Abstract
(1) Background: The microbial phase in the vaginal environment has been found to regulate the physiological activity of host cells. Studies have demonstrated that abnormal microbial growth in the vagina and a significant reduction in the proportion of lactic acid bacteria promote the occurrence of spontaneous preterm birth (sPTB). However, the contributing mechanism remains unknown. (2) Methods: This study uses extracellular vesicles (EVs) secreted by the probiotic Lactobacillus crispatus, commonly found in the vagina, to explore their potential to attenuate placental cells caused by oxidative stress induction. (3) Results: We found that L. crispatus-derived EVs improved Akt phosphorylation and attenuated both cell senescence and death in placental cells caused by oxidative stress induction. In addition, L. crispatus-derived EVs enhanced the resistance to H2O2 induction mediated by increasing mitochondrial fusion. (4) Conclusion: This is the first study to demonstrate that L. crispatus in the vagina can not only regulate the physiological functions of placental cells through the delivery of L. crispatus-EVs but also reduce cell senescence. As cell senescence is related to the occurrence of sPTB, these results indicate that maintaining the population of L. crispatus in the vaginal environment should be an adjuvant treatment strategy to avoid sPTB.
1. Introduction
Preterm birth poses a global challenge with continuously increasing incidence. Spontaneous preterm birth (sPTB) accounts for 11% of all live births worldwide. According to a follow-up survey, the complications caused by sPTB from 1990 to 2010 accounted for 35% of the global neonatal deaths. The number of newborns worldwide is ~3.1 million, this is likely because preterm infants are particularly vulnerable to nosocomial infections in the hostile environment of the neonatal intensive care unit [1,2]. Several factors associated with placental degeneration have been reported [3]. sPTB is a complex multifactorial disease with several known risk factors, such as advanced fertility, low body mass index, black ethnicity, smoking, drinking, and multiple pregnancies [4]. Conclusive explanations for such risk factors have yet to be determined. Clearly, effective strategies for predicting and preventing sPTB need to be investigated further.
It has become clear in recent years that microorganisms reside in nearly every human tissue, including the mammary glands, ovaries, uterus, and placenta. The microorganisms in the female vagina can be divided into (1) good bacteria, (2) bad bacteria, or (3) neutral bacteria. Probiotics and symbiotic bacteria are considered good bacteria. Harmful or pathogenic bacteria are aptly classified as bad bacteria. Neutral bacteria are opportunistic bacteria that change according to the environment to affect the health of the host. However, the roles of these microorganisms in host disease or infection and the regulatory mechanisms involved remain unclear.
Studies have found that the diversity of cervical microbiota in women with Chlamydia trachomatis infection completely differs from that of healthy women and predisposes the affected women to preterm birth. The microbial populations display a transition in microbial taxa away from the Lactobacillus species to anaerobes [5,6]. Having a dominance of Lactobacillus species in the cervical microbiota carries a lower risk of invasion of the amniotic cavity and chorioamnionitis after premature rupture of the membranes. Conversely, a prevalence of Gardnerella and Sneathia increases the probability [7].
The vaginal microbiome resembles that of the cervix and is physiologically dominated by Lactobacillus, but Clostridiales, Bacteroidales, and Actinomycetales are also regularly detected. Differences or shifts in bacterial community between different Lactobacillus strains are detected almost exclusively without any negative impact on pregnancy outcomes [8]. Several studies demonstrated that probiotics in the vagina play a significant role in regulating physiological functions, but the mechanism is still unclear.
Extracellular vesicles (EVs) are membrane-based structures that can carry various types of cellular cargo (lipids, proteins, and nucleic acids). EVs with a diameter ranging from 40 to 200 nm are called exosomes, whereas those with a diameter ranging from 300 to 1000 nm are called microvesicles [9]. Recently, the functional activities of EVs obtained from different species have been reported, including intestinal protection, including bacterial EVs [10]. In addition, several studies also investigated the bio-function of EVs obtained from lactic acid bacteria, the immune stimulation of monocytes treated with EVs isolated from lactic acid bacteria has been reported [11], and protection of Lactobacillus acidophilus-derived EVs against infection caused by Enterococcus faecium in Caenorhabditis elegans and human cells have been investigated [12]. Recently, L. plantarum-derived EVs have been found to regulate gut microbiota and attenuate inflammatory bowel disease [13]. EVs obtained from one species can transmit information and interact with new species. Therefore, communication between microorganisms themselves and between microorganisms and hosts can be achieved through the communication of EVs, the influence of microorganisms on the host and the interaction between microorganisms [14]. In addition, EVs are composed of a lipid bilayer structure and coated with various biological molecules, such as RNA, protein, lipopolysaccharides, peptidoglycan, and toxins. These are also considered to participate in the communication between microorganisms–hosts, microorganisms–microbes, and host–microbes [15]. Our recent study found that Lactobacillus plantarum-derived EVs can inhibit microbial growth and can be used as an antibacterial agent for improving tuna meat quality [16]. Probiotic EVs regulate the microbiota, but their impact on the health of organisms still lacks detailed research. Therefore, this study explored the protection of EVs secreted by Lactobacillus crispatus, common in the vagina, against oxidative stress-induced senescence in 3A-sub-E placental cells.
