Use of Kombucha SCOBY and Commercial Yeast as Inoculum for the Elaboration of Novel Beer
Abstract
1. Introduction
2. Material and Methods
2.1. Kombucha Preparation
2.2. Inoculum Preparation
2.3. Wort Production and Fermentation
2.4. Analysis during Fermentation and Produced Beers
2.4.1. Enumeration of Yeasts and Bacteria
2.4.2. Analysis of Carbohydrates, Organic Acids and Ethanol
2.4.3. Analysis of Volatile Compounds by GC-MS
2.4.4. Total Phenolics
2.4.5. Antioxidant Capacity Determined by the 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Test
2.4.6. Color Analysis
2.4.7. Bitterness Analysis
2.5. Statistical Analysis
3. Results and Discussion
3.1. pH and Density
3.2. Microbial Population
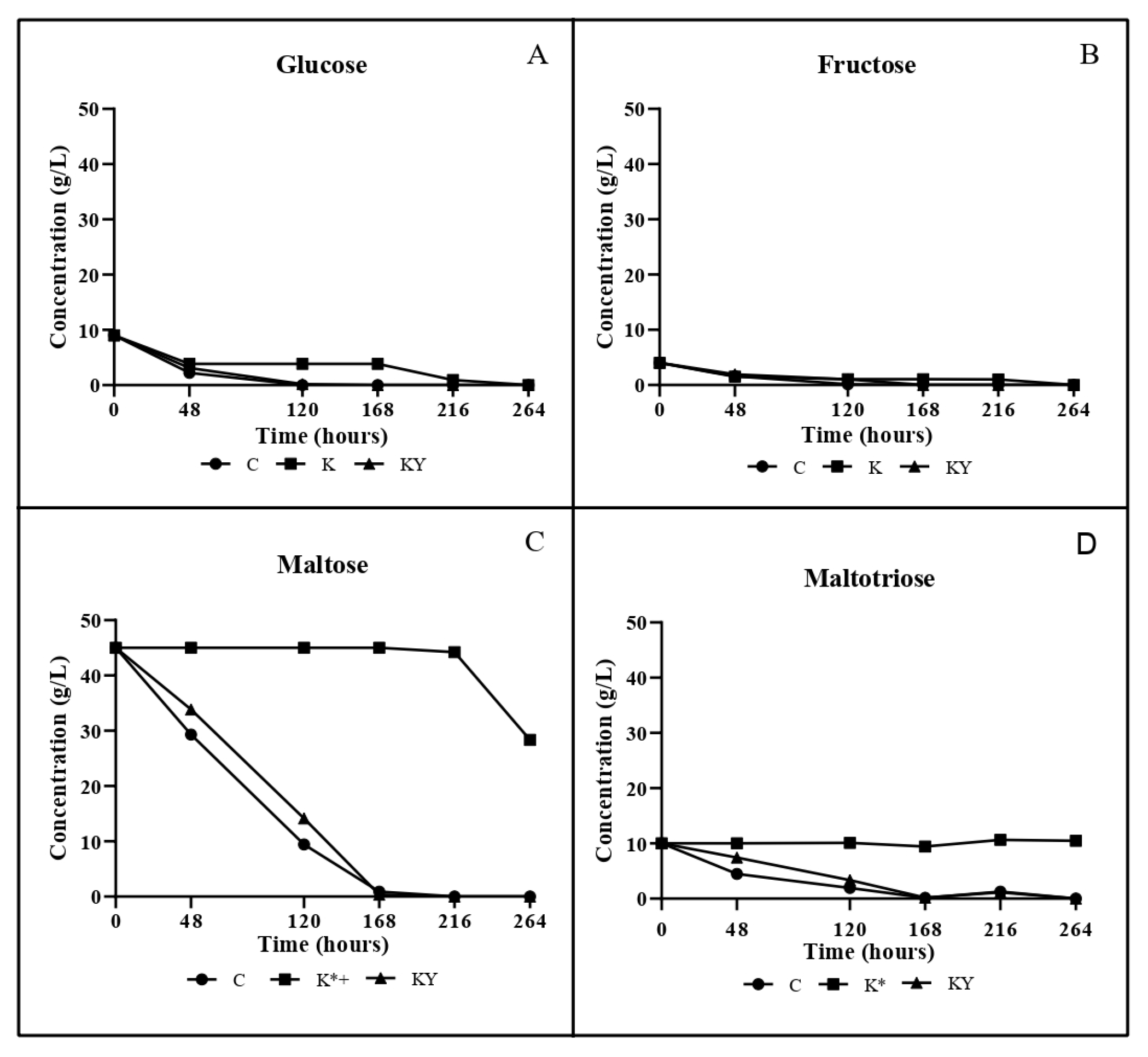
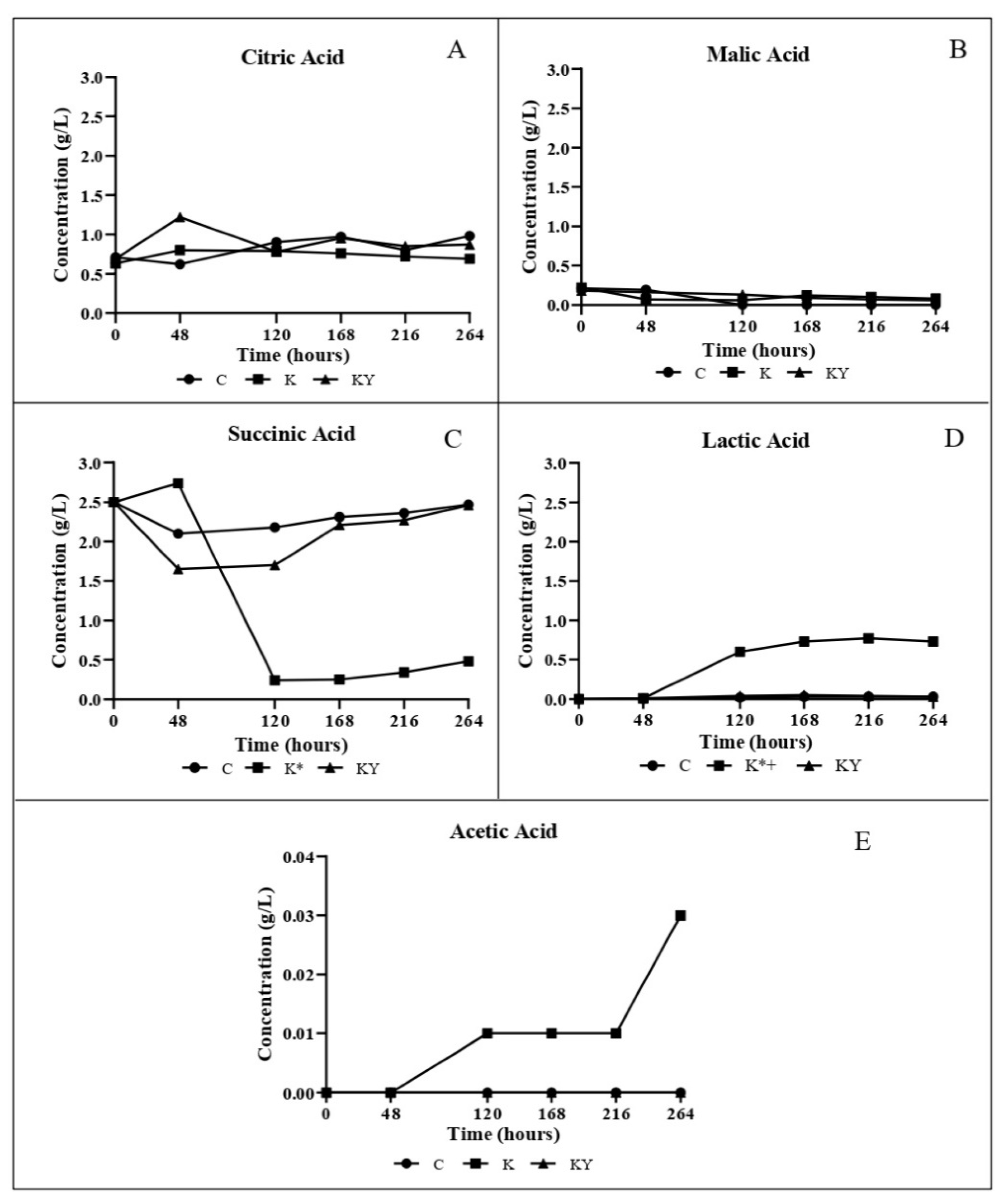
3.3. Carbohydrates, Ethanol and Organic Acids Analyses
3.4. Analysis of Volatile Compounds by GC-MS
3.5. Total Phenolic Compounds and Antioxidant Activity of the Produced Beers
3.6. Color and Bitterness of the Produced Beers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Instrução Normativa nº 41, de 17 de Setembro de 2019. Estabelece o Padrão de Identidade e Qualidade da Kombucha em todo o Território Nacional. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/instrucao-normativa-no-41-de-17-de-setembro-de-2019.pdf/view (accessed on 21 November 2022).
- Jayabalan, R.; Marimuthu, S.; Swaminathan, K. Changes in the content of organic acids and polyphenols of tea during fermentation of kombucha tea. Food Chem. 2007, 102, 392–398. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the Fermented Tea: Microbiology, Composition, and Claimed Health Effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, D.; Bhattacharya, W.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2019, 220, 63–72. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbaša, R.V.; Lončar, E.S.; Vitas, J.S.; Sathishkumar, M. A review on kombucha tea—Microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef]
- Ayed, L.; Ben Abid, S.; Hamdi, M. Development of a drink from red grape juice fermented with kombucha consortium. Ann. Microbiol. 2016, 67, 111–121. [Google Scholar] [CrossRef]
- Malbaša, R.V.; Milanović, S.; Lončar, E.S.; Djurić, M.; Carić, M.Đ.; Iličić, M.; Kolarov, L. Milk-based beverages obtained by Kombucha application. Food Chem. 2009, 112, 178–184. [Google Scholar] [CrossRef]
- Tu, C.; Tang, S.; Azi, F.; Hu, W.; Dong, M. Use of kombucha consortium to transform soy whey into a novel functional beverage. J. Funct. Foods. 2019, 52, 81–89. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Health, wellness, and safety aspects of the consumption of kombucha. J. Chem. 2015, 11, 591869. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Waisundara, V.Y. Improvement of the functional properties of coffee by fermentation by “tea fungus” (kombucha). J. Food Process. Preserv. 2015, 39, 2596–2603. [Google Scholar] [CrossRef]
- Thesseling, F.A.; Bircham, P.W.; Mertens, S.; Voordeckers, K.; Verstrepen, K.J. A hands-on guide to brewing and analyzing beer in the laboratory. Curr. Protoc. Microbiol. 2019, 54, 1–32. [Google Scholar] [CrossRef]
- Instrução Normativa nº 65, de 10 de Dezembro de 2019. Estabelece os Padrões de Identidade e Qualidade para os Produtos de Cervejaria. Available online: https://www.gov.br/agricultura/pt-br/assuntos/inspecao/produtos-vegetal/legislacao-1/biblioteca-de-normas-vinhos-e-bebidas/instrucao-normativa-no-65-de-10-de-dezembro-de-2019.pdf. (accessed on 21 November 2022).
