated at 32 °C for 48 h. Counts were performed with countable plates (30–300 colonies per plate) using a colony counter, and each count is expressed in log cfu/g.
2.5.2. Enterobacteriaceae Counts
A 0.1 mL aliquot from the appropriate dilution was spread-plated in duplicate on Violet Red Bile Glucose (VRBG) agar and incubated at 32 °C for 24 h. Then, purple/pink colored colonies surrounded by purple halos were counted as members of Enterobacteriaceae.
2.5.3. Total Coliform Counts
To enumerate total coliforms, a 0.1 mL aliquot of serially diluted sample was inoculated into trypticase soya agar (TSA) (Oxoid, London, UK), and after 30 min, overlaid with Violate Read Bile (VRBA) agar medium (BFCO). The plates were incubated at 37 °C for 24 h. Then, typical coliform colonies were counted [25].
2.5.4. Aerobic Spore-Forming Bacteria Count (ASFB)
For ASFB counts, 10 mL of the appropriate dilution was heat treated in a water bath adjusted to 80 °C for 10 min and cooled rapidly under tap water. Then, a 0.1 mL aliquot from the appropriate dilution was spread-plated on nutrient agar (NA) and incubated at 32 °C for 72 h.
2.5.5. Lactic Acid Bacteria Counts
From the appropriate dilutions, 0.1 mL aliquots were spread-plated in duplicate on pre-dried surfaces of MRS (de-Mann, Rogosa and Sharpe) agar (Oxoid) plates. The plates were incubated under anaerobic conditions using anaerobic jar (BBL, anaerobic Jar System) at 30–32 °C for 48 h [26].
2.5.6. Staphylococci Counts
A 0.1 mL aliquot from the appropriate dilution was spread-plated on mannitol salt agar and incubated at 32 °C for 36–48 h. Thereafter, yellow colonies surrounded by red color were counted as staphylococci.
2.5.7. Yeast and Mold Counts
Yeast and mold were counted by inoculating a 0.1 mL aliquot from the appropriate dilution on potato dextrose agar (PDA) medium supplemented with 200 mgL−1 chloramphenicol and incubated at 25 °C for 3–5 days [27].
2.5.8. Detection of Salmonella spp.
Pre-enrichment for Salmonella was done by mixing 25 g of a sample with 225 mL of buffered peptone water in a sterile flask and incubated at 37 °C for 24 h. Selective enrichment was done by transferring 1 mL of the pre-enrichment culture into 10 mL of selective selenite broth, homogenizing the mix and incubating the mix at 37 °C for 24 h. A loopful culture from selective enrichment broth was further transferred to selective agar media (xylose lysine deoxycholate (XLD)) agar and incubated at 37 °C [28]. Characteristic Salmonella isolates were further characterized biochemically for confirmation of whether the presumptive Salmonella isolates belonged to the genus Salmonella or not. For further confirmation of the presumptive Salmonella, the triple sugar iron (TSI) test, lysine iron test, urease production test, citrate utilization test, H2S gas test and indole production test were conducted using triple sugar iron agar, lysine iron agar, urea agar, Simmon citrate agar and sulfide indole motility medium (all Oxoid), respectively, following the standard microbiological procedure.
2.6. Characterization of LAB
Pure cultures of LAB isolates were characterized by basic morphological features, Gram reaction, catalase production, acid and gas production from glucose, growth under different salt concentrations and level of acidity following standard microbiological methods. Finally, the isolates were grouped into different genera based on their morphological, biochemical and physiological characteristics [29].
2.7. Cell Shape, Cell Arrangement and Motility Test
Overnight, pure broth cultures were smeared on clean microscopy slides, and then we analyzed them under phase contrast microscopy for their cell shapes and arrangements [21]. Motility was studied using a sulfur indole motility solution on MRS medium according to Okoro et al.’s method [30].
2.8. Gram Reaction
Isolates were grouped into either Gram-positive or Gram-negative based on their responses to the Gram reaction test [31]. Briefly, each isolate was smeared on a grease free slide, air-dried and heat fixed by passing each slide over the blue flame of a burning Bunsen burner repeatedly. Each slide was flooded with a crystal violet solution, dried for a minute, rinsed with distilled water and treated with iodine for a minute. The stained slides were decolorized in 95% ethanol (for 30 s), rinsed with distilled water and counter-stained with safranin for 30 s. The slides were rinsed again, air-dried and observed under the oil immersion objective lens (×100) of the light microscope [31]. Only those isolates that retained the original dye (crystal violet) and appeared violet were considered Gram-positive, the others being Gram-negative.
2.9. Catalase Test
The catalase test was performed by transferring an overnight activated bacterial culture to a slide containing a drop of a 3% solution of hydrogen peroxide (H2O2). The formation of bubbles within a few minutes was taken as a positive result for catalase production [32].
2.10. Acid and Gas Production from Glucose
In order to determine the homo-fermentative and hetero-fermentative characteristics of isolates, acid and CO2 production from glucose were evaluated as suggested by Seeley and Vandemark [33]. Briefly, pure bacterial isolates were separately inoculated into test tubes (inverted Durham tubes) containing 5 mL pre-sterilized glucose broth and bromocresol purple (15 mL/L 0.04 percent solution), the latter being the pH indicator. The tubes were incubated for seven days at 30 °C. The accumulation of gas in the Durham’s tube was considered a positive result for gas production, and a change in the color of the medium to yellow was considered a positive test result for acid production.
2.11. Test for Tolerance to Different Salt Concentrations
The ability of each isolate to grow under different sodium chloride concentration was tested via inoculation of a loop-full of an overnight culture into test tube containing pre-sterilized MRS broth whose sodium chloride concentration was increased by 4 or 6.5% compared to the control. Then, the overnight incubated broth culture was streaked on MRS agar and incubated at 37 °C for 24 h to observe growth or no growth [34].
2.12. Test for Tolerance to Different pH Levels
The growth of the isolates at different pH levels was determined by inoculating a loop-full of an overnight culture into MRS broth whose pH was adjusted to 2.5, 4 or 9.5. Then, the overnight activated broth culture was streaked on MRS agar and incubated at 37 °C for 24 h to observe the growth [34].
Data Analysis
Microbial counts and physicochemical properties data of Shameta samples collected from households of lactating mothers and samples of Shameta prepared in the laboratory were statistically analyzed using analysis of variance (ANOVA) and least significant difference (LSD). All samples were tested in triplicate and statistically analyzed using SAS (SAS Institute, Cary, NC, USA) version 9.3, and the significance threshold was p ≤ 0.05. Fisher’s least significant difference (LSD) was used for mean comparison tests to identify significant differences among means (p ≤ 0.05). The coefficients of variation (CV) were determined from the standard deviations and means of the parameters, and the results are expressed as mean ± standard deviation.