2. Materials and Methods
2.1. Chemicals
Fetal bovine serum (FBS) was purchased from Life Technologies (Auckland, New Zealand). Dimethyl sulfoxide was obtained from Wako Pure Chemical Industries (Saitama, Japan). Triton X-100, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), trypsin, and sodium dodecyl sulfate (SDS) were purchased from Sigma-Aldrich (St Louis, MO, USA). Dulbecco’s Modified Eagle’s Medium, streptomycin, and penicillin were purchased from HyClone Laboratories (Logan, UT, USA). Lactobacilli MRS broth was purchased from Difco Laboratories (Detroit, MI, USA). Antibodies for Akt (sc-81434), p-Akt (sc-514032), GAPDH (sc-47724), and Mfn-2 (sc-100560) were purchased from Santa Cruz (Santa Cruz, CA, USA). The PKH26 red fluorescent stain reagent was purchased from Merck (Darmstadt, Germany). The MitoTracker Deep-Red FM was purchased from Invitrogen (Carlsbad, CA, USA).
2.2. Culture of L. crispatus
L. crispatus BCRC14618 was obtained from the Bioresource Collection and Research Center (BCRC) (Hsinchu, Taiwan), and it was cultured in MRS broth under anaerobic conditions at 37 °C using an atmosphere generation system (Oxoid, Basingstoke, UK). The number of the lactobacilli was detected on MRS agar plates under anaerobic cultivation.
2.3. Isolation of L. crispatus-Derived EVs and Observation
EVs were isolated from L. crispatus using an ultracentrifugation protocol [16,17]. L. crispatus was cultured and grown to the stationary phase. Then, bacterial cells were removed via centrifugation at 3000× g for 15 min, followed by successive centrifugation at 35,000× g for 60 min to remove large bacterial debris and intact organelles. Subsequently, the supernatant was subjected to ultracentrifugation at 200,000× g for 60 min, and EVs were resuspended in PBS. The sample was then passed through a 0.22-μm filter to obtain pure EVs. The concentration of EVs as well as size distribution was detected via nanoparticles tracking analysis with Nanosight (LM10-HS, Malvern Instruments, Worcestershire, UK).
2.4. Cell Culture and Treatment
Human placental trophoblast 3A-sub-E cells (BCRC 60302) were cultured in 90% minimum essential medium (Eagle) with 0.1-mM non-essential amino acids, 1.0-mM sodium pyruvate, 10% FBS, and antibiotics (100 U/mL of penicillin and 100 μg/mL of streptomycin). The 3A-sub-E cells were induced with oxidative stress by H2O2 (5–500 μM) treatment for 24 h. Finally, cell viability was measured using the MTT assay.
2.5. Western Blot
Cells were lysed in ice-cold lysis buffer containing 20 mM of Tris–HCl (pH 7.4), % of Triton X-100, 0.1% of SDS, 2 mM of EDTA, 10 mM of NaF, 1 mM of phenylmethylsulfonyl fluoride, 500 μM of sodium-vanadate, and 10 g/mL of aprotinin overnight. Next, the cell extract was centrifuged at 12,000× g for 10 min to recover the supernatant. The supernatant was taken as the cell extract. The cell protein was resolved on 10% SDS–PAGE gel and transferred to a polyvinylidene fluoride membrane. The membranes were blocked with 5% non-fat dry milk solution for 1 h and incubated overnight (4 °C) with primary antibodies for 4 h (room temperature) (p-Akt 1:1000; Akt 1:2000; Mfn-2 1:1000; GAPDH 1:2000). Subsequently, the membrane was washed with PBST three times for 5 min each and then shaken in a solution of HRP-linked secondary antibody for 1 h (1:5000) (room temperature). The samples were washed in PBST three more times for 5 min each. Protein expression was detected with enhanced chemiluminescent (ECL) reagent (Millipore, Billerica, MA, USA). Densitometry was quantitated with SPSS Version 17.0 (SPSS Institute, Inc.), and the preparation of analysis spreadsheet by normalized band intensity values for each replicate.
