Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis
Abstract
:1. Introduction
2. Materials and Methods
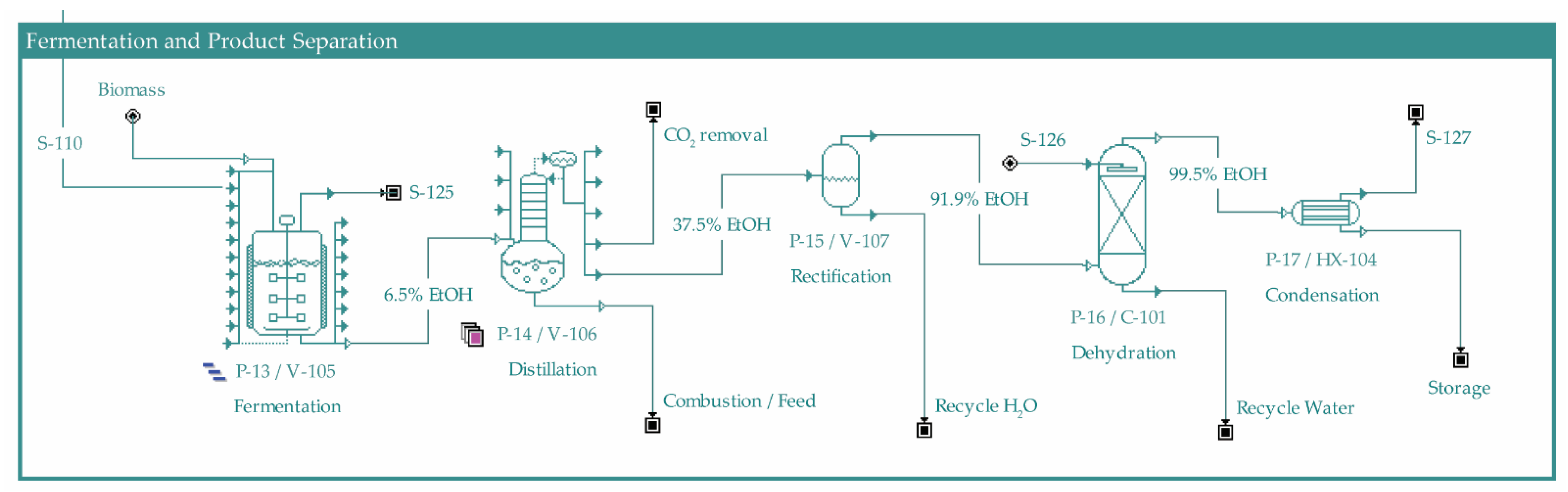
2.1. Process Overview
2.2. Process Design
3. Results and Discussion
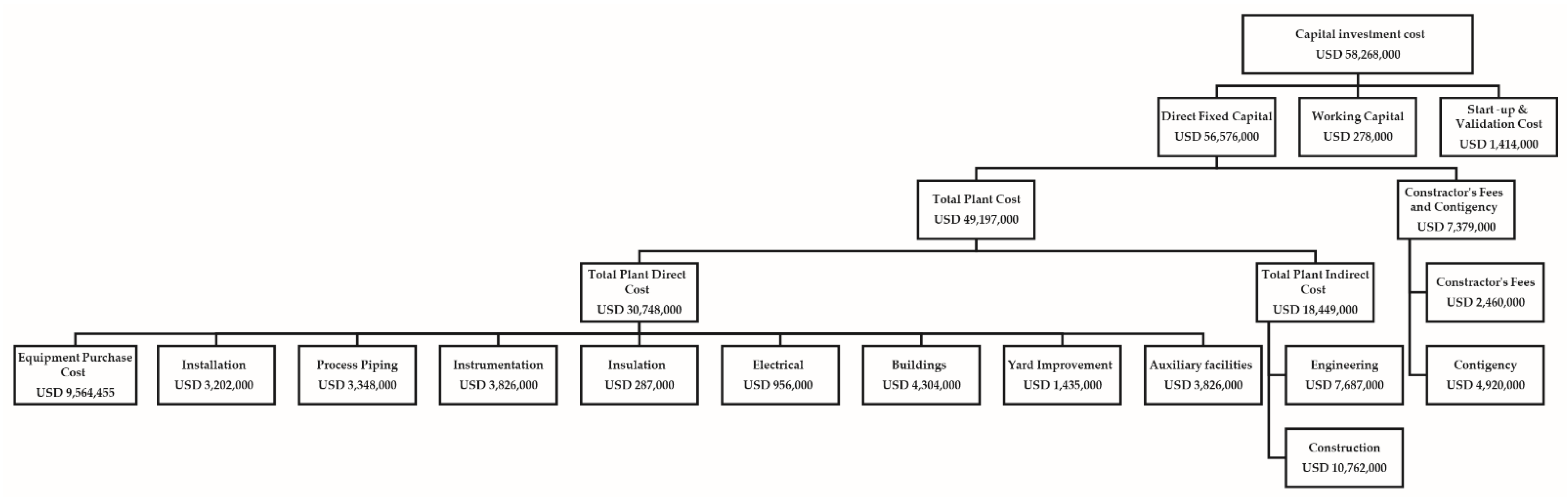
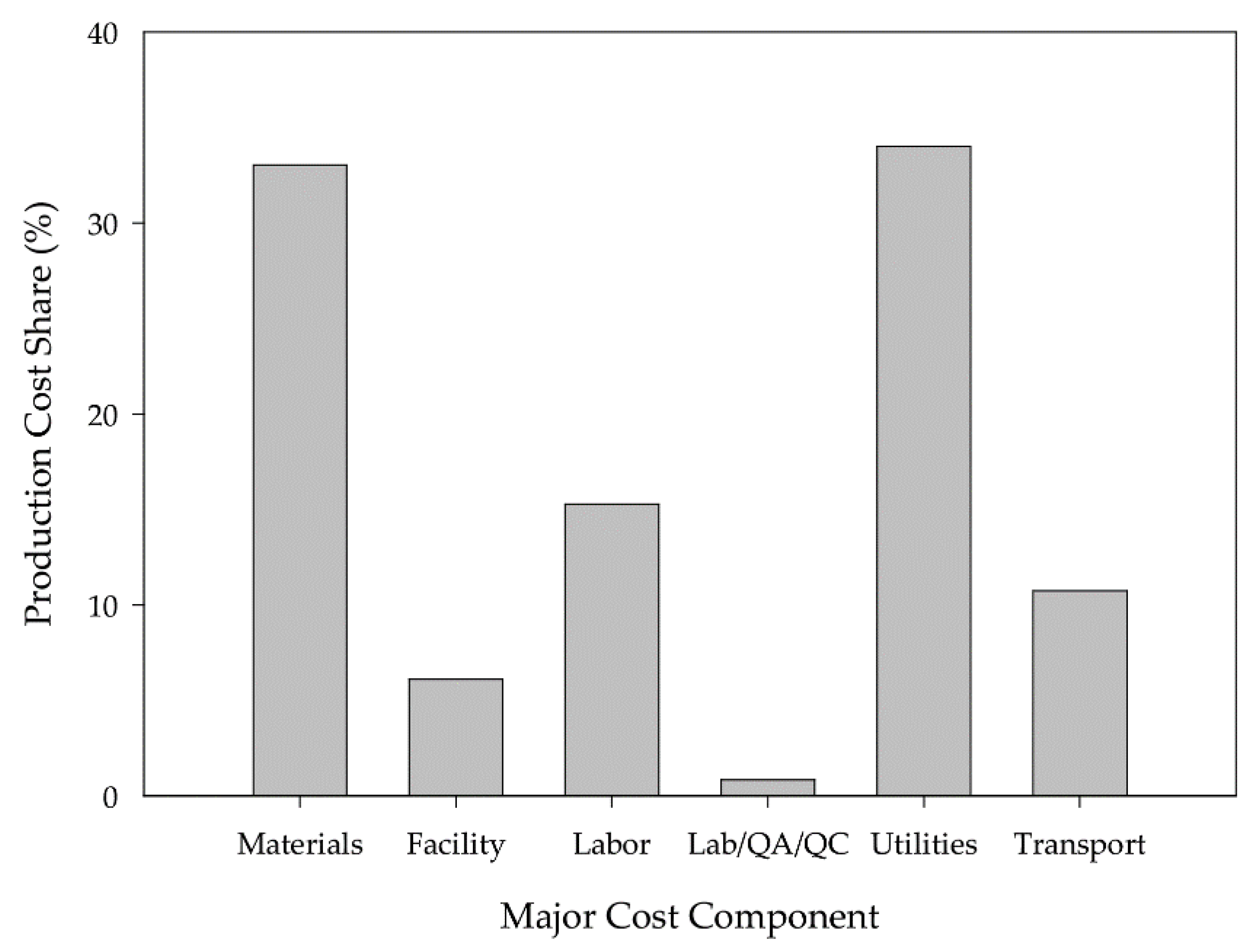
3.1. Economic Analysis
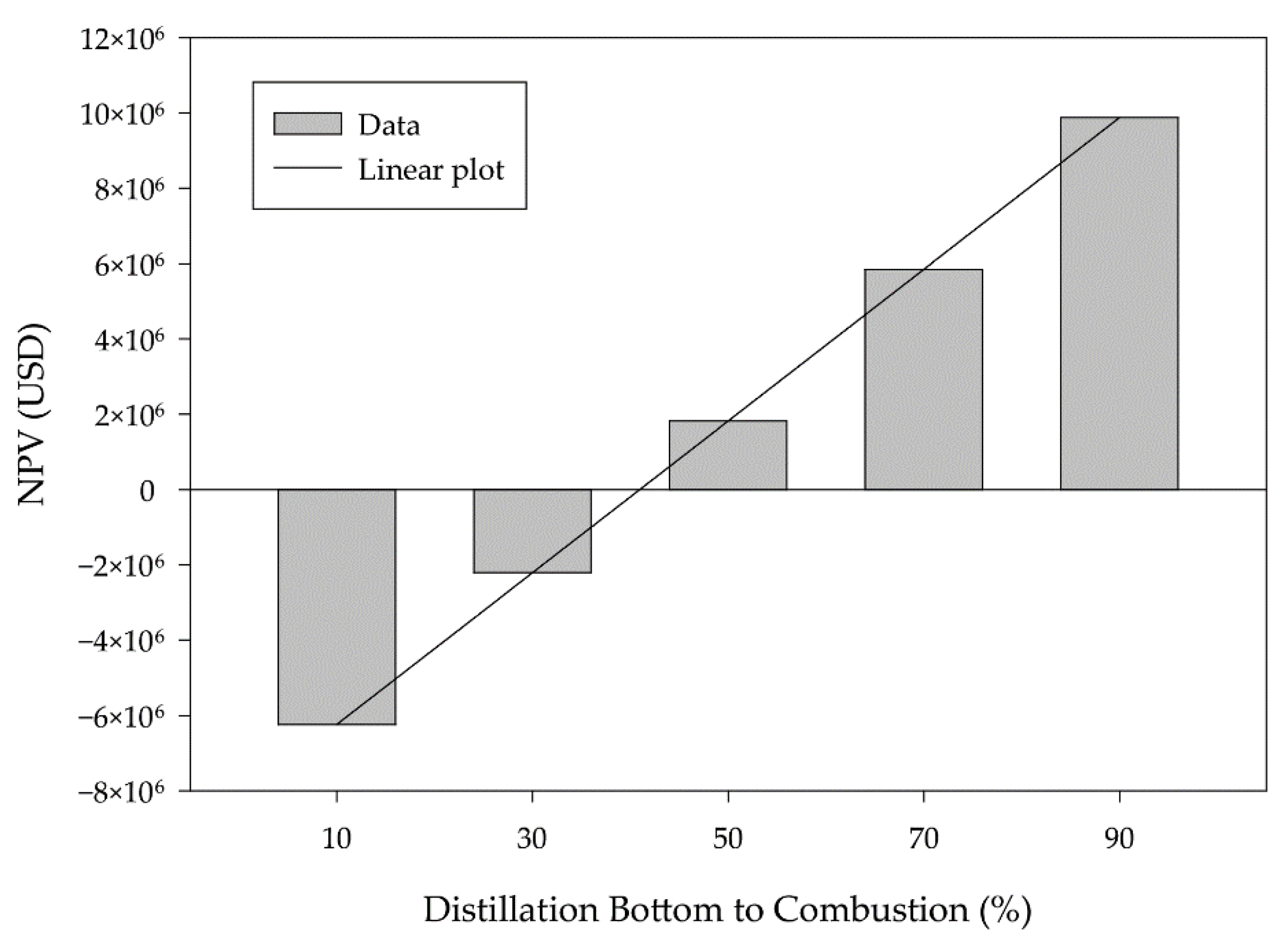
3.2. Scenario and Sensitivity Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alexandri, M.; López-Gómez, J.P.; Olszewska-Widdrat, A.; Venus, V. Valorising