3. Results and Discussion
3.1. Physicochemical and Microbial Quality of Shameta
The pH and titratable acidity (TA) values of Shameta samples collected from households of lactating mothers are as presented in Table 2. The Shameta samples were collected from four different districts but combined into nine groups based on similarity of their ingredients and durations of fermentation.

Table 2.
Physicochemical properties (pH and TA) of Shameta samples collected from households of lactating mothers, East Wollega Zone, 2020.
The highest and lowest pH values were found in BMS and MG samples, as opposed to the corresponding TA values. The variations in pH and TA of samples might be associated with differences in the duration of fermentation, ingredients used and roles and dynamics of microorganisms during the fermentation process [35]. Based upon the range of measured pH values (3.6 to 4.4), this product is categorized as an acidic food, which is an indicator of the relative safeness of the food against certain low-pH-intolerant foodborne pathogens [36,37]. The average pH (4.0) recorded in the present study is greater than those of other fermented foods in Ethiopia, such as Cheka (pH = 3.74) [38], but similar to that of Borde (pH < 4.2) [39]. Similarly, the average TA (0.78%) recorded in the present study is greater than the TA value of Azo (0.35%), but lower than that of Cheka (0.94%) [15,38]. The acids produced during fermentation lower of the pH values of Shameta, which ultimately inhibits the growth of pathogenic microbes, thereby prolonging the shelf life of the final products.
Lactic acid bacteria (LAB) mainly carry out lactic acid fermentation, which has essential roles in preservation and the wholesomeness of fermented food products [35]. In the present study, LAB were the only dominant microbes in Shameta samples. The highest mean count (8.33 log cfu/g) was recorded in the laboratory-prepared barley-based Shameta sample and the lowest (5.88 log cfu/g) in the BMS sample (Shameta made of barley blended with maize) (Table 3). The low mean count of LAB being observed in the BMS sample might have been due to the limited nutrients in the fermenting matrix and/or unfavorable biophysical environmental factors [40]. The growth pace of LAB in laboratory-prepared maize-based Shameta was faster than that of barley-based Shameta. This might have been due to variations in the nutrient contents of the substrates and their fermentability [41]. Even if the amounts of major ingredients in barley-based Shameta samples were more or less the same, there were statistically significant differences (p < 0.05) in the counts of LAB, which was also true for maize-based Shameta. This might have been due to the variations in types and amounts of spices and herbs added during Shameta preparation. In addition, the stage at which spices and herbs are added to the semi-processed product affects the counts of LAB in the fermented product. The types and amounts of bioactive compounds in spices and herbs affect the growth of microorganisms in food products to different degrees [42].

Table 3.
Mean microbial count (log of cfu/g) of “Shameta” samples collected from households of lactating mothers, East Wollega Zone, 2020.
A study in North-East Nigeria showed that the total LAB counts (log cfu/g) in commercial Fura ranged from 4.37 to 6.18 [43], which is a lower range than in the present study. However, it is a similar range to that found in a study on sorghum-based ogi, whose mean counts were 6.64 to 8.15 log cfu/g [44]. In addition to essential oils in spices used for fermentation, low pH values recorded in the present study could be attributed to the observed inhibition in the growth of pathogenic microorganisms in the laboratory-fermented Shameta, which would have practical relevance to the preservation of the product.
The present results show that the counts of aerobic mesophilic bacteria (AMB) in all samples and the counts of Enterobacteriaceae in almost all samples were >3 log cfu/g. The counts of Enterobacteriaceae were within the range of 2–4 log cfu/g, a range closer to that found in an earlier report [45]. Enterobacteriaceae are a large group of biochemically and genetically-related bacteria used to assess the general hygiene status of a food product. They are usually considered by food manufacturers as hygiene indicators and are therefore used to monitor the effectiveness of the implemented preventive measures [45,46]. In Ethiopia, the majority of ready-to-eat foods are contaminated by members of Enterobacteriaceae, such as Salmonella spp. and E. coli, due to unhygienic food handling practices, especially during transportation, handling and selling [47]. Likewise, AMB is an indicator of quality, not safety, and cannot directly contribute towards a safety assessment of ready-to-eat food; however, it can provide useful information about the remaining shelf lives of the food products [45]. In the present study, one of the major sources of contamination of Shameta with Enterobacteriaceae might be unsafe water being used for the washing of plates, forks and hands. The safety status of the food serving environment may be another.
The counts of total coliform (TC) in more than half of the samples were below the detectable level (<2 log cfu/g), and the counts of Staphylococcus spp. were 2.47–3.28 log cfu/g. The mean counts of TC and Staphylococcus spp. of the samples were much lower than in a report on commercial Fura, whose TC and Staphylococcus spp. counts ranged between 6.03 and 7.26 log cfu/g and 6.30 and 7.01 log cfu/g, respectively [43]. The low counts of TC and Staphylococcus spp. in the present study might be accounted for by the relatively good hygienic practices exercised by producers and consumers, the use of essential oils, the low pH and the processing methods (cooking) applied before the first phase fermentation of Shameta. The count of coliforms is an indicator of the degree of fecal contamination of food products [48]. Except for shiga toxin-producing Escherichia coli, which is hazardous if detected in a 25 g sample [49], the majority of coliforms are harmless or cause relatively brief diarrhea [50]. As the present study did not focus on the isolation and identification of fecal coliforms, it is difficult to generalize our findings to the safety of Shameta with respect to coliform identities.
The mean counts of Staphylococcus spp. in the present study ranged between <2 and 3.28 log cfu/g, which meets the thresholds for Staphylococcus aureus and other coagulase positive staphylococci in foods [49]. S. aureus is among the pathogens of public health concern [51,52] which contaminates the majority of ready-to-eat foods in Ethiopia due to the poor hygiene of food producers and vendors [47]. The prevalence of S. aureus in Shameta samples and laboratory-fermented Shameta needs further investigation vis-à-vis the intrinsic parameters of the products, and the current data on Staphylococcus count could only be used as baseline data and could not be used to draw sound conclusions on the safety status of this product with respect to pathogenic S. aureus.
In the current study, more than 50% of the samples had Bacillus counts below detectable levels (<2 log cfu/g). However, species of the genus Bacillus and related genera have long been troublesome microbes in food industries due to their resistant endospores [53]. Lücking et al. [54] reported that spore-forming bacteria, especially Bacillus cereus, are important contaminants in the dairy industry with a high impact on the quality and safety of dairy products. According to the Food Safety Authority of Ireland and United Kingdom, the threshold for Bacillus cereus in ready-to-foods placed on market ranges between 103 and ≤105 cfu/g [55,56]. One of the challenges of these spore-forming bacteria is that their toxins and spores are often not detected until after an outbreak of food poisoning [57]. No Salmonella spp. was detected in any Shameta sample.