- Capece, A.; Romaniello, R.; Siestoe, G.; Romano, P. Review conventional and non-conventional yeasts in beer production. Ferment. 2018, 4, 38. [Google Scholar] [CrossRef]
- Larroque, M.N.; Carrau, F.; Farina, L.; Boido, E.; Dellacassa, E.; Medina, K. Effect of Saccharomyces and non-Saccharomyces native yeasts on beer aroma compounds. Int. J. Food Microbiol. 2021, 337, 108953. [Google Scholar] [CrossRef] [PubMed]
- Callejo, M.J.; García Navas, J.J.; Alba, R.; Escott, C.; Loira, I.; González, M.C.; Morata, A. Wort fermentation and beer conditioning with selected non-Saccharomyces yeasts in craft beers. Eur. Food Res. Technol. 2019, 245, 1229–1238. [Google Scholar] [CrossRef]
- Bossaert, S.; Winne, V.; Van Opstaele, F.; Buyse, J.; Verreth, C.; Herrera-Malaver, B.; Van Geel, M.; Verstrepen, K.J.; Crauwels, S.; De Rouck, G.; et al. Description of the temporal dynamics in microbial community composition and beer chemistry in sour beer production via barrel ageing of finished beers. Int. J. Food Microbiol. 2021, 339, 109030. [Google Scholar] [CrossRef]
- Sievers, M.; Lanini, C.; Weber, A.; Schuler-Schmid, U.; Teuber, M. Microbiology and fermentation balance in a kombucha beverage obtained from a tea fungus fermentation. Syst. Appl. Microbiol. 1995, 18, 590–594. [Google Scholar] [CrossRef]
- White, C.; Zainasheff, J. Yeast: The Practical Guide to Beer Fermentation, 1st ed.; Brewers Publications: Boulder, CO, USA, 2010; pp. 1–304. [Google Scholar]
- Almeida, E.G.; Rachid, C.C.T.C.; Schwan, R.F. Microbial population present in fermented beverage cauim produced by Brazilian Amerindians. Int. J. Food Microbiol. 2007, 120, 146–151. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, R.D.; Oliveira, J.M.; Teixeira, J.A.; Almeida E Silva, J.B.; Schwan, R.F. Characterization of different fruit wines made from cacao, cupuassu, gabiroba, jabuticaba and umbu. LWT-Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Zhang, J.; Van Mullem, J.; Dias, D.R.; Schwan, R.F. The chemistry and sensory characteristics of new herbal tea-based kombuchas. J. Food Sci. 2021, 86, 740–748. [Google Scholar] [CrossRef]
- Swain, T.; Hills, W.E. The phenolic constituents of Prunus domestica. I.—The quantitative analysis of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Gibson, B.R.; Lawrence, S.J.; Leslaire, J.P.; Powdel, C.D.; Smart, K.A. Yeast responses to stresses associated with industrial brewery handling. FEMS Microbiol. Rev. 2007, 31, 535–569. [Google Scholar] [CrossRef] [PubMed]
- Doretto, D.A.; Figueira, R.; Sartori, M.M.P.; Filho, W.G.V. Análise físico-química e sensorial de cervejas comerciais brasileiras. Rev. Energia Agric. 2018, 33, 277–283. [Google Scholar]
- Beer Judge Certification Program. Style Guidelines: Beer Style Guidelines. Available online: https://www.bjcp.org/ (accessed on 21 November 2022).
- Parâmetros Cervejeiros: Quais Você Deve Acompanhar? Available online: https://consultoriamult.com.br/blog/parametros-cervejeiros/ (accessed on 21 November 2022).