2.6. Mitochondria Stain
The cells were treated with 250 nM of MitoTracker Deep-Red FM (Invitrogen) for 20 min in serum-free culture medium. After washing twice with PBS, the nuclei were stained with Hochest 33342 for 10 min. Mitochondrial morphology was observed under a confocal microscope.
2.7. Fluorescent Stain
L. crispatus-derived EVs were stained with the PKH26 reagent (excited by the 488-nm emission) for 30 min. EVs were collected by ultracentrifugation at 200,000× g for 60 min and then resuspended in PBS. The 3A-sub-E cells were treated with PKH26-labeling EVs for 12 h. These were observed under a confocal microscope.
2.8. Transmission Electron Microscopy (TEM)
EVs (2 µL) were dried onto freshly ‘glow discharged’ 300 mesh formvar/carbon- coated TEM grids (Ted Pella, Redding, CA, USA), negatively stained with aqueous uracyl acetate (2%) and observed under a TEM (JEM-2100F, JEOL, Akishima City, Japan).
2.9. Statistical Analysis
The experimental results were averaged through triplicate analysis. The data were expressed as mean ± standard deviation (SD) and analyzed using the statistical analysis system (SAS Inc., Cary, NC, USA). One-way analysis of variance was conducted using ANOVA procedures. Significant differences between means were determined via Duncan’s multiple range tests. The results were considered statistically significant at p < 0.05.
3. Results
3.1. Damage Induction by Oxidative Stress in 3A-sub-E Placental Cells Treated with H2O2
Oxidative stress is one of the main factors causing cell senescence. It is also a factor that triggers premature birth. We found that exposure to H2O2 can reduce cell survival in 3A-sub-E placental cells from 100% in control group to 78% in group treated with H2O2 at 500 μM (Figure 1) and reduce Akt phosphorylation (Figure 2). A study has indicated an association between oxidative stress and cell degeneration in placenta [2], the inhibition of Akt phosphorylation has been reported to accelerate placental cell degeneration [3]. Our results indicate that oxidative stress could result in cell degeneration.
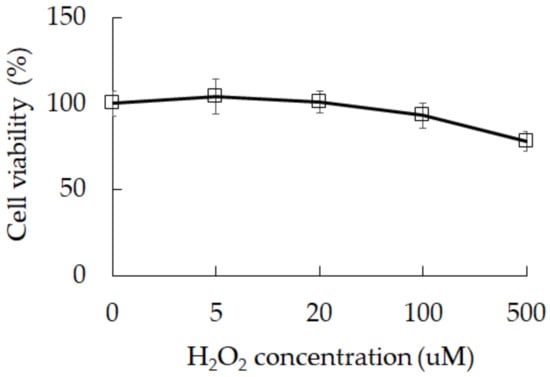
Figure 1.
The cell viability of 3A-sub-E placental cells treated with H2O2 for 24 h. Data were shown as mean ± SD (n = 3).
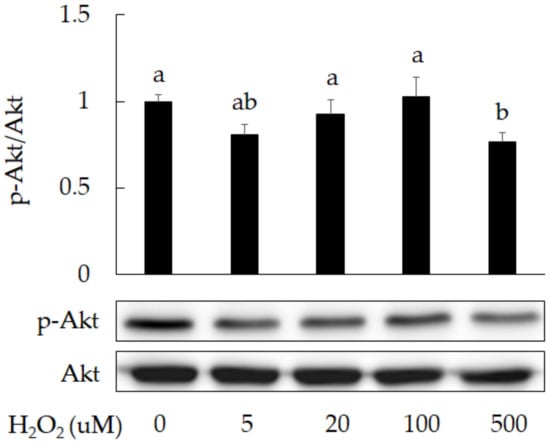
Figure 2.
The reduction of Akt phosphorylation in 3A-sub-E placental cells treated with H2O2 for 24 h. Data were shown as mean ± SD (n = 3). Significant difference (p < 0.05) were indicated by different letters (a, ab, b).
3.2. The Attenuation of Oxidative Stress-Induced in 3A-sub-E Placental Cells Treated by L. crispatus-Derived EVs
The morphology of L. crispatus-derived EVs was observed via transmission electron microscopy (TEM) as presented in Figure 3A. These images indicate that the vesicles were surrounded by a lipid bilayer. The size and production of L. crispatus-derived EVs were evaluated as presented in Figure 3B. The physicochemical characterization of these EVs was performed using a nanoparticle tracking analysis technique. The mean size distribution of L. crispatus-derived EVs was 110.8 ± 3.2 nm after cultivation for 48 h.