The counts of yeasts were >4 log cfu/g in almost all samples, and the counts of molds in more than half of the samples fell between 2.11 and 3.67 log cfu/g. The presence of molds in samples collected from households might have been due to poor handling practices and exposure to contaminants from humans, utensils and water [58]. Foods with high water content, such as meat, milk and seafood, are easily spoiled by bacteria, unlike foods with low water content. Spoilage of staple foods is usually initiated by molds or yeasts [59]. However, at optimum pH (4.5 to 8.0), fermentative bacteria multiply faster and out-compete molds, reducing their growth [59], making such a pH a good way to preserve fermented food products for longer periods of time against deterioration by molds.
3.2. Physicochemical Changes and Microbial Dynamics during Barley-Based Shameta Fermentation
The pHs, titratable acidity (TA) values and moisture contents of the laboratory-prepared barley-based Shameta samples were as shown below (Figure 3). The initial pH of the fermented sample was 5.30, and it dropped down to 3.94 at the final stage of fermentation. As a result, TA increased from 0.2 to 0.55 on the 14th day of fermentation (Figure 3a). Similarly to the present findings, drops in pH with time of fermentation were observed during the fermentation of beverages, including akamu, Borde and kisra [14,60,61]. However, during fermentation of “azo,” a traditional Ethiopian fermented condiment, the pH started to drop from day one to day six, but remained constant from the 7th day till the final stage of fermentation [15]. The absence of a change in pH in the latter could be accounted for by the depletion of fermentable ingredients in the fermenting matrix.
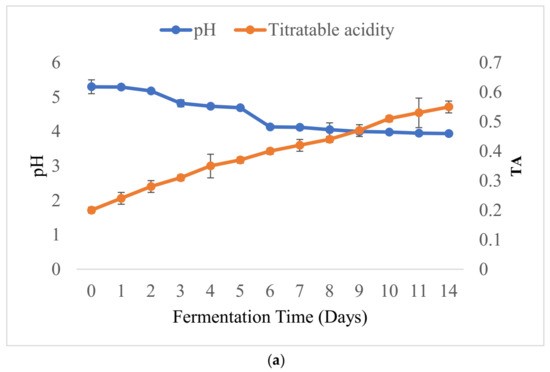
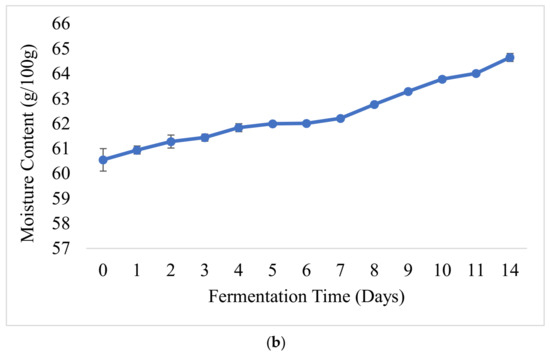
Figure 3.
pH and TA values (a) and changes in the moisture content (b) of laboratory-prepared barley-based Shameta.
The moisture content of fermenting Shameta increased from 60.55 (day 1) to 64.65 g/100 g at the final stage of fermentation (day 14) (Figure 3b). The low moisture content observed at the initial stage of fermentation might have been due to the presence of large amounts of beta-glucan and starch in the samples, as they have ability to form bonds with water molecules, making a more viscous matrix and making it difficult to evaporate all water during the drying we did to measure moisture content of samples [62,63]. However, later, as microorganisms broke down polysaccharides into simple sugars, none of the polysaccharides’ bound with water molecules; thus, free water was available, and the samples became less viscous and easily dried out. Similarly to our findings, increments in moisture content during fermentation were reported for other traditional Ethiopian fermented food products, including Azo and Borde [14,15].
The mean counts (log cfu/g) of microorganisms and their dynamics in the course of the fermentation of barley-based Shameta samples prepared in the laboratory were as presented in Figure 4. The mean counts (log cfu/g) of LAB at the beginning and the end of fermentation were 3.41 and 8.33 log cfu/g, respectively. Accordingly, LAB were the dominant microflora throughout the fermentation. Considering the relatively low count at the beginning of fermentation, the gradual increment LAB during fermentation might have been due to viscosity of the product, and the nature of the processing methods (cooking) applied before fermentation. At the initial stage of fermentation, the count of LAB was 3.41 log cfu/g, which later increased exponentially until reaching a maximum of 9.79 log cfu/g on the 10th day of fermentation. The decline in LAB after the 10th day of fermentation might have been due to the lack of available sugar, which could be attributed largely to LAB activity [64]. In agreement with the present finding, the maximum growth of LAB with counts as high as 10.09 log cfu/g was observed on the 7th day fermentation of Azo [15].
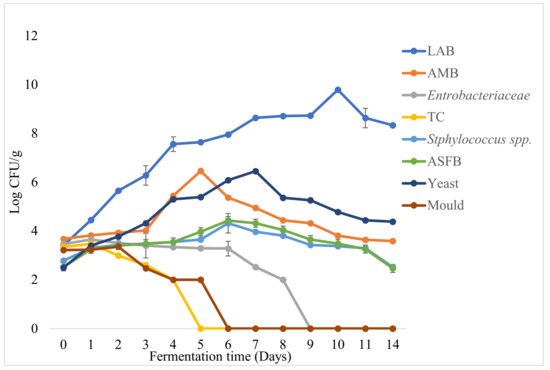
Figure 4.
Growth dynamics of various groups of microorganisms during barley-based Shameta fermentation. LAB = lactic acid bacteria, AMB = aerobic mesophilic bacteria, TC = total coliforms, ASFB = aerobic spore forming bacteria.
In the course of fermentation, the rise in the count of LAB, as compared to other microorganisms, contributed most to the increment in acidity of the fermenting Shameta. Anaerobic conditions created with time favor the growth of only facultative anaerobes, micro-aerophiles and aciduric organisms [13]. It may also have been due to the inhibitory effects of antimicrobial products, such as hydrogen peroxide, diacetyl, carbon dioxide (CO2), organic acid and bacteriocins, produced by LAB and inhibiting other competitive microorganisms in the matrix, including pathogens [12]. However, the decrement at the end of fermentation might have been due to the reductions in nutrient levels in the composited cereals.
The mean counts of AMB increased until the 5th day of fermentation and started to decrease from the 6th day onwards, which persisted till the end of the fermentation stage, leaving counts less than 4 log CFU/g. Likewise, in akamu and Azo fermentation, AMB persisted until the final stage of fermentation [15,61]. However, the count of AMB at the final stage of azo fermentation was greater than the mean count recorded in present finding. Enterobacteriaceae and TC did not survive until the final stage of Shameta fermentation, whereas Staphylococcus spp. and ASFB were detected throughout the fermentation period (Figure 4). Similarly, Enterobacteriaceae and TC did not persist until the final stage of azo and borde fermentation, even though they started the processes [14,15]. This might have been due to the fact that members of the family Enterobacteriaceae are not capable of growing under anaerobic conditions in a food product when the product’s pH is lower than 5.8 [65]. In addition, their disappearance might have been due to inhibitory activity by LAB through the production of various metabolites, including lactic acid, acetic acids, bacteriocins, diacetyl and hydrogen peroxide [12].