Agro-industrial Wastes within the Circular Bioeconomy Concept: The Case of Defatted Rice Bran with Emphasis on Bioconversion Strategies. Fermentation 2020, 6, 42. [Google Scholar] [CrossRef]
- Martins, F.; Felgueiras, C.; Smitkova, M.; Caetano, N. Analysis of Fossil Fuel Energy Consumption and Environmental Impacts in European Countries. Energies 2019, 12, 964. [Google Scholar] [CrossRef] [Green Version]
- Dodić, S.; Vučurović, D.; Popov, S.; Dodić, J.; Zavrago, Z. Concept of cleaner production in Vojvodina. Renew. Sust. Energ. Rev. 2010, 14, 1629–1634. [Google Scholar] [CrossRef]
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268. [Google Scholar] [CrossRef]
- Branco, R.H.R.; Serafim, L.S.; Xavier, A.M.R.B. Second Generation Bioethanol Production: On the Use of Pulp and Paper Industry Wastes as Feedstock. Fermentation 2019, 5, 4. [Google Scholar] [CrossRef] [Green Version]
- Directive (Eu) 2018/2001 of the European Parliament and of the Council, Official Journal of the European Union. 2018. Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 29 December 2021).
- Energy Sector Development Strategy of the Republic of Serbia until 2025 with Projections until 2030, Official Gazette of RS, No. 101/2015, Belgrade. 2015. Available online: https://www.pravno-informacioni-sistem.rs/SlGlasnikPortal/eli/rep/sgrs/skupstina/ostalo/2015/101/1/r (accessed on 29 December 2021).