- Zastrow, C.R.; Mattos, A.M.; Hollatz, C.; Stambuk, B.U. Maltotriose metabolism by Saccharomyces cerevisiae. Biotechnol. Lett. 2000, 22, 455–459. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 142. [Google Scholar] [CrossRef]
- Venturini Filho, W.G. Bebidas Alcoólicas: Ciência e Tecnologia, 2nd ed.; Blucher: São Paulo, Brasil, 2016; Volume 1, pp. 15–48. [Google Scholar]
- Bellut, K.; Michel, M.; Zarnkow, M.; Hutzler, M.; Jacob, F.; Schutter, D.; Daenen, L.; Lynch, K.; Zannini, E.; Arendt, E. Application of non-saccharomyces yeasts isolated from kombucha in the production of alcohol-free beer. Fermentation 2018, 4, 66. [Google Scholar] [CrossRef]
- Toh, D.W.K.; Chua, J.Y.; Lu, Y.; Liu, S.Q. Evaluation of the potential of commercial non-Saccharomyces yeast strains of Torulaspora delbrueckii and Lachancea thermotolerans in beer fermentation. Int. J. Food Sci. 2019, 55, 2049–2059. [Google Scholar] [CrossRef]
- Rodrigues, J.E.A.; Ernyb, G.L.; Barros, A.S.; Esteves, V.I.; Brandão, T.; Ferreira, A.A.; Cabrita, E.; Gil, A.M. Quantification of organic acids in beer by nuclear magnetic resonance (NMR)-based methods. Anal. Chim. Acta. 2010, 674, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Coton, M.; Pawtowski, A.; Taminiau, B.; Burgaud, G.; Deniel, F.; Coulloumme-Labarthe, L.; Coton, E. Unraveling microbial ecology of industrial-scale kombucha fermentations by metabarcoding and culture-based methods. FEMS Microbiol. Ecol. 2017, 93, fix048. [Google Scholar] [CrossRef]
- Marsh, A.J.; O’Sullivan, O.; Hill, C.; Ross, R.P.; Cotter, P.D. Sequence-based analysis of the bacterial and fungal compositions of multiple kombucha (tea fungus) samples. Food Microbiol. 2014, 38, 171–178. [Google Scholar] [CrossRef]
- Chidi, B.S.; Bauer, F.F.; Rossouw, D. Organic acid metabolism and the impact of fermentation practices on wine organic acid metabolism and the impact of fermen-tation practices on wine acidity: A review. S. Afr. J. Enol. Vitic. 2018, 39, 315–329. [Google Scholar]
- Aung, T.; Eun, J.B. Production and characterization of a novel beverage from laver (Porphyra dentata) through fermentation with kombucha consortium. Food Chem. 2021, 350, 129274. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Sotoa, S.A.; Beauforta, S.; Bouajilaa, J.; Soucharda, J.P.; Renardc, T.; Rollanc, S.; Taillandiera, P. Impact of fermentation conditions on the production of bioactive compounds with anticancer, anti-inflammatory and antioxidant properties in kombucha tea extracts. Process Biochem. 2019, 83, 44–54. [Google Scholar] [CrossRef]
- Villarreal-Soto, S.A.; Bouajila, J.; Pace, M.; Leech, J.; Paul, D.; Souchard, C.J.P.; Taillandier, P.; Beaufort, S. Metabolic microbiome signatures in the fermented beverage, kombucha. Int. J. Food Microbiol. 2020, 333, 108778. [Google Scholar] [CrossRef]