The sources of microorganisms detected during fermentation could have been the equipment and raw materials used for fermentation, including the spices added during fermentation (after cooking and cooling). The potential presence of spores on those utilities and raw materials could pave the way for the proliferation of other microbes, due to the simple products released by such amylolytic bacteria. Almeida et al. [66] reported that the aerobic spore-forming bacteria (Bacillus spp.) secrete a wide range of degradative enzymes, such as amylases and proteases, which convert polysaccharides and proteins to simple compounds, and then open the door for the proliferation of other non-amylolytic lactic acid bacteria.
Yeasts and molds were encountered at the early stage of fermentation (Figure 3). Counts of yeasts increased exponentially until the 7th day (6.45 log cfu/g) and then decreased until the final stage of fermentation, when there was a mean count of 4.38 log cfu/g. As in our observations, yeasts were reported to persist until the final stage during Borde fermentation [14]. In contrast to the present results, yeasts were encountered at the initial stage of Azo fermentation but eliminated on the 4th day of fermentation [15]. The presence of yeast in Shameta until the final stage of fermentation is crucial due to their ecological interactions with lactic acid bacteria. According to Steinkraus [67], yeasts afford vitamins and contribute factors for the growth of LAB. The growth paces of molds were slow, and they persisted for a few days before being eliminated after the 5th day of fermentation. The detection and growth of molds during the early stage of fermentation could be accounted for by their introduction into the fermentation system through the spices and herbs used, or possible contamination of utensils and the environments [58]. However, its subsequent disappearance after the 5th day of fermentation was probably due to the low oxygen level in the fermenting matrix, and the inhibitory effects of the antimicrobial products produced in the course of fermentation by LAB and yeasts [12,13] and those released from the spices used. Fredlund et al. [68] reported that yeasts produce some volatile organic compounds that contribute to reducing mold growth and spore germination in bacteria.
3.3. Physicochemical Changes and Microbial Dynamics during Maize-Based Shameta Fermentation
The pH and TA values at the final stage of maize-based Shameta fermentation were 3.72 and 0.75, respectively (Figure 5a). The observed values are in agreement with pH and TA values recorded for Cheka, an Ethiopian traditional cereal and vegetable-based fermented beverage with values of 3.91 and 0.77, respectively [38]. Several papers have reported that low pH values are desirable, as they inhibit the growth and survival of spoilage organisms and give the fermenting organisms an advantage [69,70,71,72]. The moisture content of the maize-based Shameta increased from its initial value of 62.32 g/100 g to 69.21 g/100 g at the final stage of fermentation (Figure 5b). Higher moisture content usually lowers the shelf life of a product, especially in developing countries where the use of physical means of food preservation, such as refrigeration, is not common [73]. Fortunately, while the moisture content increased, the pH value dropped into the acidic range, a condition that does not support the growth of non-acid-tolerant vegetative cells of pathogenic microorganisms [36,37].
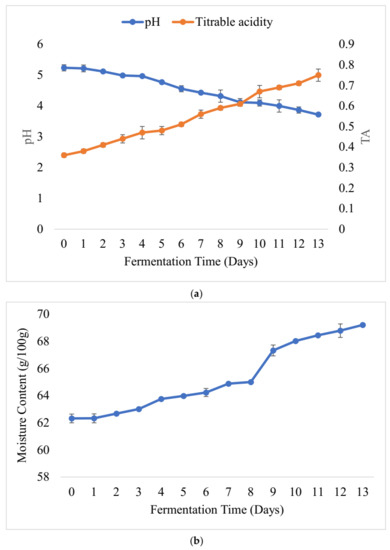
Figure 5.
pH and TA values (a) and moisture content (b) of laboratory-prepared maize-based Shameta.
The pattern of microbial dynamics of maize-based Shameta fermentation was as presented below (Figure 6). Accordingly, lactic acid bacteria (LAB), aerobic mesophilic bacteria (AMB), Staphylococcus spp., aerobic spore forming bacteria (ASFB) and yeasts were detected throughout the fermentation period. The highest mean counts were recorded for LAB (8.5 log cfu/g), followed by yeasts (6.44 log cfu/g). The highest mean counts of LAB and yeasts observed in the present finding might have been due to their in situ cell-to-cell interactions, as multiplication of yeasts is favored by the acidic environment developed by metabolic activities of LAB, and the growth of LAB is favored by the yeasts activities, as the latter provide several growth factors [74]. However, LAB are the most common microorganisms responsible for cereal fermentations. They enhance flavor, nutritional value, detoxification and lactic acid production [75].
The mean counts of Enterobacteriaceae and total coliforms (TC) increased for the first two days of fermentation. Then, there were gradual declines in counts before their total elimination from the fermentation system on the 7th and 6th days of fermentation, respectively (Figure 6). The elimination of Enterobacteriaceae and TC might have been due to the drop in pH, the inhibitory effects of essential oils in spices and production of other antimicrobial metabolites by lactic acid bacteria [12]. Accordingly, Enterobacteriaceae disappeared from the fermenting Shameta when the pH further dropped to 4.3, the result that corroborated an earlier report [60] that revealed the observation that Enterobacteriaceae cannot grow between pH 3.5 and 4.0. An early drop in the mean counts of pathogenic microorganisms (including members of Enterobacteriaceae and coliforms) leading to ultimate elimination from the fermenting product at the end of fermentation is advantageous from the consumer safety point of view, as some of these pathogens could release toxins and/or carcinogenic substances (nitrosamines) from the fermentation of nitrogen sources [76].
Morphologically distinct molds were encountered at the initial stage of fermentation with a mean count of 2.48 log cfu/g, and detected up to the 5th day of fermentation before their elimination from the fermentation system thereafter. In line with the present finding, molds were eliminated from fermenting Borde after 12 h of fermentation [14]. In other studies, molds were detected at the initial stages of fermentation, but then drastic reductions occurred in the course of fermentation, for kenkey and ogi [77,78,79]. However, during a microbial study of akamu, molds were detected at both initial and final stages of fermentation, which lasted for 72 h [61]. Detection of yeasts throughout the fermentation period has a positive role in enhancing the development of the typical flavor in the final fermented products, besides their contribution to nutrient availability, as some of them also produce enzymes such as amylase, protease and phytase [79,80]. Thapa and Tamang [81] reported that mucoraceae fungi have roles in the initial phase of fermentation, mostly in scarification of the substrates which create favorable conditions for LAB. According to Petrova et al. [82], however, LAB of the genera Lactobacillus, Lactococcus and Streptococcus are able to directly metabolize starch for the production of organic acids and other compounds, which have essential roles in the preservation and wholesomeness of food products.