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Ning, P.; Yang, G.; Hu, L.; Sun, J.; Shi, L.; Zhou, Y.; Wang, Z.; Yang, J. Recent advances in the valorization of plant biomass. Biotechnol. Biofuels 2021, 14, 102. [Google Scholar] [CrossRef]
- Rezić, T.; Oros, D.; Marković, I.; Kracher, D.; Ludwig, R.; Šantek, B. Integrated Hydrolyzation and Fermentation of Sugar Beet Pulp to Bioethanol. J. Microbiol. Biotechnol. 2013, 23, 1244–1252. [Google Scholar] [CrossRef] [Green Version]
- Gumienna, M.; Szambelan, K.; Jeleń, H.; Czarnecki, Z. Evaluation of ethanol fermentation parametersfor bioethanol production from sugar beet pulp and juice. J. Inst. Brew. 2014, 120, 543–549. [Google Scholar] [CrossRef]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Renew. Sust. Energ. Rev. 2013, 17, 77–93. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef] [PubMed]
- Awoyale, A.A.; Lokhat, D. Experimental determination of the effects of pretreatment on selected Nigerian lignocellulosic biomass in bioethanol production. Sci. Rep. 2021, 11, 557. [Google Scholar] [CrossRef] [PubMed]
- Heinzle, E.; Biwer, A.P.; Cooney, C.L. Development of Sustainable Bioprocesses: Modeling and Assessment; John Wiley & Sons: Chichester, UK, 2006. [Google Scholar]
- Sokolowski, J.A.; Banks, C.M. Principles of Modeling and Simulation a Multidisciplinary Approach; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Kumar, D.; Murthy, G.S. Impact of pretreatment and downstream processing technologies on economics and energy in cellulosic ethanol production. Biotechnol. Biofuels. 2011, 4, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mupondwa, E.; Li, X.; Tabil, L. Integrated bioethanol production from triticale grain and lignocellulosic straw in Western Canada. Ind. Crops Prod. 2018, 117, 75–87. [Google Scholar] [CrossRef]
- Barrera, I.; Amezcua-Allieri, M.A.; Estupinan, L.; Martínez, T.; Aburto, J. Technical and economical evaluation of bioethanolproduction from lignocellulosic residues in Mexico:Case of sugarcane and blue agave bagasses. Chem. Eng. Res. Des. 2016, 107, 91–101. [Google Scholar] [CrossRef]
- Abdullah, S.S.S.; Shirai, Y.; Ali, A.A.M.; Mustapha, M.; Hassan, M.A. Case study: Preliminary assessment of integrated palm biomass biorefinery for bioethanol production utilizing non-food sugars from oil palm frond petiole. Energy Convers. Manag. 2016, 108, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.D.; Guigou, M.; Lareo, C. Energy consumption evaluation of fuel bioethanol production from sweet potato. Bioresour. Technol. 2013, 136, 377–384. [Google Scholar] [CrossRef]
- Vučurović, D.; Dodić, S.; Popov, S.; Dodić, J.; Grahovac, J. Process model and economic analysis of ethanol production from sugar beet raw juice as part of the cleaner production concept. Bioresour. Technol. 2012, 104, 367–372. [Google Scholar] [CrossRef]
- Statistical Yearbook of the Republic of Serbia; Statistical Office of the Republic of Serbia: Belgrade, Serbia, 2021.
- Hutnan, M.; Drtil, M.; Mrafkova, L. Anaerobic biodegradation of sugar beet pulp. Biodegradation 2000, 11, 203–211. [Google Scholar] [CrossRef]
- Ivetić, D.; Šćiban, M.; Antov, M. Enzymatic hydrolysis of pretreated sugar beet shreds: Statistical modeling of the experimental results. Biomass Bioenergy 2012, 47, 387–394. [Google Scholar] [CrossRef]
- Rana, A.K.; Gupta, V.K.; Newbold, J.; Roberts, D.; Rees, R.M.; Krishnamurthy, S.; Thakur, V.K. Sugar beet pulp: Resurgence and trailblazing journey towards a circular bioeconomy. Fuel 2022, 312, 122953. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Oz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Zheng, Y.; Yu, C.; Cheng, Y.S.; Zhang, R.; Jenkins, B.; VanderGheynst, J.S. Effects of ensilage on storage and enzymatic degradability of sugar beet pulp. Bioresour. Technol. 2011, 102, 1489–1495. [Google Scholar] [CrossRef]
- Vohra, M.; Manwar, J.; Manmode, R.; Padgilwar, S.; Patil, S. Bioethanol production: Feedstock and current technologies. J. Environ. Chem. Eng. 2014, 2, 573–584. [Google Scholar] [CrossRef]
- Asadi, M. Beet-Sugar Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Marzo, C.; Diaz, A.B.; Caro, I.; Blandino, A. Conversion of Exhausted Sugar Beet Pulp into Fermentable Sugars from a Biorefinery Approach. Foods 2020, 9, 1351. [Google Scholar] [CrossRef] [PubMed]
- Bajić, B.; Vučurović, D.; Dodić, S.; Grahovac, J.; Dodić, J. Process model economics of xanthan production from confectionery industry wastewaters. J. Environ. Manag. 2017, 203, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Power Industry of Serbia, Annual Report for 2020. Available online: Epsdistribucija.rs/pdf/GI_2020.pdf (accessed on 29 December 2021).