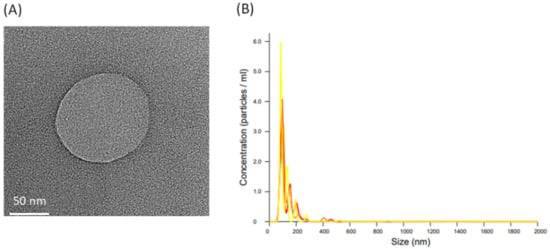
Figure 3.
Characteristics of EVs secreted from L. crispatus. (A) The morphology of L. crispatus-derived EVs was showed via transmission electron microscopy (TEM). (B) The numbers and size distribution of L. crispatus-derived EVs. L. crispatus were incubated for 48 h.
Our results indicate that L. crispatus-derived EVs (1010 particles/mL) can reduce cell death due to oxidative stress in 3A-sub-E placental cells (Figure 4). We further stained EVs with PKH26 (red fluorescent stain) and observed them entering the cells with confocal microscopy. As presented in Figure 5, L. crispatus-derived EVs (1010 particles/mL) entered the 3A-sub-E placental cells after 12 h of treatment. Moreover, L. crispatus-derived EVs (1010 particles/mL) significantly promoted Akt phosphorylation in H2O2-induced 3A-sub-E placental cells after 24 h of treatment (Figure 6). This suggests that L. crispatus-derived EVs attenuated cell senescence and elevated cell survival.
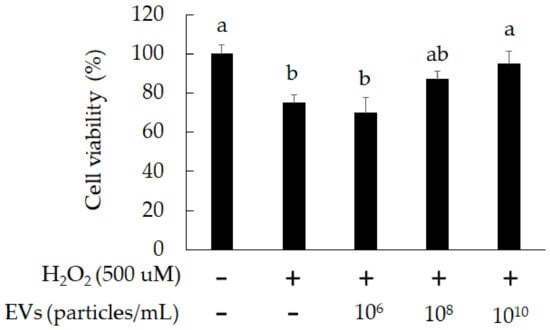
Figure 4.
The attenuation of cell death by L. crispatus-derived EVs in 3A-sub-E placental cells treated with H2O2 for 24 h. Data are expressed as mean ± SD (n = 3). Significant differences (p < 0.05) are indicated by different letters (a, ab, b).
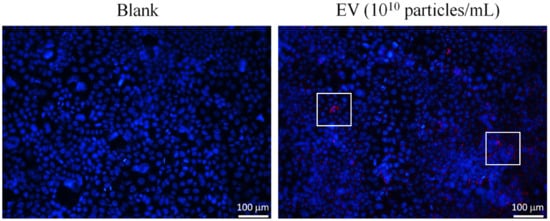
Figure 5.
Observation of L. crispatus-derived EVs (final concentration: 1010 particles/mL) entering 3A-sub-E placental cells after 12 h of treatment viewed via confocal microscopy. Red fluorescent: labelling-EV.
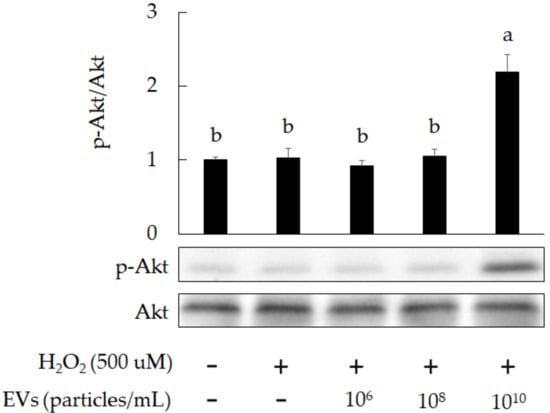
Figure 6.
The promotion of Akt phosphorylation in H2O2-induced 3A-sub-E placental cells after treatment with L. crispatus-derived EVs (final concentration: 1010 particles/mL) for 24 h. Data are expressed as mean ± SD (n = 3). Significant differences (p < 0.05) are indicated by different letters (a,b).
On the other hand, mitofusin-2 (Mfn-2) is a regulator for elevating mitochondrial activity. The results indicated that Mfn-2 expression was increased in H2O2-induced 3A-sub-E placental cells treated with L. crispatus-derived EVs (1010 particles/mL) for 24 h (Figure 7). The mitochondrial fission caused by H2O2 induction was attenuated by treatment with L. crispatus-derived EVs (1010 particles/mL) for 24 h (Figure 8).