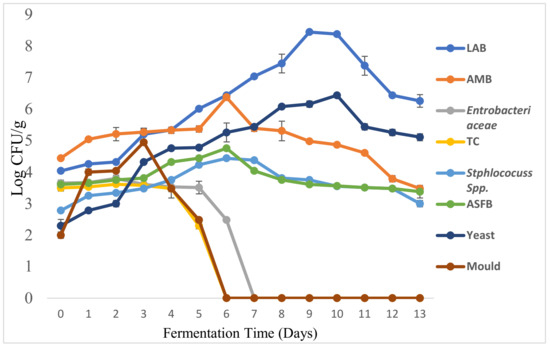
Figure 6.
Growth dynamics of various microbial groups during the fermentation of maize-based Shameta; LAB = lactic acid bacteria, AMB = aerobic mesophilic bacteria, TC = total coliforms, ASFB = aerobic spore forming bacteria.
3.4. Identification of Dominant Lactic Acid Bacteria
A total of 171 microbial isolates were isolated from Shameta samples (both laboratory-prepared and those collected from households of lactating mothers) (Table 4). Of the total strains, 73 were isolated from samples collected from households of lactating mothers, whereas 58 and 40 were isolated from laboratory-prepared barley-based and maize-based Shameta, respectively. Results of biochemical characterization of the isolates revealed that all the isolates were Gram-positive, non-motile, non-catalase producers. There were rods, cocci or cocci in tetrads. The biochemical test results confirmed that the dominant isolates were LAB, an observation that further strengthen the dominance of LAB in many cereal-based fermentations [41].

Table 4.
Morphological, physiological and biochemical characteristics of LAB isolated from Shameta samples.
As shown in Table 4, all (100%) isolates were capable of growing both at 32 °C and 37 °C, though none of them were tolerant to 10 °C. In the present results, all isolates were found to grow in 4% NaCl, but 84.21% did now grow at pH 9.6. This result is in agreement with the study on Fura samples by Owusu-Kwarteng et al. [83], although the data on growth temperature showed variations between the two results. Regarding gas production from glucose, all (100%) the isolates were homo-fermentative, contrary to earlier reports by Owusu-Kwarteng et al. [83] and Gebrelibanos [15], in which about 30 and 36% of the isolates were able to produce gas from glucose during biochemical characterization of Fura and Azo samples, respectively.
Based on the morphological and biochemical characteristics they displayed, LAB involved in Shameta fermentations were identified as members of the genera Lactobacillus, Pediococcus and Entrococcus. The isolates were dominated by Lactobacillus (74.85%), followed by Entrococcus (15.79%). Gebrelibanos [15] reported that the genus of Lactobacillus was the dominant (52%) genus in Azo samples. Among Lactobacillus spp., Lactobacillus fermentum and L. brevis have been reported to dominate the intermediate and final stages of the fermentation of fufu [84]; however, Lactobacillus spp. such as Lactobacillus buchnerii, Lactobacillus casei and Lactobacillus pentosus were among the dominant isolates in maize-based ogi [13]. In line with the present results, Lactobacilli dominated the lactic flora throughout the fermentation process of Borde [14], although Pediococcus species were predominant in the latter stage of corn dough fermentation [85]. In general, lactobacilli were the dominant LAB in the fermentation of Shameta, frequently accompanied by other genera contributing to the observed acidification of Shameta towards the end of fermentation, which indirectly contributed to the safety of the final product against acid-sensitive pathogens.
Lactobacillus is the genus that encompasses large number of GRAS species (Generally Recognized As Safe), and many of its strains are among the most important bacteria in food microbiology and are used as probiotics [86]. Enterococci are some of the lactic acid bacteria (LAB) of importance in foods for ripening and aroma development of certain traditional cheeses, ogi and sausages [87,88]. It is reported that Enterococci are used as probiotics, although they are important nosocomial pathogens that may cause bacteremia, endocarditis and other infections [86]. Like other lactic acid bacteria, Pediococcus strains are widely distributed in fermented foods, and the species are source-specific. Gebrelibanos [15] reported in Pediococcus from “Azo”, a condiment made of barley and endod leaves supplemented with different spices.
4. Conclusions
The results of this study indicated that LAB were the dominant microbial groups in all samples collected from households of lactating mothers. The detection of Enterobacteriaceae in same samples collected from households of lactating mothers with counts on the borderline of safety requirements is good indication of poor handling practices and post-production contamination at the household level in an otherwise acidic product. This calls for awareness development among the consumers to avoid possible proliferation of the potentially pathogenic microorganisms during storage and processing for consumption. On the other hand, in both laboratory-prepared barley-based Shameta and maize-based Shameta, LAB dominated the fermentation, followed by yeasts. In the courses of laboratory fermentation of barley and maize-based Shamata, AMB, Staphylococcus spp. and ASFB were detected until the end of fermentation, though with much lower counts than the two dominant groups (LAB and yeasts). However, Enterobacteriaceae, coliforms and molds failed to persist till the end of fermentation. Physicochemically, the pH of fermenting Shameta kept on dropping till the end of fermentation, and there was a corresponding rise in titratable acidity, determining the microbial profile of the final product. It is worth considering that spontaneous fermentation contributes to the growth of pathogenic bacteria, especially in the early stages of fermentation. The detection of Staphylococcus spp. in samples collected from households of lactating mothers and its persistence to the end of spontaneously fermented Shameta calls for proper hygienic practices, such as switching to controlled fermentation of the product using defined starter cultures. Proper selection of bacteria and yeast for fermentation will lower the risk of infection and diseases in mothers and babies. In general, the microbiology of Shameta fermentation was dominated by Lactobacillus, followed by Enterococcus (among LAB) and yeasts. These genera could be candidate starter cultures for large-scale production of nutritious and flavored Shameta that preserves better. Detailed characterization and molecular identification of the dominant genera are recommended before optimization and scaling up of the product.
Author Contributions
Conceptualization, D.A.K., K.B., Y.B.T., M.M., S.G. and M.G.; data curation, D.A.K., K.B. and Y.B.T.; formal analysis, D.A.K., K.B., Y.B.T., M.M., S.G. and M.G.; funding acquisition, Y.B.T. and M.M.; investigation, D.A.K., K.B., Y.B.T., S.G. and M.G.; methodology, D.A.K., K.B., Y.B.T., M.M., S.G. and M.G.; project administration, K.B. and Y.B.T.; resources, K.B., Y.B.T. and S.G.; supervision, K.B., Y.B.T., M.M. and M.G.; validation, D.A.K., K.B., Y.B.T. and S.G.; writing—original draft, D.A.K.; writing—review & editing, D.A.K., K.B., Y.B.T., M.M., S.G. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during the study are included in the manuscript.