- Holt, S.; Mukherjee, V.; Lievens, B.; Verstrepen, K.J.; Thevelein, J.M. Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol. 2018, 72, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Pires, E.J.; Teixeira, J.A.; Brányik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma—A review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef]
- Verstrepen, K.J.; Derdelinckx, G.; Dufour, J.P.; Winderickx, J.; Thevelein, J.M.; Pretorius, I.S.; Delvaux, F.R. REVIEW Flavor-Active Esters: Adding Fruitiness to Beer. J. Biosci. Bioeng. 2003, 93, 110–118. [Google Scholar] [CrossRef]
- Etschmann, M.; Huth, I.; Walisko, R.; Schuster, J.; Krull, R.; Holtmann, D.; Schrader, J. Improving 2-phenylethanol and 6-pentyl-α-pyrone production with fungi by microparticle-enhanced cultivation (MPEC). Yeast 2015, 32, 145–157. [Google Scholar] [CrossRef]
- Chan, M.Z.A.; Chua, J.Y.; Toh, M.; Liu, S.Q. Survival of probiotic strain Lactobacillus paracasei L26 during co-fermentation with S. cerevisiae for the development of a novel beer beverage. Food Microbiol. 2019, 82, 541–550. [Google Scholar] [CrossRef]
- Granato, T.M.; Romano, D.; Vigentini, I.; Foschino, R.C.; Monti, D.; Mamone, G.; Ferranti, P.; Nitride, C.; Iametti, S.; Bonomi, F.; et al. New insights on the features of the vinyl phenol reductase from the wine-spoilage yeast Dekkera/Brettanomyces bruxellensis. Ann. Microbiol. 2014, 65, 321–329. [Google Scholar] [CrossRef]
- Durello, R.S.; Silva, L.M.; Bogusz Jr, S. Hop Chemistry. Quím. Nova. 2019, 42, 900–919. [Google Scholar]
- Nance, M.R.; Setzer, W.N. Volatile components of aroma hops (Humulus lupulus L.) commonly used in beer brewing. J. Brew. Distill. 2011, 2, 16–22. [Google Scholar]
- Sun, T.Y.; Li, J.S.; Chen, C. Effects of blending wheatgrass juice on enhancing phenolic compounds and antioxidant activities of traditional kombucha beverage. J. Food Drug Anal. 2015, 23, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Ivanišová, E.; Meňhartová, K.; Terentjeva, M.; Godočíková, L.; Árvay, J.; Kačániová, M. Kombucha tea beverage: Microbiological characteristic, antioxidant activity, and phytochemical composition. Acta Aliment. 2019, 48, 324–331. [Google Scholar] [CrossRef]
- Watawana, M.I.; Jayawardena, N.; Gunawardhana, C.B.; Waisundara, V.Y. Enhancement of the antioxidant and starch hydrolase inhibitory activities of king coconut water (Cocos nucifera var. aurantiaca) by fermentation with kombucha ‘tea fungus’. Int. J. Food Sci. 2016, 51, 490–498. [Google Scholar]
- Koren, D.; Hegyesné Vecseri, B.; Kun-Farkas, G.; Urbin, A.; Nyitrai, A.; Sipos, L. How to objectively determine the color of beer? J. Food Sci. Tecnol. 2020, 57, 1183–1189. [Google Scholar] [CrossRef] [PubMed]