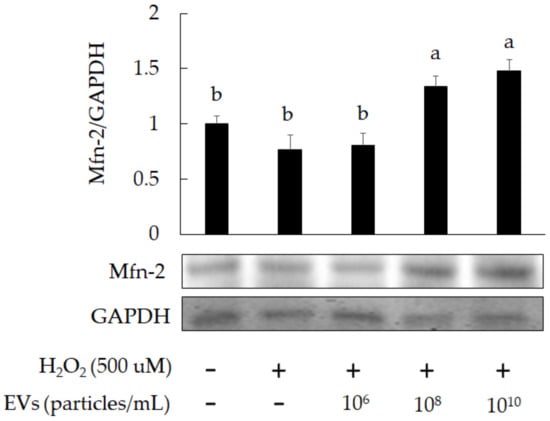
Figure 7.
The promotion of Mfn-2 expression in H2O2-induced 3A-sub-E placental cells after treatment with L. crispatus-derived EVs (final concentration: 1010 particles/mL) for 24 h. Data are expressed as mean ± SD (n = 3). Significant differences (p < 0.05) are indicated by different letters (a,b).
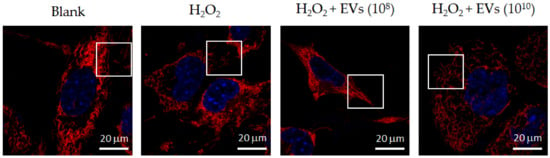
Figure 8.
Inhibition of mitochondrial fission in H2O2-induced 3A-sub-E placental cells by L. crispatus-derived EVs (final concentration: 1010 particles/mL) after treatment for 24 h, observed by MitoTracker Deep-Red staining and confocal microscopy. Mitochondrial morphology as shown in white box.
4. Discussion
There are many bacterial communities in the female vagina (genital tract). These microorganisms play a significant role in the health of newborns in the mother’s body. Indeed, an imbalance of vaginal microbiota is linked to some negative reproductive results, such as sPTB [18]. However, researchers still lack an understanding of the causes and mechanisms of sPTB. Past studies suggest that the “normal” vaginal microbiota in nonpregnant women of childbearing age is mainly composed of lactic acid bacteria. Furthermore, abnormal microbial flora (bacterial vaginosis) is characterized by a reduced content of lactic acid bacteria and overgrowth of anaerobic bacteria, such as Gardnerella vaginalis, Prevotella spp., and Bacteroides spp. in the vaginal environment [19]. Studies have also found that the microbial diversity and abundance of vaginal flora of preterm women (less than 37 weeks of gestation) are more complex compared with women who gave birth at term. In addition, the microorganisms of the Mollicutes significantly increase. Ideally, lactic acid bacteria should be able to maintain the vagina to avoid the growth of other opportunistic microorganisms and reduce the incidence of sPTB [4].
In the vaginal flora, lactic acid bacteria are the main probiotics, with the most prevalent being Lactobacillus crispatus, Lactobacillus gasseri, endogenous Lactobacillus iners, and Lactobacillus jensenii. Other microorganisms include Gardnerella vaginalis or mixed species. Research reports on the vaginal flora of pregnant women indicate that the specific microbial composition in the vagina has a significant impact on the risk of sPTB in certain women [20]. A number of studies have investigated sPTB and the vaginal flora in women of the European race and compared the data with those of women of the African race. These studies found that whether European or African, pregnant women with sPTB exhibit a significantly reduced proportion of Lactobacillus crispatus in the vagina. This shows that vaginal lactic acid bacteria are associated with the risk of sPTB [21]. Urinary tract infections, inflammation, sexual infections, and Trichomonas vaginalis infections caused by microorganisms, and the reproduction of opportunistic microorganisms are all considered to be potential causes of sPTB [22,23]. The lower genital tract to the placenta, fetal membranes, uterine cavity, as well as blood transmission of periodontal pathogens from the oral cavity, have also been cited to explain up to 40–50% of the correlation between sPTB and microbial etiology [24,25]. The above studies demonstrate that controlling vaginal microbes is one approach to prevent and treat sPTB. However, one study challenges the current hypothesis of preterm birth caused by microbial infections. This hypothesis suggests the use of antibiotics to treat vaginal microbiota disorders. However, the lactic acid bacteria in the vagina are also reduced. Furthermore, this antibiotic treatment does not improve pregnancy outcomes of preterm birth in pregnant women [26]. These findings indicate the research value of vaginal lactic acid bacteria toward the development of vaginal environment and the regulation of pregnancy. Lactic acid bacteria-derived EVs have been found to attenuate intestinal inflammation [27], influence immune-regulation [28], and affect antibacterial activity [16]. Relevant research on the regulatory effects of lactic acid bacteria-derived EVs on vaginal cells and the communication with the host is still needed. Therefore, this study investigated the protective effect of L. crispatus-derived EVs on the senescence of placental cells caused by oxidative stress.