Acknowledgments
The authors acknowledge Jimma University for allowing us to use the laboratory facilities and for the financial support of the research. We also gratefully acknowledge lactating mothers who shared samples for the laboratory study and the health workers involved during sample collection.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maria, L.M.; Dustin, H.; Sylvie, B.; Christopher, J.C.; Paul, D.C.; Benoit, F.; Michael, G.; Remco, K.; Gonca, P.; Anne, P.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar]
- Tou, E.H.; Mouquet-River, C.; Rochette, I.; Traoré, A.S.; Treche, S.; Guyot, J.P. Effect of different process combinations on the fermentation kinetics, microflora and energy density of ben-saalga, a fermented gruel from Burkina Faso. Food Chem. 2007, 100, 935–943. [Google Scholar] [CrossRef]
- Abegaz, K.; Beyene, F.; Thor, L.; Judith, A.N. Indigenous processing methods and raw materials of borde: An Ethiopian traditional fermented beverage. Afr. J. Food Sci. 2002, 7, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Binitu, B.; Zewdu, A.; Fekadu, H. Indigenous processing methods of cheka: A traditional fermented beverage in Southwestern Ethiopia. J. Food Process Technol. 2015, 7, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Holzapfel, W.H. Appropriate starter culture technologies for small-scale fermentation in developing countries. Int. J. Food Microbiol. 2002, 75, 197–212. [Google Scholar] [CrossRef]
- Mukuma, M.M. Microbiological quality and safety of the Zambian fermented cereal beverage: Chibwantu. Ph.D. Thesis, University of the Free State, Bloemfontein, South Africa, 2014. [Google Scholar]
- Katina, K.; Poutanen, K. Nutritional aspects of cereal fermentation with lactic acid bacteria and yeast. In Handbook on Sourdough Biotechnology; Gobbetti, M., Gänzle, M., Eds.; Springer: New York, NY, USA, 2013; pp. 229–244. [Google Scholar]
- Ukwuru, M.U.; Muritala, A.; Ukpomwan, S. Ecology of traditional cereal fermentation. UPI J. Chem. Life Sci. 2018, 1, 22–36. [Google Scholar]
- Admassie, M. A Review on food fermentation and the biotechnology of lactic acid bacteria. World J. Food Sci. Technol. 2018, 2, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Braide, W.; Azuwike, C.O.; Adeleye, S.A. The role of microorganisms in the production of some indigenous fermented foods in Nigeria. Int. J. Adv. Res. Biol. Sci. 2018, 5, 86–92. [Google Scholar]
- Giraffa, G. Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol. Rev. 2004, 28, 251–260. [Google Scholar] [CrossRef] [Green Version]
- Nsofor, C.A.; Ume, S.C.; Uzor, B.C. Isolation and characterization of lactic acid bacteria from ogi sold in Elele. Niger. J. Biol. Food Sci. Res. 2014, 3, 19–22. [Google Scholar]
- Mobolaji, A.O.; Ifeoma, U.O.; Obadina, A.O.; Bankole, M.O.; Ayofemi, S.O.A. Microbiological assessment of maize ogi co-fermented with pigeon pea. Food Sci. Nutr. 2018, 6, 1238–1253. [Google Scholar]
- Bacha, K.; Tetemke, M.; Ashenafi, M. The microbial dynamics of “borde” fermentation: A traditional Ethiopian fermented beverage. SINET Ethiop. J. Sci. 1998, 21, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Gebrelibanos, L. Microbiological and Physicochemical Study of Azo: A Traditional Fermented Condiment Prepared from Sorghum and Leaves of Endod (Phytolacca dodecandra) in Kafta Humera, Tigray Regional State. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2015. [Google Scholar]
- Safaye, H.A. Isolation and Characterization of the Dominant Yeast in the Traditional Beverages of Ethiopia; Tella and Tej. Master’s Thesis, Addis Ababa University, Addis Ababa, Ethiopia, 2011. [Google Scholar]
- Getnet, B.; Minyamer, T.; Masresha, G. The microbial profile and contaminants of bubugn: An Ethiopian house hold fermented beverage. Int. J. Biotechnol. 2018, 7, 17–24. [Google Scholar]
- Bacha, K.; Jonsson, H.; Ashenafi, M. Microbial Dynamics during the fermentation of wakalim: A traditional Ethiopian fermented sausage. J. Food Qual. 2010, 33, 370–390. [Google Scholar] [CrossRef]
- Central Statistical Agency (CSA). Population and Housing Census in Ethiopia. International Household Survey Network. Home/Central Data Catalog/ETH_2007_PHC_V01_M. 2007. Available online: https://microdata.worldbank.org/index.php/catalog/2747 (accessed on 11 February 2022).
- Degefa, K.; Biru, G.; Abebe, G. Farming system characterization and analysis of East Wollega Zone, Oromia, Ethiopia. Int. J. Manag. Fuzzy Syst. 2020, 6, 14–28. [Google Scholar] [CrossRef]
- Abegaz, K. Isolation, characterization and identification of lactic acid bacteria involved in traditional fermentation of borde: An Ethiopian cereal-based beverage. Afr. J. Biotechnol. 2007, 6, 1469–1478. [Google Scholar]
- Antony, U.; Chandra, T. Microbial Population and biochemical changes in fermenting finger millet (Eleusine coracana). World J. Microbiol. Biotechnol. 1997, 13, 533–537. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis of the AOAC, 18th ed.; AOAC International: Washington, DC, USA, 2000. [Google Scholar]
- Mugula, J.K.; Nnko, S.A.M.; Sorhaug, T. Changes in quality attributes during storage of togwa: A lactic acid fermented gruel. J. Food Saf. 2001, 21, 181–194. [Google Scholar] [CrossRef]
- Todd, M.S.; Elliot, T.R.; Catherine, W.D. Comparison of methods for determining coliform and Escherichia coli levels in apple cider. J. Food Prot. 1997, 4, 1302–1471. [Google Scholar]
- Soda, M.E.; Ahmed, N.; Omran, N.; Osman, G.; Morsi, A. Isolation, identification and selection of lactic acid bacteria cultures for cheese making. Emir. J. Agric. Sci. 2003, 15, 51–71. [Google Scholar] [CrossRef]