| Assays | Commercial Yeast (mL) | Kombucha (mL) | SCOBY (g) |
|---|---|---|---|
| Control (C) | 16.40 | 0 | 0 |
| Kombucha + yeast (KY) | 8.20 | 5 | 6 |
| Kombucha (K) | 0 | 10 | 12 |
| Population (CFU/mL) | ||
|---|---|---|
| Yeast | Initial Time | Final Time |
| Control (C) | 1.12 × 109 ± 1.24 | 2.25 × 1010 ± 1.62 |
| Kombucha (K) | 7.03 × 108 ± 3.29 | 3.00 × 1010 ± 2.09 |
| Kombucha + yeast (KY) | 6.50 × 108 ± 2.69 | 2.00 × 1010 ± 2.77 |
| Bacteria | ||
| Control (C) | ND | ND |
| Kombucha (K) | 7.40 × 109 ± 3.68 | 1.01 × 1010 ± 1.41 |
| Kombucha + yeast (KY) | 1.66 × 1010 ± 9.33 | 1.60 × 1010 ± 1.69 |
| C | KY | K | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Peak Area × 104 | |||||||||
| 0 h | 264 h | FM | 0 h | 264 h | FM | 0 h | 264 h | FM | |
| Acids | |||||||||
| Heptanoic acid | 2.6 | 1.8 | 2.1 | 3.2 | 1.4 | 1.4 | 1.1 | 1.4 | 2.2 |
| Nonanoic acid | 5.0 | 1.8 | 2.1 | 5.4 | 1.9 | 1.1 | 1.5 | 1.7 | 2.5 |
| n-Decanoic acid | 3.7 | 38.7 | 15.6 | 3.8 | 13.7 | 11.5 | 0.8 | 29.2 | 37.8 |
| 9-Decenoic acid | 0.9 | 1.4 | 4.1 | 1.6 | ND | 2.2 | ND | 0.4 | 5.8 |
| Dodecanoic acid | 2.3 | 5.6 | 4.5 | 2.3 | 3.2 | 5.0 | 0.3 | 9.6 | 11.3 |
| Tetradecanoic acid | 4.0 | 1.9 | 2.0 | 4.9 | 2.9 | 1.8 | 0.8 | 2.2 | 3.0 |
| Pentadecanoic acid | 0.7 | 0.4 | 0.7 | 0.8 | 0.5 | 0.2 | ND | 0.3 | 0.7 |
| n-Hexadecanoic acid | 49.5 | 12.4 | 12.3 | 45.5 | 19.7 | 7.3 | 6.6 | 9.9 | 16.9 |
| Octadecanoic acid | 1.7 | 0.7 | 0.6 | 1.7 | 0.7 | 0.6 | 1.0 | 0.8 | 0.6 |
| Hexanoic acid | 9.0 | 10.2 | 12.0 | 11.3 | 4.5 | 5.4 | 4.3 | 28.4 | 26.3 |
| Octanoic acid | 5.8 | 217.2 | 180.2 | 6.8 | 156.5 | 152.0 | 1.9 | 115.7 | 182.0 |
| Alcohols | |||||||||
| 1-Octanol | 580.9 | 41.8 | 53.5 | 1615.3 | 480.0 | 18.2 | 148.3 | 319.1 | 1155.2 |
| 1-Nonanol | 2.8 | 5.7 | 3.6 | 2.4 | 2.2 | 4.5 | 2.8 | 2.2 | 8.8 |
| L-.Alpha.-terpineol | 2.4 | 0.4 | 0.6 | 3.1 | 0.9 | 0.8 | 2.0 | 0.7 | 0.6 |
| 2-PhenyIethanol | 17.9 | 875.5 | 1049.0 | 21.5 | 626.8 | 662.5 | 13.1 | 533.2 | 672.3 |
| 1-Dodecanol | 2.6 | 2.7 | 2.9 | 2.5 | 4.9 | 4.4 | 0.6 | 4.2 | 6.0 |
| 1-Hexadecanol | ND | 1.4 | 1.3 | ND | 0.5 | 1.4 | ND | 2.5 | 4.5 |
| Benzyl alcohol | 1.3 | 0.8 | 0.9 | 1.1 | 0.8 | 0.9 | 2.0 | 0.6 | 0.7 |
| 1-Tetradecanol | ND | 2.7 | 4.4 | ND | 2.5 | 4.6 | ND | 2.4 | 3.2 |
| Esters | |||||||||
| Ethyl succinate | ND | ND | ND | ND | ND | ND | ND | 61.6 | 98.5 |
| 2-Phenylethyl acetate | ND | 112.7 | 97.4 | ND | 93.4 | 64.0 | ND | 23.7 | 21.5 |
| Ethyl decanoate | ND | 263.5 | 36.4 | ND | 237.2 | 66.5 | ND | 298.9 | 199.4 |
| Ethyl octadec-9-enoate | ND | 1.7 | 1.1 | ND | 0.3 | 1.9 | ND | 0.9 | 5.5 |
| Ethyl isopentyl succinate | ND | ND | ND | ND | ND | ND | ND | 5.2 | 6.4 |
| Ethyl pentadecanoate | ND | 0.6 | 0.6 | ND | 0.05 | 1.6 | ND | 16.4 | 5.8 |