- Global Petrol Prices. Available online: https://www.globalpetrolprices.com/ethanol_prices/ (accessed on 29 December 2021).


| Reaction | Referent Component | Reaction Extent (%) |
|---|---|---|
| Cellulose + nWater → nGlucose | Cellulose | 7.7 |
| Cellulose + 1/2nWater → 1/2nCellobiose | Cellulose | 0.7 |
| Hemicellulose + nWater → nXylose | Xylan | 92.5 |
| (Lignin)n → nSoluble Lignin | Hemicellulose | 5 |
| Reaction | Referent Component | Reaction Extent (%) |
|---|---|---|
| Cellulose + 1/2nWater → 1/2n Cellobiose | Cellulose | 1.2 |
| Cellulose + nWater → nGlucose | Cellulose | 90 |
| Cellobiose + Water → 2Glucose | Cellobiose | 100 |
| Reaction | Referent Component | Reaction Extent (%) |
|---|---|---|
| Glucose → 2Bioethanol + 2Carbon dioxide | Glucose | 90 |
| Glucose + 5.7Other compounds → 6Biomass + 2.87Oxygen + 2.4Water | Glucose | 4 |
| Glucose + 2Water → 2Glycerol + Oxygen | Glucose | 0.4 |
| Glucose + 2 Carbon dioxide → Oxygen + 2Succinic acid | Glucose | 0.6 |
| Glucose → 3Acetic acid | Glucose | 1.5 |
| Glucose → 2 Lactic acid | Glucose | 0.2 |
| 3Xylose → 5Bioethanol + 5 Carbon dioxide | Xylose | 80 |
| Xylose + 4.67 Other compounds → 5Biomass + 2.35Oxygen + 2Water | Xylose | 4 |
| 3Xylose + 5Water → 5Glycerol + 2.5Oxygen | Xylose | 0.3 |
| Xylose + Water → Xylitol + 0.5 Oxygen | Xylose | 4.6 |
| 3Xylose + 5Carbon dioxide → 2.5Oxygen + 5Succinic acid | Xylose | 0.9 |
| 2 Xylose → 5Acetic acid | Xylose | 1.4 |
| 3 Xylose → 5Lactic acid | Xylose | 0.2 |
| Project Indices | Combustion Scenario | Animal Feed Scenario |
|---|---|---|
| Gross margin (%) | 69.11 | 61.05 |
| Return on investment (%) | 16.08 | 11.27 |
| Payback time (years) | 6.22 | 8.88 |
| Internal rate of return (%) | 10.22 | 4.55 |
| Net present value at 7.00% (USD) | 11,887,591 | −8,252,807 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vučurović, D.; Bajić, B.; Vučurović, V.; Jevtić-Mučibabić, R.; Dodić, S. Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation 2022, 8, 114. https://doi.org/10.3390/fermentation8030114
Vučurović D, Bajić B, Vučurović V, Jevtić-Mučibabić R, Dodić S. Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation. 2022; 8(3):114. https://doi.org/10.3390/fermentation8030114
Chicago/Turabian StyleVučurović, Damjan, Bojana Bajić, Vesna Vučurović, Rada Jevtić-Mučibabić, and Siniša Dodić. 2022. "Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis" Fermentation 8, no. 3: 114. https://doi.org/10.3390/fermentation8030114
APA StyleVučurović, D., Bajić, B., Vučurović, V., Jevtić-Mučibabić, R., & Dodić, S. (2022). Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation, 8(3), 114. https://doi.org/10.3390/fermentation8030114