- Nemo, R.; Bacha, K. Microbial, physico-chemical and proximate analysis of selected Ethiopian traditional fermented beverage. LWT Food Sci. Technol. 2020, 131, 109713. [Google Scholar] [CrossRef]
- Idris, A.; Mehari, T.; Ashenafi, M. Some microbiological and biochemical studies on the fermentation of “awaze” and “datta”: A traditional Ethiopian condiment. Int. J. Food Sci. Nutr. 2001, 52, 5–14. [Google Scholar] [PubMed]
- Nikita, C.; Hemangi, D. Isolation, identification and characterization of lactic acid bacteria from dairy sludge sample. J. Environ. Res. Dev. 2012, 7, 234–244. [Google Scholar]
- Okoro, I.A.; Ojimelukwe, P.C.; Ekwenye, U.N.; Akaerue, B.; Atuonwu, A.C. Quality characteristics of indigenous fermented beverage; pito using Lactobacillus sake as a starter culture. Cont. J. Appl. Sci. 2011, 6, 15–20. [Google Scholar]
- Onyeagba, A.C. Laboratory Guide for Microbiology, 1st ed.; Crystal Publishers: Okigwe, Nigeria, 2004; pp. 75–94. [Google Scholar]
- Kimaryo, V.; Massawe, G.; Olasupo, N.; Holzapfel, W. The use of starter culture in the fermentation of cassava for the production of “kivunde”: A traditional Tanzanian food product. Int. J. Food Microbiol. 2000, 56, 179–190. [Google Scholar] [CrossRef]
- Seeley, H.W.; Vandemark, P.J. Microbes in Action—A Laboratory Manual of Microbiology; D.B. Taraporevala Sons and Company Pvt. Ltd.: Mumbai, India, 1970; Volume 39, pp. 85–86. [Google Scholar]
- Chowdhury, A.; Raju, K.K.; Kalurupalle, S.; Tharun, S. Both Sm-domain and C-terminal extension of Lsm1 are important for the RNA-binding Activity of the Lsm1-7-Pat1 Complex. RNA 2014, 20, 1465–1475. [Google Scholar] [CrossRef] [Green Version]
- Hasan, M.N.; Sultan, M.Z.; Mareum, M. Significance of fermented food in nutrition and food science. J. Sci. 2014, 6, 373–386. [Google Scholar] [CrossRef]
- Garbutt, J. Essentials of Food Microbiology; Hodder Headline Group: London, UK, 1997; pp. 116–170. [Google Scholar]
- Battcock, M.; Azam-Ali, S. Basic Principles of Fermentation. In Fermented Fruits and Vegetables: A Global Perspective; FAO Agricultural Services Bulletin No. 134; FAO: Rome, Italy, 1998; Chapter 2. [Google Scholar]
- Binitu, B.W.; Fekadu, H.G.; Zewdu, A.W. Nutritional and alcoholic contents of cheka: A traditional fermented beverage in Southwestern Ethiopia. Food Sci. Nutr. 2018, 6, 2466–2472. [Google Scholar] [CrossRef]
- Ashenafi, M.; Mehari, T. Some microbiological and nutritional properties of “borde” and “shamita, traditional Ethiopian fermented beverages. Ethiop. J. Health Dev. 1995, 9, 105–110. [Google Scholar]
- Lechiancole, T.; Ricciardi, A.; Parente, E. Optimization of media and fermentation conditions for the growth of Lactobacillus sakei. Ann. Microbiol. 2002, 52, 257–274. [Google Scholar]
- Liptáková, D.; Matejčeková, Z.; Valík, Ľ. Lactic acid bacteria and fermentation of cereals and Pseudocereals. In Fermentation Processes; InTech: London, UK, 2017; pp. 224–242. [Google Scholar]
- Asfaw, D. Review on use of bioactive compounds in some spices in food preservation. Food Sci. Qual. Manag. 2019, 87, 6–13. [Google Scholar]
- Chibuike, A.O.; Ify, D.A.; Ejike, M.O.; Awache, I.; James, L.A. Study on the bacteriological quality of fura sold in Wukari, North-East Nigeria. Food Microbiol. 2018, 2, 24–29. [Google Scholar]
- Ogodo, A.C.; Ugbogu, O.C.; Agwaranze, D.I.; Ihiabe, F.U. Some studies on the bacteriological quality of sorghum-based commercially prepared fermented Ogi (Akamu) in Wukari, Nigeria. Am. J. Food Sci. Nutr. 2017, 4, 48–51. [Google Scholar]
- Centre for Food Safety (CFS). Microbiological Guidelines for Food: Ready-to-Eat food in General and Specific Food Items; Centre for Food Safety, Food and Environmental Hygiene Department: Hong Kong, China, 2014. Available online: microbio-guide2014-cvr_E(cfs.gov.hk) (accessed on 20 January 2022).
- Cox, L.J.; Keller, N.; Vanschothorst, M. The use and misuse of quantitative determinations of Enterobacteriaceae in Food Microbiology. J. Appl. Bacteriol. Symp. Suppl. 1988, 17, 237S–249S. [Google Scholar] [CrossRef]
- Samuel, C.T. Street food safety, types and microbiological quality in Ethiopia: A Critical review. Am. J. Appl. Sci. Res. 2020, 6, 67–71. [Google Scholar]
- Martin, N.H.; Trmcic, A.; Hsieh, T.; Boor, K.J.; Wiedmann, M. The evolving role of coliforms as indicators of unhygienic processing conditions in dairy foods. Front. Microbial. 2016, 7, 1549. [Google Scholar] [CrossRef]
- Food Standards Australia New Zealand (FSANZ). Compendium of Microbiological Criteria for Food; Food Standards Australia New Zealand, the Terrace: Wellington, New Zealand, 2016; pp. 1–51.
- Reed, G.H. Foodborne illness (part 8): Escherichia coli. Dairy Food Environ. San. 1994, 14, 329–330. [Google Scholar]
- Ray, B. Fundamental Food Microbiology, 3rd ed.; CRC Press: New York, NY, USA, 2004; pp. 359–390. [Google Scholar]
- Ogodo, A.C.; Agwaranze, D.I.; Nwaneri, C.B.; Okoronkwo, U.C.; Ekeleme, U.G. Activity of leave and stem bark cuttings of Ocimum gratissimum extracts on foodborne pathogens. AASCIT J. Biosci. 2017, 3, 5–11. [Google Scholar]
- Tewari, A.; Abdullah, S. Bacillus cereus food poisoning: International and Indian perspective. J. Food Sci. Technol. 2015, 52, 2500–2511. [Google Scholar] [CrossRef] [Green Version]
- Lücking, G.; Stoeckel, M.; Atamer, Z.; Hinrichs, J. Characterization of aerobic spore-forming bacteria associated with industrial dairy processing environments and product spoilage. Int. J. Food Microbiol. 2013, 166, 270–279. [Google Scholar] [CrossRef]
- Health Protection Agency (HPA). Guidelines for Assessing the Microbiological Safety of Ready-to-Eat Foods; Health Protection Agency: London, UK, 2009; pp. 8–10.