| Ethyl hexadecanoate | 2.1 | 88.9 | 40.4 | 3.3 | 19.2 | 79.9 | 1.9 | 46.2 | 65.4 |
| Ethyl tetradecanoate | ND | 24.4 | 11.3 | ND | 5.2 | 23.6 | ND | 133.2 | 106.4 |
| Ethyl cinnamate | ND | 0.8 | 0.8 | ND | 0.7 | 0.9 | ND | 0.6 | 1.4 |
| Phenethyl isovalerate | ND | 3.1 | 3.2 | ND | ND | ND | ND | ND | ND |
| Ethyl octanoate | ND | 7.7 | 6.9 | ND | 2.3 | 11.4 | ND | 27.3 | 45.0 |
| Methyl 9,12-Octadecadienoate | 1.1 | 10.4 | 6.4 | 1.5 | 2.7 | 14.2 | 1.3 | 52.7 | 61.4 |
| Phenols | |||||||||
| 4-Ethyl-2-methoxy-phenol | 0.2 | 1.4 | 3.6 | 1.3 | 22.8 | 34.4 | 0.7 | 310.9 | 311.9 |
| Terpenes | |||||||||
| Citronellol | ND | 11.5 | 12.9 | ND | 12.0 | 13.0 | ND | 12.8 | 15.8 |
| Geraniol | 69.7 | 20.9 | 20.0 | 74.5 | 35.5 | 26.3 | 37.5 | 40.6 | 31.8 |
| Geranyl acetate | 5.6 | ND | ND | 4.3 | ND | ND | 3.6 | ND | ND |
| Humulene | 14.4 | 58.1 | 5.0 | 23.7 | 23.9 | 6.3 | 7.8 | 75.8 | 3.7 |
| Caryophyllene oxide | 5.9 | ND | ND | 4.6 | ND | ND | 2.9 | ND | ND |
| Analysis | Control (C) | Kombucha (K) | Kombucha + Yeast (KY) |
|---|---|---|---|
| Total phenolic compounds (mg/100 mL) | 31.64 ± 1.40 | 37.57 ± 1.59 | 33 ± 1.80 |
| DPPH (% inhibition) | 63.00 ± 6.46 | 69.04 ± 5.86 | 65.1 ± 4.79 |
| L* | 87.92 ± 0.21 | 82.56 ± 6.55 | 86.16 ± 1.79 |
| a* | 0.4 ± 0 | 1.82 ± 0.70 | 1.46 ± 1.03 |
| b* | 21.31 ± 1.07 | 23.88 ± 2.28 | 19.87 ± 0.08 |
| Bitterness (IBU %) | 27 | 27 | 27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, M.M.; de Souza, A.C.; Faria, E.R.; Molina, G.; de Andrade Neves, N.; Morais, H.A.; Dias, D.R.; Schwan, R.F.; Ramos, C.L. Use of Kombucha SCOBY and Commercial Yeast as Inoculum for the Elaboration of Novel Beer. Fermentation 2022, 8, 748. https://doi.org/10.3390/fermentation8120748
da Silva MM, de Souza AC, Faria ER, Molina G, de Andrade Neves N, Morais HA, Dias DR, Schwan RF, Ramos CL. Use of Kombucha SCOBY and Commercial Yeast as Inoculum for the Elaboration of Novel Beer. Fermentation. 2022; 8(12):748. https://doi.org/10.3390/fermentation8120748
Chicago/Turabian Styleda Silva, Mariana Muniz, Angélica Cristina de Souza, Emanuel Roberto Faria, Gustavo Molina, Nathalia de Andrade Neves, Harriman Aley Morais, Disney Ribeiro Dias, Rosane Freitas Schwan, and Cíntia Lacerda Ramos. 2022. "Use of Kombucha SCOBY and Commercial Yeast as Inoculum for the Elaboration of Novel Beer" Fermentation 8, no. 12: 748. https://doi.org/10.3390/fermentation8120748
APA Styleda Silva, M. M., de Souza, A. C., Faria, E. R., Molina, G., de Andrade Neves, N., Morais, H. A., Dias, D. R., Schwan, R. F., & Ramos, C. L. (2022). Use of Kombucha SCOBY and Commercial Yeast as Inoculum for the Elaboration of Novel Beer. Fermentation, 8(12), 748. https://doi.org/10.3390/fermentation8120748