Oxidative stress-induced damage and early senescence in preterm placenta have been associated with sPTB [29]. Our results indicate that H2O2 induction results in decreased cell survival and Akt phosphorylation (Figure 1 and Figure 2). The Akt signaling pathway plays a significant role in promoting cell proliferation, which also regulates apoptosis and cell survival [30]. We separated L. crispatus-derived EVs and found the particle sizes to measure around 110 nm (Figure 3). Moreover, L. crispatus-derived EVs recovered Akt phosphorylation to reverse cell death/senescence in placental cells caused by oxidative stress (Figure 4 and Figure 6). These probiotic-derived EVs entered into 3A-sub-E placental cells after 12 h of treatment (Figure 5). ATP production has been reported to stimulate Akt phosphorylation in a PI3K-and Gi protein-dependent manner [31]. The mitochondria also play critical roles in energy production and metabolism. They downregulate senescence and are critically involved in ATP generation [32]. One study reported that mitochondrial fission and fusion can affect a dynamic variation in senescence progress and provide potential targets for age-related disease [33]. Mitochondrial fusion/fission is thought to be crucial for mitochondrial division. Proteins such as Mfn-1, Mfn-2 (Mfn-1/2), and GTPase optic atrophy-1 (Opa-1) govern the mitochondrial membrane fusion between two individual mitochondria. On the contrary, proteins like dynamin-related protein-1 and fission protein-1 control mitochondrial fission. Given that mitochondrial morphology affects energy balance and is continuously changed through fusion and fission events, a tight coordination between mitochondrial dynamics and inter-organelle interactions is crucial [34]. In this study, we found that L. crispatus-derived EVs significantly elevated Mfn-2 levels in H2O2-induced 3A-sub-E placental cells (Figure 7) and promoted mitochondrial fusion (Figure 8). Taken together, L. crispatus-derived EVs protected against oxidative stress-induced senescence in t3A-sub-E placental cells induced by H2O2 as presented in Figure 9.
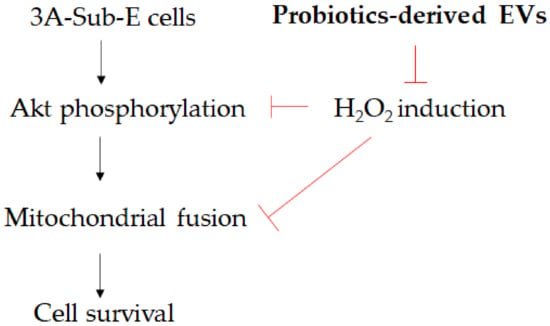
Figure 9.
The potential of L. crispatus-derived EVs to protect against oxidative stress and senescence in t3A-sub-E placental cells exposed to H2O2.
5. Conclusions
The aging of placental cell is the main cause of the development of sPTB. Such an aging is often accompanied by changes in the vaginal flora. Lactic acid bacteria, notably L. crispatus, are common beneficial microorganisms in the vagina that maintain the healthy microbial flora of the vaginal environment. This study found that L. crispatus-derived EVs have a regulatory effect on 3A-sub-E placental cells by reducing the damage of oxidative stress to placental cells by increasing mitochondrial activity and Akt phosphorylation.
Author Contributions
Conceptualization, L.-M.W. and B.-H.L.; methodology, L.-M.W.; software, W.-H.H.; formal analysis, C.-J.T.; investigation, W.-H.H. and C.-J.T.; resources, C.-J.T.; data curation, L.-M.W. and C.-Y.H.; writing—original draft preparation, L.-M.W.; writing—review and editing, W.-H.H.; project administration, C.-J.T. All authors have read and agreed to the published version of the manuscript.