- Food Safety Authority of Ireland (FSAI). Guidelines for the Interpretation of Results of Microbiological Testing of Ready-to-Eat Foods Placed on the Market; Revision 4; Guidance Note; European Comission: Brussels, Belgium, 2020. [Google Scholar]
- Rawat, S. Food Spoilage: Microorganisms and their prevention. Asian J. Plant Sci. Res. 2015, 5, 47–56. [Google Scholar]
- Ogbulie, J.N. Production of Tempeh from African Yam Bean (Sphenostylis stenocarpa Harms). Master’s Thesis, University of Port Harcourt, Port Harcourt, Nigeria, 1991; p. 33. [Google Scholar]
- Olumide, A.; Odeyemi, O.; Oluwaseun, A.; Mariyana, S.; Deyan, S. Understanding spoilage microbial community and spoilage mechanisms in foods of animal origin. Compr. Rev. Food Sci. Food Saf. 2020, 19, 311–331. [Google Scholar]
- Mohammed, S.I.; Steenson, L.R.; Kirleis, A.W. Isolation and characterization of microorganisms associated with the traditional sorghum fermentation for production of Sudanese kisra. Appl. Environ. Microbiol. 1991, 57, 2529–2533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogbonnaya, N.; Bernice, C.C. Studies on Akamu: A Traditional fermented maize food. Rev. Chil. Nutr. 2012, 39, 180–184. [Google Scholar]
- Minton, P.E. Handbook of Evaporation Technology; Noyes Publications: West Wood, NJ, USA, 1986. [Google Scholar]
- Berggren, S.; Hedren, E.; Edman, K. Water Holding Capacity and Viscosity of Ingredients from Oats. The Effect of Beta-Glucan and Starch Content, Particle Size, pH and Temperature; Linnaeus University: Växjö, Sweden, 2017. [Google Scholar]
- Halm, M.; Lillie, A.; Sorensen, A.K.; Jakobsen, M. Microbiological and aromatic characteristics of fermented maize doughs for kenkey production in Ghana. Int. J. Food Microbiol. 1993, 19, 135–143. [Google Scholar] [CrossRef]
- Alonso-Calleja, C.; Martínez-Fernández, B.; Prieto, M.; Capita-González, R. Microbiological quality of vacuum-packed retail ostrich meat in Spain. Food Microbiol. 2004, 21, 241–246. [Google Scholar] [CrossRef]
- Almeida, E.G.; Rachid, C.C.; Schwan, R.F. Microbial population present in fermented beverage ‘cauim’ produced by Brazilian Amerindians. Int. J. Food Microbiol. 2007, 120, 146–151. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Hand Book of Indigenous Fermented Foods; Revised and Expanded; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Fredlund, E.; Druvefors, U.Ä.; Olstorpe, M.N.; Passoth, V.; Schnürer, J. Influence of ethyl acetate production and ploidy on the anti-mould activity of Pichia anomala. FEMS Microbiol. Lett. 2004, 238, 133–137. [Google Scholar]
- Chelule, P.; Mokoena, M.; Gqaleni, N. Advantages of Traditional Lactic Acid Bacteria Fermentation of Food in Africa; Current Research, Technology and Education Topics in Applied Microbiology and Biotechnology; Mendez–Vilas, A., Ed.; FORMATEX Microbiology Series; Formatex Research Center: Badajoz, Spain, 2010; Volume 2. [Google Scholar]
- Anteneh, T.; Tetemke, M.; Mogessie, A. Antagonism of lactic acid bacteria against food borne pathogens during fermentation and storage of borde and shameta: Traditional Ethiopian fermented beverages. Int. Food Res. J. 2011, 18, 1189–1194. [Google Scholar]
- Achi, O.K.; Ukwuru, M. Cereal-Based fermented foods of Africa as functional foods. Int. J Microbiol. Appl. 2015, 2, 71–83. [Google Scholar]
- Ogodo, A.C.; Ugbogu, O.C.; Ugbogu, A.E.; Ezeonu, C.S. Production of mixed fruit (pawpaw, banana and watermelon) wine using Saccharomyces cerevisiae isolated from palm wine. Springer Plus 2015, 4, 683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrano, M.; Dutta, B.; Mercer, D.G.; MacLean, H.L.; Touchie, M.F. Assessment of moisture content measurement methods of dried food products in small-scale operations in developing countries: A review. Trends Food Sci. Technol. 2019, 88, 484–496. [Google Scholar] [CrossRef]
- Nout, M.J.R.; Rombouts, F.M.; Hautfast, G.J. Accelerated natural lactic fermentation of infant food formulations. Food Nutr. Bull. 1989, 11, 65–73. [Google Scholar] [CrossRef]
- Odunfa, S.A. African fermented foods. In Microbiology of Fermented Foods; Wood, B.J., Ed.; Elsevier Applied Science Publishers: London, UK; New York, NY, USA, 1985; Volume 2, pp. 155–199. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology, 7th ed.; Springer Science and Business Media Inc.: Los Angeles, CA, USA, 2005; pp. 125–143. [Google Scholar]
- Jespersen, I.; Halm, M.; Kpodo, K.; Jakobsen, M. Significance of yeasts and moulds occurring in maize dough fermentation for kenkey production. Int. J Food Microbiol. 1994, 24, 239–248. [Google Scholar] [CrossRef]
- Omemu, A.M.; Oyewole, O.B.; Bankole, M.O. Significance of yeasts in the fermentation of maize for ogi production. Food Microbiol. 2007, 24, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Omemu, A.M. Fermentation dynamics during production of ogi: A Nigerian fermented cereal porridge. Rep. Opin. 2011, 3, 8–17. [Google Scholar]
- Nout, M.J.R.; Aidoo, K.E. Asian Fungal Fermented Food; Osiewacz, H.D., Ed.; The Mycota X Industrial Applications; Springer: Berlin/Heidelberg, Germany, 2002; pp. 23–47. [Google Scholar]
- Thapa, S.; Tamang, J.P. Product characterization of kodo ko jaanr: A fermented finger millet beverage of the Himalayas. Food Microbiol. 2004, 21, 617–622. [Google Scholar] [CrossRef]
- Petrova, P.; Petrov, K.; Stoyancheva, G. Starch-modifying enzymes of lactic acid bacteria—Structures, Properties and Applications. Starch-Stärke 2013, 65, 34–47. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Tano-Debrah, K.; Glover, R.L.K.; Akabanda, F. Process characteristics and microbiology of fura produced in Ghana. J. Nat. Sci. 2010, 8, 41–51. [Google Scholar]
- Adegoke, G.O.; Babaola, A.K. Characteristics of microorganisms of importance in the fermentation of fufu and ogi: The two Nigerian fermented foods. J. Appl. Bacteriol. 1988, 65, 449–453. [Google Scholar] [CrossRef]
- Nche, P.F.; Nout, M.J.R.; Rombouts, F.M. Effect of cowpea supplementation on the quality of kenkey: A traditional Ghanaian fermented food. J. Cereal Sci. 1994, 19, 191–197. [Google Scholar] [CrossRef]
- Lukjancenko, O.; Ussery, D.W.; Wassenaar, T.M. Comparative genomics of Bifidobacterium, Lactobacillus and related probiotic genera. Microbiol. Ecol. 2012, 63, 651–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, C.M.A.P.; Stiles, M.E.; Schleifer, K.H.; Holzapfel, W.H. Enterococci in foods: A Conundrum for food safety. Int. J. Food Microbiol. 2003, 88, 105–122. [Google Scholar] [CrossRef]
- Olatunde, O.O.; Obadina, A.O.; Omemu, A.M.; Oyewole, O.B.; Olugbile, A.; Olukomaiya, O.O. Screening and molecular identification of potential probiotic lactic acid bacteria in effluents generated during ogi production. Ann. Microbiol. 2018, 68, 433–443. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).