Funding
Acknowledgement for this research work and subsidiary spending were supported by the Taipei Municipal Wan Fang Hospital (111-wf-phd-08) (Taiwan, R.O.C.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Azizia, M.; Lloyd, J.; Allen, M.; Klein, N.; Peebles, D. Immune status in very preterm neonates. Pediatrics 2012, 129, e967–e974. [Google Scholar] [CrossRef] [Green Version]
- Tjoa, M.L.; Cindrova-Davies, T.; Spasic-Boskovic, O.; Bianchi, D.W.; Burton, G.J. Trophoblastic oxidative stress and the release of cell-free feto-placental DNA. Am. J. Pathol. 2006, 169, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Hung, T.H.; Wu, C.P.; Chen, S.F. Differential changes in Akt and AMPK phosphorylation regulating mTOR activity in the placentas of pregnancies complicated by fetal growth restriction and gestational diabetes mellitus with large-for-gestational age infants. Front. Med. 2021, 8, 788969. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Bocking, A.; Hill, J.E.; Money, D.M. Increased richness and diversity of the vaginal microbiota and spontaneous preterm birth. Microbiome 2018, 6, 117. [Google Scholar] [CrossRef]
- Andrews, W.W.; Goldenberg, R.L.; Mercer, B.; Iams, J.; Meis, P.; Moawad, A.; Das, A.; Vandorsten, J.P.; Caritis, S.N.; Thurnau, G.; et al. The preterm prediction study: Association of second-trimester genitourinary chlamydia infection with subsequent spontaneous preterm birth. Am. J. Obstet. Gynecol. 2000, 183, 662–668. [Google Scholar] [CrossRef]
- Filardo, S.; Di Pietro, M.; Porpora, M.G.; Recine, N.; Farcomeni, A.; Latino, M.A.; Sessa, R. Diversity of cervical microbiota in asymptomatic chlamydia trachomatis genital infection: A pilot study. Front. Cell. Infect. Microbiol. 2017, 7, 321. [Google Scholar] [CrossRef]
- Kacerovsky, M.; Vrbacky, F.; Kutova, R.; Pliskova, L.; Andrys, C.; Musilova, I.; Menon, R.; Lamont, R.; Nekvindova, J. Cervical microbiota in women with preterm prelabor rupture of membranes. PLoS ONE 2015, 10, e0126884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staude, B.; Oehmke, F.; Lauer, T.; Behnke, J.; Gopel, W.; Schloter, M.; Schulz, H.; Krauss-Etschmann, S.; Ehrhardt, H. The microbiome and preterm birth: A change in paradigm with profound implications for pathophysiologic concepts and novel therapeutic strategies. BioMed Res. Int. 2018, 2018, 7218187. [Google Scholar] [CrossRef]
- Johnstone, R.M. Revisiting the road to the discovery of exosomes. Blood Cells Mol. Dis. 2005, 34, 214–219. [Google Scholar] [CrossRef]
- Liao, Y.; Du, X.; Lonnerdal, B. Human milk exosomes and their microRNAs survive digestion in vitro and are taken up by human intestinal cells. Mol. Nutr. Food Res. 2017, 2017, 1700082. [Google Scholar] [CrossRef] [PubMed]
- Forsberg, M.M.; Bjorkander, S.; Pang, Y.; Lundqvist, L.; Ndi, M.; Ott, M.; Escriba, I.B.; Jaeger, M.C.; Roos, S.; Sverremark-Ekstrom, E. Extracellular membrane vesicles from Lactobacilli dampen IFN-γ responses in a moocyte-dependent manner. Sci. Rep. 2019, 9, 17109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; Lee, K.; Hsu, M.; Nau, G.; Mylonakis, E.; Ramratnam, B. Lactobacillus-derived extracellular vesicles enhance hos immune responses against vancomycin-resistant enterococci. BMC Microbiol. 2017, 17, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hao, H.; Zhang, X.; Tong, L.; Liu, Q.; Liang, X.; Bu, Y.; Gong, P.; Liu, T.; Zhang, L.; Xia, Y.; et al. Effect of extracellular vesicles derived from Lactobacillus plantarum Q7 on gut microbiota and ulcerative colitis in mice. Front. Immunol. 2021, 12, 777147. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lee, J.; Park, J.; Gho, Y.S. Gram-negative and Gram-positive bacterial extracellular vesicles. Semin. Cell Dev. Biol. 2015, 40, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Bitto, N.J.; Kaparakis-Liaskos, M. The therapeutic benefit of bacterial membrane vesicles. Int. J. Mol. Sci. 2017, 18, 1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, B.H.; Wu, S.C.; Shen, T.L.; Hsu, Y.Y.; Chen, C.H.; Hsu, W.H. The applications of Lactobacillus plantarum-derived extracellular vesicles as a novel natural antibacterial agent for improving quality and safety in tuna meat. Food Chem. 2021, 340, 128104. [Google Scholar] [CrossRef]
- Yu, S.; Zhao, Z.; Sun, L.; Li, P. Fermentation results in quantitative changes in milk-derived exosomes and different effects on cell growth and survival. J. Agric. Food Chem. 2017, 65, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Hill, G.B. The microbiology of bacterial vaginosis. Am. J. Obstet. Gynecol. 1993, 169, 450–454. [Google Scholar] [CrossRef]
- Kindinger, L.M.; Bennett, P.B.; Lee, Y.S.; Marchesi, J.R.; Smith, A.; Cacciatore, S.; Holmes, E.; Nicholson, J.K.; Teoh, T.G.; Maclntyre, D.A. The interaction between vaginal microbiota, cervical length, and vaginal progesterone treatment for preterm birth risk. Microbiome 2017, 5, 6. [Google Scholar] [CrossRef] [Green Version]
- Callahan, B.J.; Daniel, B.; Daniela, S.; Goltsman, A.; Sun, C.L.; Costello, E.K.; Jeganathan, P.; Biggio, J.R.; Wong, R.J.; Druzin, M.L.; et al. Replication and refinement of a vaginal microbial signature of preterm birth in two racially distinct cohorts of US women. Proc. Natl. Acad. Sci. USA 2017, 114, 9966–9971. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, R.L.; Culhane, J.R.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Donders, G.G.; Van Calsteren, K.; Bellen, G.; Reybrouck, R.; Van den Bosch, T.; Riphagen, I.; Van Lierde, S. Predictive value for preterm birth of abnormal vaginal fora, bacterial vaginosis and aerobic vaginitis during the first trimester of pregnancy. Br. J. Obstet. Gynecol. 2009, 116, 1315–1324. [Google Scholar] [CrossRef]
- Lamont, R.F. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. Br. J. Obstet. Gynecol. 2003, 110, 71–75. [Google Scholar] [CrossRef]
- Lockwood, C.J. Predicting premature delivery—No easy task. N. Engl. J. Med. 2002, 346, 282–284. [Google Scholar] [CrossRef]
- Bostwick, D.G.; Woody, J.; Hunt, C.; Budd, W. Antimicrobial resistance genes and modelling of treatment failure in bacterial vaginosis: Clinical study of 289 symptomatic women. J. Med. Microbiol. 2016, 65, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Moon, C.M.; Shin, T.S.; Kim, E.K.; McDowell, A.; Jo, M.K.; Joo, Y.H.; Kim, S.E.; Jung, H.K.; Shim, K.N.; et al. Lactobacillus-paracasei-derived extracellular vesicles attenuate the intestinal inflammatory response by augmenting the endoplasmic reticulum stress pathway. Exp. Mol. Med. 2020, 52, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Choi, S.J.; Choi, H.I.; Choi, J.P.; Park, H.K.; Kim, E.K.; Kim, M.J.; Moon, B.S.; Min, T.K.; Rho, M.; et al. Lactobacillus plantarum-derived extracellular vesicles protect atopicdermatitis induced by Staphylococcus aureus-derived extracellular vesicles. Allergy Asthma Immunol. Res. 2018, 10, 516–532. [Google Scholar] [CrossRef] [PubMed]
- Saroyo, Y.B.; Wibowo, N.; Irwinda, R.; Prijanti, A.R.; Yunihastuti, E.; Bardosono, S.; Krisnadi, S.R.; Permata, P.I.; Wijaya, S.; Andika Santawi, V.P. Oxidative stress induced damage and early senescence in preterm placenta. J. Pregnancy 2021, 2021, 9923761. [Google Scholar] [CrossRef] [PubMed]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerasimovskaya, E.V.; Tucker, D.A.; Weiser-Evans, M.; Wenzlau, J.M.; Klemm, D.J.; Banks, M.; Stenmark, K.R. Extracellular ATP-induced proliferation of adventitial fibroblasts requires phosphoinositide 3-kinase, Akt, mammalian target of rapamycin, and p70 S6 kinase signaling pathways. J. Biol. Chem. 2005, 280, 1838–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh-Choudhary, S.K.; Liu, J.; Finkel, T. The role of mitochondrial in cellular senescence. FASEB J. 2021, 35, e21991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Mcintyre, R.L.; Janssens, G.E.; Houtkooper, R.H. Mitochondrial fission and fusion: A dynamic role in aging and potential target for age-related disease. Mech. Aging Dev. 2020, 186, 111212. [Google Scholar] [CrossRef]
- Hsu, W.H.; Lee, B.H.; Pan, T.M. Leptin-induced mitochondrial fusion mediates hepatic lipid accumulation. Int. J. Obes. 2015, 39, 1750–1756. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).